Genetic Dissection of Anthocyanin Accumulation in Tomato Using GWAS and Hybridization Probe Melting (HPM) for Marker-Assisted Breeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. GBS (Genotyping-by-Sequencing) Library Preparation
2.3. DNA Extraction
2.4. Preparation of Libraries for Next-Generation Sequencing
2.5. Preprocessing
2.6. Alignment and Detection of SNPs and InDels
2.7. GWAS Analysis
2.8. HPM Analysis
3. Results
3.1. Phenotypic Analysis of Seedling Growth and Fruit Trait
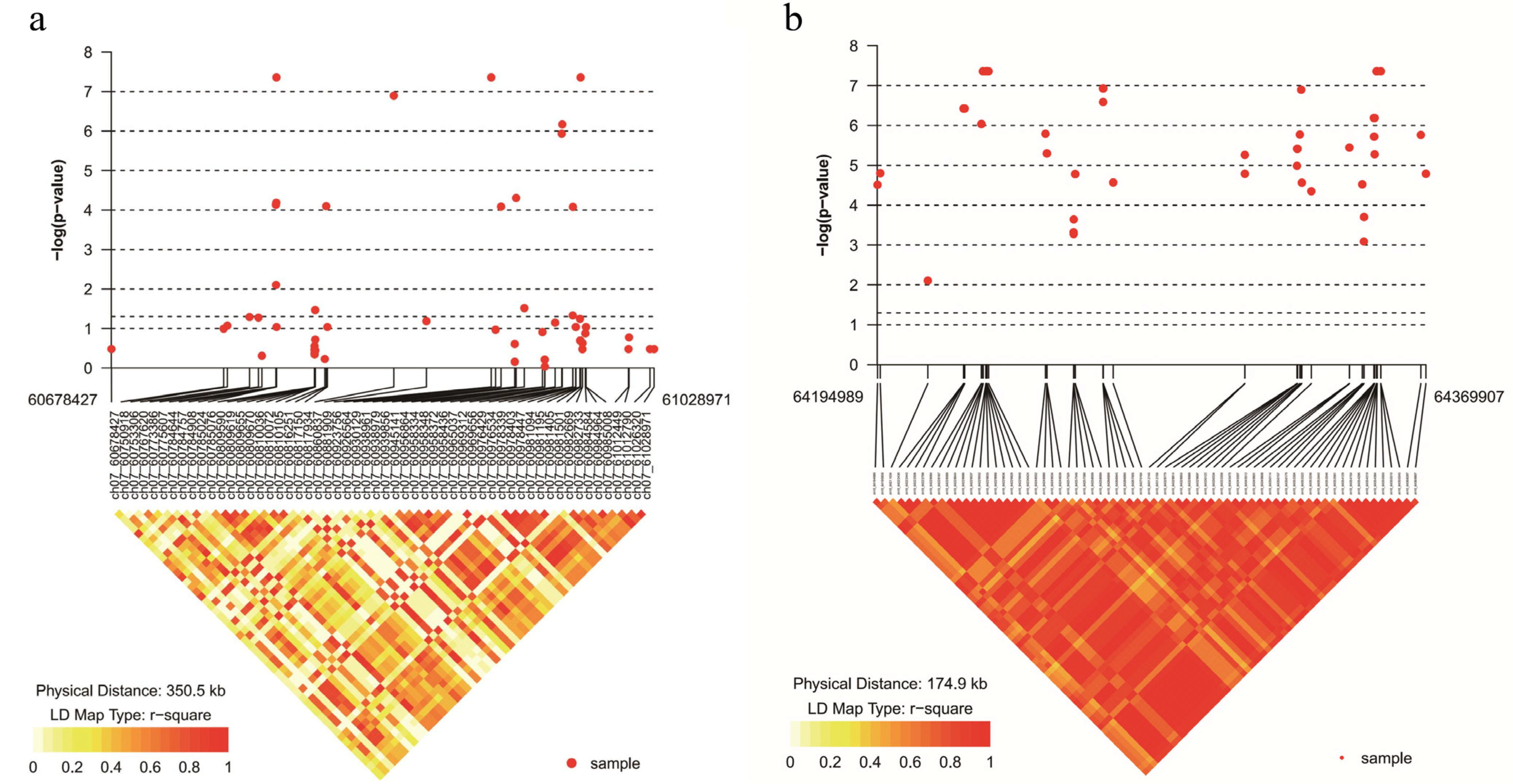
3.2. Identification of Makeres Related to Anthosyanin Content by GWAS Analysis
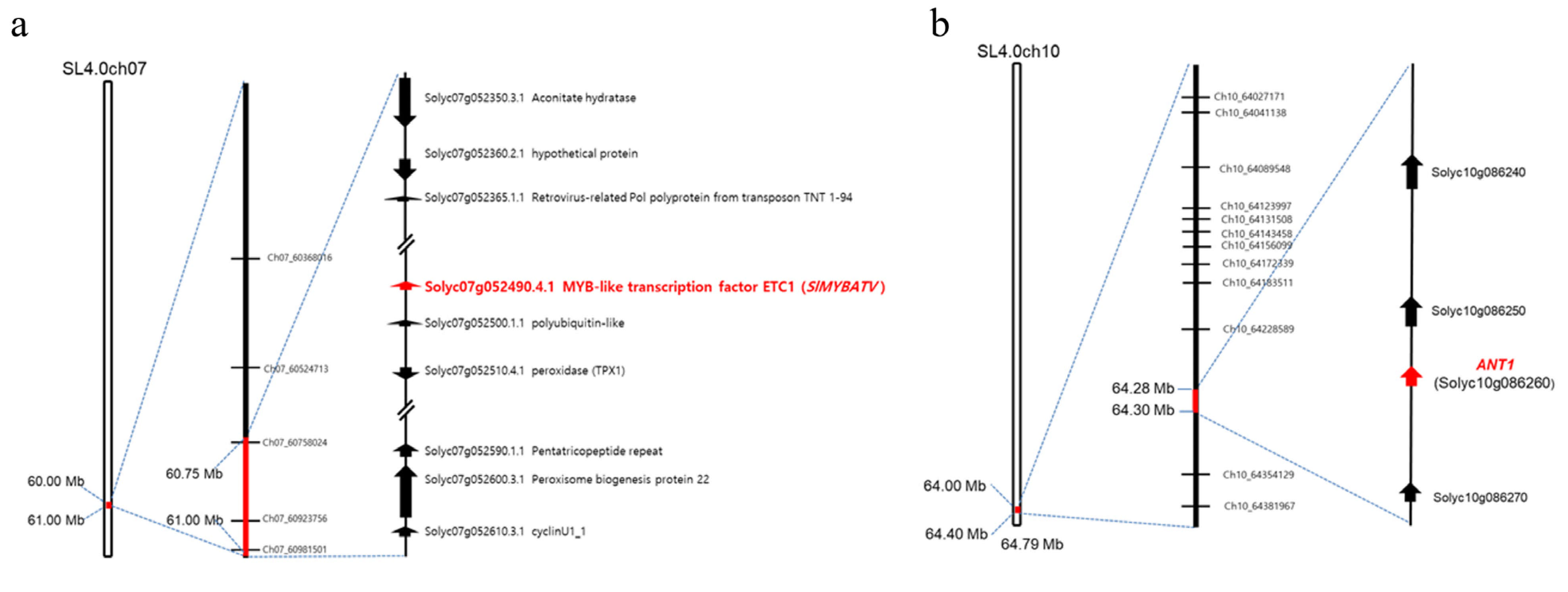
3.3. Mapping of Significantly Associated Genes
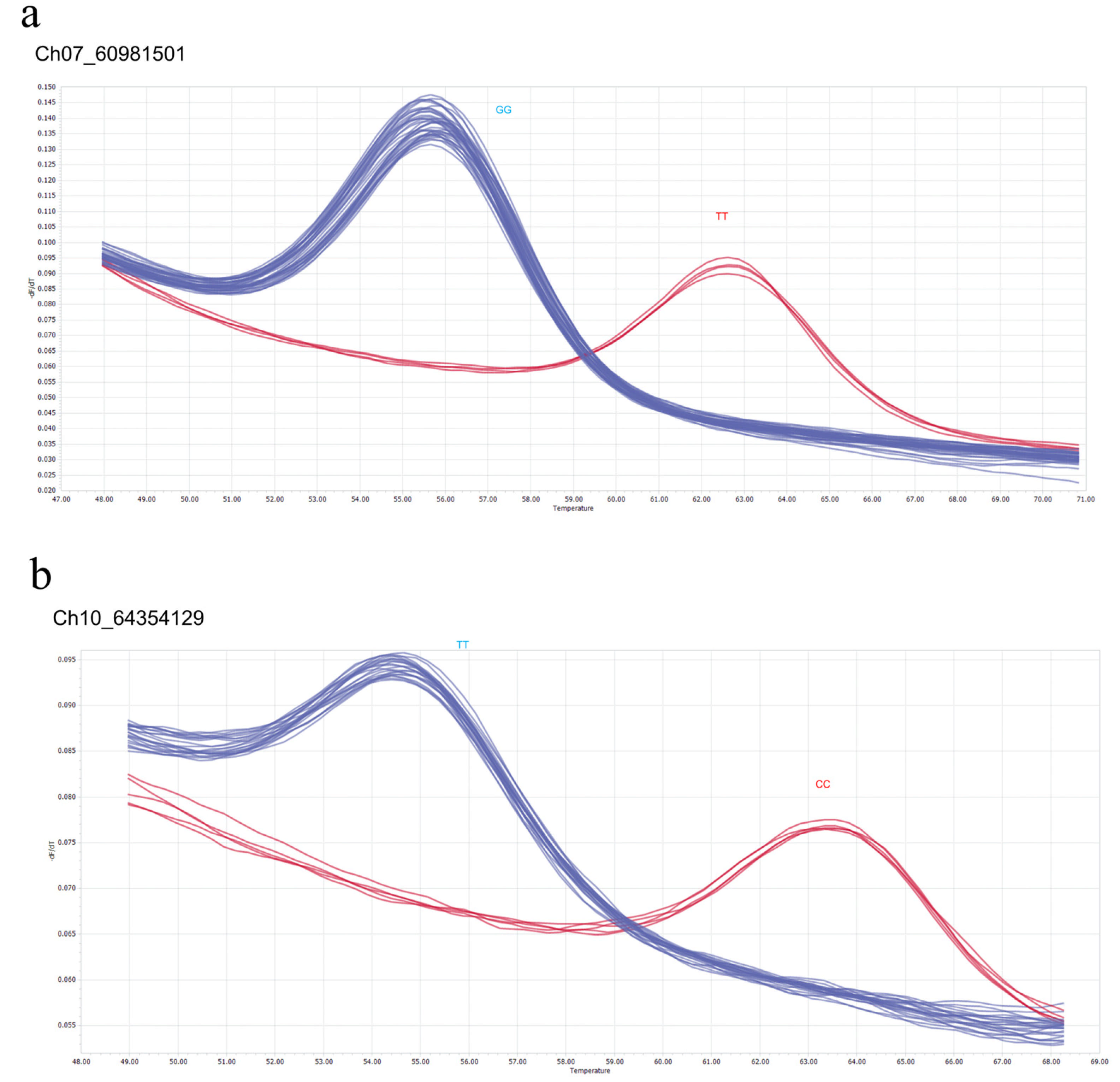
3.4. Identification of Markers for SNP Genotyping
3.5. Genotyping of Purple and Non-Purple Cultivars by Hybridization Probe Melting (HPM)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akotowanou, O.; Adjou, E.; Olubi, A.; Kougblenou, S.; Ahoussi, E.; Sohounhloué, D. The Tomato (Solanum lycopersicum L.) in Community Development: An Overview Focused on Nutritional Properties, Agronomic Constraints, Recent Achievements and Future Prospective. Int. J. Front. Biol. Pharm. Res. 2022, 3, 8–16. [Google Scholar] [CrossRef]
- Joshi, T.; Singh, A. Supply Chain of Tomato: Linking Indian Farmers to International Consumers. Int. J. Agric. Sci. 2021, 17, 650–657. [Google Scholar] [CrossRef]
- Sah, S.K.; Singh, A.K.; Singh, B.K.; Barman, K.; Pal, A.K.; Kumar, A. Heterosis Studies for Growth and Yield Traits in Tomato (Solanum lycopersicum L.). Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2732–2738. [Google Scholar] [CrossRef]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Bhowmik, D.; Kumar, K.P.S.; Paswan, S.; Srivastava, S. Tomato-A Natural Medicine and Its Health Benefits. Phytojournal 2012, 1, 33–43. [Google Scholar]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial Effects and Potential Risks of Tomato Consumption for Human Health: An Overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef]
