Abstract
In the transitional region between agriculture and livestock rearing in northern China, planting forage crops in rows among fruit trees as feed in orchards represents an effective strategy for enhancing the ecological environment while addressing the increasing demand for livestock feed. Nonetheless, the impact of short-term mowing of cover forage crops for livestock feed on the quality of soil remains unclear. A two-year field experiment was conducted in Ziziphus jujuba cv. “Lingwuchangzao” orchards located in Lingwu County, Ningxia Hui Autonomous Region, in arid and semi-arid Northwest China. The experiment consisted of four treatments: (A) clean tillage (CK), (B) plantation with Lolium perenne (LP), (C) plantation with Trifolium repens (TR), and (D) plantation with Vicia villosa (VV).The results showed that short-term intercropping of forage crops may lead to a reduction in most soil nutrients in Z. jujuba cv. “Lingwuchangzao” orchards, particularly in the 0–20 cm soil layer. However, intercropping with TR can mitigate this declining trend and may even enhance nutrient levels within the 0–40 cm depth. Furthermore, intercropping of forage crops had a more pronounced effect on the α-diversity of fungal communities than on bacterial communities. This practice also altered the relative abundance of bacterial genera such as Sphingomonas, Bacillus, and Flavobacterium in the 20–40 cm depth and dominant fungal genera Fusarium and Mortierella in the 0–20 cm soil layer. The effects of soil physicochemical properties on bacterial communities were more significant than those on fungal communities.These results indicate that the short-term intercropping of forage crops in Z. jujuba cv. “Lingwuchangzao” orchards in arid and semi-arid Northwest China have varying impacts depending on the type of forage crop used.
1. Introduction
Healthy soil forms the foundation for high-quality fruit production. The practice of growing grass in orchards originated in the United States in the 19th century and was subsequently adopted in Europe, South America, and Japan [1]. However, it was not until the 1990s that grass cultivation in orchards was recognized as an ecological orchard-construction initiative and promoted in China [2]. Planting grass in orchards plays a crucial role in maintaining and improving soil health. When properly managed, grass in orchards can boost soil fertility [3,4], effectively prevent soil-borne diseases and pests [5], reduce reliance on fertilizers and pesticides, promote environmental sustainability, and significantly improve the yield and quality of fruit trees [4,6]. Therefore, cultivating grass in orchards represents an effective strategy for promoting the sustainable development of fruit cultivation. Previous studies have demonstrated that grass cultivation impacts not only soil properties—including soil temperature, humidity, porosity, permeability, pH, organic matter content [7], and nutrient levels [8,9,10]—but also biological processes, including soil enzyme activity and overall soil biological activity [9,11]. Grass cultivation offers significant ecological and environmental benefits for soil health, including the enhancement of soil microorganisms [9,10,11,12,13,14,15], soil enzymes [9,11,16], tree growth, nutrient uptake, photosynthetic efficiency, fruit yield and quality [17,18], and orchard microclimate [19,20]. However, the influence of grass cultivation on soil can vary significantly depending on the type of fruit tree and grass species, as well as regional conditions, leading to inconsistent and sometimes contradictory results. For example, the effect of cover crop cultivation on tree growth correlates with regional precipitation levels. Monteiro et al. [21] reported that in a 15-year-old non-irrigated vineyard, sward treatments reduced vine vegetative growth and decreased must acidity in berries. Linares et al. [22] found that grass cultivation had no significant impact on the growth of citrus trees.
The Ziziphus jujuba cv. “Lingwuchangzao” is a premium jujube variety cultivated in China [23]. This unique variety, found in Ningxia, boasts the highest vitamin C content among all jujube varieties, exceeding even Chinese kiwifruit by more than 10-fold. By the end of 2021, the cultivated area of Z. jujuba cv. “Lingwuchangzao” spanned 68,300 mu and supported over 30,000 jujube farmers [24,25]. The cultivation of Z. jujuba cv. “Lingwuchangzao” serves as a nexus between farming and livestock activities in Northwest China. In recent years, forage shortages have intensified due to the increasing number of dairy cows. Consequently, intercropping of grass between rows in the expansive Z. jujuba cv. “Lingwuchangzao” orchards has emerged as a viable strategy to mitigate forage shortages by optimizing resource use. Historically, the primary objective of planting grass in orchards across northern China was to enhance the ecological environment of orchards. This includes regulating the microclimate, enriching orchard soil nutrient content [7], promoting the growth of fruit trees, and improving both fruit yield and quality [4,17,18]. For instance, Wang et al. [26] reported that intercropping ryegrass in Z. jujuba Mill. cv. “Lingwuchangzao” orchards enhanced both plant productivity and soil nutrition. Studies on grass yield in orchards have largely been overlooked. Furthermore, most studies have focused on long-term experiments, with forest grass often utilized as cover crops by being plowed into the soil [18,27,28]. However, there is a lack of research on the effects of cutting and harvesting forest grass on forage yields during the same growing season. This study aimed to assess the short-term effects of intercropping forage crops on soil nutrition and the microbial community in Z. jujuba cv. “Lingwuchangzao” orchards located in arid and semi-arid Northwest China.
2. Material and Methods
2.1. Study Field
Field experiments were performed in a Z. jujuba cv. “Lingwuchangzao” orchard managed by the “Fucheng” Jujube Industry Cooperative, located in Linwu City, Ningxia Hui Autonomous Region, Northwest China (106.331370° E, 38.121889° N), at an altitude of 1250 m. The region experiences a typical continental monsoon climate, characterized by an annual sunshine duration of 3080.2 h, an average frost-free period of 157 days, and a plant growth period of 170 days. The yearly mean temperature is 8.8 °C, while the mean annual precipitation ranges from 206.2 to 25.5 mm. The soil is deep, fertile, sandy loam irrigated using water from the Yellow River. Jujube cultivation in this area began in 2018.
