Effect of Light Intensity on the Functional Traits of Two Ormosia Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Site
2.2. Materials
2.3. Experimental Methods
2.3.1. Determination of Growth Index
2.3.2. Determination of Light Response Curve
2.3.3. Determination of CO2 Response Curve
2.3.4. Determination of Leaf Microstructure
2.3.5. Determination of Photosynthetic Pigments
2.3.6. Plasticity Index and Correlation Analysis
2.4. Data Processing
3. Results
3.1. Changes in Growth Indicators
3.2. Photosynthetic Characteristics
3.2.1. Light Response Curves
3.2.2. CO2 Response Curves
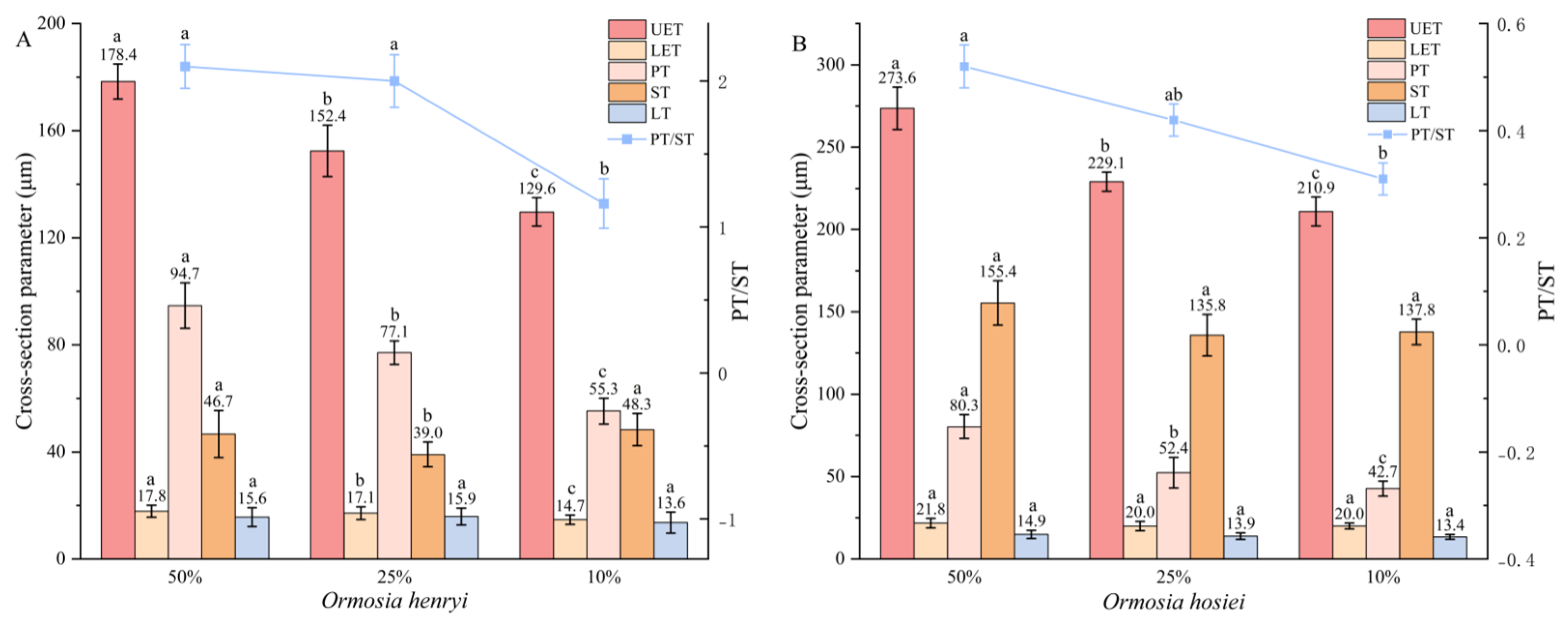
3.3. Leaf Microscopic Structure
3.3.1. Leaf Anatomical Structure
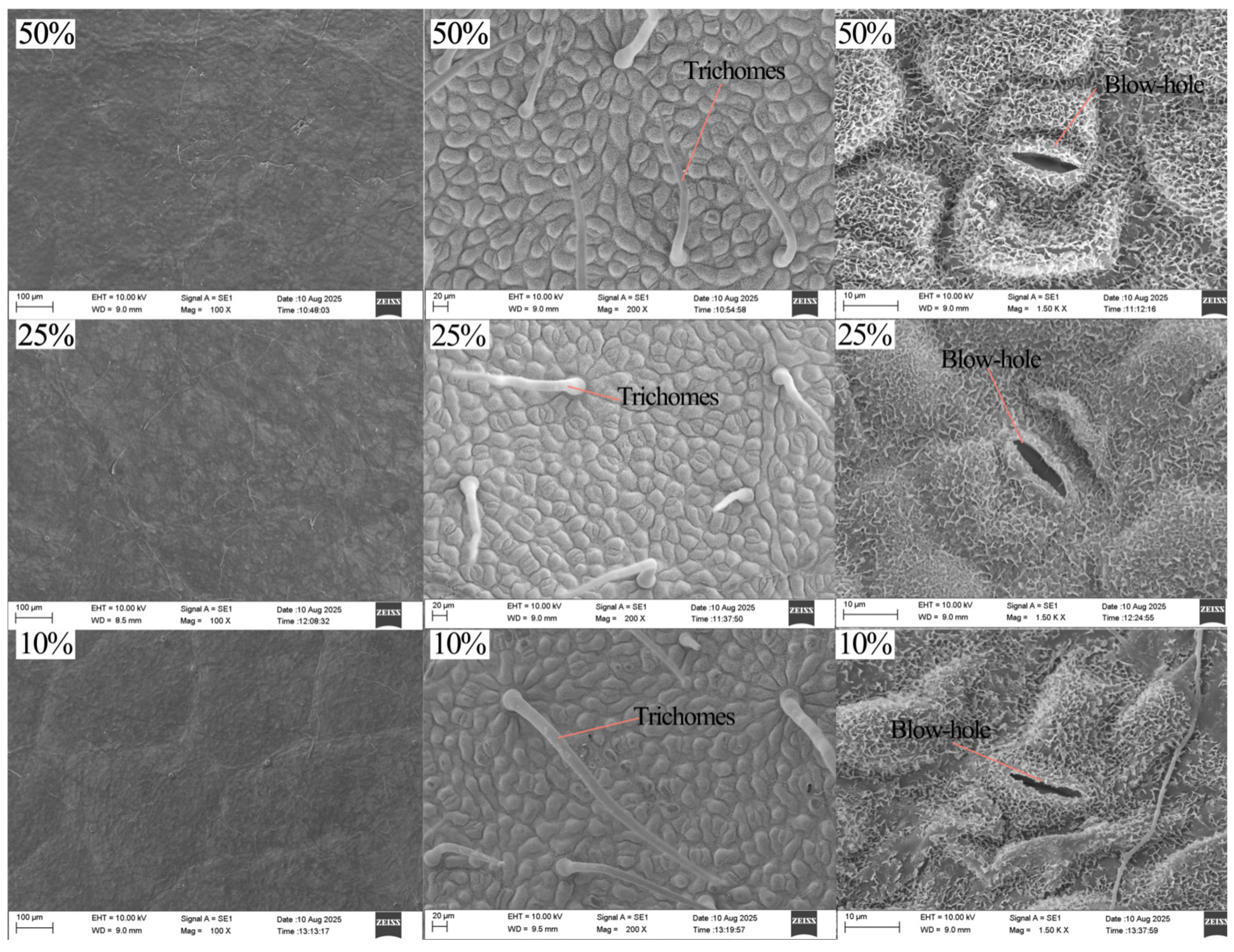
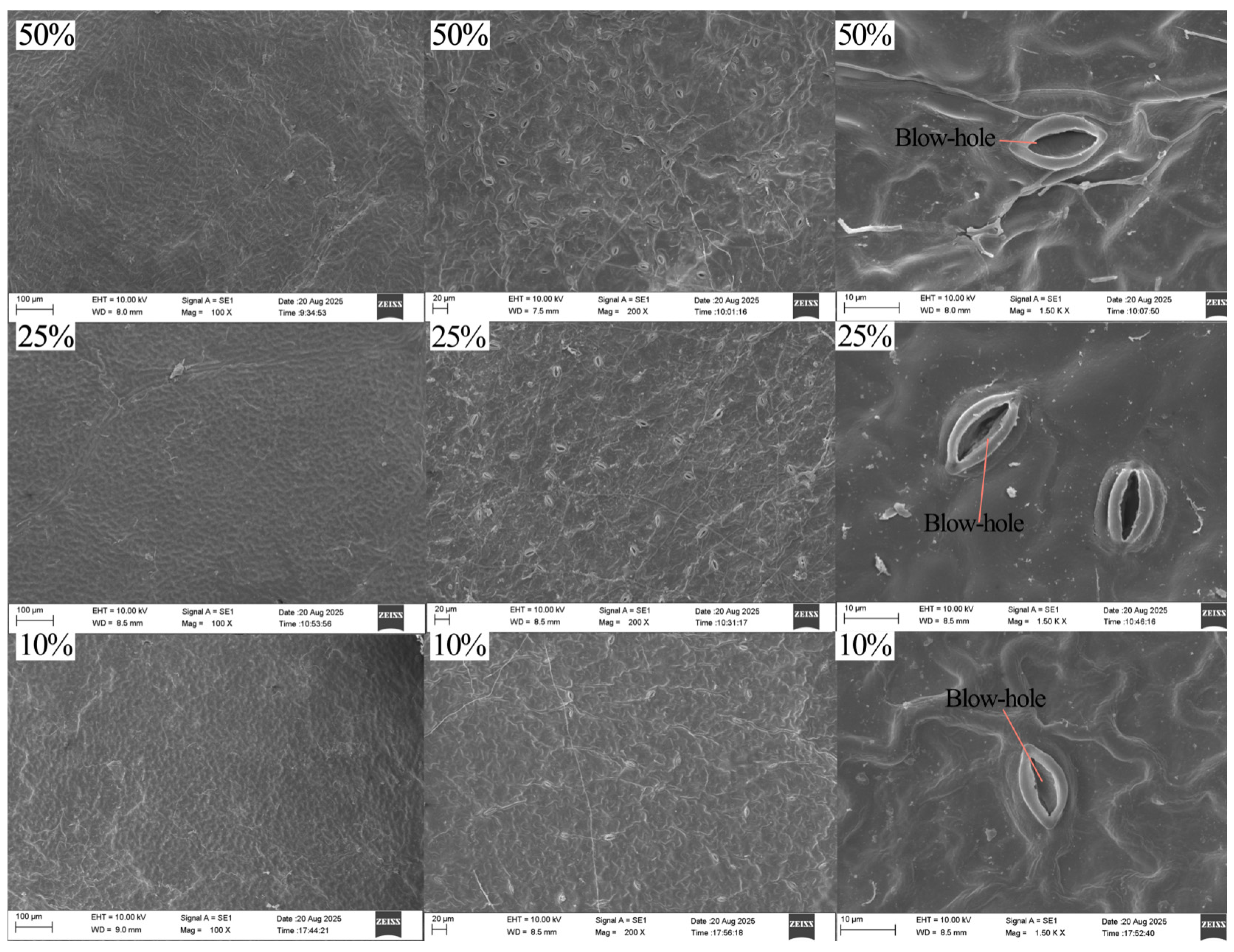
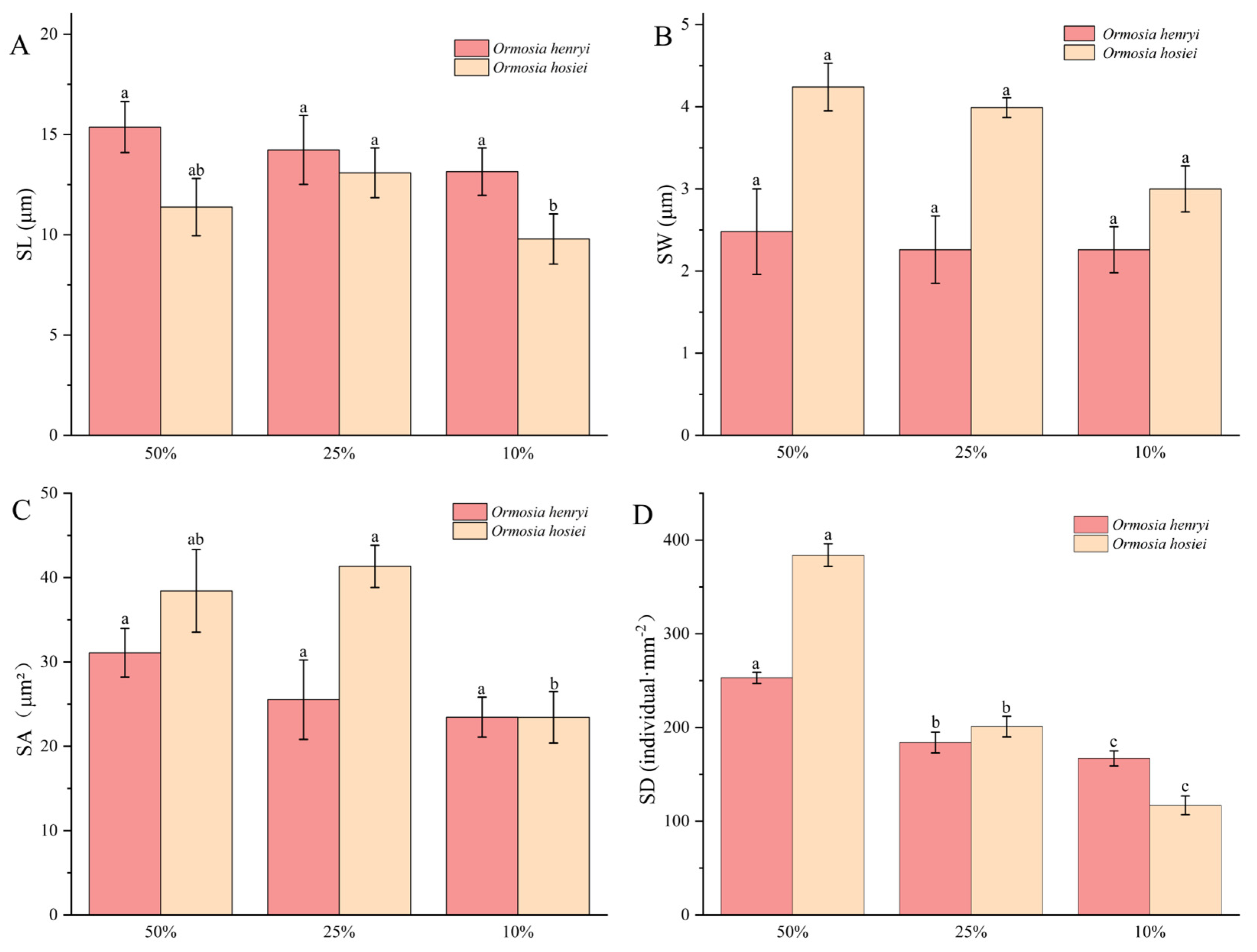
3.3.2. Leaf Epidermal Traits
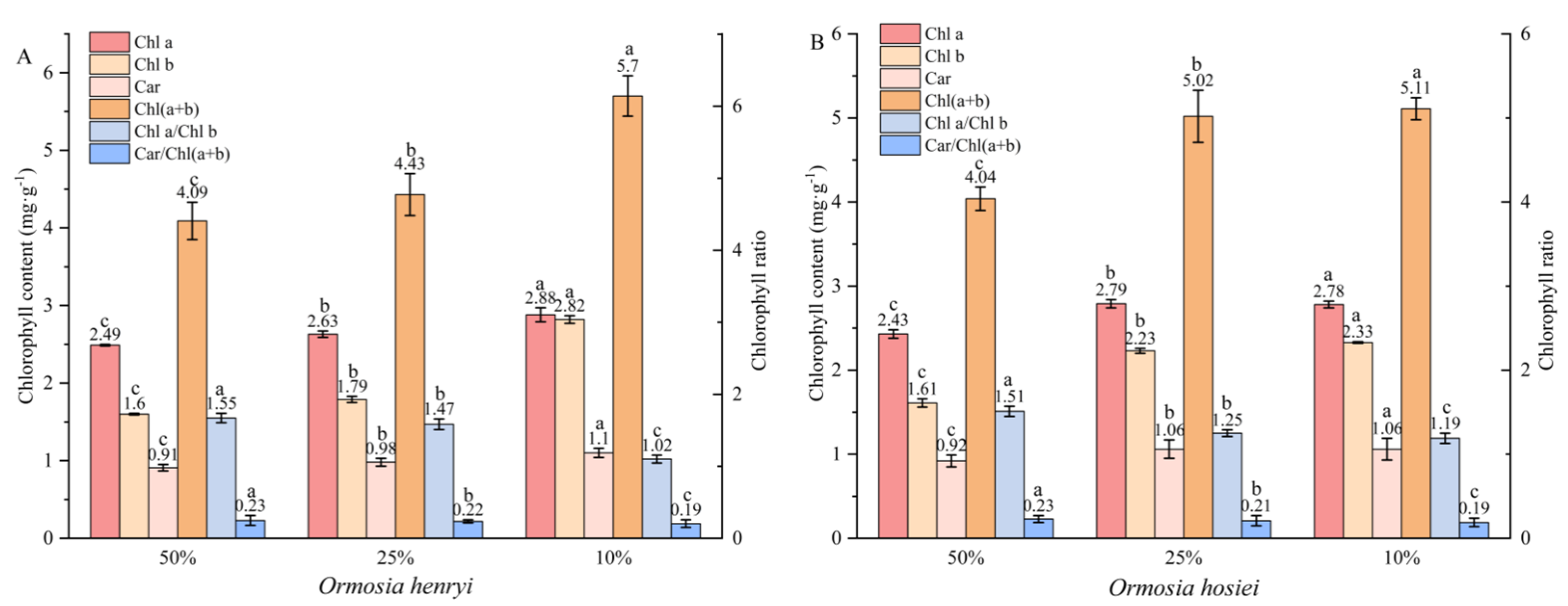
3.4. Chlorophyll Content
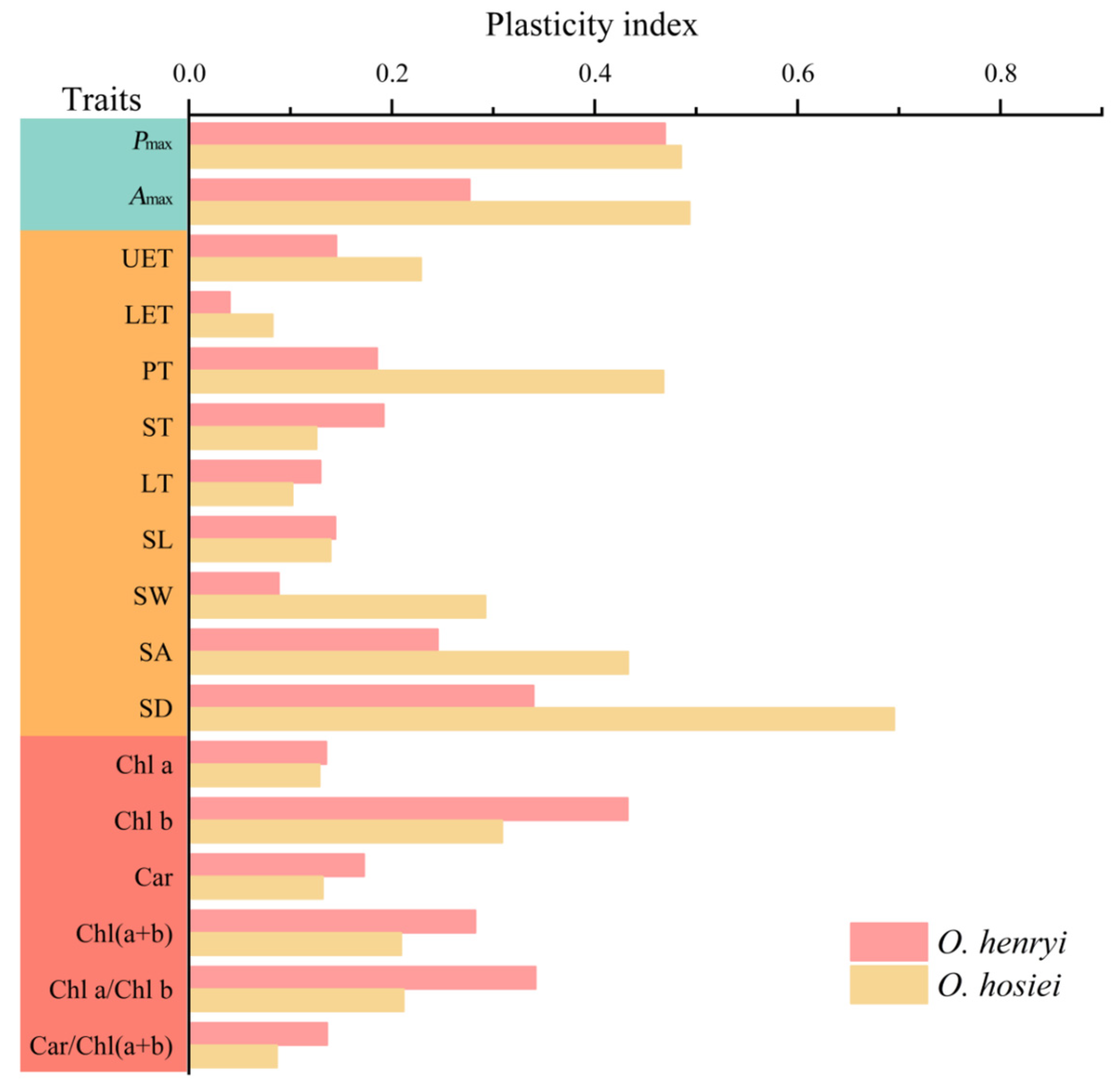
3.5. Plasticity Indices Under Different Light Conditions
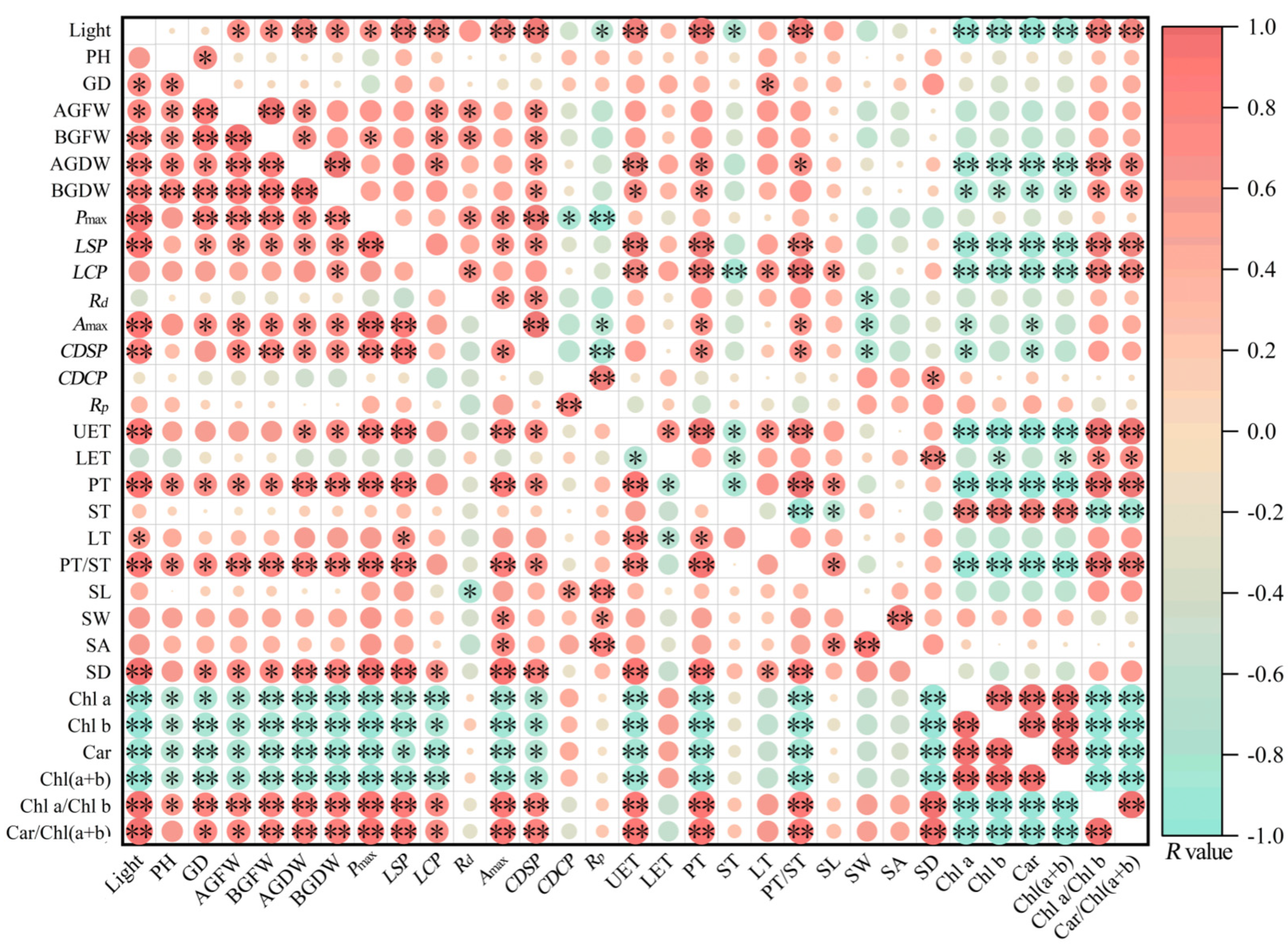
3.6. Correlation Analysis of Functional Traits in Ormosia Species
4. Discussion
4.1. Responses of Chlorophyll to Different Light Conditions
4.2. Responses of Photosynthetic Traits to Different Light Conditions
4.3. Responses of Leaf Microscopic Structure to Different Light Conditions
4.4. Response of Growth Index to Different Light Conditions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China: Vol.10; Science Press: Beijing, China, 2010; pp. 73–85. [Google Scholar]
- Liu, D.; Liu, D.D.; Ma, L.; Yun, X.L.; Xiang, Y.; Nie, P.; Zeng, G.R.; Guo, J.S. Ormosia henryi prain leaf extract alleviates cognitive impairment in chronic unpredictable mild stress mice. Prog. Biochem. Biophys. 2020, 47, 768–779. [Google Scholar]
- Zhang, L.J.; Zhou, W.J.; Ni, L.; Huang, M.Q.; Zhang, X.Q.; Xu, H.Y. A review on chemical constituents and pharmacological activities of Ormosia. Chin. Herb. Med. 2021, 52, 4433–4442. [Google Scholar] [CrossRef]
- Yao, J.; Yang, B. Excellent landscape tree species—Livistona chinensis. China Urban For. 2007, 1, 65. [Google Scholar]
