Cytological Characterization of vrnp 1, a Pollen-Free Male Sterile Mutant in Mung Bean (Vigna radiata)
Abstract
1. Introduction
1.1. The Significance of the Tapetum and Pollen Wall for Plant Fertility
1.2. Investigation on Male Sterility and Hybrid Vigor in Mung Bean Mutants
2. Materials and Methods
2.1. Plant Materials and EMS Mutagenesis
2.2. Genetic Analysis of Male Sterile Mutants
2.3. Observation of Anther Paraffin Sections
2.4. Observation of Pollen Grains
2.5. Observation of Pollen Wall
3. Results
3.1. Male Sterility in the vrnp 1 Mutant Is Controlled by a Single Gene
3.2. The vrnp 1 Mutant Is Completely Sterile
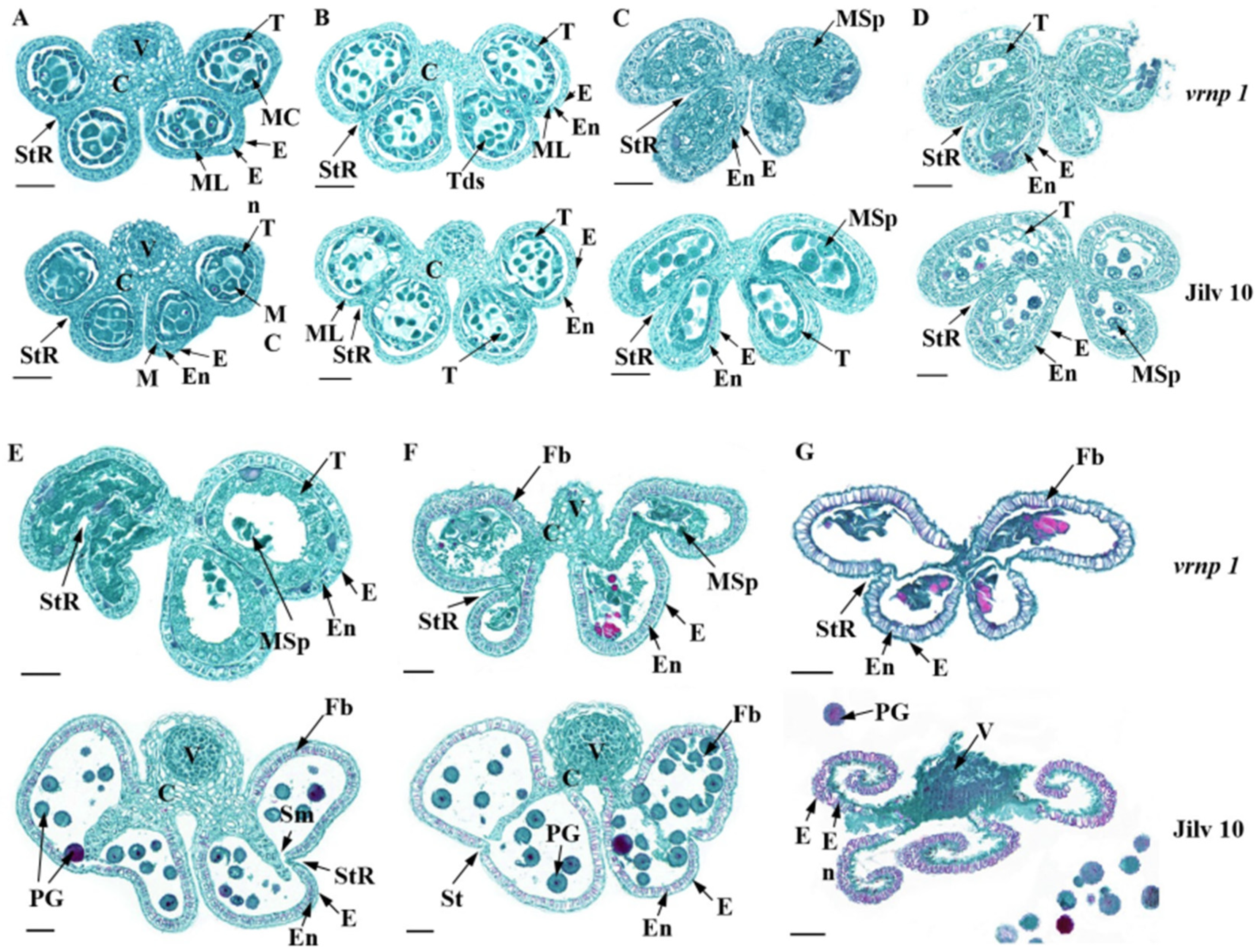
3.3. Anther Development in the vrnp-1 Mutant Is Affected
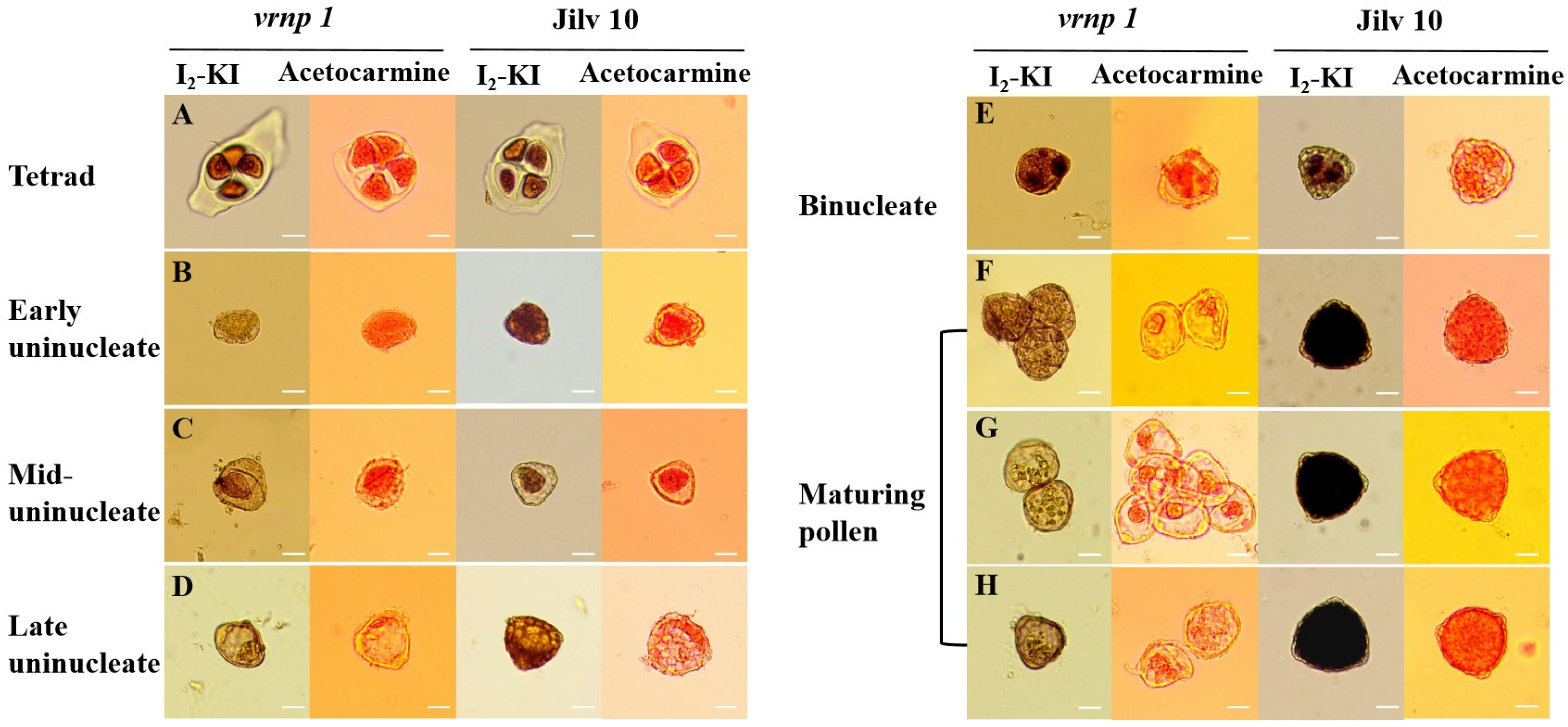
3.4. Pollen Development in the vrnp 1 Mutant Is Affected at the Early Uninucleate Stage
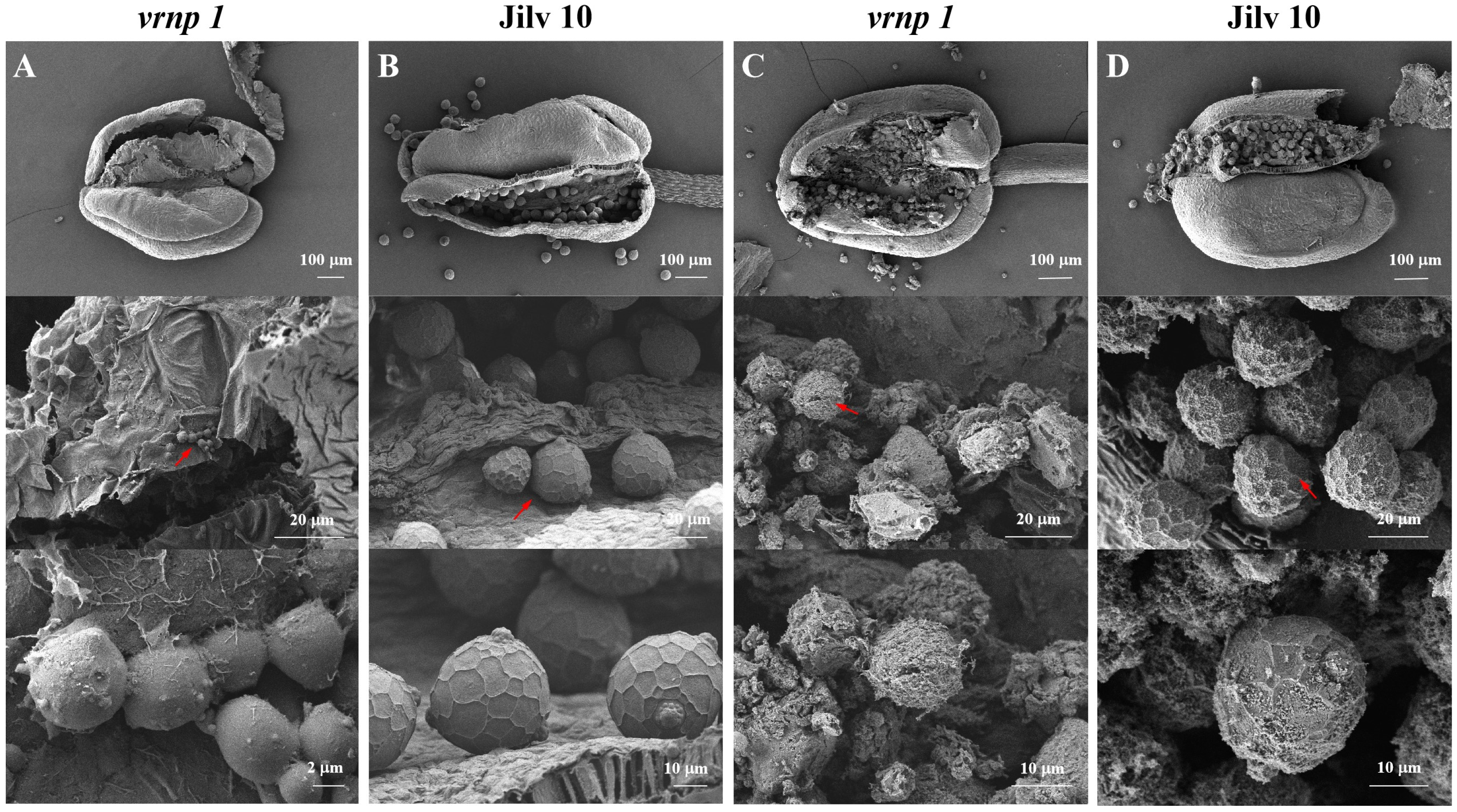
3.5. Scanning Electron Microscopy Observations of the Anther Interior
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sangiri, C.; Kaga, A.; Tomooka, N.; Vaughan, D.; Srinives, P. Genetic diversity of the mungbean (Vigna radiata, Leguminosae) genepool on the basis of microsatellite analysis. Aust. J. Bot. 2007, 55, 837–847. [Google Scholar] [CrossRef]
- Chen, T.X.; Hu, L.L.; Wang, S.H.; Wang, L.X.; Cheng, X.Z.; Chen, H.L. Construction of high-density genetic map and identification of a bruchid resistance locus in mung bean (Vigna radiata L.). Front. Genet. 2022, 13, 903267. [Google Scholar] [CrossRef] [PubMed]
- Sorajjapinun, W.; Srinives, P. Chasmogamous mutant, a novel character enabling commercial hybrid seed production in mungbean. Euphytica 2011, 181, 217–222. [Google Scholar] [CrossRef]
- Lin, Y.; Laosatit, K.; Chen, J.B.; Yuan, X.X.; Wu, R.R.; Amkul, K.; Chen, X.; Somta, P. Mapping and functional characterization of Stigma Exposed 1, a DUF1005 gene controlling petal and stigma cells in mungbean (Vigna radiata). Front. Plant Sci. 2020, 11, 575922. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Chen, B.R.; Cheng, Y.X.; Su, Y.F.; Song, M.Y.; Guo, R.Q.; Wang, M.H.; Deng, K.P.; Lan, T.J.; Bao, S.Y.; et al. Integration of GWAS and RNA-seq analysis to identify SNPs and candidate genes associated with alkali stress tolerance at the germination stage in mung bean. Genes 2023, 14, 1294. