Effect of Drought and High-Light Stress on Volatile Compounds and Quality of Welsh Onion (Allium fistulosum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation
2.2. Experimental Design
2.3. Determination of Nutritional Quality
2.4. Determination of Volatile Compounds
2.5. Determination of Mineral Elements
2.6. Statistical Analysis
3. Results
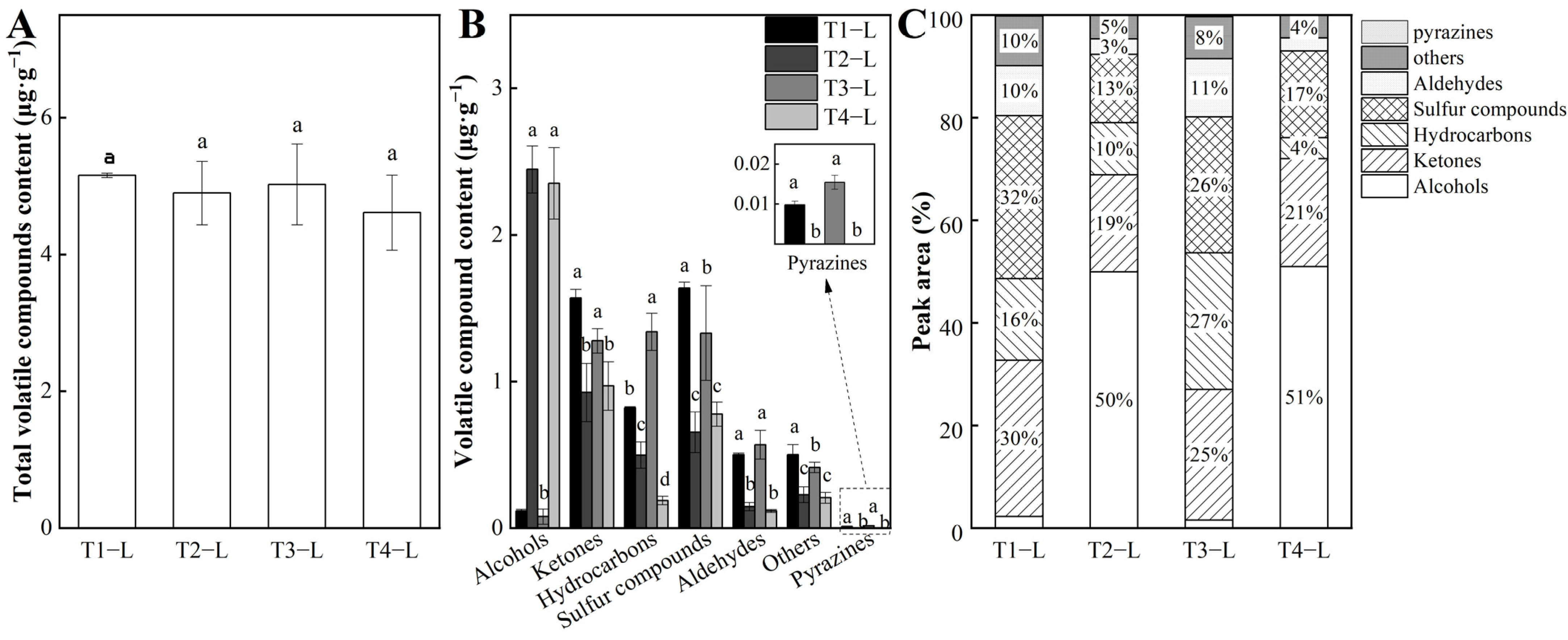
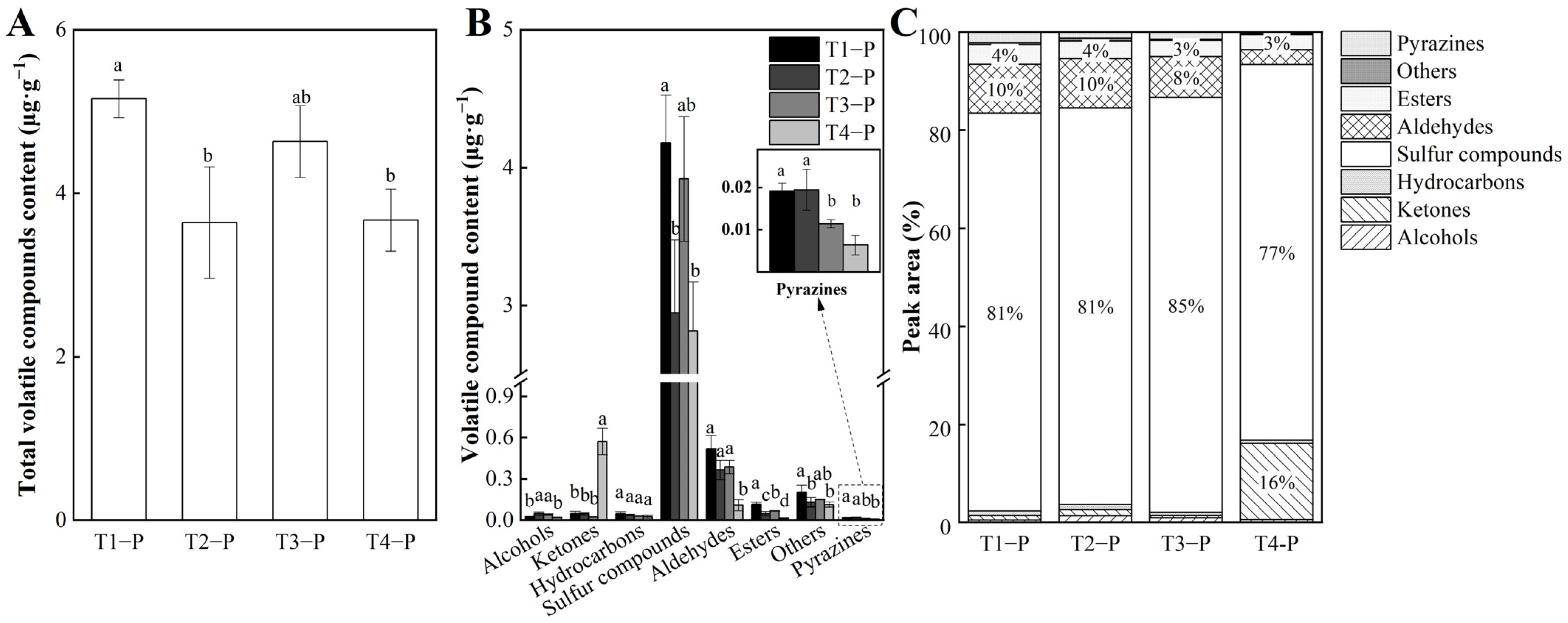
3.1. Identification and Analysis of Volatile Compounds
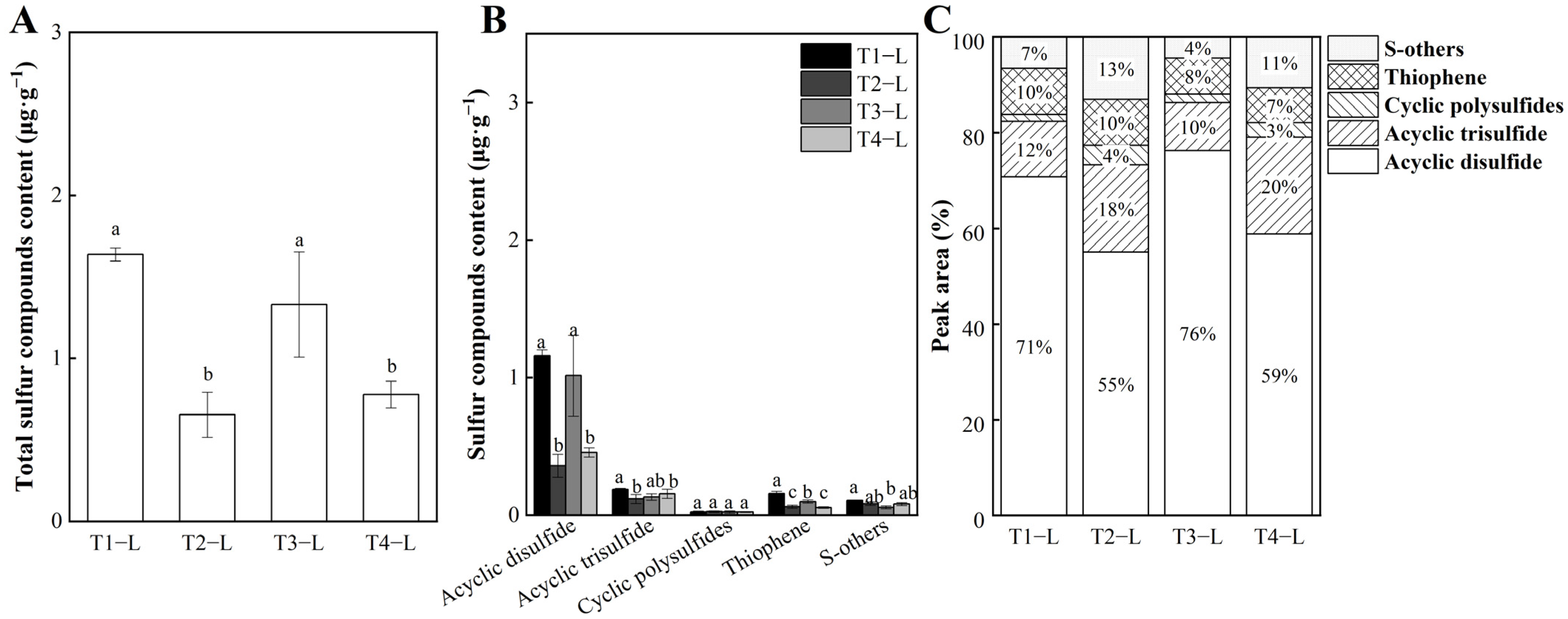
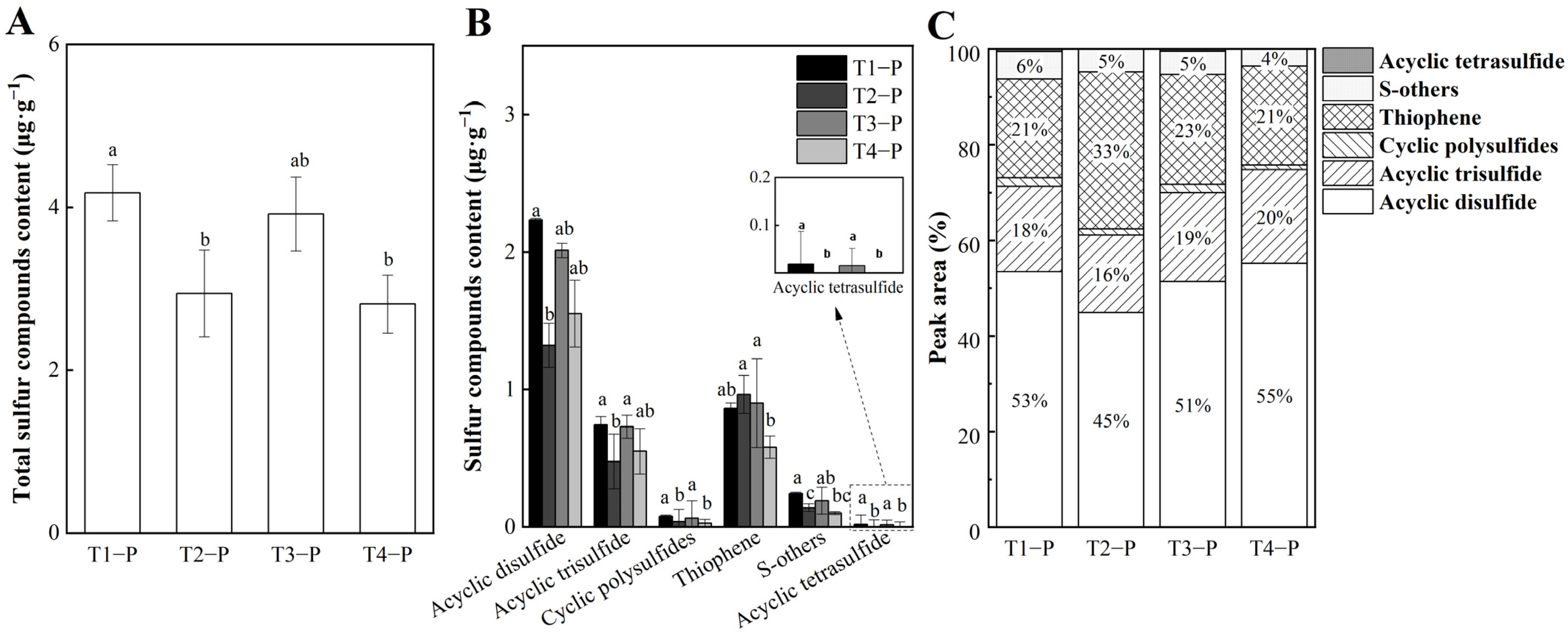
3.2. Analysis of Sulfur Compounds
3.3. Nutritional Quality of Welsh Onion
3.4. Analysis of Mineral Elements
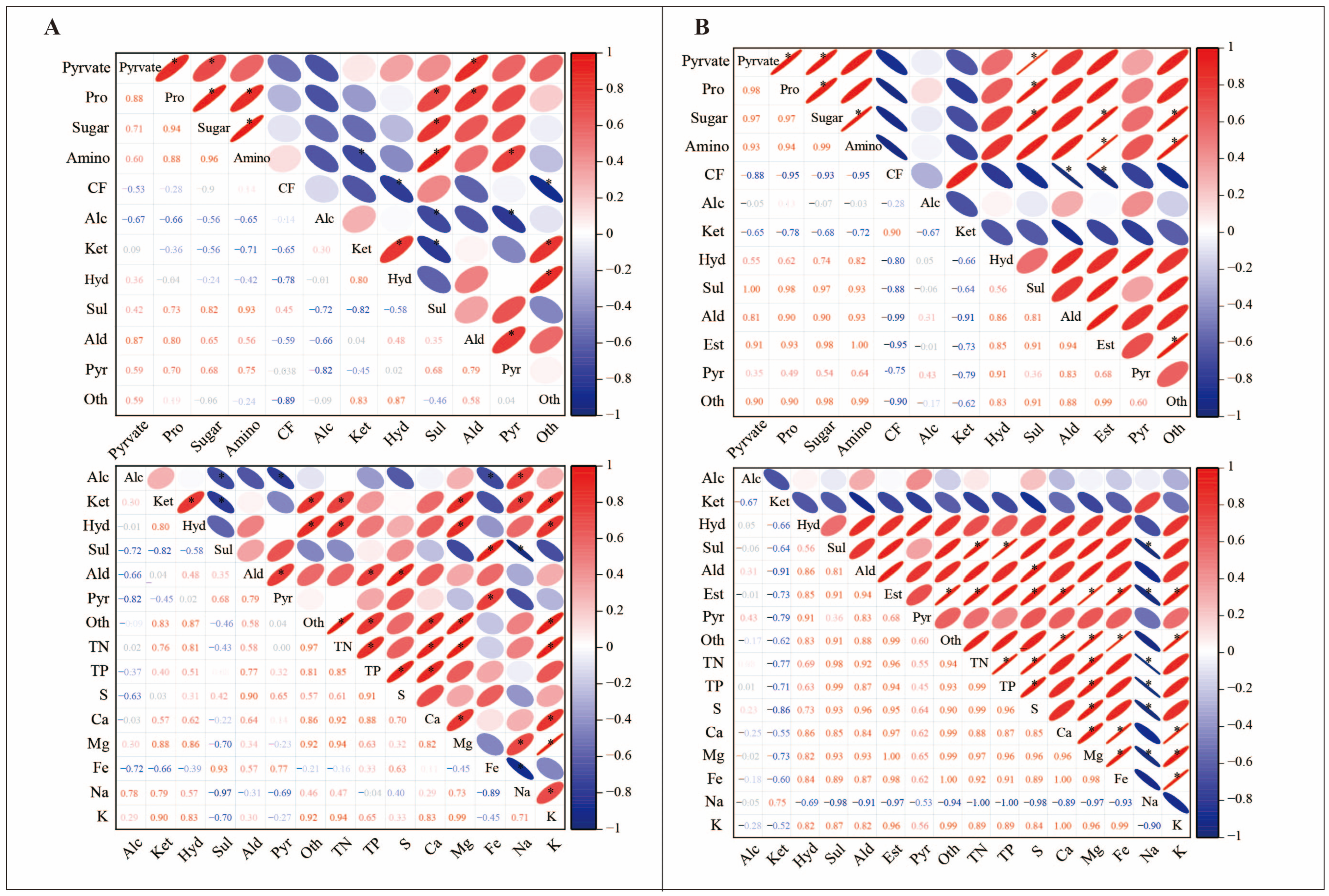
3.5. Correlation Between Volatile Compounds, Nutritional Quality, and Mineral Elements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, P.; Weng, R.; Sheng, X.; Wang, X.; Zhang, W.; Qian, Y.; Qiu, J. Profiling of organosulfur compounds and amino acids in garlic from different regions of China. Food Chem. 2020, 305, 125499. [Google Scholar] [CrossRef]
- Yan, J.-K.; Zhu, J.; Liu, Y.; Chen, X.; Wang, W.; Zhang, H.; Li, L. Recent advances in research on Allium plants: Functional ingredients, physiological activities, and applications in agricultural and food sciences. Crit. Rev. Food Sci. Nutr. 2023, 63, 8107–8135. [Google Scholar] [CrossRef]
- Gupta, A.J.; Kaldate, S.; Volaguthala, S.; Mahajan, V. Onion nutritional and nutraceutical composition and therapeutic potential of its phytochemicals assessed through preclinical and clinical studies. J. Funct. Foods 2025, 129, 106889. [Google Scholar] [CrossRef]
- Zhao, X.-X.; Lin, F.-J.; Li, H.; Li, H.-B.; Wu, D.-T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.-H.; Gan, R.-Y. Recent Advances in Bioactive Compounds, Health Functions, and Safety Concerns of Onion (Allium cepa L.). Front. Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef]
- Bommareddy, A.; VanWert, A.L.; McCune, D.F.; Brozena, S.L.; Witczak, Z.; Singh, S.V. The Role of Organosulfur Compounds Derived from Allium Vegetables in Cancer Prevention and Therapy. In Critical Dietary Factors in Cancer Chemoprevention; Ullah, M.F., Ahmad, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 111–152. [Google Scholar]