- Wei, X.; He, G.H.; Tang, J.M.; Feng, S.; Li, X.; Lu, L.; Cai, X.R. Advances in Conservation Biology of Endangered Ormosia Specie. Guangxi Sci. 2025, 32, 226–235. [Google Scholar] [CrossRef]
- Tang, J.M.; Wei, X.; Chai, S.F.; Zou, R.; Ding, T.; Wu, L.F.; Deng, L.L. Key National Protected Wild Plants in Guangxi; China Forestry Press: Beijing, China, 2023; pp. 340–375. [Google Scholar]
- Duan, R.Y.; Wei, X.L.; An, C.R.; Zhang, L. Physiological response of Ormosia henryi seedlings with inoculating different rhizobium strains to moderate drought stress. For. Sci. Res. 2018, 31, 61–69. [Google Scholar] [CrossRef]
- Jia, Q.Q.; Deng, H.Y.; Mo, X.Y.; Liu, L.T. Growth rhythms of three Ormosia species seedlings of different provenances. Rev. Árvore 2019, 43, e430606. [Google Scholar] [CrossRef]
- Zhou, C.C.; Xia, S.Q.; Wen, Q.; Song, Y.; Jia, Q.; Wang, T.; Liu, L.; Ouyang, T. Genetic structure of an endangered species Ormosia henryi in southern China and implications for conservation. BMC Plant Biol. 2023, 23, 220. [Google Scholar] [CrossRef]
- Liu, H.S.; Su, Z.H.; Yu, S.Q.; Liu, J.; Yin, X.; Zhang, G.; Liu, W.; Li, B. Genome comparison reveals mutation hotspots in the chloroplast genome and phylogenetic relationships of Ormosia species. BioMed Res. Int. 2019, 2019, 7265030. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, S.; He, Y.; Peng, C.; Wang, Z.; Tang, Q. Phytochemical profile and antidepressant effect of Ormosia henryi Prain leaf ethanol extract. Int. J. Mol. Sci. 2019, 20, 3396. [Google Scholar] [CrossRef]
- Zhai, D.C.; Fang, Z.; Wang, Y.; Zhang, M.L.; Hu, Z.H.; Li, Q.; Bai, X.H. Chemical constituents of the seed of Ormosia hosiei and its antioxidant and antimicrobial activity. Nat. Prod. Res. Dev. 2019, 31, 946–951. [Google Scholar] [CrossRef]
- Li, X.S. Seed sowing, nursery, and density experiments of Livistona chinensis under different storage methods. For. Surv. Des. 2018, 38, 36–38. [Google Scholar]
- Gui, P.; Wei, X.L.; Qiao, D.; Wu, G.Y. Establishment of plantlet regeneration system of tissue Culture of Ormosia henryi. Seed 2018, 37, 135–139. [Google Scholar] [CrossRef]