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Xue, C.C.; Lin, Y.; Yan, Q.; Chen, J.B.; Wu, R.R.; Zhang, X.Y.; Chen, X.; Yuan, X.X. Genetic analysis and identification of VrFRO8, a salt tolerance-related gene in mungbean. Gene 2022, 836, 146658. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Kim, S.K.; Kim, M.Y.; Lestari, P.; Kim, K.H.; Ha, B.K.; Jun, T.H.; Hwang, W.J.; Lee, T.; Lee, J.; et al. Genome sequence of mungbean and insights into evolution within Vigna species. Nat. Commun. 2014, 5, 5443. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.M.; Yang, R.; Easdown, W.J.; Thavarajah, D.; Thavarajah, P.; Hughes, J.; Keatinge, J.D. Biofortification of mungbean (Vigna radiata) as a whole food to enhance human health. J. Sci. Food Agric. 2013, 93, 1805–1813. [Google Scholar] [CrossRef]
- Somta, P.; Laosatit, K.; Yuan, X.X.; Chen, X. Thirty years of mungbean genome research: Where do we stand and what have we learned? Front. Plant Sci. 2022, 13, 944721. [Google Scholar] [CrossRef] [PubMed]
- Schnable, P.S.; Springer, N.M. Progress toward understanding heterosis in crop plants. Annu. Rev. Plant Biol. 2013, 64, 71–88. [Google Scholar] [CrossRef]
- Wang, D.P.; Mu, Y.Y.; Hu, X.J.; Ma, B.; Wang, Z.B.; Zhu, L.; Xu, J.; Huang, C.L.; Pan, Y.H. Comparative proteomic analysis reveals that the heterosis of two maize hybrids is related to enhancement of stress response and photosynthesis respectively. BMC Plant Biol. 2021, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Ramlal, A.; Nautiyal, A.; Baweja, P.; Mahto, R.K.; Mehta, S.; Mallikarunja, B.P.; Vijayan, R.; Saluja, S.; Kumar, V.; Dhiman, S.K.; et al. Harnessing heterosis and male sterility in soybean [Glycine max (L.) Merrill]: A critical revisit. Front. Plant Sci. 2022, 13, 981768. [Google Scholar] [CrossRef] [PubMed]
- Barad, H.R.; Pithia, M.S.; Vachhani, J. Heterosis and combining ability studies for economic traits in genetically diverse lines of mungbean [Vigna radiata (L.) wilczek]. Legume Res. 2008, 31, 68–71. [Google Scholar]
