Preliminary Investigation on Resistance of Beckmannia syzigachne to Clodinafop-Propargyl and Mesosulfuron-Methyl from China
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Detection of Resistance Level to Clodinafop-Propargyl and Mesosulfuron-Methyl
2.3. Identification of ACCase and ALS Gene Mutations in Resistant Populations
3. Results
3.1. Resistance Levels and Distribution of B. syzigachne to Clodinafop-Propargyl
3.2. Resistance Levels and Distribution of B. syzigachne to Mesosulfuron-Methyl
3.3. Analysis of the Multiple Resistance to Clodinafop-Propargyl and Mesosulfuron-Methyl in B. syzigachne
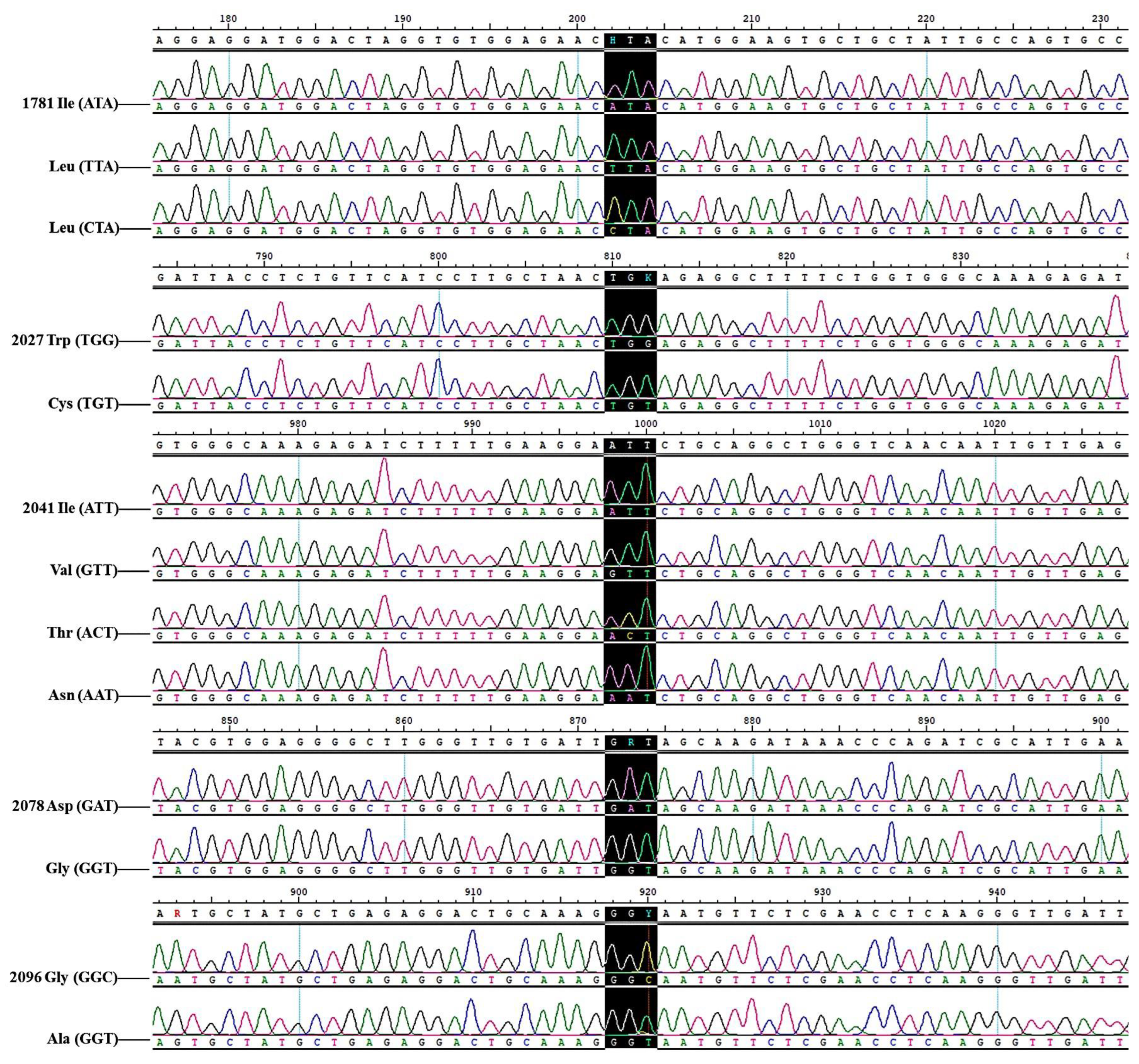
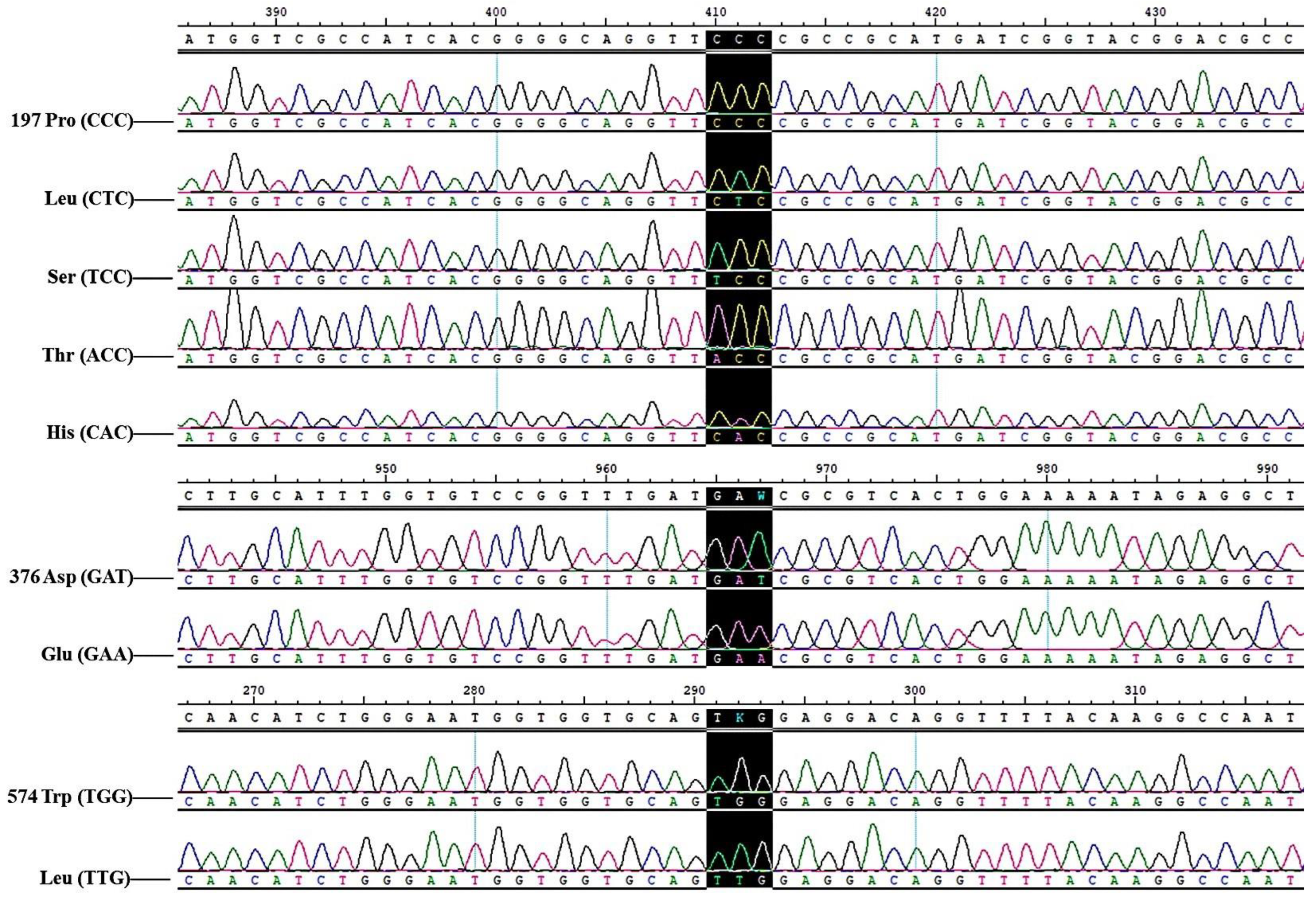
3.4. The Detection Results of ACCase and ALS Gene Mutations in B. syzigachne Populations Resistant to Clodinafop-Propargyl and Mesosulfuron-Methyl
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qu, X.; Zhang, Z.; Gao, P.; Chen, W.; Qiang, S. Intra- and cross-field dispersal of seed by a combine harvester. Pest Manag. Sci. 2021, 77, 4109–4116. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Chauhan, B.S.; Saud, S.; Wu, C.; Hassan, S.; Tanveer, M.; Jan, A.; Huang, J. Weed growth and crop yield loss in wheat as influenced by row spacing and weed emergence times. Crop Prot. 2015, 71, 101–108. [Google Scholar] [CrossRef]
- Van der Meulen, A.; Chauhan, B.S. A review of weed management in wheat using crop competition. Crop Prot. 2017, 95, 38–44. [Google Scholar] [CrossRef]
- Kraehmer, H.; Laber, B.; Rosinger, C.; Schulz, A. Herbicides as weed control agents: State of the art: I. Weed control research and safener technology: The path to modern agriculture. Plant Physiol. 2014, 166, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Jamal, R.Q. Herbicide Resistant Weeds: The technology and weed management. In Herbicides; Andrew, J.P., Jessica, A.K., Eds.; IntechOpen: Rijeka, Yugoslavia, 2013. [Google Scholar] [CrossRef]
- Riechers, D.E.; Soltani, N.; Chauhan, B.S.; Concepcion, J.C.T.; Geddes, C.M.; Jugulam, M.; Kaundun, S.S.; Preston, C.; Wuerrfel, R.J.; Sikkema, P.H. Herbicide resistance is complex: A global review of cross-resistance in weeds within herbicide groups. Weed Sci. 2024, 72, 465–486. [Google Scholar] [CrossRef]