- Zhang, J.F.; Ge, J.R.; Dayananda, B.; Li, J.Q. Effect of light intensities on the photosynthesis, growth and physiological performances of two maple species. Front. Plant Sci. 2022, 13, 999026. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Li, H.; Zhou, P.; Guo, J.; Yuan, J.; Wang, J.; Liu, Y.; Zhang, Z.; Lei, Y. Leaf plasticity responses of four urban garden plants to low-light environments under viaducts. Forests 2025, 16, 651. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light signaling and UV-B-mediated plant growth regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, S.; Yu, D. The role of light quality in regulating early seedling development. Plants 2023, 12, 2746. [Google Scholar] [CrossRef]
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Li, X.; Huang, B.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The role of light in regulating plant growth, development and sugar metabolism: A review. Front. Plant Sci. 2025, 15, 1507628. [Google Scholar] [CrossRef]
- Chen, C.-I.; Lin, K.-H.; Lin, T.-C.; Huang, M.-Y.; Chen, Y.-C.; Huang, C.-C.; Wang, C.-W. Responses of photosynthesis and chlorophyll fluorescence during light induction in different seedling ages of Mahonia oiwakensis. Bot. Stud. 2023, 64, 5. [Google Scholar] [CrossRef]
- Lu, D.; Xu, B.; Yu, Q.; Liu, Z.; Ren, M.; Wang, Y.; Zhang, S.; Wu, C.; Shen, Y. Identification of potential light deficiency response regulators in endangered species Magnolia sinostellata. Sci. Rep. 2022, 12, 22536. [Google Scholar] [CrossRef]
- Hu, Y.; Javed, H.H.; Liu, L.; Liu, Y.; Yang, X.; Xu, F.; Liu, Y.; Peng, X.; Wu, Y. Impact of low light on photosynthetic characteristics, antioxidant activity, and yield of Brassica napus L. Agronomy 2025, 15, 214. [Google Scholar] [CrossRef]
- Arsovski, A.A.; Galstyan, A.; Guseman, J.M.; Nemhauser, J.L. Photomorphogenesis. Arab. Book 2012, 10, e0147. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Z.Y.; Chen, Y.; Yang, Q.S.; Busso, C.A. The spatial-temporal heterogeneity of understory light availability in a temperate forest of North China. Phyton-Int. J. Exp. Bot. 2021, 90, 1633–1644. [Google Scholar] [CrossRef]