- Rout, K.; Mishra, T.K.; Bastia, D.N.; Pradhan, B. Studies on heterosis for yield and yield components in mungbean [Vigna radiata (L.) Wilczek]. Res. Crops 2010, 11, 87–90. [Google Scholar]
- Tantasawat, P.A.; Khajudparn, P.; Prajongjai, T.; Poolsawat, O. Heterosis for the improvement of yield in mungbean [Vigna radiata (L.) Wilczek]. Genet. Mol. Res. 2015, 14, 10444–10451. [Google Scholar] [CrossRef]
- Nie, Z.X.; Zhao, T.J.; Liu, M.F.; Dai, J.Y.; He, T.T.; Lyu, D.; Zhao, J.M.; Yang, S.P.; Gai, J.Y. Molecular mapping of a novel male-sterile gene msNJ in soybean [Glycine max (L.) Merr.]. Plant Reprod. 2019, 32, 371–380. [Google Scholar] [CrossRef]
- Johnson, M.A.; Harper, J.F.; Palanivelu, R. A fruitful journey: Pollen tube navigation from germination to fertilization. Annu. Rev. Plant Biol. 2019, 70, 809–837. [Google Scholar] [CrossRef] [PubMed]
- Sze, H.; Palanivelu, R.; Harper, J.F.; Johnson, M.A. Holistic insights from pollen omics: Co-opting stress-responsive genes and ER-mediated proteostasis for male fertility. Plant Physiol. 2021, 187, 2361–2380. [Google Scholar] [CrossRef]
- Paxson-Sowders, D.M.; Dodrill, C.H.; Owen, H.A.; Makaroff, C.A. DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol. 2001, 127, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Sanders, P.M.; Bui, A.Q.; Weterings, K.; McIntire, K.N.; Hsu, Y.; Lee, P.Y.; Truong, M.T.; Beals, T.P.; Goldberg, R.B. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 1999, 11, 297–322. [Google Scholar] [CrossRef]
- Wang, B.; Xue, J.S.; Yu, Y.H.; Liu, S.Q.; Zhang, J.X.; Yao, X.Z.; Liu, Z.X.; Xu, X.F.; Yang, Z.N. Fine regulation of ARF17 for anther development and pollen formation. BMC Plant Biol. 2017, 17, 243. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.F.; Talle, B.; Wilson, Z.A. Anther and pollen development: A conserved developmental pathway. Title of the article. J. Integr. Plant Biol. 2015, 57, 876–891. [Google Scholar] [CrossRef]
- Lou, Y.; Zhou, H.S.; Han, Y.; Zeng, Q.Y.; Zhu, J.; Yang, Z.N. Positive regulation of AMS by TDF1 and the formation of a TDF1-AMS complex are required for anther development in Arabidopsis thaliana. New Phytol. 2018, 217, 378–391. [Google Scholar] [CrossRef]