- Marefati, N.; Ghorani, V.; Shakeri, F.; Boskabady, M.; Kianian, F.; Rezaee, R.; Boskabady, M.H. A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharm. Biol. 2021, 59, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological Properties and Bioactive Components of Allium cepa L.: Focus on Potential Benefits in the Treatment of Obesity and Related Comorbidities. Molecules 2019, 24, 119. [Google Scholar] [CrossRef] [PubMed]
- Randle, W.; Lancaster, J.E. Sulphur Compounds in Alliums in Relation to Flavour Quality; CABI Publishing: Wallingford, United Kingdom, 2002; pp. 329–356. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Locatelli, D.A.; González, R.E.; Cavagnaro, P.F.; Camargo, A.B. Analytical methods for bioactive sulfur compounds in Allium: An integrated review and future directions. J. Food Compos. Anal. 2017, 61, 4–19. [Google Scholar] [CrossRef]
- Barba, F.J.; Orlien, V. Processing, bioaccessibility and bioavailability of bioactive sulfur compounds: Facts and gaps. J. Food Compos. Anal. 2017, 61, 1–3. [Google Scholar] [CrossRef]
- Gunther, W.H.H. Garlic and other alliums—The lore and the science. J. Sulfur Chem. 2013, 34, 208. [Google Scholar] [CrossRef]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef]
- Krest, I.; Glodek, J.; Keusgen, M. Cysteine Sulfoxides and Alliinase Activity of Some Allium Species. J. Agric. Food Chem. 2000, 48, 3753–3760. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Hori, Y.; Myoda, T. Volatile compounds of fresh and processed garlic (Review). Exp. Ther. Med. 2020, 19, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Doran, J.A.; O’Donnell, J.S.; Lairson, L.L.; McDonald, M.R.; Schwan, A.L.; Grodzinski, B. S-Alk(en)yl-L-cysteine Sulfoxides and Relative Pungency Measurements of Photosynthetic and Nonphotosynthetic Tissues of Allium porrum. J. Agric. Food Chem. 2007, 55, 8243–8250. [Google Scholar] [CrossRef] [PubMed]
- Bernaert, N.; Goetghebeur, L.; De Clercq, H.; De Loose, M.; Daeseleire, E.; Van Pamel, E.; Van Bockstaele, E.; Van Droogenbroeck, B. Influence of Cultivar and Harvest Time on the Amounts of Isoalliin and Methiin in Leek (Allium ampeloprasum var. porrum). J. Agric. Food Chem. 2012, 60, 10910–10919. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C.F.R. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef]
- Freeman, G.G.; Mossadeghi, N. Studies on relationship between water regime and flavour strength in watercress (Rorippa nasturtium-aquaticum (L) Hayek), cabbage (Brassica oleracea capitata) and onion (Allium cepa). J. Hortic. Sci. 1973, 48, 365–378. [Google Scholar] [CrossRef]
