Comparative Study on Photosynthetic Characteristics and Leaf Structure of Paphiopedilum parishii in Different Growth Periods
Abstract
1. Introduction
2. Materials and Methods
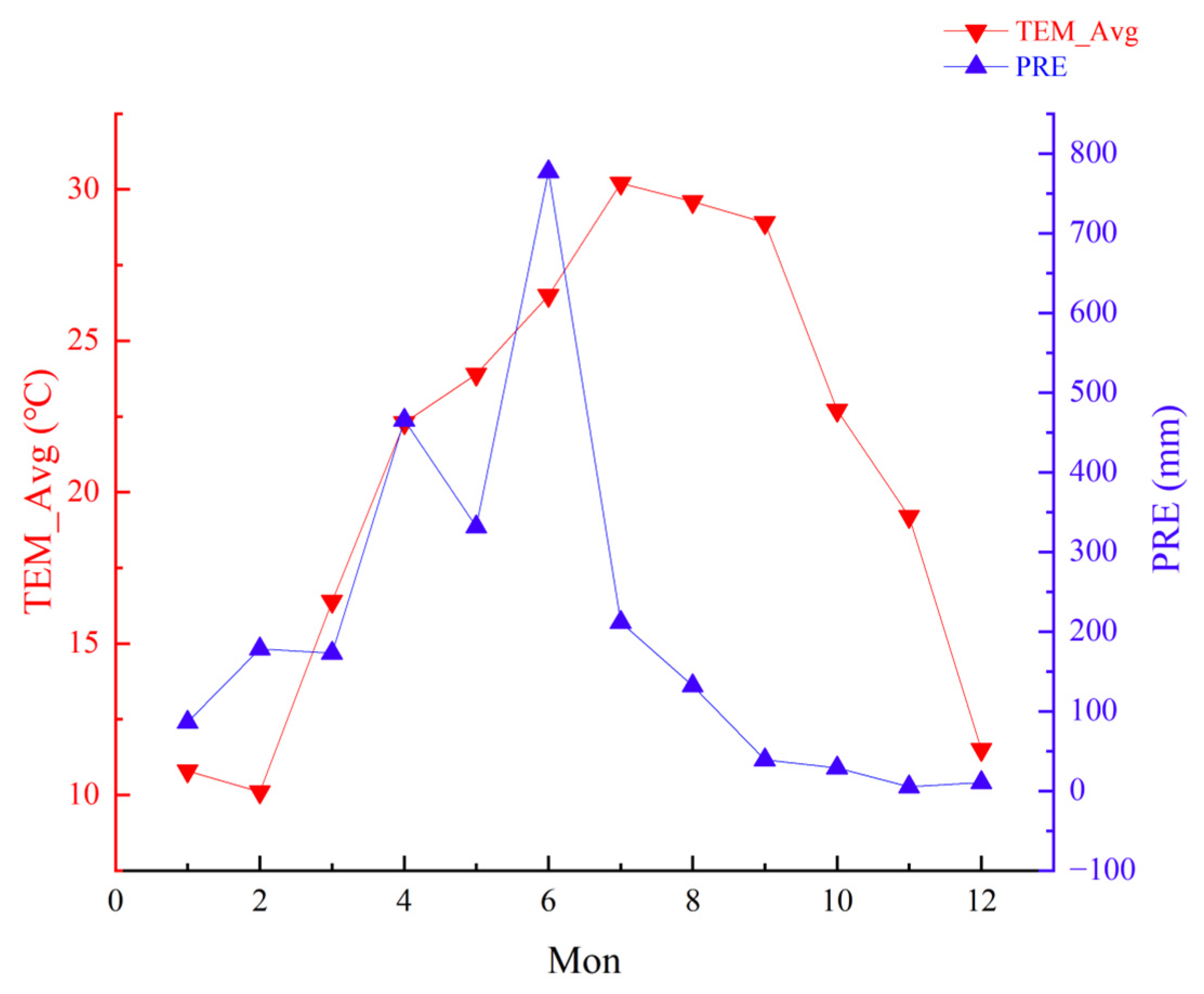
2.1. Overview of the Study Site
2.2. Experimental Methods
2.2.1. Determination of Light Response Curve
2.2.2. Foliar Surface Features
2.2.3. Leaf Anatomical Structure
2.2.4. Determination of Photosynthetic Pigment Content in Leaves
2.3. Data Processing
3. Results
3.1. Comparison of Light Response Curves in P. parishii at Different Growth Periods
3.2. Comparison of Light Response Characteristic Parameters
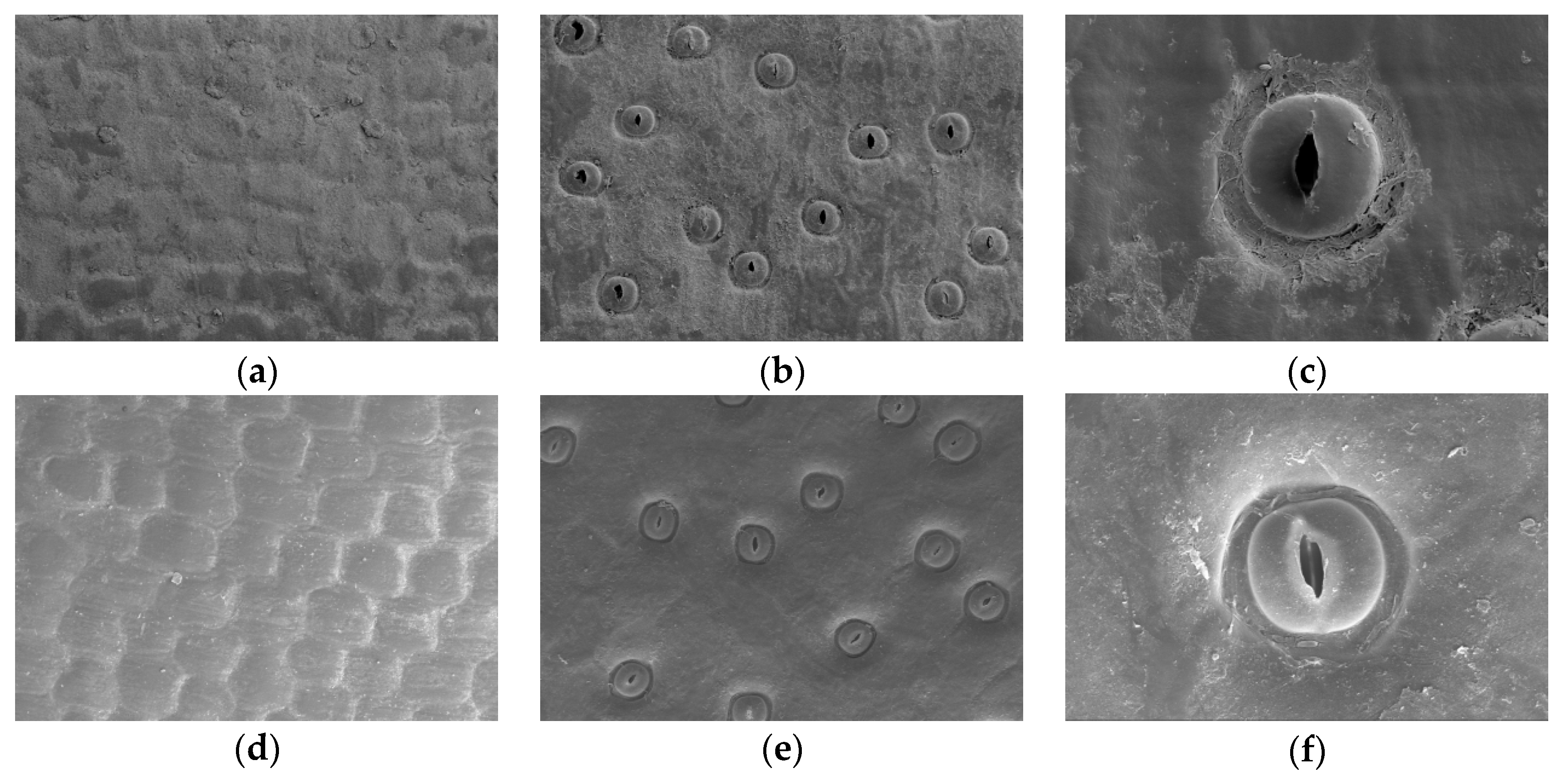
3.3. Comparison of Leaf Epidermal Characteristics
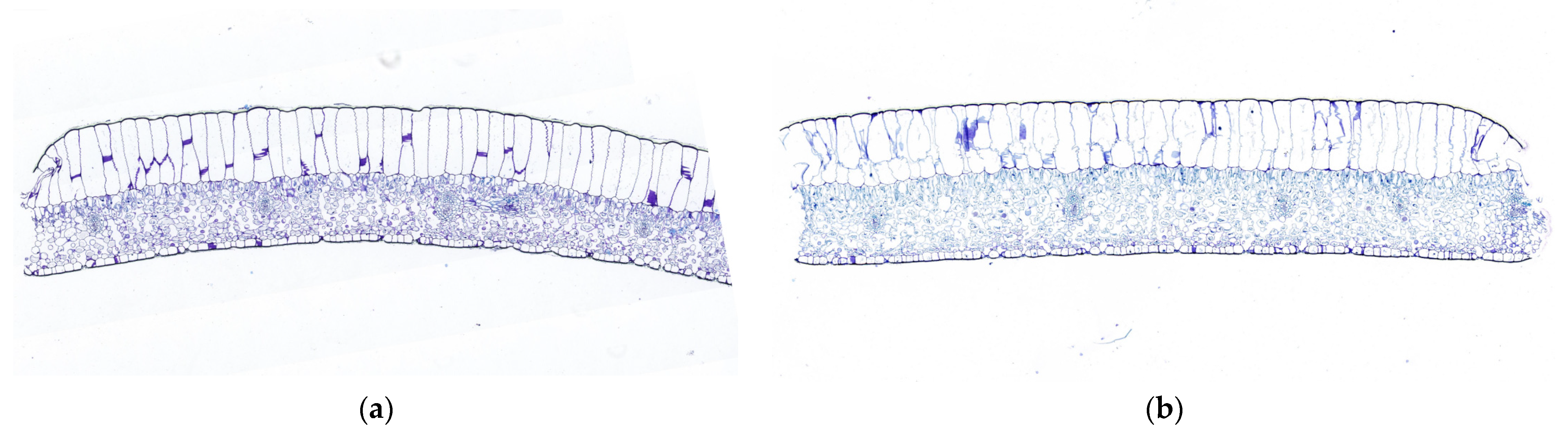
3.4. Comparison of Leaf Anatomical Structure
3.5. Comparison of Leaf Pigment Content
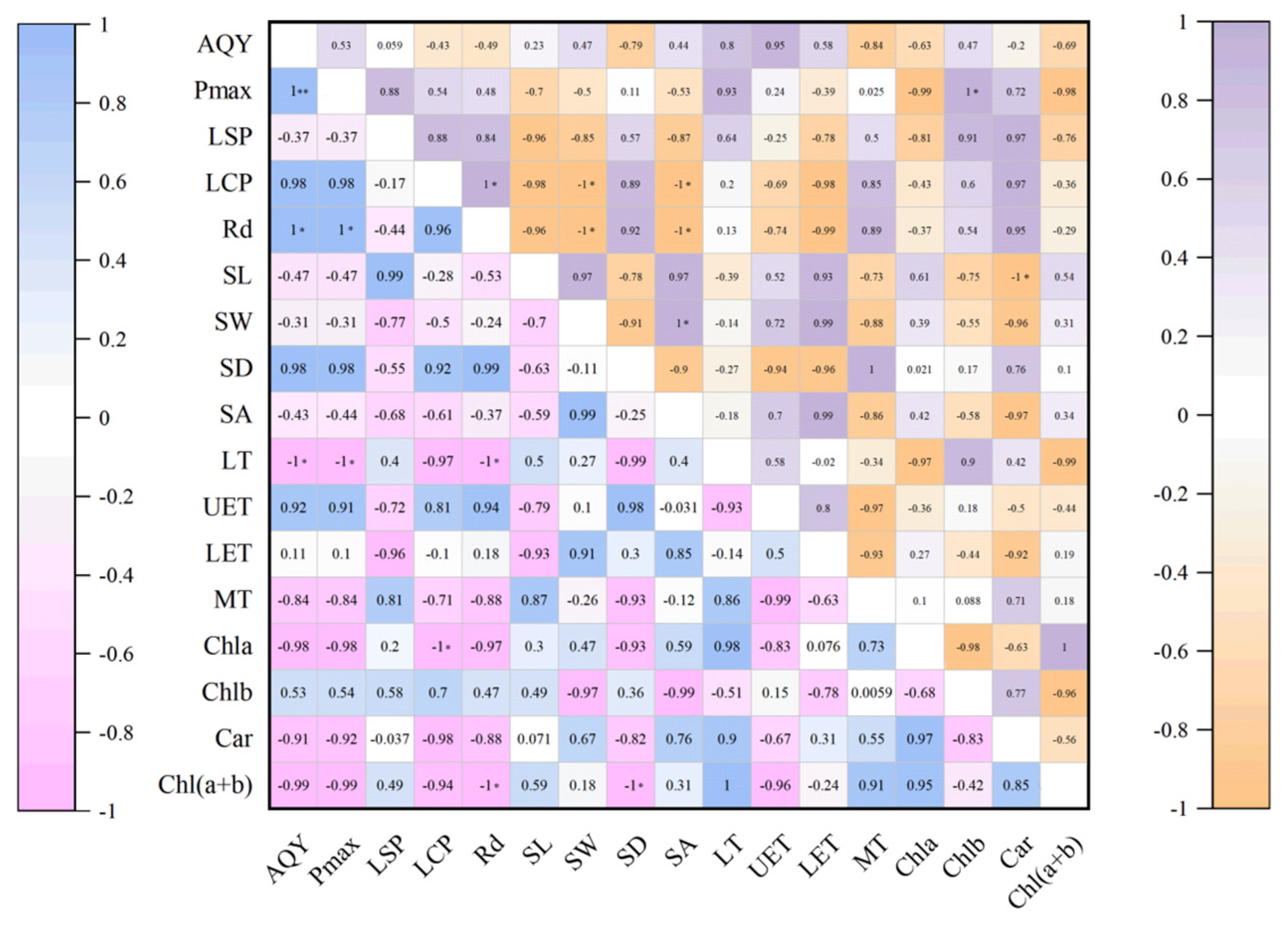
3.6. Correlation Analysis of Leaf Structure and Photosynthetic Physiology of P. parishii in Different Growth Periods
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| P. parishii | Paphiopedilum parishii (Rchb.f.) Stein |
| TEM_Avg | Monthly average temperature |
| PRE | Total monthly precipitation |
| PAR | Photosyntetically Active Radiation |
| Pn | Net Photosynthetic Rate |
| AQY | Apparent Quantum Efficiency |
| LSP | Light Saturation Point |
| LCP | Light Compensation Point |
| Pmax | Maximum Net Photosynthetic Rate |
| Rd | Dark Respiration Rate |
| SL | The long axis of Stomata |