- Jugulam, M.; Shyam, C. Non-target-site resistance to herbicides: Recent developments. Plants 2019, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Torra, J.; Montull, J.M.; Taberner, A.; Onkokesung, N.; Boonham, N.; Edwards, R. Target-site and non-target-site resistance mechanisms confer multiple and cross-resistance to ALS and ACCase inhibiting herbicides in Lolium rigidum from Spain. Front. Plant Sci. 2021, 12, 625138. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, R.; Wang, D.; Valverde, B.E.; Qiang, S. Floating dynamics of Beckmannia syzigachne seed dispersal via irrigation water in a rice field. Agric. Ecosyst. Environ. 2019, 277, 36–43. [Google Scholar] [CrossRef]
- Murphy, B.P.; Tranel, P.J. Target-site mutations conferring herbicide resistance. Plants 2019, 8, 382. [Google Scholar] [CrossRef]
- González-Torralva, F.; Norsworthy, J.K. Overexpression of Acetyl CoA Carboxylase 1 and 3 (ACCase1 and ACCase3), and CYP81A21 were related to cyhalofop resistance in a barnyardgrass accession from Arkansas. Plant Signal. Behav. 2023, 18, 2172517. [Google Scholar] [CrossRef]
- Rigon, C.A.; Gaines, T.A.; Küpper, A.; Dayan, F.E. Metabolism-based herbicide resistance, the major threat among the non-target site resistance mechanisms. Outlooks Pest Manag. 2020, 31, 162–168. [Google Scholar] [CrossRef]
- Li, Y.; Mei, C.; Li, Y.; Tang, R.; Zhai, Q.; Liu, N. Studies on resistance of weeds Backmannia syzigachne and Alopecurus japonicum to herbicide chlorsulfuron. Jiangsu J. Agric. Sci. 1996, 12, 34–38. [Google Scholar]
- Liu, B.; Ding, F.; Wang, M.; Wang, F.; Luo, X.; Li, L. Cross-resistance pattern to ACCase-inhibiting herbicides in a novel Trp1999Leu mutation American sloughgrass (Beckmannia syzigachne) population. Pestic. Biochem. Physiol. 2019, 159, 80–84. [Google Scholar] [CrossRef]
- Wang, J.; Qi, J.; Ouyang, Y.; Zhou, S.; Qin, L.; Zhang, B.; Bai, L.; Pan, L. The mutation Asp-376-Glu in the ALS gene confers resistance to mesosulfuron-methyl in Beckmannia syzigachne. Plant Physiol. Biochem. 2024, 215, 109083. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.; Li, X.; Li, D.; Li, Z.; Cui, H. Pro-197-Ser Mutation in ALS and high-level GST Activities: Multiple resistance to ALS and ACCase inhibitors in Beckmannia syzigachne. Front. Plant Sci. 2020, 11, 572610. [Google Scholar] [CrossRef]