- Rosam, J.R.; Warman, L.; Ostertag, R.; Perroy, R.; Cordell, S. Light quality and spatial variability influences on seedling regeneration in Hawaiian lowland wet forests. J. Appl. Ecol. 2024, 61, 2638–2652. [Google Scholar] [CrossRef]
- Méndez-Dewar, G.; González-Espinosa, M.; Equihua, M. From seedling to sapling: Tree species responses to spatial and temporal understory light heterogeneity in disturbed tropical montane forests. Bot. Sci. 2015, 93, 719–729. [Google Scholar] [CrossRef]
- Modolo, G.S.; dos Santos, V.A.H.F.; Ferreira, M.J. Testing for functional significance of traits: Effect of the light environment in tropical tree saplings. Ecol. Evol. 2021, 11, 6480–6492. [Google Scholar] [CrossRef]
- Ye, Z.P. A review on modeling of responses of photosynthesis to light and CO2. Acta Ecol. Sin. 2010, 34, 727–740. [Google Scholar]
- Pan, L.P.; Tang, J.M.; Jiang, H.D.; Zou, R.; Chai, S.F.; Wei, X. Comparison of photosynthesis and structure of Leaves between Manglietia aromatica seedlings and adult plants. Mol. Plant Breed. 2023, 1–11. [Google Scholar]
- Li, Z.L. Plant Sectioning Techniques, 2nd ed.; Science Press: Beijing, China, 1987. [Google Scholar]
- Li, H.S. Principles and Techniques of Plant Physiology and Biochemistry Experiments; Higher Education Press: Beijing, China, 2000; pp. 134–137. [Google Scholar]
- Valladares, F.; Wright, S.J.; Lasso, E.; Kitajima, K.; Pearcy, R.W. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 2000, 81, 1925–1936. [Google Scholar] [CrossRef]
- Ma, Z.; Li, S.; Zhang, M.; Jiang, S.; Xiao, Y. Light intensity affects growth, photosynthetic capability, and total flavonoid accumulation of Anoectochilus plants. HortScience 2010, 45, 863–867. [Google Scholar] [CrossRef]
- Kong, D.X.; Li, Y.Q.; Wang, M.L.; Bai, M.; Zou, R.; Tang, H.; Wu, H. Effects of light intensity on leaf photosynthetic characteristics, chloroplast structure, and alkaloid content of Mahonia bodinieri (Gagnep.) Laferr. Acta Physiol. Plant 2016, 38, 120. [Google Scholar] [CrossRef]