The jujube tree plantation was established at a density of approximately 800 plants per hectare, with plant spacing of 3 m and row spacing of 4 m. The primary fertilizers used included 22–30 tons of organic fertilizer (fermented sheep feces containing 30–37% organic matter, 0.75% N, 0.5% P2O5, and 0.45% K2O; Putianke [Zhongwei] Organic Fertilizer Co., Ltd., Zhongwei, Ningxia, China), 150–300 kg of diammonium phosphate (total phosphorus [TP] content ≥ 46%, available phosphorus [AP] content ≥ 62%, and water content ≤ 2%; Yuntianhua Co., Ltd., Kunming, Yunnan, China), 150 kg of urea (total nitrogen [TN] content ≥ 46%; Luxi Chemical Industrial Group Co., Ltd., Liaocheng, Shandong, China), and 300 kg of nitrogen–phosphorus–potassium compound fertilizer (water content ≤ 2%, TN content ≥ 14%, AP content ≥ 22%, and available potassium [AK] content ≥ 10%; Sinofert Fertilizer [Holdings] Co., Ltd., Beijing, China) per hectare. Fertilizers were applied three times annually, consisting of one base application and two top dressings. Organic fertilizer was applied immediately after the previous year’s fruit harvest or as soon as the soil thawed in the current year. Chemical fertilizers were applied in late April and early May and from mid-July to mid-August, in conjunction with irrigation.
2.2. Experimental Design
Perennial ryegrass (Lolium perenne L., LP), white clover (Trifolium repens L., TR), and Vicia villosa Roth. (VV) were planted in a Z. jujuba cv. “Lingwuchangzao” orchard. Seeds were sown in the inter-row spaces of each row of fruit trees in May 2023, using a line-sowing method with a spacing of 20 cm (30–45 kg ha−1). The grass was allowed to grow naturally and was not removed. In contrast, weeds in the Z. jujuba cv. “Lingwuchangzao” orchard under clean tillage (CK) were manually eliminated. Other field management practices, such as the type and timing of fertilizer application, were consistent across all experimental sites. By 2023, the intercropped forage crops yielded as follows: LP was mowed once, producing a fresh forage yield of approximately 1 kg/m2; TR was mowed once, with a fresh forage yield of approximately 0.5 kg/m2; and VV was mowed twice, yielding approximately 5 kg/m2. The distance between plots exceeded 50 m, and each treatment included five replicates for analysis.
2.3. Soil Sampling
Soil samples were gathered from Z. jujuba cv. “Lingwuchangzao” orchards in April 2024 (Figure 1). Samples were obtained from two soil depths—0–20 cm (labeled as “a”) and 20–40 cm (labeled as “b”)—in each plot, using a five-point sampling method. These were then combined into a single mixed sample for each soil layer. For each treatment, five soil samples were collected from both the 0–20 cm and 20–40 cm depths. In total, 40 soil samples were collected using four mulching methods, two soil layers, and five replicates. The soil samples were sieved through a 2 mm sieve and divided into two portions. One portion was stored at −80 °C for microbial community analysis, while the other was air-dried for the assessment of soil properties.

Figure 1.
Schematic representation of the experimental plots. (A) Clean tillage (CK). (B) L. perenne (LP) plantation. (C) T. repens (TR) plantation. (D) V. villosa (VV) plantation.
2.4. Physicochemical Properties of Soil
Soil organic carbon (SOC) was determined using the wet oxidation method. TN and available nitrogen (AN) were analyzed using the alkaline permanganate method. TP, AP, total potassium (TK), and AK were measured using the Olsen method and the ammonium acetate extractable method proposed by Bao (2000) [29].
2.5. Isolation of DNA, Library Preparation, and Metagenome Analysis Through Sequencing
DNA was isolated from the soil samples with HiPure Soil DNA Kits or HiPure Stool DNA Kits (Magen, Guangzhou, China). The V5-V6-V7 variable regions of the 16S rRNA genes and the fungal ITS2 variable regions were amplified using the 314F/806R primer pair [30] and fungal ITS3_KYO2/ITS4 [31].
Sequencing libraries were prepared using an Illumina DNA Prep Kit (Illumina, San Diego, CA, USA). Sequencing was performed on the NovaSeq 6000 platform (Illumina Inc., San Diego, CA, USA) by Gene Denovo Biotechnology Co., Ltd. (Guangzhou, China). The resulting raw reads were uploaded to the NCBI Sequence Read Archive (SRA) under accession numbers PRJNA1195000 and PRJNA1195014.
2.6. Bioinformatics and Statistical Analyses
Paired-end reads were combined into single sequences using FLASH [32]. Representative amplicon sequence variants (ASVs) were identified and classified using a naïve Bayesian model via the Ribosomal Database Project (RDP) classifier [33] (version 2.2) or the UNITE database [34] (version 8.3). Diversity metrics, including the Chao1, Shannon, Simpson, and Pielou evenness indices, were calculated using QIIME [35] (version 1.9.1). Principal coordinate analysis (PCoA) was conducted based on Bray–Curtis distances with the Vegan package (version 2.5-7) in R and visualized through ggplot2 (version 2.2.1). Redundancy analysis (RDA) and canonical correspondence analysis (CCA) were performed using the Vegan package in R (version 2.5.3) to explore how environmental factors influenced community composition. Welch’s t-test and the Wilcoxon rank test were applied for pairwise comparisons, while Tukey’s honestly significant difference (HSD) test and the Kruskal–Wallis H test were used for comparisons among multiple groups, all implemented in the Vegan package [36] (version 2.5-7). The data were analyzed on the free online OmicSmart platform (https://www.omicsmart.com) (accessed on 25 July 2024).
3. Results
3.1. Effects of Intercropping of Forage Crops on Soil Physicochemical Properties
The physicochemical characteristics of soil samples gathered from various intercropping forage crops were analyzed (Table 1). The TK levels in the 0–20 cm and 20–40 cm soil layers did not differ significantly across the intercropping systems. The highest levels of SOC and TN in both the 0–20 cm and 20–40 cm soil layers, as well as AN and AP in the 20–40 cm soil layers, were observed in TR intercropping. Conversely, the highest levels of AK and TP in both the 0–20 cm and 20–40 cm soil layers, along with AP and AN in the 0–20 cm soil layers, were recorded in CK. Intercropping with grass did not significantly (p > 0.05) affect TK levels in either soil layer, but it did lead to a reduction in AK levels across both layers. Notably, intercropping with TR helped mitigate the declining trend of AK content at the 20–40 cm depth. With respect to SOC, TN, AN, and AP, the decreasing trend in the 0–20 cm soil layer could be alleviated by intercropping all types of forage grass. Furthermore, these parameters increased in the 20–40 cm layer with TR intercropping. The trend for TP was similar to that for AK. Therefore, intercropping forage crops may result in a reduction in most soil nutrients in Z. jujuba cv. “Lingwuchangzao” orchards, particularly in the 0–20 cm layer. Only intercropping with TR could alleviate this declining trend and potentially increase nutrient levels in the 0–40 cm soil layer.

