How to Minimize the Impact of Biochar on Soil Salinity in Drylands? Lessons from a Data Synthesis †
Abstract
1. Introduction
2. Materials and Methods
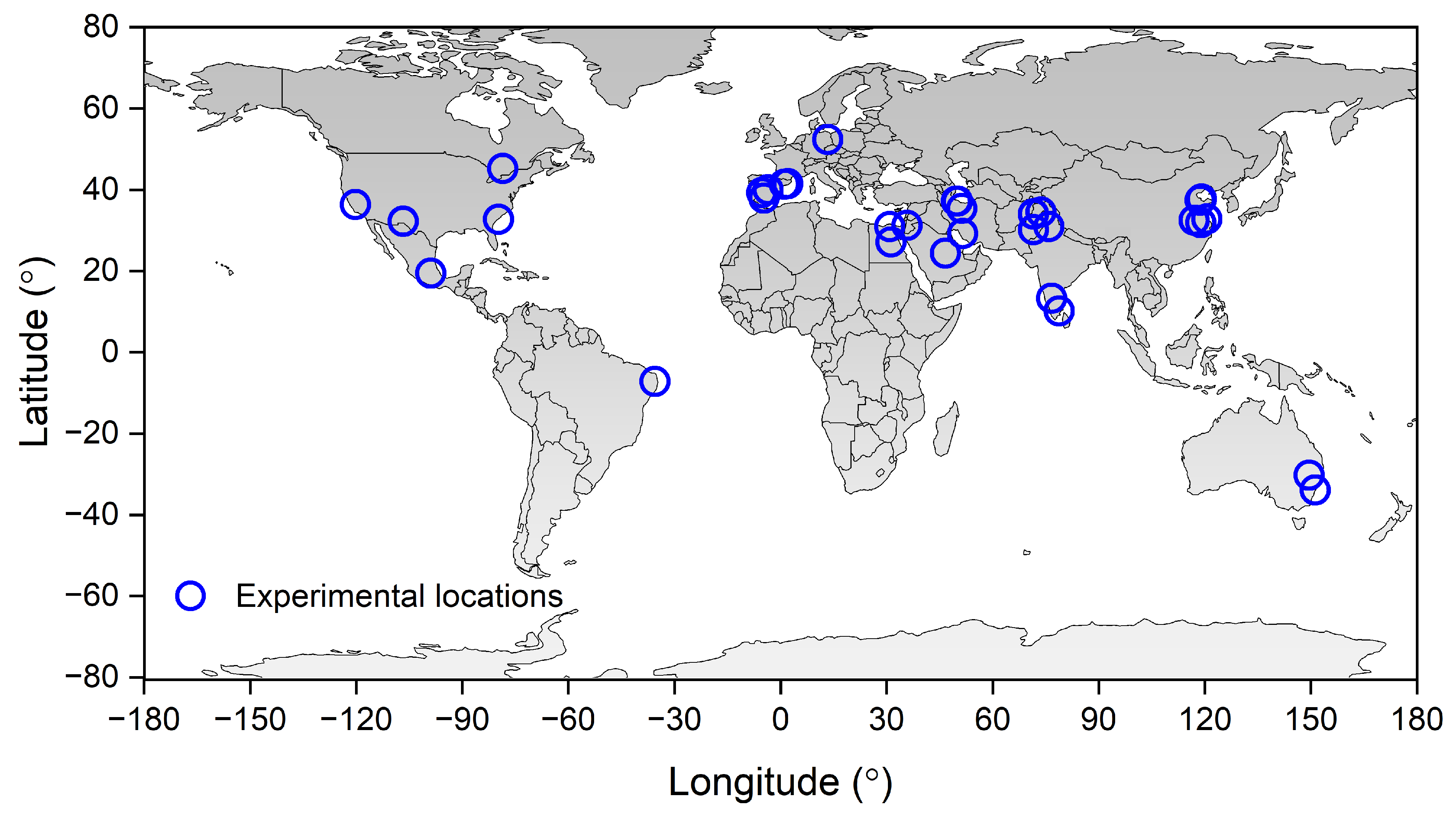
2.1. Data Collection
2.2. Meta-Analysis
2.3. Statistical Analysis
3. Results
3.1. Effects of Biochar on the Grand Mean
3.2. Effects of Climatic Conditions and Irrigation Practices
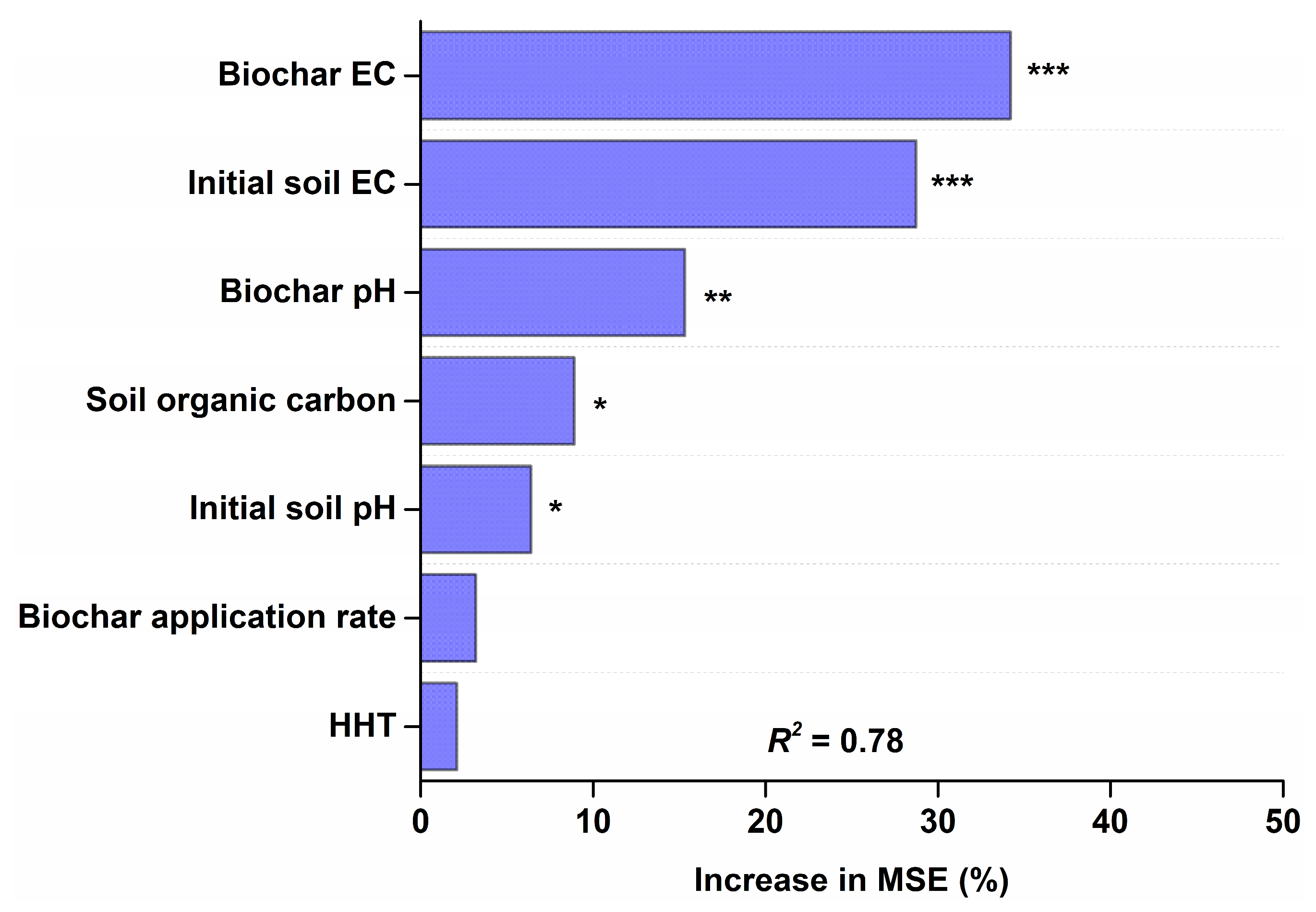
3.3. Effects of Initial Soil Properties
3.4. Effects of Biochar Properties
3.5. Effects of Biochar Application Rates and Simultaneous Addition of Other Amendments
3.6. Relationship Between the Effects of Bochar and Environmental Factors
4. Discussion
4.1. Is the Type of Recipient Soil Important?
4.2. Selection and Application of a Biochar to Dryland Soils
4.3. Limitations and Looking Forward
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in climate change mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Bo, X.; Zhang, Z.; Wang, J.; Guo, S.; Li, Z.; Lin, H.; Huang, Y.; Han, Z.; Kuzyakov, Y.; Zou, J. Benefits and limitations of biochar for climate-smart agriculture: A review and case study from China. Biochar 2023, 5, 77. [Google Scholar] [CrossRef]
- Yang, J.; Xia, L.; van Groenigen, K.J.; Zhao, X.; Ti, C.; Wang, W.; Du, Z.; Fan, M.; Zhuang, M.; Smith, P. Sustained benefits of long-term biochar application for food security and climate change mitigation. Proc. Natl. Acad. Sci. USA 2025, 122, e2509237122. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts towards overcoming drought stress in crops: Revisiting the mechanisms employed by plant growth-promoting bacteria. Front. Microbiol. 2022, 13, 962427. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; Al-Huqail, A.A.; El-Gamal, S.M.A. Potential role of biochar and silicon in improving physio-biochemical and yield characteristics of borage plants under different irrigation regimes. Plants 2023, 12, 1605. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. Glob. Change Biol. Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hockaday, W.C.; Dean, M.R.; Zygourakis, K.; Barnes, R.T. Hydrologic properties of biochars produced at different temperatures. Biomass Bioenergy 2012, 41, 34–43. [Google Scholar] [CrossRef]