| SW | The short axis of Stomata |
| SD | Stomatal Density |
| SA | Single Stomatal Area |
| LT | Leaf thickness |
| UET | Upper epidermal thickness |
| LET | Lower epidermal thickness |
| MT | Mesophyll tissue thickness |
| Chla | Chlorophyll a |
| Chlb | Chlorophyll b |
| Car | Carotenoids |
| Chl(a+b) | Chlorophyll(a+b) |
| Chla/Chlb | The ratio of chlorophyll a to b |
| Car/Chl | The carotenoid-to-total-chlorophyll ratio |
References
- Liu, J.; Ge, Y.Y.; Hong, Z.; Zhang, Y.; Zhai, W.; Xiang, X.; Tao, Y. Photosynthetic Characteristics of Different Needle Ages in Pinus dabeshanensis Plantation. Anhui Agric. Sci. Bull. 2024, 30, 36–44. [Google Scholar] [CrossRef]
- Pan, L.P.; Tang, J.M.; Jiang, H.D.; Zou, R.; Chai, S.; Wei, X. Comparison of Leaf Photosynthesis and Structure between Seedlings and Adult Plants of the Endangered Plant Manglietia aromatica. Mol. Plant Breed. 2023, 1–11. [Google Scholar]
- Zhao, L.J.; Quan, J.H.; Zhu, L.Q.; Huang, T.; Jin, Y. Ecological Adaptation of Saplings of the Endangered Plant Bhesa robusta Under Different Habitats. Guihaia 2022, 42, 501–509. [Google Scholar] [CrossRef]
- Zhang, J.R.; Fang, L.; Zeng, J.J.; Li, L.; Wu, K.; Zeng, S. Species and Geographical Distribution of Paphiopedilum. Chin. J. Trop. Crops 2024, 45, 1572–1584. [Google Scholar] [CrossRef]
- Wang, Z.; Cong, L.; Liu, Y. Research Status of Paphiopedilum. Sci. Silvae Sin. 2006, 7, 113–119. [Google Scholar]