- Pan, L.; Gao, H.; Wu, H.; Dong, L. Molecular basis of multiple resistance to herbicides inhibiting acetyl-CoA carboxylase and acetolactate synthase in American sloughgrass (Beckmannia syzigachne) from China. Crop Pasture Sci. 2016, 67, 1208–1214. [Google Scholar] [CrossRef]
- Pan, L.; Li, J.; Zhang, T.; Zhang, D.; Dong, L.Y. Cross-resistance patterns to acetyl coenzyme A carboxylase (ACCase) inhibitors associated with different ACCase mutations in Beckmannia syzigachne. Weed Res. 2015, 55, 609–620. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Li, D.; Han, Y.; Li, Z.; Yu, H.; Cui, H. Non-target-site and target-site resistance to AHAS inhibitors in American sloughgrass (Beckmannia syzigachne). J. Integr. Agric. 2018, 17, 2714–2723. [Google Scholar] [CrossRef]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-logistic analysis of herbicide dose-response relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, H.; Quan, Z.; Liang, R.; Ren, Z. Technical code of practice for monitoring of herbicide resistance—Broad leaf weeds in wheat. Agric. Ind. Stand. People’s Repub. China 2020, ICS 65.020.01. [Google Scholar]
- Yin, F.; Jiang, J.; Liao, M.; Cao, H.; Huang, Z.; Zhao, N. Fenoxaprop-P-ethyl, mesosulfuron-ethyl, and isoproturon resistance status in Beckmannia syzigachne from wheat fields across Anhui Province, China. Pestic. Biochem. Physiol. 2024, 198, 105711. [Google Scholar] [CrossRef] [PubMed]
- Nakka, S.; Jugulam, M.; Peterson, D.; Asif, M. Herbicide resistance: Development of wheat production systems and current status of resistant weeds in wheat cropping systems. Crop J. 2019, 7, 750–760. [Google Scholar] [CrossRef]
- Owen, M.D.; Zelaya, I.A. Herbicide-resistant crops and weed resistance to herbicides. Pest Manag. Sci. 2005, 61, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Délye, C.; Zhang, X.Q.; Michel, S.; Matéjicek, A.; Powles, S.B. Molecular bases for sensitivity to acetyl-coenzyme A carboxylase inhibitors in black-grass. Plant Physiol. 2005, 137, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, W.; Chi, Y.; Guo, W.; Luo, X.; Wang, J. Molecular mechanism of mesosulfuron-methyl resistance in multiply-resistant American Sloughgrass (Beckmannia syzigachne). Weed Sci. 2015, 63, 781–787. [Google Scholar] [CrossRef]