- Hien, T.T.T.; Tsubota, T.; Taniguchi, T.; Shinogi, Y. Enhancing soil water holding capacity and provision of a potassium source via optimization of the pyrolysis of bamboo biochar. Biochar 2021, 3, 51–61. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Improving plant available water holding capacity of soil by solid and chemically modified biochars. Rhizosphere 2022, 21, 100469. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.Q.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202, 183–191. [Google Scholar] [CrossRef]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does biochar influence soil physical properties and soil water availability? Plant Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Amonette, J.E.; Ippolito, J.A.; Lima, I.M.; Gaskin, J.; Das, K.C.; Steiner, C.; Ahmedna, M.; et al. Biochars impact on soil-moisture storage in an ultisol and two aridisols. Soil Sci. 2012, 177, 310–320. [Google Scholar] [CrossRef]
- Thomas, S.C.; Frye, S.; Gale, N.; Garmon, M.; Launchbury, R.; Machado, N.; Melamed, S.; Murray, J.; Petroff, A.; Winsborough, C. Biochar mitigates negative effects of salt additions on two herbaceous plant species. J. Environ. Manag. 2013, 129, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Hammer, E.C.; Forstreuter, M.; Rillig, M.C.; Kohler, J. Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl. Soil Ecol. 2015, 96, 114–121. [Google Scholar] [CrossRef]
- Amini, S.; Ghadiri, H.; Chen, C.R.; Marschner, P. Salt-affected soils, reclamation, carbon dynamics, and biochar: A review. J. Soils Sediments 2016, 16, 939–953. [Google Scholar] [CrossRef]
- Sigua, G.C.; Novak, J.M.; Watts, D.W.; Johnson, M.G.; Spokas, K. Efficacies of designer biochars in improving biomass and nutrient uptake of winter wheat grown in a hard setting subsoil layer. Chemosphere 2016, 142, 176–183. [Google Scholar] [CrossRef]