Table 1.
Physicochemical characteristics of different soil layers under intercropping with different forage crops.
3.2. Effects of Intercropping of Forage Crops on Soil Bacterial and Fungal Community Diversity in Different Layers in a Z. jujuba cv. “Lingwuchangzao” Orchard
The α-diversity indices, including the Sob, Shannon, Chao1, Pielou, and Coverage indices, were employed to quantify the diversity, richness, evenness, and sequencing depth of the microbial community across various soil layers under intercropping with various forage crops. The coverage indices for both microbial groups across the two soil layers and four forage crop management practices were approximately 0.9, indicating sufficient sequencing depth (Table S1). No significant differences were observed in the richness, evenness, or diversity indices of the bacterial communities between the 0–20 and 20–40 cm depths across the different forage crops. In the 0–20 cm depth, marked variations were noted in the diversity, richness, and evenness of the fungal communities among the four intercropping forage crops. Notably, the diversity, richness, and evenness indices of TR were significantly higher than those of the other forage crops. In the 20–40 cm soil layer, TR also exhibited significantly greater diversity and richness than the other treatments, although the evenness index did not show significant differences among the groups. Intercropping with different forage crops had minimal effects on most of the α-diversity indices, including the Sob, Shannon, Chao1, and Pielou indices, in both bacterial and fungal communities associated with various forage crops, excluding TR, across the 0–20 cm and 20–40 cm depths in the Z. jujuba cv. “Lingwuchangzao” orchard. Conversely, the intercropping of TR markedly elevated the Sob, Shannon, Chao1, and Pielou indices for fungal communities, particularly at the 0–20 cm depth.
The β-diversity of bacterial and fungal communities across different soil layers in the Z. jujuba cv. “Lingwuchangzao” orchard was compared using PCoA. PCoA of the 16S rDNA sequencing data showed that 18.31% of the variation in bacterial communities was attributed to the 0–20 cm depth, whereas 23.63% of the variation was observed at the 20–40 cm depth, as indicated by PCoA1 and PCoA2, respectively (Figure 2A,B). In addition, Adonis multivariate analysis of variance indicated that the bacterial communities in the 0–20 cm and 20–40 cm soil layers differed significantly (p = 0.001; Figure 2C,D). PCoA results for the ITS region showed that 27.93% of the variation in fungal communities was attributed to the 0–20 cm soil layer, whereas 34.98% was attributed to the 20–40 cm soil layer, as shown by PCoA1 and PCoA2, respectively (Figure 2E,F). Moreover, multivariate analysis based on PCoA further confirmed significant differences in fungal communities between the 0–20 cm and 20–40 cm soil layers (p = 0.001; Figure 2G,H).
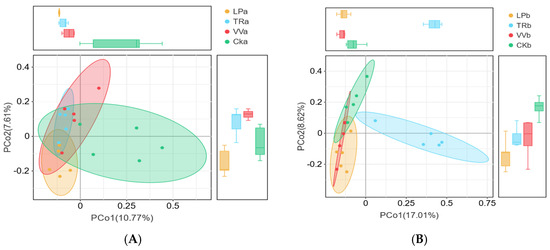
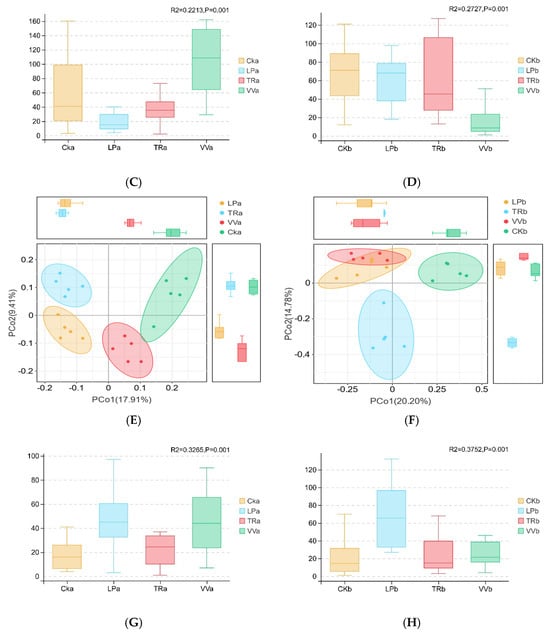
Figure 2.
PCoA based on Bray–Curtis distances for bacterial and fungal communities in different soil layers of a Z. jujuba cv. “Lingwuchangzao” orchard under intercropping with different forage crops. (A) Distribution of bacterial communities in the 0–20 cm soil layer. (B) Distribution of bacterial communities in the 20–40 cm soil layer. (C) Adonis multivariate analysis of variance of bacterial communities in the 0–20 cm soil layer. (D) Adonis multivariate analysis of variance of bacterial communities in the 20–40 cm soil layers. (E) Distribution of fungal communities in the 0–20 cm soil layer. (F) Distribution of fungal communities in the 20–40 cm soil layer. (G) Adonis multivariate analysis of variance of fungal communities in the 0–20 cm soil layer. (H) Adonis multivariate analysis of variance of fungal communities in the 20–40 cm soil layer. CKa, 0–20 cm soil layers of clean tillage; CKb, 20–40 cm soil layers of clean tillage; LPa, 0–20 cm soil layers planted with L. perenne; LPb, 20–40 cm soil layers planted with L. perenne; TRa, 0–20 cm soil layers planted with T. repens; TRb, 0–20 cm soil layers planted with T. repens; VVa, 0–20 cm soil layers planted with V. villosa; VVb, 20–40 cm soil layers planted with V. villosa.