- Sharma, L.; Kumar, R.; Paul, V.; Pandey, R.; Lal, M.K. Purple Tomato–Importance and Scope: A Review. Agric. Rev. 2024, 45, 464–471. [Google Scholar] [CrossRef]
- Gonzali, S.; Mazzucato, A.; Perata, P. Purple as a Tomato: Towards High Anthocyanin Tomatoes. Trends Plant Sci. 2009, 14, 237–241. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phyther. Res. 2016, 1286, 1265–1286. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health Benefits of Anthocyanins and Molecular Mechanisms: Update from Recent Decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary Anthocyanin-Rich Plants: Biochemical Basis and Recent Progress in Health Benefits Studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; Carvalho, L.; Bernstein, P.S.; Muir, G.J.; Zhao, D.Y.; Katz, N.B. Chemistry, Distribution, and Metabolism of Tomato Carotenoids and Their Impact on Human Health. Exp. Biol. Med. 2002, 227, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Mes, P.J.; Boches, P.; Myers, J.R.; Durst, R. Characterization of Tomatoes Expressing Anthocyanin in the Fruit. J. Am. Soc. Hortic. Sci. 2008, 133, 262–269. [Google Scholar] [CrossRef]
- Pervaiz, T.; Songtao, J.; Faghihi, F.; Haider, M.S.; Fang, J. Naturally Occurring Anthocyanin, Structure, Functions and Biosynthetic Pathway in Fruit Plants. J. Plant Biochem. Physiol. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Mazza, G. Anthocyanins in Fruits, Vegetables, and Grains; Mazza, G., Miniati, E., Eds.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351069700. [Google Scholar]
- Jones, C.M.; Mes, P.; Myers, J.R. Characterization and Inheritance of the Anthocyanin Fruit (Aft) Tomato. J. Hered. 2003, 94, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Povero, G.; Gonzali, S.; Bassolino, L.; Mazzucato, A.; Perata, P. Transcriptional Analysis in High-Anthocyanin Tomatoes Reveals Synergistic Effect of Aft and Atv Genes. J. Plant Physiol. 2011, 168, 270–279. [Google Scholar] [CrossRef]