- Freeman, G.G.; Mossadeghi, N. Effect of sulphate nutrition on flavour components of onion (Allium cepa). J. Sci. Food Agric. 1970, 21, 610–615. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Schnug, E. Influence of Nitrogen and Sulfur Fertilization on the Alliin Content of Onions and Garlic. J. Plant Nutr. 2005, 27, 1827–1839. [Google Scholar] [CrossRef]
- Lee, E.J.; Yoo, K.S.; Jifon, J.; Patil, B.S. Application of extra sulfur to high-sulfur soils does not increase pungency and related compounds in shortday onions. Sci. Hortic. 2009, 123, 178–183. [Google Scholar] [CrossRef]
- Barrales-Heredia, S.M.; Grimaldo-Juárez, O.; Suárez-Hernández, Á.M.; González-Vega, R.I.; Díaz-Ramírez, J.; García-López, A.M.; Soto-Ortiz, R.; González-Mendoza, D.; Iturralde-García, R.D.; Dórame-Miranda, R.F.; et al. Effects of Different Irrigation Regimes and Nitrogen Fertilization on the Physicochemical and Bioactive Characteristics of onion (Allium cepa L.). Horticulturae 2023, 9, 344. [Google Scholar] [CrossRef]
- Randle, W.M. Increasing nitrogen concentration in hydroponic solutions affects onion flavor and bulb quality. J. Am. Soc. Hortic. Sci. 2000, 125, 254–259. [Google Scholar] [CrossRef]
- Ling-jun, K.; Kun, X.U.; Lei, W.; Ping, H.E.; Yong-zheng, Z. Influence of nitrogen and sulfur interaction on growth and quality of Chinese spring onion. J. Plant Nutr. Fertil. 2013, 19, 1272–1278. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, Y.; Zhang, W.; Lang, D.; Zhang, X.; Li, Z.; Zhang, X. Response of Carbon and Nitrogen Metabolism and Secondary Metabolites to Drought Stress and Salt Stress in Plants. J. Plant Biol. 2019, 62, 387–399. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, W.; Ma, J.; Cheng, Z.; Zhang, X.; Liu, X.; Wu, X.; Zhang, J. An Integrated Framework for Drought Stress in Plants. Int. J. Mol. Sci. 2024, 25, 9347. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; Yang, X.; Feng, K.; Wang, G.; Shi, Q.; Wang, X.; Yuan, X.; Ren, J. Hydrogen Sulfide Increases Drought Tolerance by Modulating Carbon and Nitrogen Metabolism in Foxtail Millet Seedlings. Agronomy 2024, 14, 1080. [Google Scholar] [CrossRef]
- Ren, J.; Xie, T.; Wang, Y.; Li, H.; Liu, T.; Zhang, S.; Yin, L.; Wang, S.; Deng, X.-P.; Ke, Q. Coordinated regulation of carbon and nitrogen assimilation confers drought tolerance in maize (Zea mays L.). Environ. Exp. Bot. 2020, 176, 104086. [Google Scholar] [CrossRef]