- Bai, Z.Y.; Ding, X.L.; Zhang, R.J.; Yang, Y.H.; Wei, B.G.; Yang, S.P.; Gai, J.Y. Transcriptome analysis reveals the genes related to pollen abortion in a cytoplasmic male-sterile soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2022, 23, 12227. [Google Scholar] [CrossRef]
- Xiang, X.J.; Sun, L.P.; Yu, P.; Yang, Z.F.; Zhang, P.P.; Zhang, Y.X.; Wu, W.X.; Chen, D.B.; Zhan, X.D.; Khan, R.M.; et al. The MYB transcription factor Baymax1 plays a critical role in rice male fertility. Theor. Appl. Genet. 2021, 134, 453–471. [Google Scholar] [CrossRef]
- Ariizumi, T.; Toriyama, K. Genetic regulation of sporopollenin synthesis and pollen exine development. Annu. Rev. Plant Biol. 2011, 62, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Long, T.; Wang, Y.F.; Tong, X.H.; Tang, J.; Li, J.L.; Wang, H.M.; Tang, L.Q.; Li, Z.Y.; Shu, Y.Z.; et al. RMS2 encoding a GDSL lipase mediates lipid homeostasis in anthers to determine rice male fertility. Plant Physiol. 2020, 182, 2047–2064. [Google Scholar] [CrossRef]
- Fang, X.L.; Feng, X.C.; Sun, X.Y.; Yang, X.D.; Li, Q.; Yang, X.L.; Xu, J.; Zhou, M.H.; Lin, C.J.; Sui, Y.; et al. Natural variation of MS2 confers male fertility and drives hybrid breeding in soybean. Plant Biotechnol. J. 2023, 21, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sun, X.H.; Zhang, Z.G.; Feng, D.; Zhang, Q.; Han, L.D.; Wu, J.X.; Lu, T.G. GLUCAN SYNTHASE-LIKE 5 (GSL5) plays an essential role in male fertility by regulating callose metabolism during microsporogenesis in rice. Plant Cell Physiol. 2015, 56, 497–509. [Google Scholar] [CrossRef]
- Quilichini, T.D.; Douglas, C.J.; Samuels, A.L. New views of tapetum ultrastructure and pollen exine development in Arabidopsis thaliana. Ann. Bot. 2014, 114, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Parish, R.W.; Li, S.F. Death of a tapetum: A programme of developmental altruism. Plant Sci. 2010, 178, 73–89. [Google Scholar] [CrossRef]
- Xie, H.T.; Wan, Z.Y.; Li, S.; Zhang, Y. Spatiotemporal production of reactive oxygen species by NADPH oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell 2014, 26, 2007–2023. [Google Scholar] [CrossRef]