- Kiferle, C.; Fantini, E.; Bassolino, L.; Povero, G.; Spelt, C.; Buti, S.; Giuliano, G.; Quattrocchio, F.; Koes, R.; Perata, P.; et al. Tomato R2R3-MYB Proteins SlANT1 and SlAN2: Same Protein Activity, Different Roles. PLoS ONE 2015, 10, e0136365. [Google Scholar] [CrossRef]
- Colanero, S.; Tagliani, A.; Perata, P.; Gonzali, S. Alternative Splicing in the Anthocyanin Fruit Gene Encoding an R2R3 MYB Transcription Factor Affects Anthocyanin Biosynthesis in Tomato Fruits. Plant Commun. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Liu, C.C.; Chi, C.; Jin, L.J.; Zhu, J.; Yu, J.Q.; Zhou, Y.H. The BZip Transcription Factor HY5 Mediates CRY1a-Induced Anthocyanin Biosynthesis in Tomato. Plant Cell Environ. 2018, 41, 1762–1775. [Google Scholar] [CrossRef]
- Maligeppagol, M.; Sharath Chandra, G.; Navale, P.M.; Deepa, H.; Rajeev, P.R.; Asokan, R.; Prasad Babu, K.; Bujji Babu, C.S.; Keshava Rao, V.; Krishna Kumar, N.K. Anthocyanin Enrichment of Tomato (Solanum lycopersicum L.) Fruit by Metabolic Engineering. Curr. Sci. 2013, 105, 72–80. [Google Scholar]
- Bovy, A.; De Vos, R.; Kemper, M.; Schijlen, E.; Almenar Pertejo, M.; Muir, S.; Collins, G.; Robinson, S.; Verhoeyen, M.; Hughes, S.; et al. High-Flavonol Tomatoes Resulting from the Heterologous Expression of the Maize Transcription Factor Genes LC and C1. Plant Cell 2002, 14, 2509–2526. [Google Scholar] [CrossRef]
- Zuluaga, D.L.; Gonzali, S.; Loreti, E.; Pucciariello, C.; Degl’Innocenti, E.; Guidi, L.; Alpi, A.; Perata, P. Arabidopsis Thaliana MYB75/PAP1 Transcription Factor Induces Anthocyanin Production in Transgenic Tomato Plants. Funct. Plant Biol. 2008, 35, 606–618. [Google Scholar] [CrossRef]
- Cerqueira, J.V.A.; Zhu, F.; Mendes, K.; Nunes-Nesi, A.; Martins, S.C.V.; Benedito, V.; Fernie, A.R.; Zsögön, A. Promoter Replacement of ANT1 Induces Anthocyanin Accumulation and Triggers the Shade Avoidance Response through Developmental, Physiological and Metabolic Reprogramming in Tomato. Hortic. Res. 2023, 10, uhac254. [Google Scholar] [CrossRef]
- Mathews, H.; Clendennen, S.K.; Caldwell, C.G.; Liu, X.L.; Connors, K.; Matheis, N.; Schuster, D.K.; Menasco, D.J.; Wagoner, W.; Lightner, J.; et al. Activation Tagging in Tomato Identifies a Transcriptional Regulator of Anthocyanin Biosynthesis, Modification, and Transport. Plant Cell 2003, 15, 1689–1703. [Google Scholar] [CrossRef]