- Ghasemi, S.; Kumleh, H.H.; Kordrostami, M.; Rezadoost, M.H. Drought stress-mediated alterations in secondary metabolites and biosynthetic gene expression in cumin plants: Insights from gene-specific and metabolite-level analyses. Plant Stress 2023, 10, 100241. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Wu, W.; Wu, H.; Liang, R.; Huang, S.; Meng, L.; Zhang, M.; Xie, F.; Zhu, H. Light regulates the synthesis and accumulation of plant secondary metabolites. Front. Plant Sci. 2025, 16, 1644472. [Google Scholar] [CrossRef]
- Alsanius, B.W.; Karlsson, M.; Rosberg, A.K.; Dorais, M.; Naznin, M.T.; Khalil, S.; Bergstrand, K.-J. Light and Microbial Lifestyle: The Impact of Light Quality on Plant–Microbe Interactions in Horticultural Production Systems—A Review. Horticulturae 2019, 5, 41. [Google Scholar] [CrossRef]
- Gao, S.; Kong, Y.; Lv, Y.; Cao, B.; Chen, Z.; Xu, K. Effect of different LED light quality combination on the content of vitamin C, soluble sugar, organic acids, amino acids, antioxidant capacity and mineral elements in green onion (Allium fistulosum L.). Food Res. Int. 2022, 156, 111329. [Google Scholar] [CrossRef]
- Gao, S.; Liu, X.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. The Spectral Irradiance, Growth, Photosynthetic Characteristics, Antioxidant System, and Nutritional Status of Green Onion (Allium fistulosum L.) Grown Under Different Photo-Selective Nets. Front. Plant Sci. 2021, 12, 650471. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, K.; Li, N.; Lv, Y.; Cao, B.; Chen, Z.; Xu, K. The growth and photosynthetic responses of white LEDs with supplemental blue light in green onion (Allium fistulosum L.) unveiled by Illumina and single-molecule real-time (SMRT) RNA-sequencing. Environ. Exp. Bot. 2022, 197, 104835. [Google Scholar] [CrossRef]
- Van Gestel, N.C.; Nesbit, A.D.; Gordon, E.P.; Green, C.; Paré, P.W.; Thompson, L.; Peffley, E.B.; Tissue, D.T. Continuous light may induce photosynthetic downregulation in onion—Consequences for growth and biomass partitioning. Physiol. Plant. 2005, 125, 235–246. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Minhas, P.S.; Meena, K.K.; Singh, N.P.; Hegade, P.M.; Sorty, A.M. Growth, bulb yield, water productivity and quality of onion (Allium cepa L.) as affected by deficit irrigation regimes and exogenous application of plant bio–regulators. Agric. Water Manag. 2018, 199, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Gao, S.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. Alterations in leaf photosynthetic electron transport in Welsh onion (Allium fistulosum L.) under different light intensity and soil water conditions. Plant Biol. 2021, 23, 83–90. [Google Scholar] [CrossRef]