- Yin, Y.Y.; Fang, L.; Li, L.; Chen, X.; Fu, W.; Wu, K.; Zeng, S. Research Advances in Flowering Regulation of Paphiopedilum. Chin. J. Trop. Crops 2022, 43, 769–778. [Google Scholar] [CrossRef]
- Ran, J.C.; Yu, R.; Liu, J.; Lu, C. Ethnomedicinal Orchids in Maolan Reserve and Their Conservation Strategies. J. Guizhou Norm. Univ. (Nat. Sci.) 2012, 30, 1–5. [Google Scholar] [CrossRef]
- Huang, B.Y.; Lyu, H.Z.; Huang, X.Y.; Wei, Y.; Zhang, Z. Investigation on New Resources of Medicinal Orchids in Guangxi. Southwest China J. Agric. Sci. 2012, 25, 1940–1943. [Google Scholar] [CrossRef]
- CITES Secretariat. CITES Appendices I, II and III. Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). Available online: https://cites.org/eng/app/appendices.php (accessed on 7 February 2025).
- Luo, Y.B.; Jia, J.S.; Wang, C.L. A Preliminary Discussion on Conservation Strategies and Potential Resource Advantages of Paphiopedilum in China. Biodivers. Sci. 2003, 6, 491–498. [Google Scholar]
- Zhang, Y.; An, M.T.; Wu, J.Y.; Yang, Y.; Li, Z.; Ye, C. Survival Status and Conservation Effectiveness of Subgenus Brachypetalum of Paphiopedilum in China. China Environ. Sci. 2022, 42, 3461–3472. [Google Scholar] [CrossRef]
- Flora of China Editorial Committee. Flora of China; Science Press: Beijing, China, 2006; Volume 18. [Google Scholar]
- Wei, X.; Zhu, S.J.; Yang, Y.S. Advances in Physiological Ecology and Conservation Genetics of Paphiopedilum (Orchidaceae) in China. Guangxi Sci. 2025, 32, 218–225. [Google Scholar] [CrossRef]