- Nishimura, M.; Sakurai, H.; Takamiya, A. Wavelength dependency of the inhibition of Hill reaction and the analysis of the process by flashing light. Biochim. Biophys. Acta 1964, 79, 241–248. [Google Scholar] [CrossRef]
- Qiu, N.W.; Jiang, D.C.; Wang, X.S.; Wang, B.S.; Zhou, F. Advances in the members and biosynthesis of chlorophyll family. Photosynthetica 2019, 57, 974–984. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A.; Nicola, S. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiol. Biochem. 2016, 106, 141–148. [Google Scholar] [CrossRef]
- Dziwulska-Hunek, A.; Myśliwa-Kurdziel, B.; Matwijczuk, A.; Szymanek, M. A case study in photosynthetic parameters of perennial plants growing in natural conditions. BMC Plant Biol. 2025, 25, 1044. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.; Zhang, W.J.; Ding, Y.F.; Zhang, J.W.; Cambula, E.D.; Weng, F.; Liu, Z.H.; Ding, C.Q.; Tang, S.; Chen, L.; et al. Shading contributes to the reduction of stem mechanical strength by decreasing cell wall synthesis in Japonica rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 881. [Google Scholar] [CrossRef]
- Wei, Z.M.; Qin, D.W.; Wu, M.; Qin, W.M. Effects of different light intensities on growth and physiological traits of Neolitsea aurata seedlings. West China For. Sci. 2018, 47, 48–53. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, J.; He, W.K.; Zhou, X. Effects of different light intensities on chlorophyll fluorescence and photosynthetic physiology of four Ocimum species. Henan Agric. Sci. 2025, 54, 113–123. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J. Effects of light intensity and temperature on photosynthesis characteristics and yield of lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, K.M.; Yu, H.F. Photosynthetic characteristics of ten cultivars of autumn chrysanthemum (Dendranthema morifolium) and correlation analysis between net photosynthetic rate and some physio-ecological factors. J. Plant Resour. Environ. 2012, 21, 70–76. [Google Scholar]