- Wu, X.; Hao, Z.; Zhang, X.; Hao, F. Distribution and trend of compound hot and dry events during summer in China. Water Resour. Hydropower Eng. 2021, 52, 90–98. [Google Scholar] [CrossRef]
- Borthakur, U.; Roy, M.; Handique, N.; Purkait, K.; Gautam, S.; Sarma, G.; Choudhury, D.; Sarma, N. Assessment of chromosomal aberrations in onion root cells under polyethylene glycol(PEG 6000) induced osmotic stress. CAHIERS MAGELLANES-NS 2024, 6, 4115–4125. [Google Scholar]
- Tang, J.; Qi, X.; Chen, Z.; Shen, H.; Gao, L.; Yang, X. Study on Effect of Drought Stress with PEG-6000 on Physiological and Biochemical Indicators in Kadsura coccinea. ACS Agric. Sci. Technol. 2016, 17, 2059–2061. [Google Scholar]
- Wan, Y.-Y.; Chen, S.-Y.; Huang, Y.-W.; Li, X.; Zhang, Y.; Wang, X.-J.; Bai, J.-G. Caffeic acid pretreatment enhances dehydration tolerance in cucumber seedlings by increasing antioxidant enzyme activity and proline and soluble sugar contents. Sci. Hortic. 2014, 173, 54–64. [Google Scholar] [CrossRef]
- Li, H. Principle and Technology of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2002. [Google Scholar]
- Randle, W.M.; Bussard, M.; Warnock, D. Ontogeny and sulfur fertility affect leaf sulfur in short-day onions. J. Am. Soc. Hortic. Sci. 1993, 118, 762–765. [Google Scholar] [CrossRef]
- Kremr, D.; Bajerová, P.; Bajer, T.; Eisner, A.; Adam, M.; Ventura, K. Using headspace solid-phase microextraction for comparison of volatile sulphur compounds of fresh plants belonging to families Alliaceae and Brassicaceae. J. Food Sci. Technol. 2015, 52, 5727–5735. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Suzuno, H.; Sugiyama, N.; Innami, S.; Tadokoro, T.; Maekawa, A. Nutritive evaluation on chemical components of leaves, stalks and stems of sweet potatoes (Ipomoea batatas poir). Food Chem. 2000, 68, 359–367. [Google Scholar] [CrossRef]
- Pai, S.; Yang, C.; Riley, J.P. Effects of acidity and molybdate concentration on the kinetics of the formation of the phosphoantimonylmolybdenum blue complex. Anal. Chim. Acta 1990, 229, 115–120. [Google Scholar] [CrossRef]
- Liu, X.; Guo, J.; Chen, Z.; Xu, K.; Xu, K. Detection of Volatile Compounds and Their Contribution to the Nutritional Quality of Chinese and Japanese Welsh Onions (Allium fistulosum L.). Horticulturae 2024, 10, 446. [Google Scholar] [CrossRef]