- Wang, J.; Cao, W.; Guo, Q.; Yang, Y.; Bai, L.; Pan, L. Resistance to mesosulfuron-methyl in Beckmannia syzigachne may involve ROS burst and non-target-site resistance mechanisms. Ecotoxicol. Environ. Saf. 2022, 229, 113072. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Li, X.; Cui, H. RNA-Seq transcriptome analysis to identify candidate genes involved in non-target site-based mesosulfuron-methyl resistance in Beckmannia syzigachne. Pestic. Biochem. Physiol. 2021, 171, 104738. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, B.; Li, Y.; Luo, X.; Li, L. Non–target site based resistance to the ALS-inhibiting herbicide mesosulfuron-methyl in American sloughgrass (Beckmannia syzigachne). Weed Sci. 2019, 67, 527–533. [Google Scholar] [CrossRef]
- Beckie, H.J. Herbicide resistance in plants. Plants 2020, 9, 435. [Google Scholar] [CrossRef]
- Huang, Y.; Li, N.; Wang, D.; Du, J.; Liu, W.; Wang, J.; Li, W. Overexpression of a glycosyltransferase gene from a metabolically poly-resistant Beckmannia syzigachne population alters growth and confers herbicide resistance to brachypodium distachyon. Phyton 2022, 91, 761. [Google Scholar] [CrossRef]
- Bai, S.; Yin, M.; Lyu, Q.; Jiang, B.; Li, L. Cytochrome P450 BsCYP99A44 and BsCYP704A177 confer metabolic resistance to ALS herbicides in Beckmannia syzigachne. Int. J. Mol. Sci. 2022, 23, 12175. [Google Scholar] [CrossRef] [PubMed]
- Depetris, M.B.; Muñiz Padilla, E.; Ayala, F.; Tuesca, D.; Breccia, G. Resistance to acetyl-CoA carboxylase (ACCase)-inhibiting herbicides in Lolium multiflorum Lam. populations of Argentina. Pest Manag. Sci. 2024, 80, 6600–6606. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhou, F.; Zhang, Y.; Chen, J. Resistance of American sloughgrass (Bechmannia syzigachne) populations to ACCase-inhibiting herbicides involves three different target site mutations from China. Pestic. Biochem. Physiol. 2015, 124, 93–96. [Google Scholar] [CrossRef]
- Pan, L.; Li, J.; Zhang, W.N.; Dong, L. Detection of the I1781L mutation in fenoxaprop-p-ethyl-resistant American sloughgrass (Beckmannia syzigachne Steud.), based on the loop-mediated isothermal amplification method. Pest Manag. Sci. 2015, 71, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Gao, H.; Xia, W.; Zhang, T.; Dong, L. Establishing a herbicide-metabolizing enzyme library in Beckmannia syzigachne to identify genes associated with metabolic resistance. J. Exp. Bot. 2016, 67, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 22 January 2025).