According to the 16S rDNA data, the four bacterial communities in the 0–20 cm soil layer were indistinguishable; however, TRb was separated from the other three groups in the 20–40 cm soil layer along the x-axis. Based on ITS gene sequencing, the CKa and VVa fungal communities from the 0–20 cm soil layer clustered on the positive side of the x-axis, whereas LPa and TRa clustered on the negative side. In addition, CKa and TRa grouped on the positive side of the y-axis, whereas LPa and VVa grouped on the negative side. In the 20–40 cm soil layer, TRb was distinct from the other three fungal communities, appearing on the negative y-axis, while CKb was separated from the other two groups that clustered on the negative side of the x-axis. Therefore, the effect of intercropping different forage crops on microbial communities was more pronounced in the 0–20 cm soil layer than in the 20–40 cm layer and had a greater impact on bacterial communities than on fungal communities.
3.3. Distribution of Bacterial and Fungal Populations in Different Soil Layers of a Z. jujuba cv. “Lingwuchangzao” Orchard Under Intercropping with Different Forage Crops
The composition of bacterial and fungal community structures was compared at both the phylum and genus levels across different soil layers in the Z. jujuba cv. “Lingwuchangzao” orchard under conditions of intercropping with various forage crops. The dominant bacterial phyla in the 0–20 cm and 20–40 cm soil layers were Proteobacteria, Acidobacteria, Bacteroidota, and Chloroflexi (Figure 3A). At the genus level in the 0–20 cm soil layer, the predominant groups included RB41, MND1, Sphingomonas, Flavobacterium, Nitrospira, and Pseudomonas; these genera did not exhibit significant differences across the intercropping systems with various forage crops (Figure 3B). At the 20–40 cm depth, the dominant genera were MND1, RB41, Pseudomonas, Nitrospira, Sphingomonas, Bacillus, and Flavobacterium. Notably, Sphingomonas, Bacillus, and Flavobacterium exhibited significant differences among the intercropped forage crops. The relative abundance of the Sphingomona genus was the lowest in LPb, whereas that of Bacillus was highest in LPb and lowest in TRb. Additionally, the relative abundance of Flavobacterium was higher in both LPb and TRb than in CKb and VVb (Figure 3C).
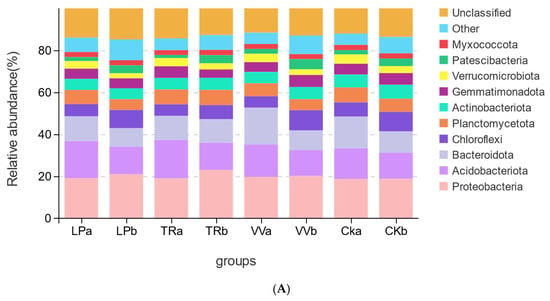
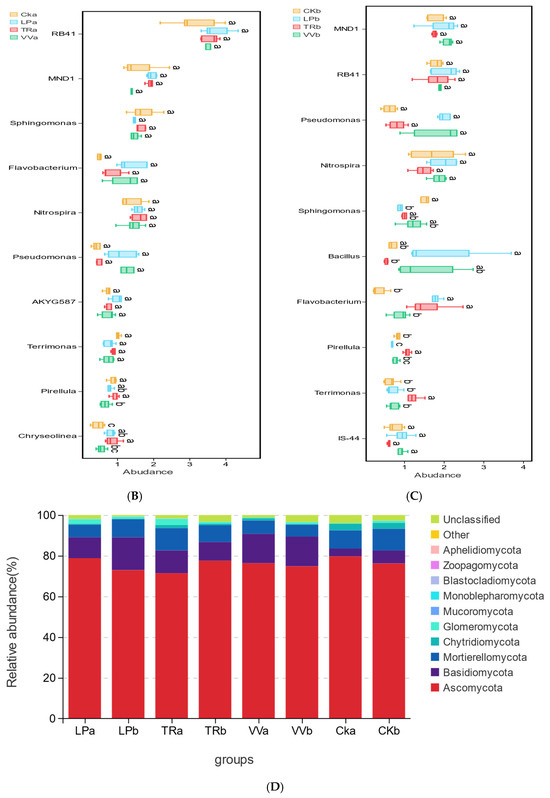
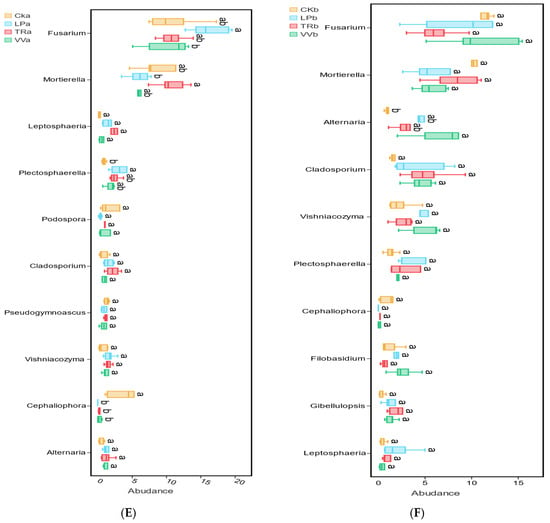
Figure 3.
Relative abundances of bacterial and fungal communities in different soil layers of a Z. jujuba cv. “Lingwuchangzao” orchard under intercropping with different forage crops. (A) Relative abundance of dominant bacterial communities at the phylum level in the 0–20 cm and 20–40 cm soil layers. (B) Relative abundance of the top 10 bacterial communities at the genus level in the 0–20 cm soil layer. (C) Relative abundance of the top 10 bacterial communities at the genus level in the 20–40 cm soil layer. (D) Relative abundance of dominant fungal communities at the phylum level in the 0–20 cm and 20–40 cm soil layers. (E) Relative abundance of the top 10 fungal communities at the genus level in the 0–20 cm soil layer. (F) Relative abundance of the top 10 fungal communities at the genus level in the 20–40 cm soil layer. Note: The different lowercase indicates the significant differences at 0.05 level.