- Chen, L.J.; Liu, Z.J. Orchid Mating: The Anther Steps onto the Stigma. Plant Signal. Behav. 2014, 9, e976484. [Google Scholar] [CrossRef]
- Kao, H.X.; Zhao, Y.X.; Yang, M.Q.; Sun, Y.; Cheng, J. The Complete Chloroplast Genome Sequences of an Endangered Orchid Species Paphiopedilum parishii (Orchidaceae). Mitochondrial DNA Part B Resour. 2021, 6, 2521–2522. [Google Scholar] [CrossRef]
- Gao, X.Z.; Tang, L.; Wang, Y.; Shao, S.; Luo, Y. Intraspecific Variation of the Complete Chloroplast Genome of the Rare and Endangered Paphiopedilum parishii. Guihaia 2024, 1, 1–14. [Google Scholar] [CrossRef]
- Zhu, G.F.; Yang, Z.J.; Wang, B.Q.; Zhang, X. Karyotype Analysis of 12 Species in Subgenus Paphiopedilum. J. Trop. Subtrop. Bot. 2011, 19, 152–158. [Google Scholar] [CrossRef]
- Gao, J.; Wang, F.; Wu, J.R. Study on the Microstructure of Mycorrhizae in Four Terrestrial Orchids. J. Northwest A&F Univ. (Nat. Sci. Ed.) 2014, 42, 133–140. [Google Scholar] [CrossRef]
- Zeng, S.J.; Xia, N.H.; Chen, Z.L.; Wu, K.; Duan, J. Ornamental Value Evaluation of Native Paphiopedilum Species in China and Their Application Prospects in South China. Chin. Wild Plant Res. 2011, 30, 9–13. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, Z.X.; Guo, S.L.; Guan, M.; Wang, X. Photosynthetic Eco-Physiological Characteristics of the Endangered Plant Amentotaxus argotaenia. Acta Ecol. Sin. 2014, 34, 6460–6470. [Google Scholar] [CrossRef][Green Version]
- Li, Z.L. Plant Microtechnique, 2nd ed.; Science Press: Beijing, China, 1987. [Google Scholar]