- Porri, A.; Panozzo, S.; Tekeste Sisay, M.; Scarabel, L.; Lerchl, J.; Milani, A. 3D structure of acetolactate synthase explains why the Asp-376-Glu point mutation does not give the same resistance level to different imidazolinone herbicides. Pestic. Biochem. Physiol. 2024, 204, 106070. [Google Scholar] [CrossRef]
- Zakaria, N.; Ruzmi, R.; Moosa, S.; Asib, N.; Zulperi, D.; Ismail, S.I.; Ahmad-Hamdani, M.S. Asp-376-Glu substitution endows target-site resistance to AHAS inhibitors in Limnocharis flava, an invasive weed in tropical rice fields. Physiol. Mol. Biol. Plants 2021, 27, 969–983. [Google Scholar] [CrossRef]


| Herbicides | Doses (g a.i. ha−1) |
|---|---|
| clodinafop-propargyl (15%, WP, Syngenta) | S: 0, 8.44 (1/8X), 16.88 (1/4X), 33.75 (1/2X), 67.5 (1X), 135 (2X) SR: 0, 33.75 (1/2X), 67.5 (1X), 135 (2X), 270 (4X), 540 (8X), 1080 (16X) |
| mesosulfuron-methyl (30 g/L, OD, Bayer) | S: 0, 1.69 (1/8X), 3.38 (1/4X), 6.75 (1/2X), 13.5 (1X), 27 (2X) SR: 0, 6.75 (1/2X), 13.5 (1X), 27 (2X), 54 (4X), 108 (8X), 216 (16X) |
| Collection Province | Total Number | The Population Number of Each Resistance Level to Both Herbicides | |||||||||||||||
| RIC = 1 | RIC = 1 | RIC = 1 | RIC = 1 | 1 < RIC ≤ 3 | 1 < RIC ≤ 3 | 1 < RIC ≤ 3 | 1 < RIC ≤ 3 | 3 < RIC ≤ 10 | 3 < RIC ≤ 10 | 3 < RIC ≤ 10 | 3 < RIC ≤ 10 | RIC ≥ 10 | RIC ≥ 10 | RIC ≥ 10 | RIC ≥ 10 | ||
| RIM = 1 | 1 < RIM ≤ 3 | 3 < RIM ≤ 1 | RIM ≥ 10 | RIM = 1 | 1 < RIM ≤ 3 | 3 < RIM ≤ 10 | RIM ≥ 10 | RIM = 1 | 1 < RIM ≤ 3 | 3 < RIM ≤ 10 | RIM ≥ 10 | RIM = 1 | 1 < RIM ≤ 3 | 3 < RIM ≤ 10 | RIM ≥ 10 | ||
| Anhui | 46 | 0 | 3 | 0 | 0 | 1 | 9 | 2 | 0 | 0 | 1 | 2 | 1 | 1 | 22 | 4 | 0 |
| Hubei | 13 | 1 | 6 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Jiangsu | 59 | 0 | 0 | 0 | 0 | 0 | 4 | 4 | 2 | 0 | 4 | 1 | 0 | 1 | 22 | 15 | 6 |
| Shandong | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Total | 120 | 1 | 9 | 0 | 0 | 2 | 15 | 7 | 2 | 1 | 5 | 3 | 1 | 2 | 46 | 19 | 7 |
| Mutations | The Population Number a of Different Provinces | Total | |||
|---|---|---|---|---|---|
| Anhui | Hubei | Jiangsu | Shandong | ||
| Number of detection populations | 43 | 6 | 59 | 2 | 110 |