Ascomycota, Mortierellomycota, and Basidiomycota were the predominant fungal phyla at both the 0–20 cm and 20–40 cm depths (Figure 3D). Fusarium and Mortierella were the dominant fungal genera in these layers. At the 0–20 cm depth, the abundance of these genera varied significantly depending on the intercropping of different forage crops. Fusarium exhibited the highest relative abundance in LPa and the lowest in VVa, whereas Mortierella had the highest relative abundance in TRa and the lowest in LPa (Figure 3E). However, in the 20–40 cm soil layer, no significant differences in the abundance of Fusarium and Mortierella were observed across the intercropping of various forage crops (Figure 3F). Venn diagrams illustrate the common and specific ASVs of bacterial and fungal communities across different soil layers in the Z. jujuba cv. “Lingwuchangzao” orchard under conditions of intercropping with various forage crops. In the bacterial communities of both the 0–20 cm and 20–40 cm soil layers, the specific ASVs for each forage crop constituted the largest proportion of the total ASVs, surpassing the number of common ASVs. Additionally, the number of common ASVs among the four forage crops was greater than that of the common ASVs shared by any two or three crops. In the fungal communities of both the 0–20 cm and 20–40 cm soil layers, the number of specific ASVs for each forage crop was also higher than the number of common ASVs. However, the ratio of specific-to-common ASVs in the fungal communities was lower than that observed in the bacterial communities. Furthermore, the number of common ASVs among the four different forage crops was greater than the number of common ASVs shared by any two or three crops in the fungal communities (Figure S1).
3.4. Inter-Relationships Between Bacterial and Fungal Communities in Different Soil Layers of a Z. jujuba cv. “Lingwuchangzao” Orchard Under Intercropping with Different Forage Crops
The network analysis revealed that LP, TR, and VV exhibited lower total edge counts and average degrees of bacterial co-occurrence in the 0–20 cm soil layer than CK, with LP exhibiting the lowest values. Among the forage crops, VV had the highest proportion of positive edges. For the bacterial co-occurrence network at the 20–40 cm depth, TR had the highest total edge count and average degree among the different forage crops, whereas LP had the lowest values. Regarding the fungal co-occurrence network at the 0–20 cm depth, TR had the lowest total edge count and average degree, whereas VV had the highest. In the 20–40 cm soil layer, TR, again, demonstrated the highest total edge count and average degree (Table S2; Figure 4).
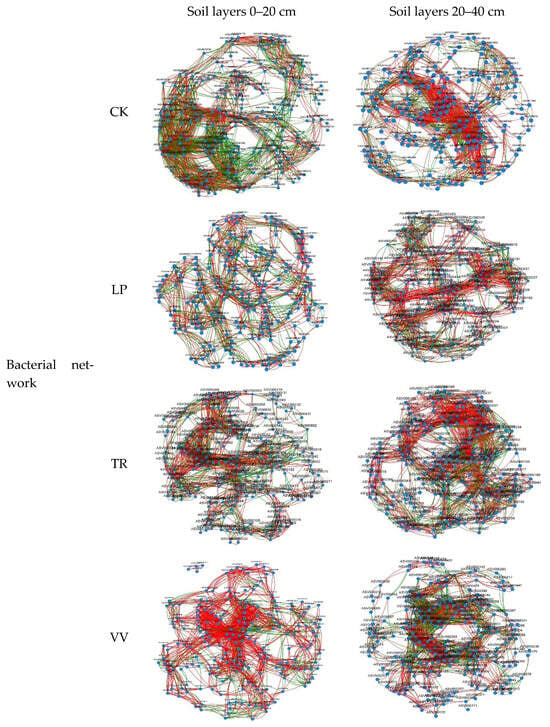
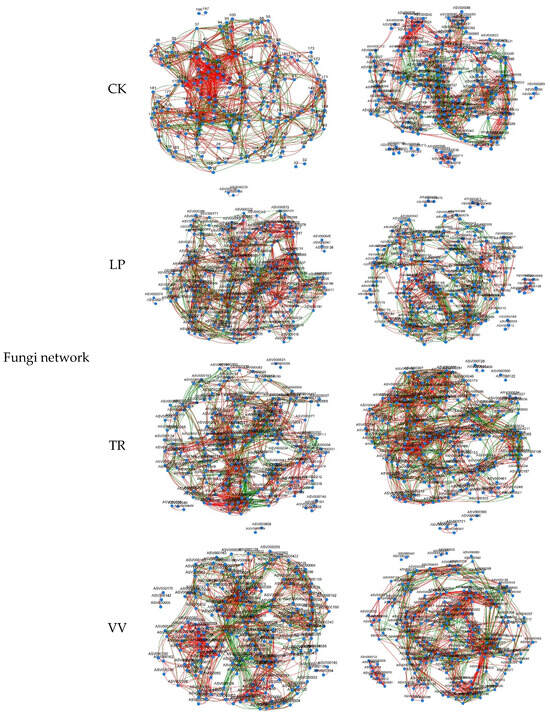
Figure 4.
Co-occurrence networks based o the top 200 ASVs. Red lines represent significant positive relationships, and blue lines denote negative relationships.
3.5. Factors Shaping Bacterial and Fungal Communities in Soil
The effects of soil physicochemical properties on bacterial and fungal communities were analyzed using RDA. In both the 0–20 cm and 20–40 cm soil layers, the impact of these properties on bacterial communities was significantly greater than on fungal communities. Additionally, fungal communities showed minimal response to the physicochemical properties of the soil (Figure 5).
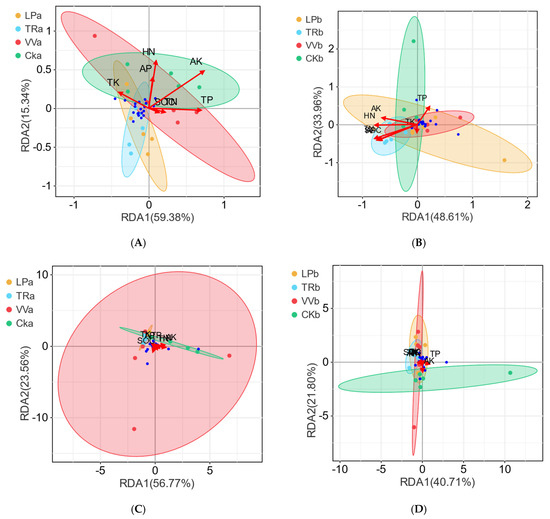
Figure 5.
RDA ordination plot of bacterial and fungal communities constrained by soil physicochemical properties. (A,B) Bacterial communities.(C,D) Fungal communities a, 0–20 cm soil layer; b, 20–40 cm soil layer o.