- Li, H.S. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2000; pp. 134–137. [Google Scholar]
- Yang, M.H.; Zeng, X.L.; You, M.H.; Liu, J.; Yang, L. Effects of Drought Intensity on Sexual Reproduction Index and Seed Yield of Arthraxon xinanensis. J. Grassland 2024, 1, 30–35. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Zhao, W.; Wang, F.F.; Hou, Z.; Cao, Z.; Wang, K.; Han, Y.; Yang, Y. Seasonal Dynamics of Morphology and Physiological Characteristics of Paeonia ostii. Spec. Wild Econ. Anim. Plant Res. 2022, 44, 98–103. [Google Scholar] [CrossRef]
- Jiang, G.M. Plant Physiological Ecology; Higher Education Press: Beijing, China, 2004; pp. 55–58. [Google Scholar]
- Taylor, S.H.; Franks, P.J.; Hulme, S.P.; Spriggs, E.; Christin, P.A.; Edwards, E.J.; Woodward, F.I.; Osborne, C.P. Photosynthetic Pathway and Ecological Adaptation Explain Stomatal Trait Diversity Amongst Grasses. New Phytol. 2012, 193, 387–396. [Google Scholar] [CrossRef]
- Shi, S.J.; Fu, H.; Liang, Z.L.; Li, B.; Lou, X. Effects of Photoperiod on Stomatal Characteristics and Photosynthetic Properties of Blueberry Leaves. China Fruit Veg. 2025, 45, 26–35. [Google Scholar] [CrossRef]
- Li, J.W.; Bai, B.; Hou, Y.Q.; Zhang, L.; He, R.; Guo, Y.; Du, X. Analysis of Stomatal and Photosynthetic Physiological Characteristics of Dryland Winter Wheat Varieties in Semi-Arid Rainfed Area of Northwest China. J. Triticeae Crops 2025, 45, 593–607. [Google Scholar]