- Lyu, C.Y.; Liu, Y.H. Provenance difference in growth traits and photosynthetic characteristics of Acer catalpifolium seedlings under different shading conditions. Ying Yong Sheng Tai Xue Bao 2018, 29, 2307–2314. [Google Scholar] [CrossRef]
- Taylor, S.H.; Gonzalez-Escobar, E.; Page, R.; Parry, M.A.J.; Long, S.P.; Carmo-Silva, E. Faster than expected Rubisco deactivation in shade reduces cowpea photosynthetic potential in variable light conditions. Nat. Plants 2022, 8, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.Y.; Wang, J.X.; Wu, M.; Zou, Z.Y.; Cheng, S.; Li, Y.H. Leaf structure and photosynthetic characteristics of two species of Hesperis. Grassl. Sci. 2022, 31, 172–184. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, W.; Wang, Y.; Yong, T.; Yang, F.; Liu, W.; Wu, X.; Du, J.; Shu, K.; Liu, J.; et al. Leaf area and photosynthesis of newly emerged trifoliolate leaves are regulated by mature leaves in soybean. J. Plant Res. 2018, 131, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Luan, X.Y.; Tang, Z.H.; Han, A.Z.; Guo, L. Evaluation of drought resistance of apricot germplasm resources based on leaf microstructure. Acta Hortic. Sin. 2025, 52, 2300–2316. [Google Scholar] [CrossRef]
- Wei, H.; Kong, D.; Yang, J.; Wang, H. Drought resistance evaluation of apricot germplasm resources based on leaf microstructure. Plant Commun. 2020, 1, 100030. [Google Scholar] [CrossRef]
- Gay, A.P.; Hurd, R.G. The influence of light on stomatal density in tomato. New Phytol. 1975, 75, 37–46. [Google Scholar] [CrossRef]
- Lake, J.A.; Quick, W.P.; Beerling, D.J.; Woodward, F.I. Signals from mature to new leaves. Nature 2001, 411, 154. [Google Scholar] [CrossRef]
- Tang, W.; Guo, H.P.; Baskin, C.C.; Xiong, W.D.; Yang, C.; Li, Z.Y.; Song, H.; Wang, T.R.; Yin, J.N.; Wu, X.L.; et al. Effect of light intensity on morphology, photosynthesis and carbon metabolism of Medicago sativa seedlings. Plants 2022, 11, 1688. [Google Scholar] [CrossRef]
- Feng, L.; Raza, M.A.; Li, Z.; Chen, Y.; Khalid, M.H.B.; Du, J.; Liu, W.; Wu, X.; Song, C.; Yu, L.; et al. Influence of light intensity and leaf movement on photosynthesis characteristics and carbon balance of soybean. Front. Plant Sci. 2019, 9, 1952. [Google Scholar] [CrossRef]