- Blok, C.; van der Salm, C.; Hofland-Zijlstra, J.; Streminska, M.; Eveleens, B.; Regelink, I.; Fryda, L.; Visser, R. Biochar for horticultural rooting media improvement: Evaluation of biochar from gasification and slow pyrolysis. Agronomy 2017, 7, 6. [Google Scholar] [CrossRef]
- Luo, X.X.; Liu, G.C.; Xia, Y.; Chen, L.; Jiang, Z.X.; Zheng, H.; Wang, Z.Y. Use of biochar-compost to improve properties and productivity of the degraded coastal soil in the Yellow River Delta, China. J. Soils Sediments 2017, 17, 780–789. [Google Scholar] [CrossRef]
- Zheng, R.L.; Sun, G.X.; Li, C.; Reid, B.J.; Xie, Z.B.; Zhang, B.; Wang, Q.H. Mitigating cadmium accumulation in greenhouse lettuce production using biochar. Environ. Sci. Pollut. Res. 2017, 24, 6532–6542. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R. Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar-soil mixtures. Geoderma 2013, 193, 122–130. [Google Scholar] [CrossRef]
- Sigua, G.C.; Stone, K.C.; Hunt, P.G.; Cantrell, K.B.; Novak, J.M. Increasing biomass of winter wheat using sorghum biochars. Agron. Sustain. Dev. 2015, 35, 739–748. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Bachmann, R.T.; Rafiq, M.T.; Shang, Z.H.; Joseph, S.; Long, R.J. Influence of pyrolysis temperature on physico-chemical properties of corn stover (Zea mays L.) biochar and feasibility for carbon capture and energy balance. PLoS ONE 2016, 11, e0156894. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.P. Soil properties influencing apparent electrical conductivity: A review. Comput. Electron. Agric. 2005, 46, 45–70. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F.L. Biochar mitigates salinity stress in potato. J. Agron. Crop Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F.L. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Naveed, M.; Zahir, Z.A.; Liu, F.L. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 2015, 42, 770–781. [Google Scholar] [CrossRef]
- Yue, Y.; Guo, W.N.; Lin, Q.M.; Li, G.T.; Zhao, X.R. Improving salt leaching in a simulated saline soil column by three biochars derived from rice straw (Oryza sativa L.), sunflower straw (Helianthus annuus), and cow manure. J. Soil Water Conserv. 2016, 71, 467–475. [Google Scholar] [CrossRef]