- Peng, L.H.; Chen, N.; Yang, Z.; Jiang, H.; Wei, X.; Chai, S.; Zeng, D. Comparative Study on Photosynthetic Characteristics and Leaf Anatomical Structure of Three Rare and Endangered Dendrobium Species. J. Chin. Med. Mater. 2024, 47, 2966–2973. [Google Scholar] [CrossRef]
- Mu, F.; Feng, B.L.; Gao, X.L.; Miao, F.; Liu, P.T.; Xia, M.Y. Ultrastructure of Leaves at Different Stem Nodes and Growth Stages in Broomcorn Millet. Acta Bot. Boreali-Occident. Sin. 2009, 29, 1–8. [Google Scholar]
- Zhang, C.; Wang, X.Y.; Wang, X.R.; Duan, Y.; Zhang, M.; Shi, D.; Zhu, Y.; Song, Y.; Chai, Z.; Li, L. Changes of Photosynthesis and Endogenous Hormones in Andromonoecious Osmanthus fragrans During Different Floral Stages. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 46, 75–80. [Google Scholar] [CrossRef]
- Yao, X.M.; Ou, C.; Zhang, Y.L.; Yang, L.; Xu, M.; Wang, Q.; Qu, C. Effects of Abscisic Acid on Ion Absorption and Photosynthesis of Toona sinensis Seedlings Under Salt Stress. J. Northeast For. Univ. 2020, 48, 27–32. [Google Scholar] [CrossRef]
- Corrêa de Souza, T.; Magalhães, P.C.; Mauro de Castro, E.; Pereira de Albuquerque, P.E.; Marabesi, M.A. The Influence of ABA on Water Relation, Photosynthesis Parameters, and Chlorophyll Fluorescence Under Drought Conditions in Two Maize Hybrids with Contrasting Drought Resistance. Acta Physiol. Plant. 2013, 35, 515–527. [Google Scholar] [CrossRef]
- Zhang, T.D.; Wu, K.; Du, Z.W.; Mei, H.; Liu, Y.; Zheng, Y. Study on Photosynthetic Characteristics and Chlorophyll Content of Sesame at Flowering Stage. Seed 2019, 38, 7–10+17. [Google Scholar] [CrossRef]