- Shi, J.X.; Cui, M.H.; Yang, L.; Kim, Y.J.; Zhang, D.B. Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Izhar, S.; Frankle, R. Mechanism of male sterility in Petunia: The relationship between pH, callase activity in the anthers, and the breakdown of the microsorogenesis. Theor. Appl. Genet. 1971, 41, 104–108. [Google Scholar] [CrossRef]
- Mepham, R.H. Development of the pollen grain wall: Further work with Tradescantia Bracteata. Protoplasma 1970, 71, 39–54. [Google Scholar] [CrossRef]
- Pacini, E. Tapetum character states: Analytical keys for tapetum types and activities. Can. J. Bot. 1997, 75, 1448–1459. [Google Scholar] [CrossRef]
- Dickinson, H.G.; Elleman, C.J.; Doughty, J. Pollen coatings—Chimaeric genetics and new functions. Sex. Plant Reprod. 2000, 12, 302–309. [Google Scholar] [CrossRef]
- Ma, X.F.; Wu, Y.; Zhang, G.F. Formation pattern and regulatory mechanisms of pollen wall in Arabidopsis. J. Plant Physiol. 2021, 260, 153388. [Google Scholar] [CrossRef]
- Yang, C.Y.; Vizcay-Barrena, G.; Conner, K.; Wilson, Z.A. MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell 2007, 19, 3530–3548. [Google Scholar] [CrossRef]
- Lu, J.Y.; Xiong, S.X.; Yin, W.Z.; Teng, X.D.; Lou, Y.; Zhu, J.; Zhang, C.; Gu, J.N.; Wilson, Z.A.; Yang, Z.N. MS1, a direct target of MS188, regulates the expression of key sporophytic pollen coat protein genes in Arabidopsis. J. Exp. Bot. 2020, 71, 4877–4889. [Google Scholar] [CrossRef] [PubMed]
- Wilson, Z.A.; Morroll, S.M.; Dawson, J.; Swarup, R.; Tighe, P.J. The Arabidopsis MALE STERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J. 2001, 28, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, Y.J.; Timofejeva, L.; Chen, C.B.; Grossniklaus, U.; Ma, H. Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development 2006, 133, 3085–3095. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Xu, X.F.; Zhu, J.; Gu, J.N.; Blackmore, S.; Yang, Z.N. The tapetal AHL family protein TEK determines nexine formation in the pollen wall. Nat. Commun. 2014, 5, 3855. [Google Scholar] [CrossRef] [PubMed]
- Aboulela, M.; Nakagawa, T.; Oshima, A.; Nishimura, K.; Tanaka, Y. The Arabidopsis COPII components, AtSEC23A and AtSEC23D, are essential for pollen wall development and exine patterning. J. Exp. Bot. 2018, 69, 1615–1633. [Google Scholar] [CrossRef]