4. Discussion
This study assessed the effects of short-term intercropping of forage crops on the soil microbial communities in a Z. jujuba cv. “Lingwuchangzao” orchard in arid and semi-arid Northwest China. We found that short-term intercropping of forage crops may deplete most soil nutrients in Z. jujuba cv. “Lingwuchangzao” orchards in arid and semi-arid Northwest China. TR has the potential to mitigate this nutrient decline and may even enhance nutrient levels in the 0–40 cm layer. These results provide a new perspective for the integrated development of orchard agroecosystems.
At present, planting forage crops between tree rows can influence soil nutrient content, particularly when cover crops are incorporated, thereby enhancing soil fertility [8,9,17]. In this study, we found that intercropping of forage crops may reduce soil nutrient levels in Z. jujuba cv. “Lingwuchangzao” orchards, particularly at the 0–20 cm depth. Similar to our results, Fu et al. [17] demonstrated that intercropping of forage crops significantly reduced most soil nutrient contents, particularly those of AN, TP, and AP. This may be due to potential nutrient competition between forage crops and fruit trees, particularly when the forage crops are mowed for livestock feed. Mowing these crops can result in the loss of chemical nutrients that are absorbed from the soil during growth, ultimately leading to a decrease in soil nutrient content. However, certain plants can help mitigate this declining trend through the contribution of litter, root sediments, and residual materials, potentially increasing soil nutrient content [37]. Notably, TR was the only forage crop that effectively alleviated the decrease in nutrient levels and enhanced nutrient availability at the 0–40 cm depth. Compared to other forage crops, the intercropping of TR resulted in a lower forage yield, which may have led to the removal of fewer nutrients from the soil. Conversely, this is closely related to the nitrogen fixation ability of TR as a leguminous plant [14,38,39].
Diversity, richness, and evenness are critical characteristics of microbial communities. Intercropping forage crops between tree rows can influence not only the nutrient content of the soil but also the diversity, richness, and evenness of soil microbial communities [9,11,12,13,14,15]. In this study, we found that intercropping forage crops did not significantly affect most indices of α-diversity, such as the Sob, Shannon, Chao1, and Pielou indices, in the bacterial and fungal communities associated with forage crops, except for TR, across both the 0–20 cm and 20–40 cm soil layers in a Z. jujuba cv. “Lingwuchangzao” orchard. However, intercropping TR led to increases in the Sob, Shannon, Chao1, and Pielou indices of fungal communities, particularly at the 0–20 cm depth. Therefore, the practice of intercropping forage crops had a more pronounced effect on the α-diversity of fungal communities than on that of bacterial communities. Regarding β-diversity, the PCoA results further supported the finding that intercropping forage crops also had a greater effect on fungal communities than on bacterial communities, with the intercropping of TR exerting the strongest influence on the β-diversity of both bacterial and fungal communities. Liu et al. [14] also demonstrated that cover crop treatments had a more complex microbial co-occurrence network in fungal communities than in bacterial communities. A possible explanation for this result is that fungal communities are more responsive to shifts in vegetation than bacterial populations. Fungi are primary consumers and facilitate the use of some difficult-to-decompose carbon sources input by plants better than bacteria [40]. The significant variability in fungal activity observed with TR may be associated with its physiology, rooting activity, and root exudates, which are more suitable for fungi.
RB41, MND1, Sphingomonas, Flavobacterium, Bacillus, Nitrospira, and Pseudomonas were the dominant genera in the bacterial community of both the 0–20 cm and 20–40 cm depths. The depth of the soil layers influenced the relative abundance of these genera. In the 0–20 cm soil layers, no significant differences were observed in the abundance of these genera across intercropping systems with different forage crops. However, at the 20–40 cm depth, significant differences were observed in the abundance of Sphingomonas, Bacillus, and Flavobacterium depending on the intercropping system. Fusarium and Mortierella were the two predominant fungal genera in both the 0–20 cm and 20–40 cm soil layers, with their abundance showing no significant differences in the 20–40 cm layer between intercropping systems. In contrast, at the 0–20 cm depth, significant differences in their abundances were found based on the intercropping system. Fusarium is a well-known pathogen associated with soil-borne plant diseases [41]. No root diseases were observed in the extensively grown Z. jujuba cv. “Lingwuchangzao” orchard, which may be attributed to the dominance of bacterial genera such as Sphingomonas [42], Flavobacterium [43], Bacillus [44], and Pseudomonas [45], which are known for their antagonistic properties against Fusarium. Mortierella spp. is a common saprophytic fungus, with Ozimek and Hanaka [46] reporting that Mortierella spp. are saprotrophic microorganisms isolated from forest litter, where they play a crucial role as notable decomposers in agricultural soils. Li et al. [47] examined the effect of the saprophytic fungus Mortierella capitata K on the chemical composition of soil organic matter.
Changes in soil nutrition are influenced by biological activity [9,11], enzyme activity [9,11,16], and soil microorganisms [9,11,12,13,14,15]. This study focused exclusively on soil microorganisms and did not examine soil enzyme activity. Moreover, one of the primary objectives of planting grass in orchards is to enhance fruit yield and quality [17,18], a factor that was beyond the scope of this study. Further research in this area is required.
5. Conclusions
The results of this study indicated that intercropping forage crops may deplete most soil nutrients in a Z. jujuba cv. “Lingwuchangzao” orchard, particularly in the 0–20 cm layer. However, the intercropping of TR appeared to mitigate this decline and could even enhance nutrient content at the 0–40 cm depth. Furthermore, the effects of intercropping forage crops on the α-diversity of fungal communities were more pronounced than their effects on bacterial communities. These practices altered the relative abundance of bacterial genera such as Sphingomonas, Bacillus, and Flavobacterium at the 20–40 cm depth and dominant fungal genera Fusarium and Mortierella in the 0–20 cm layer. The effect of soil physicochemical properties on bacterial communities was more significant than their effect on fungal communities. This study revealed that short-term intercropping of forage crops can lead to competition with fruit trees for nutrients in a Z. jujuba cv. “Lingwuchangzao” orchard. In arid and semi-arid regions, intercropped herbage serves primarily as a cover crop and mulch. Additionally, leguminous forages are the preferred choice.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15020319/s1, Figure S1: Venn diagram based on ASV in bacterial and fungal communities of different soil layers in a Z. jujuba cv. “Lingwuchangzao” orchard under intercropping with different forage crops; Table S1: Diversity indices of soil bacterial and fungal communities in different soil layers after intercropping with different forage crops; Table S2: Co-occurrence network parameters of bacterial and fungal communities in different soil layers under intercropping with different forage crops.