- Santanoo, S.; Lontom, W.; Dongsansuk, A.; Vongcharoen, K.; Theerakulpisut, P. Photosynthesis Performance at Different Growth Stages, Growth, and Yield of Rice in Saline Fields. Plants 2023, 12, 1903. [Google Scholar] [CrossRef]
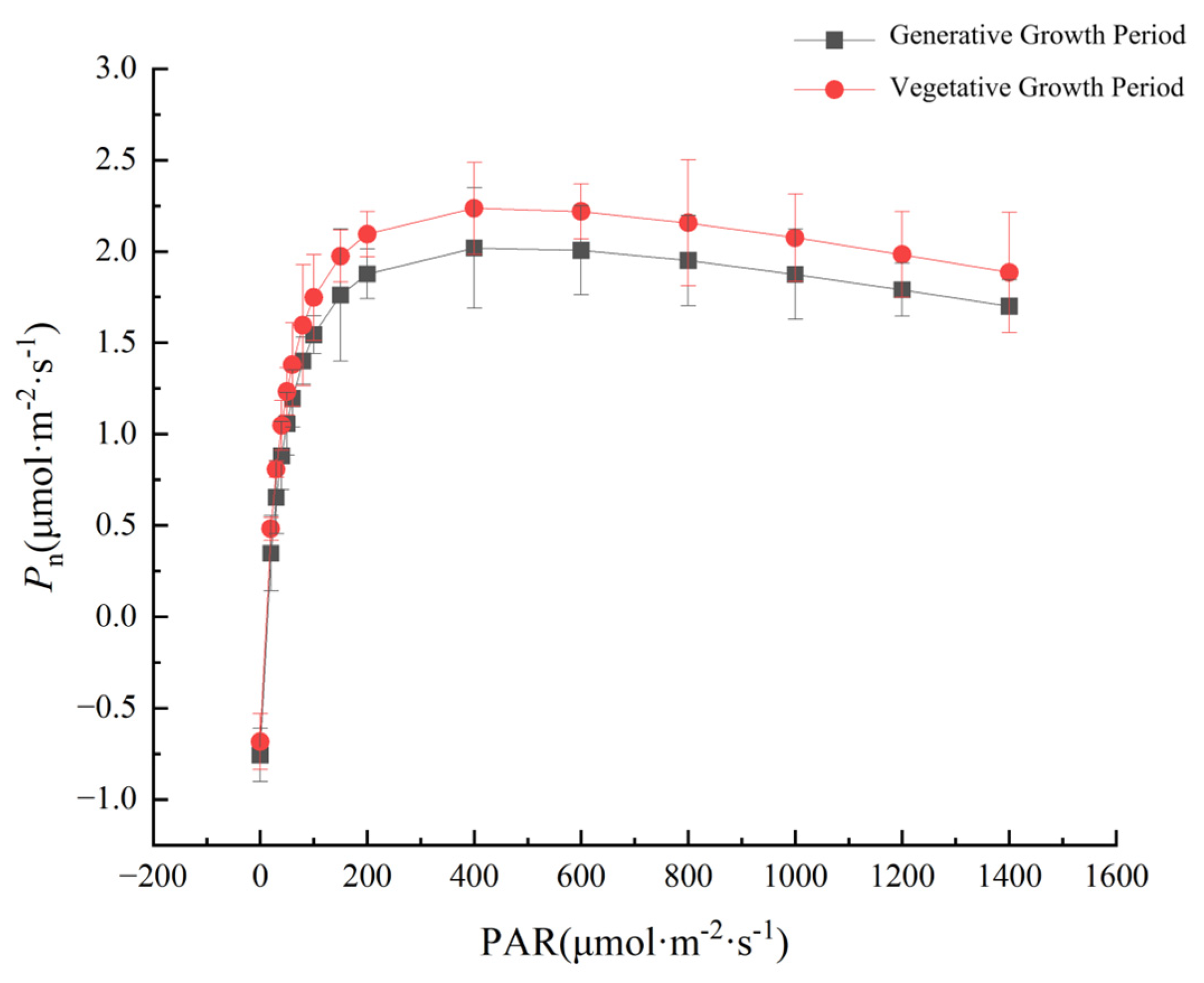
| Growth Period | AQY (mol·mol−1) | Pmax (μmol·m−2·s−1) | LSP (μmol·m−2·s−1) | LCP (μmol·m−2·s−1) | Rd (μmol·m−2·s−1) |
|---|---|---|---|---|---|
| Nutrient | 0.0103 ± 0.0012 b | 2.0274 ± 0.0103 b | 440.5724 ± 8.1323 b | 9.6067 ± 1.4269 a | 0.6633 ± 0.1254 a |
| Reproductive | 0.0120 ± 0.0020 a | 2.2391 ± 0.0514 a | 448.4288 ± 6.6514 a | 9.6102 ± 1.6677 a | 0.6850 ± 0.1526 a |
| Growth Period | SL (μm) | SW (μm) | SD (Individual·mm−2) | SA (μm2) |
|---|---|---|---|---|
| Nutrient | 41.014 ± 2.0896 a | 38.719 ± 2.4801 a | 78.730 ± 6.8252 b | 1248.354 ± 122.9003 a |
| Reproductive | 40.009 ± 1.5160 b | 37.586 ± 2.7082 b | 84.444 ± 13.4020 a | 1182.354 ± 117.7894 b |
| Growth Period | Leaf Thickness (LT, μm) | Upper Epidermal Thickness (UET, μm) | Lower Epidermal Thickness (LET, μm) | Mesophyll Tissue Thickness (MT, μm) |
|---|---|---|---|---|
| Nutrient | 1236.40 ± 20.34 b | 629.03 ± 30.04 a | 69.61 ± 7.04 a | 538.24 ± 28.86 b |
| Reproductive | 1334.89 ± 19.22 a | 638.86 ± 41.12 a | 74.05 ± 7.16 a | 623.32 ± 30.64 a |
| Growth Period | Chla (mg·g−1) | Chlb (mg·g−1) | Car (mg·g−1) | Chl (a + b) (mg·g−1) | Chla/Chlb | Car/Chl |