- Blackmore, S.; Wortley, A.H.; Skvarla, J.J.; Rowley, J.R. Pollen wall development in flowering plants. New Phytol. 2007, 174, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ding, Z.W.; Vizcay-Barrena, G.; Shi, J.X.; Liang, W.Q.; Yuan, Z.; Werck-Reichhart, D.; Schreiber, L.; Wilson, Z.A.; Zhang, D.B. ABORTED MICROSPORES acts as a master regulator of pollen wall formation in Arabidopsis. Plant Cell 2014, 26, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Edlund, A.F.; Swanson, R.; Preuss, D. Pollen and stigma structure and function: The role of diversity in pollination. Plant Cell 2004, 16 (Suppl. S1), S84–S97. [Google Scholar] [CrossRef] [PubMed]
- Quilichini, T.D.; Friedmann, M.C.; Samuels, A.L.; Douglas, C.J. ATP-binding cassette transporter G26 is required for male fertility and pollen exine formation in Arabidopsis. Plant Physiol. 2010, 154, 678–690e. [Google Scholar] [CrossRef]
- Quilichini, T.D.; Samuels, A.L.; Douglas, C.J. ABCG26-mediated polyketide trafficking and hydroxycinnamoyl spermidines contribute to pollen wall exine formation in Arabidopsis. Plant Cell 2014, 26, 4483–4498. [Google Scholar] [CrossRef]
- Dou, X.Y.; Yang, K.Z.; Zhang, Y.; Wang, W.; Liu, X.L.; Chen, L.Q.; Zhang, X.Q.; Ye, D. WBC27, an adenosine Tri-phosphate-binding cassette protein, controls pollen wall formation and patterning in Arabidopsis. J. Integr. Plant Biol. 2011, 53, 74–88. [Google Scholar] [CrossRef]
- Ariizumi, T.; Hatakeyama, K.; Hinata, K.; Sato, S.; Kato, T.; Tabata, S.; Toriyama, K. The HKM gene, which is identical to the MS1 gene of Arabidopsis thaliana, is essential for primexine formation and exine pattern formation. Sex. Plant Reprod. 2005, 18, 1–7. [Google Scholar] [CrossRef]
- Vizcay-Barrena, G.; Wilson, Z.A. Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. J. Exp. Bot. 2006, 57, 2709–2717. [Google Scholar] [CrossRef]
- Xing, S.P.; Zachgo, S. ROXY1 and ROXY2, two Arabidopsis glutaredoxin genes, are required for anther development. Plant J. 2008, 53, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.Y.; Wu, S.W.; Li, Z.W.; Dong, Z.Y.; An, X.L.; Ma, B.; Tian, Y.H.; Li, J.P. Maize genic male-sterility genes and their applications in hybrid breeding: Progress and perspectives. Mol. Plant 2019, 12, 321–342. [Google Scholar] [CrossRef]