- Nandakumar, R.; Eyres, G.T.; Burritt, D.J.; Kebede, B.; Leus, M.; Oey, I. Impact of Pulsed Electric Fields on the Volatile Compounds Produced in Whole Onions (Allium cepa and Allium fistulosum). Foods 2018, 7, 183. [Google Scholar] [CrossRef]
- Kusano, M.; Kobayashi, M.; Iizuka, Y.; Fukushima, A.; Saito, K. Unbiased profiling of volatile organic compounds in the headspace of Allium plants using an in-tube extraction device. BMC Res. Notes 2016, 9, 133. [Google Scholar] [CrossRef]
- Xu, L.; Zang, E.; Sun, S.; Li, M. Main flavor compounds and molecular regulation mechanisms in fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2023, 63, 11859–11879. [Google Scholar] [CrossRef]
- Naeem, M.Y. The Impact of Drought Stress on the Nutritional Quality of Vegetables. In Drought Stress: Review and Recommendations; Chaudhry, U.K., Öztürk, Z.N., Gökçe, A.F., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 143–158. [Google Scholar]
- Yasmin, H.; Rashid, U.; Hassan, M.N.; Nosheen, A.; Naz, R.; Ilyas, N.; Sajjad, M.; Azmat, A.; Alyemeni, M.N. Volatile organic compounds produced by Pseudomonas pseudoalcaligenes alleviated drought stress by modulating defense system in maize (Zea mays L.). Physiol. Plant. 2021, 172, 896–911. [Google Scholar] [CrossRef]
- Schreiner, M.; Beyene, B.; Krumbein, A.; Stützel, H. Ontogenetic Changes of 2-Propenyl and 3-Indolylmethyl Glucosinolates in Brassica carinata Leaves as Affected by Water Supply. J. Agric. Food Chem. 2009, 57, 7259–7263. [Google Scholar] [CrossRef]
- Yoo, K.S.; Pike, L.M. Development of an automated system for pyruvic acid analysis in onion breeding. Sci. Hortic. 1999, 82, 193–201. [Google Scholar] [CrossRef]
- Filella, I.; Peñuelas, J.; Seco, R. Short-chained oxygenated VOC emissions in Pinus halepensis in response to changes in water availability. Acta Physiol. Plant. 2009, 31, 311–318. [Google Scholar] [CrossRef]
- Gîtin, L.; Dinică, R.; Neagu, C.; Dumitrascu, L. Sulfur compounds identification and quantification from Allium spp. fresh leaves. J. Food Drug Anal. 2014, 22, 425–430. [Google Scholar] [CrossRef]
- McGorrin, R.J. The Significance of Volatile Sulfur Compounds in Food Flavors. In Volatile Sulfur Compounds in Food; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Volume 1068, pp. 3–31. [Google Scholar]
- Ianni, F.; Maura, M.; Stefania, S.; Roccaldo, S.; Natalini, B. Quantitative Evaluation of the Pyruvic Acid Content in Onion Samples with a Fully Validated High-Performance Liquid Chromatography Method. Int. J. Food Prop. 2016, 19, 752–759. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, G.; Zhou, L.; Zhang, S.; Su, L.; Sun, X.; Borrás-Hidalgo, O.; Li, K.; Yue, Q.; Zhao, L. Effects of nitrogen levels on gene expression and amino acid metabolism in Welsh onion. BMC Genom. 2021, 22, 803. [Google Scholar] [CrossRef]