- Naveed, M.; Bansal, U.; Kaiser, B.N. Impact of low light intensity on biomass partitioning and genetic diversity in a chickpea mapping population. Front. Plant Sci. 2024, 15, 1292753. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Guo, S.; Li, M.Q.; Tian, Z.F.; Han, B.; Tang, X.H.; Liu, B. Effects of light intensity on seedling emergence and early growth of Liquidambar formosana Hance. Forests 2023, 14, 867. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Zhang, J.F.; Deng, X.J.; Luo, Y.H.; Yan, X.F. Responses of growth and physiological characteristics of Quercus wutaishanica seedlings to the light intensity. J. Cent. South For. Univ. 2021, 41, 73–81. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Scopel, A.L.; Sánchez, R.A. Far-red radiation reflected from adjacent leaves: An early signal of competition in plant canopies. Science 1990, 247, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Pierik, R.; de Wit, M. Shade avoidance: Phytochrome signalling and other aboveground neighbour detection cues. J. Exp. Bot. 2014, 65, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Mašková, T.; Herben, T. Root:Shoot ratio in developing seedlings: How seedlings change their allocation in response to seed mass and ambient nutrient supply. Ecol. Evol. 2018, 8, 7143–7150. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Funct. Plant Biol. 2000, 27, 1191. [Google Scholar] [CrossRef]
- Shipley, B.; Meziane, D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]

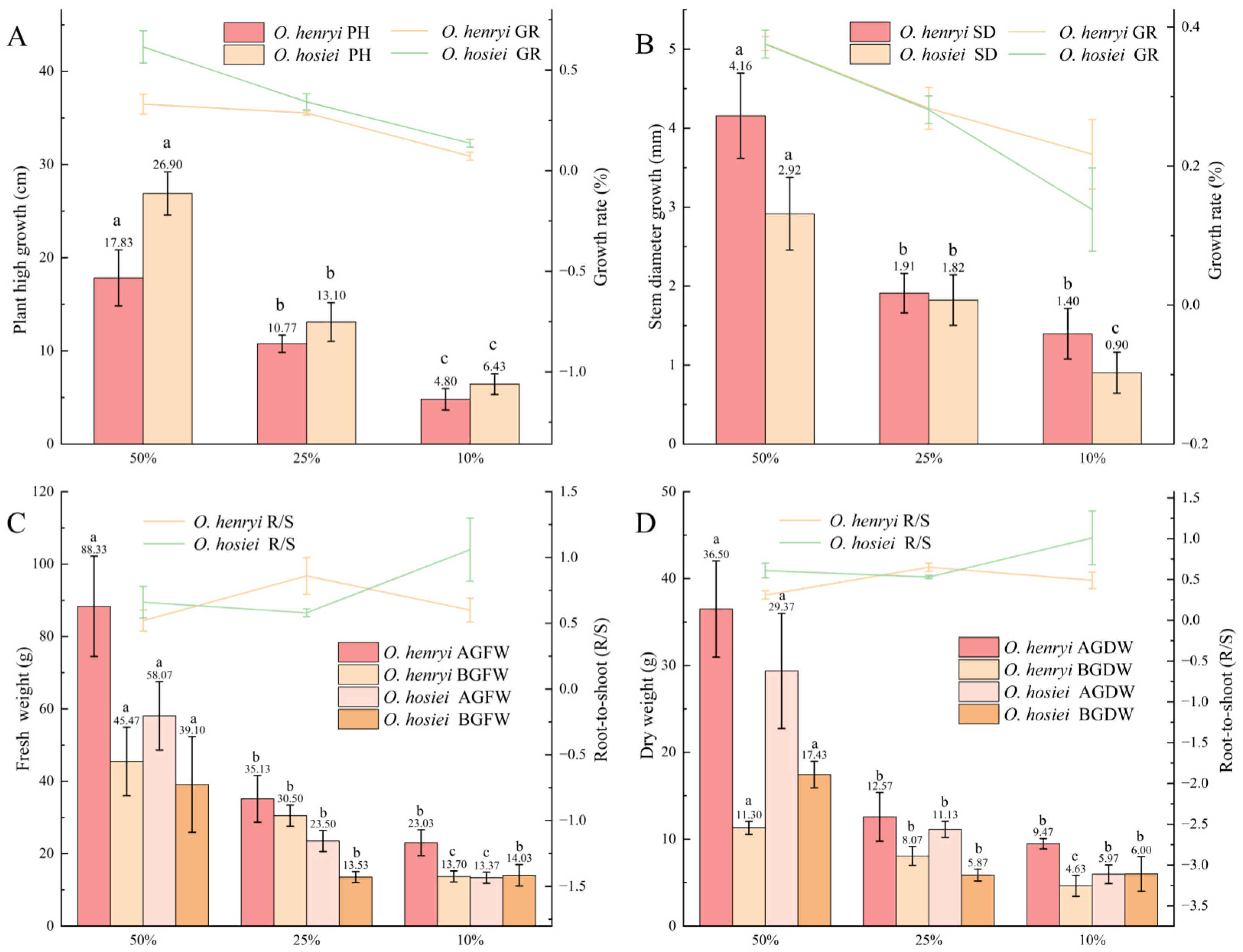
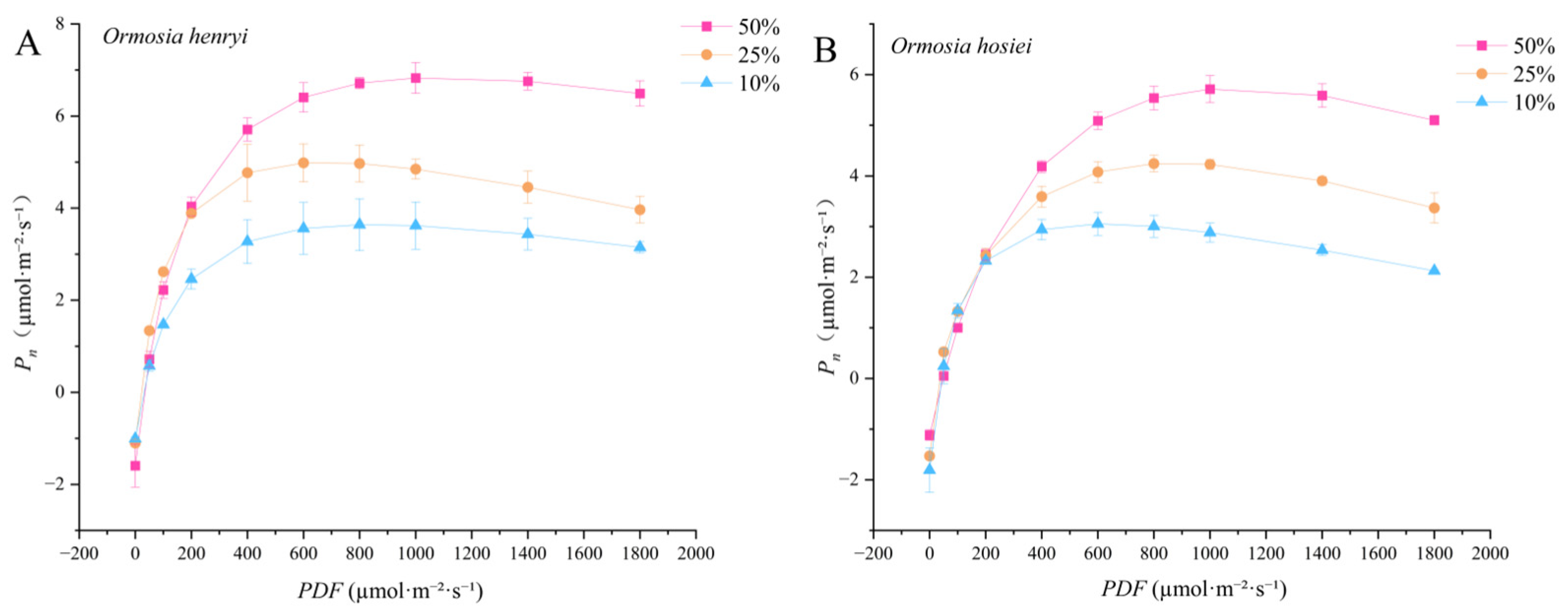
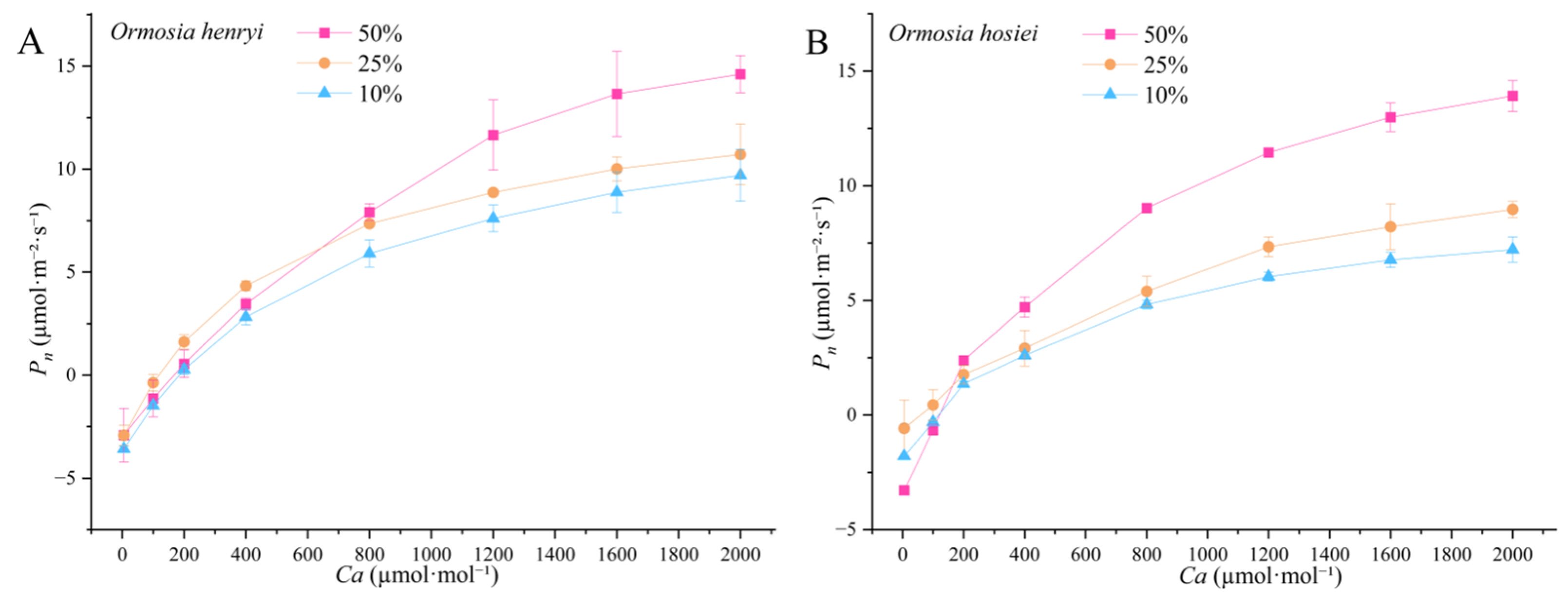
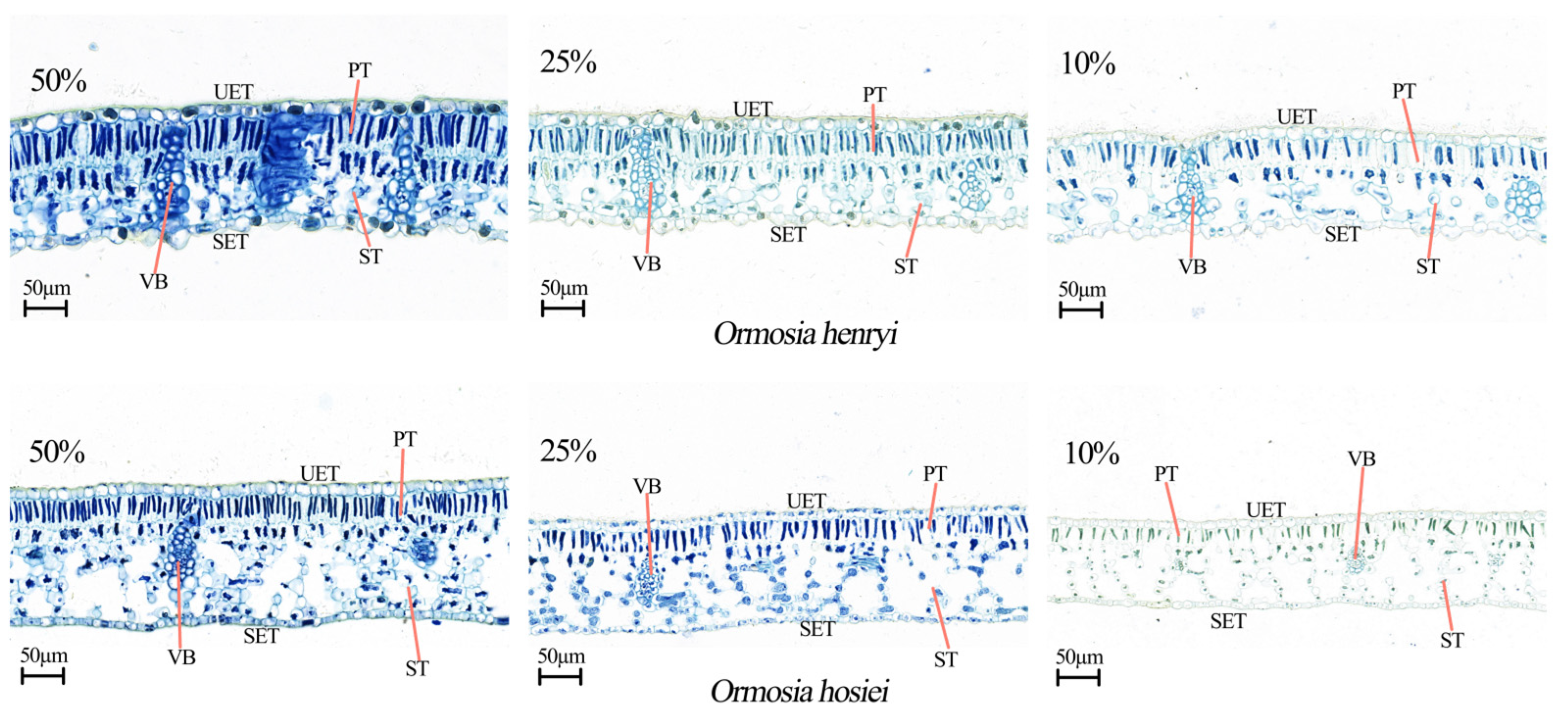
| Species | Relative Light Intensity | AQY | Pmax | LSP | LCP | Rd |
|---|---|---|---|---|---|---|
| O. henryi | 50% | 0.055 ± 0.007 b | 6.863 ± 0.182 a | 1087.43 ± 40.19 a | 33.22 ± 6.8 a | 1.585 ± 0.454 a |
| 25% | 0.043 ± 0.007 b | 4.998 ± 0.009 b | 909.81 ± 168.78 a | 28.26 ± 4.22 a | 1.004 ± 0.033 a | |