- Burghardt, L.T.; Young, N.D.; Tiffin, P. A Guide to Genome-Wide Association Mapping in Plants. Curr. Protoc. Plant Biol. 2017, 2, 22–38. [Google Scholar] [CrossRef]
- Belzile, F.; Torkamaneh, D. Designing a Genome-Wide Association Study: Main Steps and Critical Decisions. Methods Mol. Biol. 2022, 2481, 3–12. [Google Scholar] [PubMed]
- Tibbs Cortes, L.; Zhang, Z.; Yu, J. Status and Prospects of Genome-Wide Association Studies in Plants. Plant Genome 2021, 14, e20077. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Scintu, A.; Posadinu, C.M.; Xu, Y.; Nguyen, C.V.; Sun, H.; Bitocchi, E.; Bellucci, E.; Papa, R.; Fei, Z.; et al. Gwas Based on Rna-Seq Snps and High-Throughput Phenotyping Combined with Climatic Data Highlights the Reservoir of Valuable Genetic Diversity in Regional Tomato Landraces. Genes 2020, 11, 1387. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Q.; Cregan, P.B.; Nelson, R.L.; Wang, X.; Wu, J.; Jiang, G.L. Genome-Wide Association Study for Flowering Time, Maturity Dates and Plant Height in Early Maturing Soybean (Glycine Max) Germplasm. BMC Genom. 2015, 16, 217. [Google Scholar] [CrossRef]
- Sauvage, C.; Segura, V.; Bauchet, G.; Stevens, R.; Do, P.T.; Nikoloski, Z.; Fernie, A.R.; Causse, M. Genome-Wide Association in Tomato Reveals 44 Candidate Loci for Fruit Metabolic Traits. Plant Physiol. 2014, 165, 1120–1132. [Google Scholar] [CrossRef]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A Robust, Simple Genotyping-by-Sequencing (GBS) Approach for High Diversity Species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Cox, M.P.; Peterson, D.A.; Biggs, P.J. SolexaQA: At-a-Glance Quality Assessment of Illumina Second. BMC Bioinform. 2010, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M. Twelve Years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Kasampalis, D.; Tsouvaltzis, P.; Siomos, A. Tomato Fruit Quality in Relation to Growing Season, Harvest Period, Ripening Stage and Postharvest Storage. Emir. J. Food Agric. 2021, 33, 130–138. [Google Scholar] [CrossRef]