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. [Google Scholar] [CrossRef]
- Smider, B.; Singh, B. Agronomic performance of a high ash biochar in two contrasting soils. Agric. Ecosyst. Environ. 2014, 191, 99–107. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Shen, W.; Yao, H.; Meng, X.; Zeng, J.; Zhang, G.; Zamanien, K. A meta-analysis of ecological functions and economic benefits of co-culture models in paddy fields. Agric. Ecosyst. Environ. 2023, 341, 108195. [Google Scholar] [CrossRef]
- Conyers, M.K.; Davey, B.G. Observations on some routine methods for soil pH determination. Soil Sci. 1988, 145, 29–36. [Google Scholar] [CrossRef]
- Kabała, C.; Musztyfaga, E.; Gałka, B.; Łabuńska, D.; Mańczyńska, P. Conversion of Soil pH 1: 2.5 KCl and 1: 2.5 H2O to 1: 5 H2O: Conclusions for Soil Management, Environmental Monitoring, and International Soil Databases. Pol. J. Environ. Stud. 2016, 25, 647. [Google Scholar] [CrossRef] [PubMed]
- Lierop, W.V. Conversion of organic soil pH values measured in water, 0.01 M CaCl2 or 1 N KCl. Can. J. Soil Sci. 1981, 61, 577–579. [Google Scholar] [CrossRef]
- Pan, G.; Li, Y.; Luo, M.; Wang, L. Study on determination of soluble salts by electrical conductivity method in loess-paleosol sequence. J. Univ. Chin. Acad. Sci. 2014, 31, 791. [Google Scholar]
- Al-Busaidi, A.; Yamamoto, T.; Bakheit, C.; Cookson, P. Soil salinity assessment by some destructive and non destructive methods in calcareous soils. J. Jpn. Soc. Soil Phys. 2006, 104, 27–40. [Google Scholar]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Singh, B.; Dolk, M.M.; Shen, Q.; Camps-Arbestain, M. Biochar pH, electrical conductivity and liming potential. In Biochar: A Guide to Analytical Methods; CRC Press: Boca Raton, FL, USA, 2017; Volume 23. [Google Scholar]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Rosenberg, M.S.; Adams, D.C.; Gurevitch, J. Metawin, Version 2.1; Statistical Software for Meta-Analysis; Sinauer Associates: Sunderland, MA, USA, 2000.