- Liu, X.Z.; Zhang, S.W.; Jiang, Y.L.; Yan, T.W.; Fang, C.W.; Hou, Q.C.; Wu, S.W.; Xie, K.; An, X.L.; Wan, X.Y. Use of CRISPR/Cas9-based gene editing to simultaneously mutate multiple homologous genes required for pollen development and male fertility in maize. Cells 2022, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Zhuang, J.Y.; Fan, Y.Y.; Du, J.H.; Cao, L.Y. Progress in research and development on hybrid rice: A super-domesticate in China. Ann. Bot. 2007, 100, 959–966. [Google Scholar] [CrossRef]
- Fan, Y.R.; Zhang, Q.F. Genetic and molecular characterization of photoperiod and thermo-sensitive male sterility in rice. Plant Reprod. 2018, 31, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sorajjapinun, W.; Reiwhongchum, S.; Srinives, P. Identification of parental mungbean lines for production of hybrid varieties Chiang Mai Univ. J. 2003, 2, 97–105. [Google Scholar]
- Chen, J.B.; Somta, P.; Chen, X.; Cui, X.Y.; Yuan, X.X.; Srinives, P. Gene mapping of a mutant mungbean (Vigna radiata L.) using new molecular markers suggests a gene encoding a YUC4-like protein regulates the chasmogamous flower trait. Front. Plant Sci. 2016, 7, 830. [Google Scholar] [CrossRef]
- Barrett, S.C. Mating strategies in flowering plants: The outcrossing-selfing paradigm and beyond. Philos. Trans. R. Soc. Lond. B 2003, 358, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Lin, Y.; Chen, J.B.; Xue, C.C.; Wu, R.R.; Yan, Q.; Chen, X.; Yuan, X.X. Identification and clarification of VrCYCA1: A key genic male sterility-related gene in mungbean by multi-omics analysis. Agriculture 2022, 12, 686. [Google Scholar] [CrossRef]
- Zhu, J.; Lou, Y.; Xu, X.F.; Yang, Z.N. A genetic pathway for tapetum development and function in Arabidopsis. J. Integr. Plant Biol. 2011, 53, 892–900. [Google Scholar] [CrossRef]
- Hord, C.L.; Chen, C.B.; Deyoung, B.J.; Clark, S.E.; Ma, H. The BAM1/BAM2 receptor-like kinases are important regulators of Arabidopsis early anther development. Plant Cell 2006, 18, 1667–1680. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, J.; Ye, N.H.; Cao, J.M.; Tan, M.P.; Zhang, J.H.; Jiang, M.Y. ZmMPK5 is required for the NADPH oxidase-mediated self-propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defence in leaves of maize. J. Exp. Bot. 2010, 61, 4399–4411. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.N.; Zhu, J.; Yu, Y.; Teng, X.D.; Lou, Y.; Xu, X.F.; Liu, J.L.; Yang, Z.N. DYT1 directly regulates the expression of TDF1 for tapetum development and pollen wall formation in Arabidopsis. Plant J. 2014, 80, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Ariizumi, T.; Hatakeyama, K.; Hinata, K.; Inatsugi, R.; Nishida, I.; Sato, S.; Kato, T.; Tabata, S.; Toriyama, K. Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J. 2004, 39, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Nagata, N.; Yoshiba, Y.; Ohme-Takagi, M.; Ma, H.; Shinozaki, K. Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. Plant Cell 2007, 19, 3549–3562. [Google Scholar] [CrossRef] [PubMed]
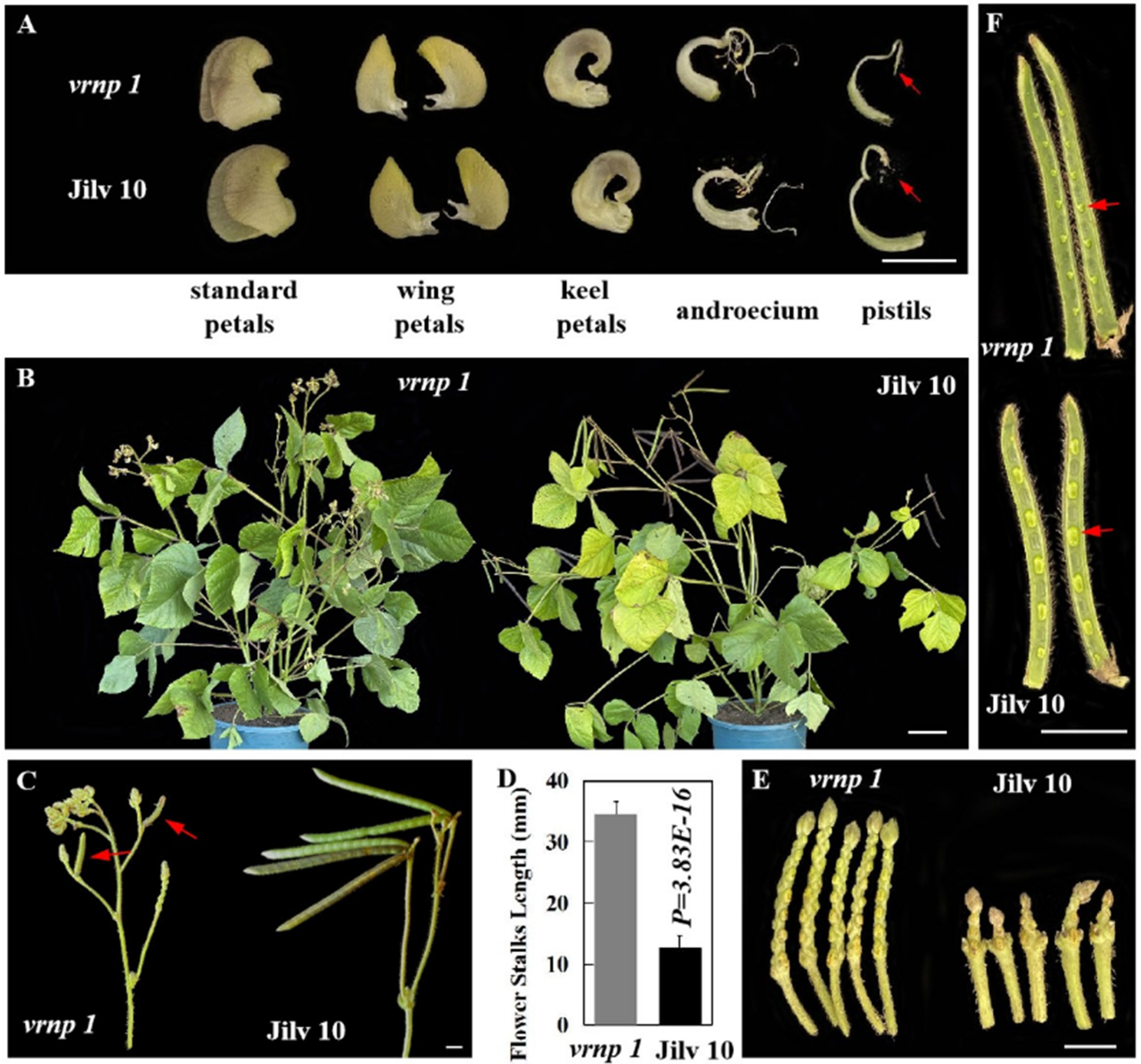
| Phenotype | Theoretical Number | Observed Number | X2(3:1) | P0.05 |
|---|---|---|---|---|
| Fertile | 214.5 | 214 | 3.841 | 0.0047 |
| Sterile | 71.5 | 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Lan, T.; Deng, K.; Wang, M.; Bao, S.; Han, D.; Xu, Y.; Wang, H.; Xu, N.; Guo, Z. Cytological Characterization of vrnp 1, a Pollen-Free Male Sterile Mutant in Mung Bean (Vigna radiata). Agronomy 2025, 15, 312. https://doi.org/10.3390/agronomy15020312
Cheng Y, Lan T, Deng K, Wang M, Bao S, Han D, Xu Y, Wang H, Xu N, Guo Z. Cytological Characterization of vrnp 1, a Pollen-Free Male Sterile Mutant in Mung Bean (Vigna radiata). Agronomy. 2025; 15(2):312. https://doi.org/10.3390/agronomy15020312
Chicago/Turabian StyleCheng, Yuxin, Tianjiao Lan, Kunpeng Deng, Minghai Wang, Shuying Bao, Dan Han, Yapeng Xu, Han Wang, Ning Xu, and Zhongxiao Guo. 2025. "Cytological Characterization of vrnp 1, a Pollen-Free Male Sterile Mutant in Mung Bean (Vigna radiata)" Agronomy 15, no. 2: 312. https://doi.org/10.3390/agronomy15020312
APA StyleCheng, Y., Lan, T., Deng, K., Wang, M., Bao, S., Han, D., Xu, Y., Wang, H., Xu, N., & Guo, Z. (2025). Cytological Characterization of vrnp 1, a Pollen-Free Male Sterile Mutant in Mung Bean (Vigna radiata). Agronomy, 15(2), 312. https://doi.org/10.3390/agronomy15020312