- Kang, S.I.; Hwang, I.; Goswami, G.; Jung, H.J.; Nath, U.K.; Yoo, H.J.; Lee, J.M.; Nou, I.S. Molecular Insights Reveal Psy1, Sgr, and Slmyb12 Genes Are Associated with Diverse Fruit Color Pigments in Tomato (Solanum lycopersicum L.). Molecules 2017, 22, 2180. [Google Scholar] [CrossRef]
- Gonzali, S.; Perata, P. Fruit Colour and Novel Mechanisms of Genetic Regulation of Pigment Production in Tomato Fruits. Horticulturae 2021, 7, 259. [Google Scholar] [CrossRef]
- Cao, X.; Qiu, Z.; Wang, X.; Van Giang, T.; Liu, X.; Wang, J.; Wang, X.; Gao, J.; Guo, Y.; Du, Y.; et al. A Putative R3 MYB Repressor Is the Candidate Gene Underlying Atroviolacium, a Locus for Anthocyanin Pigmentation in Tomato Fruit. J. Exp. Bot. 2017, 68, 5745–5758. [Google Scholar] [CrossRef]
- Vu, A.T.; Lee, J.M. Genetic Variations Underlying Anthocyanin Accumulation in Tomato Fruits. Euphytica 2019, 215, 196. [Google Scholar] [CrossRef]
- Kang, S.I.; Rahim, M.A.; Afrin, K.S.; Jung, H.J.; Kim, H.T.; Park, J.I.; Nou, I.S. Expression of Anthocyanin Biosynthesis-Related Genes Reflects the Peel Color in Purple Tomato. Hortic. Environ. Biotechnol. 2018, 59, 435–445. [Google Scholar] [CrossRef]
- Shi, L.; Li, X.; Fu, Y.; Li, C. Environmental Stimuli and Phytohormones in Anthocyanin Biosynthesis: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 16415. [Google Scholar] [CrossRef]
- Khan, R.A.; Abbas, N. Role of Epigenetic and Post-Translational Modifications in Anthocyanin Biosynthesis: A Review. Gene 2023, 887, 147694. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, W.; Xu, J.; Lu, X.; Liu, Y. Anthocyanin Biosynthesis Induced by MYB Transcription Factors in Plants. Int. J. Mol. Sci. 2022, 23, 11701. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, F.; Marinelli, A.; Toccaceli, M.; Tonelli, C.; Petroni, K. Anthocyanins: From Mechanisms of Regulation in Plants to Health Benefits in Foods. Front. Plant Sci. 2021, 12, 748049. [Google Scholar] [CrossRef]