Author Contributions
Investigation, writing—original draft, revision, and final approval of the version to be published: H.Z.; investigation, revision, and final approval of the version to be published: M.B., Y.G., T.C. and J.W.; formal analysis, revision, and final approval of the version to be published: Y.L., S.L. and X.S.; writing—original draft, revision, and final approval of the version to be published: Y.Q., X.G. and X.Z.; writing—review and editing, revision, and final approval of the version to be published: J.L. (Jingyu Li) and J.L. (Jianli Li); funding acquisition, revision, and final approval of the version to be published: J.Z. and T.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Project of Ningxia Academy of Agricultural Sciences Agricultural High-Quality Development and Ecological Protection Technology Innovation Demonstration (No. NGSB-2021-1-02), the Innovation Team for Genetic Improvement of Economic Forests (No. 2022QCXTD04), the Science and Technology Leading Talent Project of Ningxia (No. 2022GKLRLX06), and the Talent Highland for the Research and Development of Engineering Technologies for Ecological Restoration in Arid Areas (No. 2023RCGD07).
Informed Consent Statement
Written informed consent was obtained from all the participants prior to their enrolment in this study.
Data Availability Statement
The data presented in this paper were deposited at https://www.ncbi.nlm.nih.gov/ (accession number: PRJNA1195000 and PRJNA1195014) (accessed on 5 December 2024).
Acknowledgments
The authors appreciate the insightful feedback and recommendations provided by the editors and reviewers.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Wang, J.; Qin, X.; Tan, Y.; Du, Y.; Tudi, Y.; Yang, Y.; Ping, X. Impact of grass cover on the soil physicochemical properties in China’s orchards: A meta-analysis. Agrofor. Syst. 2024, 98, 1745–1758. [Google Scholar] [CrossRef]
- Ma, X.; Liao, J.; Zhao, J. A meta-analysis of the effects on soil quality in XinJiang (China) orchards after grass cultivation. Appl. Ecol. Environ. Res. 2023, 21, 1891–1902. [Google Scholar] [CrossRef]
- Tang, W.; Yang, H.; Wang, W.; Wang, C.; Pang, Y.; Chen, D.; Hu, X. Effects of living grass mulch on soil properties and assessment of soil quality in Chinese apple orchards: A meta-analysis. Agronomy 2022, 12, 1974. [Google Scholar] [CrossRef]
- Ma, X.; Liao, J.; Zhao, J. Experiment and meta-analysis on the effects of grass cultivation in the orchard on fruit yield and quality. Food Sci. Technol. 2022, 43, e95122. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, D.; Lei, Y.; Lozano-Torres, J.L.; Deng, Y.; Xu, J.; Hu, L. Cover crop rotation suppresses root-knot nematode infection by shaping soil microbiota. New Phytol. 2024, 245, 363–377. [Google Scholar] [CrossRef]
- Ren, J.; Li, F.; Yin, C. Orchard grass safeguards sustainable development of fruit industry in China. J. Clean. Prod. 2023, 382, 135291. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, K.; Zhang, J.; Li, D.; Zhang, Y.; Xiang, H. Grass cultivation alters soil organic carbon fractions in a subtropical orchard of southern China. Soil. Tillage Res. 2018, 181, 110–116. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, R.; Fu, L.; Tao, S.; Bao, J. Effects of orchard grass on soil fertility and nutritional status of fruit trees in Korla fragrant pear orchard. Horticulturae 2023, 9, 903. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Kamran, M.; Chen, X.; Tan, H.; Long, M. Effects of grass inter-planting on soil nutrients, enzyme activity, and bacterial community diversity in an apple orchard. Front. Plant. Sci. 2022, 13, 901143. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, W.; Goodwin, P.H.; Wang, Y.; Zheng, S.-J.; Li, X. Effect of cover crop on soil fertility and bacterial diversity in a banana plantation in southwestern China. Soil. Tillage Res. 2024, 240, 106092. [Google Scholar] [CrossRef]
- Xiang, Y.; Chang, S.X.; Shen, Y.; Chen, G.; Liu, Y.; Yao, B.; Xue, J.; Li, Y. Grass cover increases soil microbial abundance and diversity and extracellular enzyme activities in orchards: A synthesis across China. Appl. Soil. Ecol. 2023, 182, 104720. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, J.; Sainju, U.M.; Zhang, S.; Zhao, F.; Khan, A. Cover cropping enhances soil microbial biomass and affects microbial community structure: A meta-analysis. Geoderma 2021, 381, 114696. [Google Scholar] [CrossRef]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R.; Hart, M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016, 36, 48. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Chen, X.; Tan, H.; Jin, X.; Lu, Q.; He, S.; Long, M. Cover cropping increases soil fungal-bacterial community diversity and network complexity in apple orchards on the Loess Plateau, China. Front. Environ. Sci. 2022, 10, 916288. [Google Scholar] [CrossRef]
- Wei, Z.; Zeng, Q.; Tan, W. Cover cropping impacts soil microbial communities and functions in mango orchards. Agriculture 2021, 11, 343. [Google Scholar] [CrossRef]
- Wang, N.; Li, L.; Gou, M.; Jian, Z.; Hu, J.; Chen, H.; Xiao, W.; Liu, C. Living grass mulching improves soil enzyme activities through enhanced available nutrients in citrus orchards in subtropical China. Front. Plant. Sci. 2022, 13, 1053009. [Google Scholar] [CrossRef]
- Fu, H.; Chen, H.; Ma, Q.; Han, K.; Wu, S.; Wu, L. Effect of planting and mowing cover crops as livestock feed on soil quality and pear production. Front. Plant. Sci. 2023, 13, 1105308. [Google Scholar] [CrossRef] [PubMed]