- Yu, H.; Zhu, R.; Zhang, X.; Meng, X.; Kong, C.; Zhang, G.; Liu, X.; Li, Y.; Yu, Y.; Yao, H. Responses of yield, CH4 and N2O emissions to ratoon rice cropping and different management practices. Field Crops Res. 2024, 319, 109622. [Google Scholar] [CrossRef]
- Saifullah; Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Uchimiya, M.; Abiven, S.; Schmidt, M.W. Evolution of biochar properties in soil. In Biochar for Environmental Management; Routledge: Abingdon, UK, 2015; pp. 195–233. [Google Scholar]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Matar, A.; Torrent, J.; Ryan, J. Soil and fertilizer phosphorus and crop responses in the dryland Mediterranean zone. In Advances in Soil Science; Springer: Berlin/Heidelberg, Germany, 1992; Volume 18, pp. 81–146. [Google Scholar]
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Soil Sci. Plant Nutr. 2015, 15, 333–352. [Google Scholar] [CrossRef]
- Camps-Arbestain, M.; Amonette, J.E.; Singh, B.; Wang, T.; Schmidt, H.P. A biochar classification system and associated test methods. In Biochar for Environmental Management; Routledge: Abingdon, UK, 2015; pp. 165–193. [Google Scholar]
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterisation and evaluation of biochars for their application as a soil amendment. Soil Res. 2010, 48, 516–525. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef]
- Kong, C. Designing technosols to reduce salinity and water stress of crops growing under arid conditions. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2021. [Google Scholar]
- Li, J.; Song, M.; Yin, J.; Gao, L.; Tian, Y. The beneficial effects of biochar on overall soil quality and plant performance are dose-dependent and are closely associated with soil pH. Pedosphere 2025, in press. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Bennett, J.M.; Wu, L.; Li, H. Effects of sodium adsorption ratio and electrolyte concentration on soil saturated hydraulic conductivity. Geoderma 2022, 414, 115772. [Google Scholar] [CrossRef]
- Wang, X.; Ding, J.; Han, L.; Tan, J.; Ge, X.; Nan, Q. Biochar addition reduces salinity in salt-affected soils with no impact on soil pH: A meta-analysis. Geoderma 2024, 443, 116845. [Google Scholar] [CrossRef]
- Feng, J.; Yu, D.L.; Sinsabaugh, R.L.; Moorhead, D.L.; Andersen, M.N.; Smith, P.; Song, Y.T.; Li, X.Q.; Huang, Q.Y.; Liu, Y.R.; et al. Trade-offs in carbon-degrading enzyme activities limit long-term soil carbon sequestration with biochar addition. Biol. Rev. 2023, 98, 1184–1199. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xue, L.; Zheng, L.; Bao, S.; Liu, Y.; Fang, T.; Xing, B. Biomass-derived N/S dual-doped hierarchically porous carbon material as effective adsorbent for the removal of bisphenol F and bisphenol S. J. Hazard. Mater. 2021, 416, 126126. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabaiai, A.; Menezes-Blackburn, D.; Al-Ismaily, S.; Janke, R.; Pracejus, B.; Al-Alawi, A.; Al-Kindi, M.; Bol, R. Customized biochar for soil applications in arid land: Effect of feedstock type and pyrolysis temperature on soil microbial enumeration and respiration. J. Anal. Appl. Pyrolysis 2022, 168, 105693. [Google Scholar] [CrossRef]