- Ayvaz, H.; Santos, A.M.; Rodriguez-Saona, L.E. Understanding Tomato Peelability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Zhao, W.; Ahmad, N.; Zhao, L. Beyond Green and Red: Unlocking the Genetic Orchestration of Tomato Fruit Color and Pigmentation. Funct. Integr. Genom. 2023, 23, 243. [Google Scholar] [CrossRef]
- Rubatzky, V.E.; Yamaguchi, M. Tomatoes, Peppers, Eggplants, and Other Solanaceous Vegetables. In World Vegetables; Springer: Boston, MA, USA, 1997; pp. 532–576. [Google Scholar] [CrossRef]
- Vogele, A.C. Effect of Environmental Factors Upon the Color of the Tomato and the Watermelon. Plant Physiol. 1937, 12, 929–955. [Google Scholar] [CrossRef]
- López Camelo, A.F.; Gómez, P.A. Comparison of Color Indexes for Tomato Ripening. Hortic. Bras. 2004, 22, 534–537. [Google Scholar] [CrossRef]
- Cammareri, M.; Frary, A.; Frary, A.; Grandillo, S. Genetic and Biotechnological Approaches to Improve Fruit Bioactive Content: A Focus on Eggplant and Tomato Anthocyanins. Int. J. Mol. Sci. 2024, 25, 6811. [Google Scholar] [CrossRef]
- Dono, G.; Rambla, J.L.; Frusciante, S.; Fabene, E.; Gómez-Cadenas, A.; Granell, A.; Diretto, G.; Mazzucato, A. Pigment-Related Mutations Greatly Affect Berry Metabolome in San Marzano Tomatoes. Horticulturae 2022, 8, 120. [Google Scholar] [CrossRef]
- Georgiev, C. Anthocyanin Fruit (Af). Rep. Tomato Genet. Coop. 1972, 22, 10. [Google Scholar]
- Rick, C.M.; Reeves, A.F.; Zobel, R.W. Inheritance and Linkage Relations of Four New Mutants. Rep. Tomato Genet. Coop. 1968, 18, 34–35. [Google Scholar]
- Myers, J.R. Breeding Tomato for Increased Fruit Phenolics. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2009. [Google Scholar]
- Colanero, S.; Perata, P.; Gonzali, S. What’s behind Purple Tomatoes? Insight into the Mechanisms of Anthocyanin Synthesis in Tomato Fruits. Plant Physiol. 2020, 182, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, G.; Reuveni, M.; Evenor, D.; Oren-Shamir, M.; Ovadia, R.; Sapir-Mir, M.; Bootbool-Man, A.; Nahon, S.; Shlomo, H.; Chen, L.; et al. Anthocyanin1 from Solanum Chilense Is More Efficient in Accumulating Anthocyanin Metabolites than Its Solanum lycopersicum Counterpart in Association with the Anthocyanin Fruit Phenotype of Tomato. Theor. Appl. Genet. 2012, 124, 295–307. [Google Scholar] [CrossRef]