- Golubkina, N.; Amalfitano, C.; Sekara, A.; Tallarita, A.; Pokluda, R.; Stoleru, V.; Cuciniello, A.; Agafonov, A.F.; Kalisz, A.; Hamburdă, S.B.; et al. Yield and bulb quality of storage onion cultivars as affected by farming system and nitrogen dose. Sci. Hortic. 2022, 293, 110751. [Google Scholar] [CrossRef]
- Coolong, T.W.; Randle, W.M. The Effects of Calcium Chloride and Ammonium Sulfate on Onion Bulb Quality at Harvest and During Storage. HortScience 2008, 43, 465–471. [Google Scholar] [CrossRef]
- Liu, S.; He, H.; Feng, G.; Chen, Q. Effect of nitrogen and sulfur interaction on growth and pungency of different pseudostem types of Chinese spring onion (Allium fistulosum L.). Sci. Hortic. 2009, 121, 12–18. [Google Scholar] [CrossRef]
- McCallum, J.; Porter, N.; Searle, B.; Shaw, M.; Bettjeman, B.; McManus, M. Sulfur and nitrogen fertility affects flavour of field-grown onions. Plant Soil 2005, 269, 151–158. [Google Scholar] [CrossRef]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Mallor, C.; Balcells, M.; Mallor, F.; Sales, E. Genetic variation for bulb size, soluble solids content and pungency in the Spanish sweet onion variety Fuentes de Ebro. Response to selection for low pungency. Plant Breed. 2011, 130, 55–59. [Google Scholar] [CrossRef]
- Mallor, C.; Sales, E. Yield and traits of bulb quality in the Spanish sweet onion cultivar ‘Fuentes de Ebro’ after selection for low pungency. Sci. Hortic. 2012, 140, 60–65. [Google Scholar] [CrossRef]
- Simon, P.W. Genetic analysis of pungency and soluble solids in long-storage onions. Euphytica 1995, 82, 1–8. [Google Scholar] [CrossRef]
| Treatment | Pyruvate (mg/g) | Soluble Protein (mg/g) | Soluble Sugar (%) | Free Amino Acid (mg/g) | Crude Fiber (mg/g) | |
|---|---|---|---|---|---|---|
| Leaf | T1 | 0.97 ± 0.01 a | 1.02 ± 0.01 a | 0.84 ± 0.01 a | 0.66 ± 0.02 a | 0.31 ± 0.01 d |
| T2 | 0.58 ± 0.01 c | 0.61 ± 0.01 c | 0.65 ± 0.01 c | 0.49 ± 0.01 c | 0.56 ± 0.02 b | |
| T3 | 0.81 ± 0.01 b | 0.84 ± 0.01 b | 0.75 ± 0.01 b | 0.58 ± 0.01 b | 0.39 ± 0.01 c | |
| T4 | 0.45 ± 0.03 d | 0.50 ± 0.04 d | 0.51 ± 0.02 d | 0.45 ± 0.01 d | 0.70 ± 0.02 a | |
| Pseudo-stem | T1 | 0.88 ± 0.01 a | 1.2 ± 0.024 a | 1.39 ± 0.04 a | 1.1 ± 0.01 a | 0.65 ± 0.01 d |