- Ma, C.; Li, G.; Yue, X.; Chen, X.; Yang, Y.; Lam, S.S.; Gu, H.; Peng, W.; Dang, Y. Advancement of Climate Mitigation through Biochar Applications in Agriculture. Eng. Sci. 2025, 36, 1619. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-Ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. 2018, 25, 25668–25680. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-Ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Abrishamkesh, S.; Gorji, M.; Asadi, H.; Bagheri-Marandi, G.H.; Pourbabaee, A.A. Effects of rice husk biochar application on the properties of alkaline soil and lentil growth. Plant Soil Environ. 2015, 61, 475–482. [Google Scholar] [CrossRef]
- Agbna, G.H.D.; She, D.; Liu, Z.; Elshaikh, N.A.; Shao, G.; Timm, L.C. Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci. Hortic. 2017, 222, 90–101. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Calero, J.M.; Barrón, V.; Torrent, J.; Del Campillo, M.C.; Gallardo, A.; Villar, R. Effects of biochars produced from different feedstocks on soil properties and sunflower growth. J. Plant Nutr. Soil Sci. 2014, 177, 16–25. [Google Scholar] [CrossRef]
- Andrés, P.; Rosell-Melé, A.; Colomer-Ventura, F.; Denef, K.; Cotrufo, M.F.; Riba, M.; Alcañiz, J.M. Belowground biota responses to maize biochar addition to the soil of a Mediterranean vineyard. Sci. Total. Environ. 2019, 660, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Ali, K.; Jan, M.T.; Shah, Z.; Jones, D.L.; Quilliam, R.S. Integration of biochar with animal manure and nitrogen for improving maize yields and soil properties in calcareous semi-arid agroecosystems. Field Crops Res. 2016, 195, 28–35. [Google Scholar] [CrossRef]
- Chaganti, V.N.; Crohn, D.M. Evaluating the relative contribution of physiochemical and biological factors in ameliorating a saline–sodic soil amended with composts and biochar and leached with reclaimed water. Geoderma 2015, 259, 45–55. [Google Scholar] [CrossRef]
- Chaganti, V.N.; Crohn, D.M.; Šimůnek, J. Leaching and reclamation of a biochar and compost amended saline–sodic soil with moderate SAR reclaimed water. Agric. Water Manag. 2015, 158, 255–265. [Google Scholar] [CrossRef]
- Chávez-García, E.; Siebe, C. Rehabilitation of a highly saline-sodic soil using a rubble barrier and organic amendments. Soil Tillage Res. 2019, 189, 176–188. [Google Scholar] [CrossRef]
- de Vasconcelos, A.C.F.; Chaves, L.H.G.; Gheyi, H.R.; Fernandes, J.D.; Tito, G.A. Crambe growth in a soil amended with biochar and under saline irrigation. Commun. Soil Sci. Plant Anal. 2017, 48, 1291–1300. [Google Scholar] [CrossRef]
- Elshaikh, N.A.; Liu, Z.; She, D.; Timm, L.A. Increasing the okra salt threshold value with biochar amendments. J. Plant Interact. 2018, 13, 51–63. [Google Scholar] [CrossRef]
- Elshaikh, N.A.; She, D. Decreasing the salt leaching fraction and enhancing water-use efficiency for okra using biochar amendments. Commun. Soil Sci. Plant Anal. 2018, 49, 225–236. [Google Scholar] [CrossRef]
- Ghorbani, M.; Asadi, H.; Abrishamkesh, S. Effects of rice husk biochar on selected soil properties and nitrate leaching in loamy sand and clay soil. Int. Soil Water Conserv. Res. 2019, 7, 258–265. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Z.; Zhu, C.; Zhai, Y.; Lu, P. Effect of biochar on sweet corn and soil salinity under conjunctive irrigation with brackish water in coastal saline soil. Sci. Hortic. 2019, 250, 405–413. [Google Scholar] [CrossRef]
- Irfan, M.; Hussain, Q.; Khan, K.S.; Akmal, M.; Ijaz, S.S.; Hayat, R.; Khalid, A.; Azeem, M.; Rashid, M. Response of soil microbial biomass and enzymatic activity to biochar amendment in the organic carbon deficient arid soil: A 2-year field study. Arab. J. Geosci. 2019, 12, 95. [Google Scholar] [CrossRef]