- Colanero, S.; Perata, P.; Gonzali, S. The Atroviolacea Gene Encodes an R3-MYB Protein Repressing Anthocyanin Synthesis in Tomato Plants. Front. Plant Sci. 2018, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Deng, L.; Du, M.; Zhao, J.; Chen, Q.; Huang, T.; Jiang, H.; Li, C.B.; Li, C. A Transcriptional Network Promotes Anthocyanin Biosynthesis in Tomato Flesh. Mol. Plant 2020, 13, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Erali, M.; Voelkerding, K.V.; Wittwer, C.T. High Resolution Melting Applications for Clinical Laboratory Medicine. Exp. Mol. Pathol. 2008, 85, 50–58. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, A.; Rajendran, S.; Noh, S.; Kwon, D.; Kim, C.M.; Lee, S.-H.; Nam, M.; Lee, B. Genetic Dissection of Anthocyanin Accumulation in Tomato Using GWAS and Hybridization Probe Melting (HPM) for Marker-Assisted Breeding. Agronomy 2025, 15, 295. https://doi.org/10.3390/agronomy15020295
Jeong A, Rajendran S, Noh S, Kwon D, Kim CM, Lee S-H, Nam M, Lee B. Genetic Dissection of Anthocyanin Accumulation in Tomato Using GWAS and Hybridization Probe Melting (HPM) for Marker-Assisted Breeding. Agronomy. 2025; 15(2):295. https://doi.org/10.3390/agronomy15020295
Chicago/Turabian StyleJeong, Areum, Sujeevan Rajendran, Sara Noh, Dohyeon Kwon, Chul Min Kim, Sang-Hoon Lee, Moon Nam, and Bumkyu Lee. 2025. "Genetic Dissection of Anthocyanin Accumulation in Tomato Using GWAS and Hybridization Probe Melting (HPM) for Marker-Assisted Breeding" Agronomy 15, no. 2: 295. https://doi.org/10.3390/agronomy15020295
APA StyleJeong, A., Rajendran, S., Noh, S., Kwon, D., Kim, C. M., Lee, S.-H., Nam, M., & Lee, B. (2025). Genetic Dissection of Anthocyanin Accumulation in Tomato Using GWAS and Hybridization Probe Melting (HPM) for Marker-Assisted Breeding. Agronomy, 15(2), 295. https://doi.org/10.3390/agronomy15020295