- Tu, A.; Xie, S.; Zheng, H.; Li, H.; Li, Y.; Mo, M. Long-term effects of living grass mulching on soil and water conservation and fruit yield of citrus orchard in south China. Agric. Water Manag. 2021, 252, 106897. [Google Scholar] [CrossRef]
- Fu, H.; Chen, H.; Ma, Q.; Chen, B.; Wang, F.; Wu, L. Planting and mowing cover crops as livestock feed to synergistically optimize soil properties, economic profit, and environmental burden on pear orchards in the Yangtze River Basin. J. Sci. Food Agric. 2023, 103, 6680–6688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, T.; Wei, W.; Shen, L.; Wang, X.; Tuertia, T.; Li, L.; Zhang, W. In arid regions, forage mulching between fruit trees rows enhances fruit tree light and lowers soil salinity. Agriculture 2022, 12, 1895. [Google Scholar] [CrossRef]
- Monteiro, A.; Lopes, C.M. Influence of cover crop on water use and performance of vineyard in Mediterranean Portugal. Agric. Ecosyst. Environ. 2007, 121, 336–342. [Google Scholar] [CrossRef]
- Linares, J.; Scholberg, J.; Graetz, D.; Boote, K.; McSorley, R.; Chase, C. Effects of perennial peanut and common bermudagrass on nitrogen and water uptake of young citrus trees. J. Plant Nutr. 2010, 33, 200–218. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, D.; Wang, Z.; Song, L.; Cao, B. Fruit morphology measurements of jujube cultivar ‘Lingwu Changzao’(Ziziphus jujuba Mill. cv. Lingwuchangzao) during fruit development. Horticulturae 2021, 7, 26. [Google Scholar] [CrossRef]
- Wang, X.; Cao, B.; Zou, J.; Chen, W. Composition and environmental interpretation of the weed communities in the main planting base of jujube (Ziziphus jujuba Mill. cv.‘LingwuChangzao’), Ningxia province of China. Peer J. 2022, 10, e13583. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, L.; Han, Y.; Cao, B.; Song, L. Effects of elevated temperature and drought stress on fruit coloration in the jujube variety ‘Lingwuchangzao’(Ziziphus jujube cv. Lingwuchangzao). Sci. Hortic. 2020, 274, 109667. [Google Scholar] [CrossRef]
- Wang, X.; Cao, B.; Zou, J.; Xu, A.; Feng, X. Intercropping Gramineae Herbage in Semiarid Jujube Cultivar ‘LingwuChangzao’(Ziziphus jujuba Mill. cv. LingwuChangzao) Orchard Improves Productivity, Plant Nutritional Quality, and Soil Quality. Horticulturae 2022, 8, 834. [Google Scholar] [CrossRef]
- Atucha, A.; Merwin, I.A.; Brown, M.G. Long-term effects of four groundcover management systems in an apple orchard. Hort. Sci. 2011, 46, 1176–1183. [Google Scholar] [CrossRef]
- Bunce, J.A. Long-term growth of alfalfa and orchard grass plots at elevated carbon dioxide. J. Biogeogr. 1995, 22, 341–348. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Agriculture Publication: Beijing, China, 2000; pp. 355–356. [Google Scholar]
- Guo, M.; Wu, F.; Hao, G.; Qi, Q.; Li, R.; Li, N.; Wei, L.; Chai, T. Bacillus subtilis improves immunity and disease resistance in rabbits. Front. Immunol. 2017, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.5-7; R Project for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Dung, T.V.; Ngoc, N.P.; Dang, L.V.; Hung, N.N. Impact of cover crop and mulching on soil physical properties and soil nutrients in a citrus orchard. PeerJ 2022, 10, e14170. [Google Scholar] [CrossRef]
- Ordóñez-Fernández, R.; de Torres, M.A.R.-R.; Márquez-García, J.; Moreno-García, M.; Carbonell-Bojollo, R.M. Legumes used as cover crops to reduce fertilisation problems improving soil nitrate in an organic orchard. Eur. J. Agron. 2018, 95, 1–13. [Google Scholar] [CrossRef]
- Capri, C.; Gatti, M.; Fiorini, A.; Ardenti, F.; Tabaglio, V.; Poni, S. A comparative study of fifteen cover crop species for orchard soil management: Water uptake, root density traits and soil aggregate stability. Sci. Rep. 2023, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Roncero, M.I.G.; Hera, C.; Ruiz-Rubio, M.; Maceira, F.I.G.; Madrid, M.P.; Caracuel, Z.; Calero, F.; Delgado-Jarana, J.; Roldán-Rodrıguez, R.; Martınez-Rocha, A.L. Fusarium as a model for studying virulence in soilborne plant pathogens. Physiol. Mol. Plant Pathol. 2003, 62, 87–98. [Google Scholar] [CrossRef]
- Wachowska, U.; Irzykowski, W.; Jędryczka, M.; Stasiulewicz-Paluch, A.D.; Głowacka, K. Biological control of winter wheat pathogens with the use of antagonistic Sphingomonas bacteria under greenhouse conditions. Biocontrol Sci. Technol. 2013, 23, 1110–1122. [Google Scholar] [CrossRef]
- Soltani, A.-A.; Khavazi, K.; Asadi-Rahmani, H.; Alikhani, H.-A.; Omidvari, M.; Dahaji, P.A. Evaluation of biological control traits in some isolates of fluorescent Pseudomonads and Flavobacterium. J. Agric. Sci. 2012, 4, 164. [Google Scholar] [CrossRef]
- Shobha, G.; Kumudini, B. Antagonistic effect of the newly isolated PGPR Bacillus spp. on Fusarium oxysporum. Int. J. Appl. Sci. Eng. Res. 2012, 1, 463–474. [Google Scholar]
- Khalifa, M.W.; Rouag, N.; Bouhadida, M. Evaluation of the antagonistic effect of Pseudomonas rhizobacteria on Fusarium wilt of chickpea. Agriculture 2022, 12, 429. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2020, 11, 7. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Zhao, Z.; Li, Y.; Yu, H.; Wang, Y.; Zhang, J.; Han, Y. The changes of chemical molecular components in soil organic matter are associated with fungus Mortierella capitata K. Soil. Tillage Res. 2023, 227, 105598. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).