| Number of mutation populations | 36 | 5 | 54 | 2 | 97 |
| Single-point mutation | |||||
| Ile-1781-Leu | 16 | 1 | 17 | 2 | 36 |
| Trp-2027-Cys | 4 | 1 | 7 | 0 | 12 |
| Ile-2041-Asn | 12 | 2 | 27 | 0 | 41 |
| Ile-2041-Val | 1 | 0 | 0 | 0 | 1 |
| Asp-2078-Gly | 15 | 1 | 25 | 0 | 41 |
| Gly-2096-Ala | 1 | 0 | 5 | 0 | 6 |
| Double-site mutation | |||||
| Ile-1781-Leu and Trp-2027-Cys | 1 | 1 | 0 | 0 | 2 |
| Ile-1781-Leu and Ile-2041-Asn | 3 | 0 | 2 | 0 | 5 |
| Ile-1781-Leu and Asp-2078-Gly | 3 | 0 | 2 | 0 | 5 |
| Trp-2027-Cys and Ile-2041-Asn | 0 | 0 | 1 | 0 | 1 |
| Trp-2027-Cys and Asp-2078-Gly | 0 | 0 | 1 | 0 | 1 |
| Ile-2041-Asn and Asp-2078-Gly | 0 | 0 | 2 | 0 | 2 |
| Mutations | The Population Number a of Different Provinces | Total | |||
|---|---|---|---|---|---|
| Anhui | Hubei | Jiangsu | Shandong | ||
| Number of detection populations | 44 | 10 | 58 | 2 | 114 |
| Number of mutation populations | 3 | 1 | 20 | 1 | 25 |
| Pro-197-Ser | 1 | 0 | 6 | 0 | 7 |
| Pro-197-Thr | 1 | 0 | 8 | 0 | 9 |
| Pro-197-His | 1 | 0 | 1 | 0 | 2 |
| Pro-197-Leu | 0 | 0 | 2 | 0 | 2 |
| Asp-376-Glu | 0 | 0 | 1 | 0 | 1 |
| Trp-574-Leu | 0 | 1 | 2 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Li, X.; Zhang, S.; Guo, X.; Li, Z.; Chen, J.; Wei, S.; Cui, H. Preliminary Investigation on Resistance of Beckmannia syzigachne to Clodinafop-Propargyl and Mesosulfuron-Methyl from China. Agronomy 2025, 15, 314. https://doi.org/10.3390/agronomy15020314
Peng L, Li X, Zhang S, Guo X, Li Z, Chen J, Wei S, Cui H. Preliminary Investigation on Resistance of Beckmannia syzigachne to Clodinafop-Propargyl and Mesosulfuron-Methyl from China. Agronomy. 2025; 15(2):314. https://doi.org/10.3390/agronomy15020314
Chicago/Turabian StylePeng, Licun, Xiangju Li, Shuai Zhang, Xiaotong Guo, Zheng Li, Jingchao Chen, Shouhui Wei, and Hailan Cui. 2025. "Preliminary Investigation on Resistance of Beckmannia syzigachne to Clodinafop-Propargyl and Mesosulfuron-Methyl from China" Agronomy 15, no. 2: 314. https://doi.org/10.3390/agronomy15020314
APA StylePeng, L., Li, X., Zhang, S., Guo, X., Li, Z., Chen, J., Wei, S., & Cui, H. (2025). Preliminary Investigation on Resistance of Beckmannia syzigachne to Clodinafop-Propargyl and Mesosulfuron-Methyl from China. Agronomy, 15(2), 314. https://doi.org/10.3390/agronomy15020314