| 10% | 0.071 ± 0.001 a | 3.649 ± 0.547 c | 674.91 ± 14.1 b | 18.1 ± 1.25 b | 1.101 ± 0.076 a | |
| O. hosiei | 50% | 0.019 ± 0.001 b | 5.933 ± 0.197 a | 1119.94 ± 17.84 a | 65.97 ± 7.08 a | 1.175 ± 0.059 b |
| 25% | 0.024 ± 0.001 b | 4.264 ± 0.137 b | 890.83 ± 97.47 b | 23.17 ± 1.62 c | 0.529 ± 0.064 c | |
| 10% | 0.075 ± 0.011 a | 3.053 ± 0.226 c | 611.69 ± 1.3 c | 41.66 ± 11.62 b | 2.141 ± 0.74 a |
| Species | Relative Light Intensity | α | Amax | CDSP | CDCP | Rp |
|---|---|---|---|---|---|---|
| O. henryi | 50% | 0.039 ± 0.001 b | 15.659 ± 1.124 a | 1232.27 ± 15.73 a | 158.02 ± 1.14 b | 5.636 ± 0.114 b |
| 25% | 0.047 ± 0.003 a | 11.324 ± 1.16 b | 804.15 ± 10.97 b | 181.15 ± 5.12 a | 7.521 ± 0.333 a | |
| 10% | 0.045 ± 0.001 a | 11.575 ± 0.826 b | 820.41 ± 61.5 b | 167.39 ± 10.54 ab | 6.826 ± 0.561 a | |
| O. hosiei | 50% | 0.037 ± 0.006 a | 13.37 ± 0.535 a | 1195.88 ± 93.34 a | 143.86 ± 3.6 b | 4.829 ± 0.711 b |
| 25% | 0.034 ± 0.013 a | 9.978 ± 1.308 b | 980.99 ± 173.78 ab | 191.01 ± 33.45 a | 6.068 ± 2.909 a | |
| 10% | 0.019 ± 0.002 b | 6.77 ± 0.685 c | 755.47 ± 100.88 c | 149.52 ± 7.82 b | 2.558 ± 0.441 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, G.; Jiang, H.; Li, X.; Lu, L.; Feng, S.; Zou, R.; Wei, X.; Tang, J. Effect of Light Intensity on the Functional Traits of Two Ormosia Species. Agronomy 2025, 15, 2729. https://doi.org/10.3390/agronomy15122729
He G, Jiang H, Li X, Lu L, Feng S, Zou R, Wei X, Tang J. Effect of Light Intensity on the Functional Traits of Two Ormosia Species. Agronomy. 2025; 15(12):2729. https://doi.org/10.3390/agronomy15122729
Chicago/Turabian StyleHe, Guohua, Haiying Jiang, Xi Li, Li Lu, Shuo Feng, Rong Zou, Xiao Wei, and Jianmin Tang. 2025. "Effect of Light Intensity on the Functional Traits of Two Ormosia Species" Agronomy 15, no. 12: 2729. https://doi.org/10.3390/agronomy15122729
APA StyleHe, G., Jiang, H., Li, X., Lu, L., Feng, S., Zou, R., Wei, X., & Tang, J. (2025). Effect of Light Intensity on the Functional Traits of Two Ormosia Species. Agronomy, 15(12), 2729. https://doi.org/10.3390/agronomy15122729