| T2 | 0.62 ± 0.01 c | 0.81 ± 0.01 c | 0.80 ± 0.01 c | 0.77 ± 0.01 c | 0.88 ± 0.02 b | |
| T3 | 0.83 ± 0.01 b | 1.16 ± 0.01 b | 1.10 ± 0.01 b | 0.89 ± 0.01 b | 0.78 ± 0.03 c | |
| T4 | 0.59 ± 0.01 d | 0.61 ± 0.02 d | 0.64 ± 0.02 d | 0.63 ± 0.01 d | 1.17 ± 0.02 a | |
| Treatment | TN (mg/g) | TP (mg/g) | K (mg/g) | S (mg/g) | Ca (mg/g) | Mg (mg/g) | Fe (mg/g) | Na (mg/g) | |
|---|---|---|---|---|---|---|---|---|---|
| Leaf | T1 | 31.27 ± 0.36 a | 6.11 ± 0.02 a | 45.32 ± 0.26 a | 8.78 ± 0.06 a | 10.39 ± 0.03 a | 3.06 ± 0.04 a | 0.20 ± 0.01 a | 2.54 ± 0.05 d |
| T2 | 20.17 ± 0.34 c | 3.77 ± 0.03 c | 34.60 ± 0.22 c | 7.92 ± 0.08 c | 7.50 ± 0.05 c | 2.72 ± 0.04 c | 0.15 ± 0.01 c | 3.18 ± 0.14 b | |
| T3 | 25.96 ± 0.55 b | 4.58 ± 0.03 b | 37.03 ± 0.64 b | 8.35 ± 0.08 b | 8.35 ± 0.05 b | 2.91 ± 0.04 b | 0.18 ± 0.01 b | 2.87 ± 0.08 c | |
| T4 | 17.28 ± 0.11 d | 3.27 ± 0.01 d | 27.13 ± 0.34 d | 7.68 ± 0.03 d | 6.63 ± 0.04 d | 2.48 ± 0.03 d | 0.14 ± 0.01 d | 3.39 ± 0.01 a | |
| Pseudo-stem | T1 | 18.23 ± 0.30 a | 4.76 ± 0.09 a | 20.32 ± 0.4 a | 8.59 ± 0.03 a | 8.86 ± 0.11 a | 2.20 ± 0.03 a | 0.30 ± 0.01 a | 1.38 ± 0.06 c |
| T2 | 12.22 ± 0.19 c | 3.40 ± 0.06 c | 13.41 ± 0.57 c | 8.16 ± 0.05 c | 5.42 ± 0.26 c | 1.95 ± 0.0 bc | 0.22 ± 0.01 c | 1.64 ± 0.01 b | |
| T3 | 16.19 ± 0.12 b | 4.40 ± 0.03 b | 15.09 ± 0.2 b | 8.44 ± 0.02 b | 6.00 ± 0.07 b | 2.04 ± 0.01 b | 0.24 ± 0.01 b | 1.47 ± 0.05 c | |
| T4 | 9.51 ± 0.20 d | 3.05 ± 0.03 d | 12.56 ± 0.21 c | 7.79 ± 0.04 d | 4.71 ± 0.19 d | 1.84 ± 0.08 c | 0.20 ± 0.01 c | 1.74 ± 0.01 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chen, Z.; Xu, K.; Xu, K. Effect of Drought and High-Light Stress on Volatile Compounds and Quality of Welsh Onion (Allium fistulosum L.). Agronomy 2025, 15, 2349. https://doi.org/10.3390/agronomy15102349
Liu X, Chen Z, Xu K, Xu K. Effect of Drought and High-Light Stress on Volatile Compounds and Quality of Welsh Onion (Allium fistulosum L.). Agronomy. 2025; 15(10):2349. https://doi.org/10.3390/agronomy15102349
Chicago/Turabian StyleLiu, Xuena, Zijing Chen, Kun Xu, and Kang Xu. 2025. "Effect of Drought and High-Light Stress on Volatile Compounds and Quality of Welsh Onion (Allium fistulosum L.)" Agronomy 15, no. 10: 2349. https://doi.org/10.3390/agronomy15102349
APA StyleLiu, X., Chen, Z., Xu, K., & Xu, K. (2025). Effect of Drought and High-Light Stress on Volatile Compounds and Quality of Welsh Onion (Allium fistulosum L.). Agronomy, 15(10), 2349. https://doi.org/10.3390/agronomy15102349