- Mahmoud, E.; El-Beshbeshy, T.; El-Kader, N.A.; El Shal, R.; Khalafallah, N. Impacts of biochar application on soil fertility, plant nutrients uptake and maize (Zea mays L.) yield in saline sodic soil. Arab. J. Geosci. 2019, 12, 719. [Google Scholar] [CrossRef]
- Martos, S.; Mattana, S.; Ribas, A.; Albanell, E.; Domene, X. Biochar application as a win-win strategy to mitigate soil nitrate pollution without compromising crop yields: A case study in a Mediterranean calcareous soil. J. Soils Sediments 2020, 20, 220–233. [Google Scholar] [CrossRef]
- Maucieri, C.; Zhang, Y.; McDaniel, M.; Borin, M.; Adams, M. Short-term effects of biochar and salinity on soil greenhouse gas emissions from a semi-arid Australian soil after re-wetting. Geoderma 2017, 307, 267–276. [Google Scholar] [CrossRef]
- Mickan, B.S.; Abbott, L.K.; Stefanova, K.; Solaiman, Z.M. Interactions between biochar and mycorrhizal fungi in a water-stressed agricultural soil. Mycorrhiza 2016, 26, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Mohawesh, O.; Coolong, T.; Aliedeh, M.; Alqaraleh, S. Greenhouse evaluation of biochar to enhance soil properties and plant growth performance under arid environment. Bulg. J. Agric. Sci. 2018, 24, 1012–1019. [Google Scholar]
- Mokhtar, Z.B.; Abdolmajid, R.; Reza, G.F.; Yasrebi, J. Influence of poultry manure derived biochars on nutrients bioavailability and chemical properties of a calcareous soil. Arch. Agron. Soil Sci. 2016, 62, 1578–1591. [Google Scholar] [CrossRef]
- Pandian, K.; Subramaniayan, P.; Gnasekaran, P.; Chitraputhirapillai, S. Effect of biochar amendment on soil physical, chemical and biological properties and groundnut yield in rainfed Alfisol of semi-arid tropics. Arch. Agron. Soil Sci. 2016, 62, 1293–1310. [Google Scholar] [CrossRef]
- Awad, M.; Rekaby, S.A.; Hegab, S.A.; Eissa, M.A. Effect of biochar application on barley plants grown on calcareous sandy soils irrigated by saline water. Sci. J. Agric. Sci. 2019, 1, 52–61. [Google Scholar] [CrossRef][Green Version]
- Sekar, S.; Hottle, R.D.; Lal, R. Effects of biochar and anaerobic digester effluent on soil quality and crop growth in Karnataka, India. Agric. Res. 2014, 3, 137–147. [Google Scholar] [CrossRef]
- Shah, T.; Khan, S.; Shah, Z. Soil respiration, pH and EC as influenced by biochar. Soil Environ. 2017, 36, 77–83. [Google Scholar] [CrossRef]
- She, D.; Sun, X.; Gamareldawla, A.H.D.; Nazar, E.A.; Hu, W.; Edith, K.; Yu, S. Benefits of soil biochar amendments to tomato growth under saline water irrigation. Sci. Rep. 2018, 8, 14743. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Mavi, M.S.; Choudhary, O.P. Saline soils can be ameliorated by adding biochar generated from rice-residue waste. CLEAN—Soil Air Water 2019, 47, 1700656. [Google Scholar] [CrossRef]
- Teutscherova, N.; Lojka, B.; Houška, J.; Masaguer, A.; Benito, M.; Vazquez, E. Application of holm oak biochar alters dynamics of enzymatic and microbial activity in two contrasting Mediterranean soils. Eur. J. Soil Biol. 2018, 88, 15–26. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Al-Wabel, M.I.; Ok, Y.S.; Al-Harbi, A.; Wahb-Allah, M.; El-Naggar, A.H.; Ahmad, M.; Al-Faraj, A.; Al-Omran, A. Conocarpus biochar induces changes in soil nutrient availability and tomato growth under saline irrigation. Pedosphere 2016, 26, 27–38. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, G.; Shao, H.B. Furfural and its biochar improve the general properties of a saline soil. Solid Earth 2014, 5, 665–671. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Z.; Huang, J.; Hussain, S.; Zhao, F.; Zhu, C.; Zhu, L.; Cao, X.; Jin, Q. Biochar alleviated the salt stress of induced saline paddy soil and improved the biochemical characteristics of rice seedlings differing in salt tolerance. Soil Tillage Res. 2019, 195, 104372. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Wang, H.; Su, L.; Zhao, C. Biochar addition alleviate the negative effects of drought and salinity stress on soybean productivity and water use efficiency. BMC Plant Biol. 2020, 20, 288. [Google Scholar] [CrossRef]