|---|---|---|---|---|---|---|
| Nutrient | 2.4183 ± 0.0054 b | 1.5136 ± 0.0017 b | 0.3957 ± 0.0041 b | 3.9319 ± 0.0038 b | 1.5976 ± 0.0053 b | 0.1006 ± 0.0011 a |
| Reproductive | 2.4971 ± 0.0032 a | 1.5264 ± 0.0011 a | 0.4095 ± 0.0016 a | 4.0235 ± 0.0026 a | 1.6360 ± 0.0030 a | 0.1018 ± 0.0003 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Jiang, H.; Cai, X.; Li, X.; He, G.; Feng, S.; Wei, X.; Tang, J. Comparative Study on Photosynthetic Characteristics and Leaf Structure of Paphiopedilum parishii in Different Growth Periods. Agronomy 2025, 15, 2713. https://doi.org/10.3390/agronomy15122713
Lu L, Jiang H, Cai X, Li X, He G, Feng S, Wei X, Tang J. Comparative Study on Photosynthetic Characteristics and Leaf Structure of Paphiopedilum parishii in Different Growth Periods. Agronomy. 2025; 15(12):2713. https://doi.org/10.3390/agronomy15122713
Chicago/Turabian StyleLu, Li, Haiying Jiang, Xinru Cai, Xi Li, Guohua He, Shuo Feng, Xiao Wei, and Jianmin Tang. 2025. "Comparative Study on Photosynthetic Characteristics and Leaf Structure of Paphiopedilum parishii in Different Growth Periods" Agronomy 15, no. 12: 2713. https://doi.org/10.3390/agronomy15122713
APA StyleLu, L., Jiang, H., Cai, X., Li, X., He, G., Feng, S., Wei, X., & Tang, J. (2025). Comparative Study on Photosynthetic Characteristics and Leaf Structure of Paphiopedilum parishii in Different Growth Periods. Agronomy, 15(12), 2713. https://doi.org/10.3390/agronomy15122713