- Zhang, Y.; Idowu, O.J.; Brewer, C.E. Using agricultural residue biochar to improve soil quality of desert soils. Agriculture 2016, 6, 10. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Chen, L.; Wang, Z.; Xia, Y.; Zhang, Y.; Wang, H.; Luo, X.; Xing, B. Enhanced growth of halophyte plants in biochar-amended coastal soil: Roles of nutrient availability and rhizosphere microbial modulation. Plant Cell Environ. 2018, 41, 517–532. [Google Scholar] [CrossRef]


| Categorical Variable | Groups and Ranges | Notes |
|---|---|---|
| (i) Experimental Climate | ① Arid & Semi-arid ② Mediterranean ③ Greenhouse & Chambers | |
| (ii) Soil texture | ① Loam ② Sandy ③ Clay ④ Unknown | |
| (iii) Initial soil pH | ① ≤6.5 ② >6.5 |
|
| (iv) Initial soil OC | ① ≤5 g kg−1 ② 5–10 g kg−1 ③ >10 g kg−1 ④ Unknown |
|
| (v) Initial soil EC | ① ≤0.4 dS m−1 ② >0.4 dS m−1 |
|
| (vi) Type of feedstock | ① Ligneous materials ② Animal and human wastes ③ Cereal residues ④ Green waste ⑤ Mixed materials ⑥ Unknown |
|
| (vii) Pyrolysis highest heating temperature (HHT) | ① ≤400 °C ② 400–550 °C ③ >550 °C ④ Unknown |
|
| (viii) Biochar pH | ① ≤9.0 ② >9.0 ③ Unknown | |
| (ix) Biochar EC | ① ≤2 dS m−1 ② >2 dS m−1 ③ Unknown |
|
| (x) Leaching fraction | ① Without the leaching fraction ② With the leaching fraction | |
| (xi) Biochar application rate | ① ≤20 t ha−1 yr−1 ② 20–40 t ha−1 yr−1 ③ 40–80 t ha−1 yr−1 ④ >80 t ha−1 yr−1 | |
| (xii) Treatments | ① BC ② BC + IF ③ BC + OA ④ BC + IF + OA |
| Category | Groups of HHT | n | Change (%) | Lower 95CIs (%) | Upper 95CIs (%) |
|---|---|---|---|---|---|
| Ligneous material | ≤400 °C | 14 | 19.23 | 4.22 | 38.00 |
| 400–550 °C | 41 | 19.63 | 5.94 | 36.07 | |
| >550 °C | 13 | 6.46 | −3.02 | 16.92 | |
| Cereal residue | ≤400 °C | 27 | 38.83 | 19.20 | 62.94 |
| 400–550 °C | 22 | 16.51 | −6.85 | 47.24 | |
| >550 °C | 2 | −12.81 | −16.93 | −8.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Feng, B.; Dong, Y.; Song, X.; Sun, X.; Song, X.; Li, X.; Guo, G.; Bai, D.; Kong, C. How to Minimize the Impact of Biochar on Soil Salinity in Drylands? Lessons from a Data Synthesis. Agronomy 2025, 15, 2609. https://doi.org/10.3390/agronomy15112609
Yu H, Feng B, Dong Y, Song X, Sun X, Song X, Li X, Guo G, Bai D, Kong C. How to Minimize the Impact of Biochar on Soil Salinity in Drylands? Lessons from a Data Synthesis. Agronomy. 2025; 15(11):2609. https://doi.org/10.3390/agronomy15112609
Chicago/Turabian StyleYu, Haiyang, Biyun Feng, Yuanyuan Dong, Xinyue Song, Xiaojing Sun, Xiaoyue Song, Xiaojing Li, Guomei Guo, Dezhi Bai, and Chao Kong. 2025. "How to Minimize the Impact of Biochar on Soil Salinity in Drylands? Lessons from a Data Synthesis" Agronomy 15, no. 11: 2609. https://doi.org/10.3390/agronomy15112609
APA StyleYu, H., Feng, B., Dong, Y., Song, X., Sun, X., Song, X., Li, X., Guo, G., Bai, D., & Kong, C. (2025). How to Minimize the Impact of Biochar on Soil Salinity in Drylands? Lessons from a Data Synthesis. Agronomy, 15(11), 2609. https://doi.org/10.3390/agronomy15112609





