Analysis of Morphological Traits, Essential Oil Yield, and Secondary Metabolites in Seven Lavandins and Lavenders Grown in Two Pedoclimatic Areas in Tuscany (Italy)
Abstract
1. Introduction
2. Materials and Methods
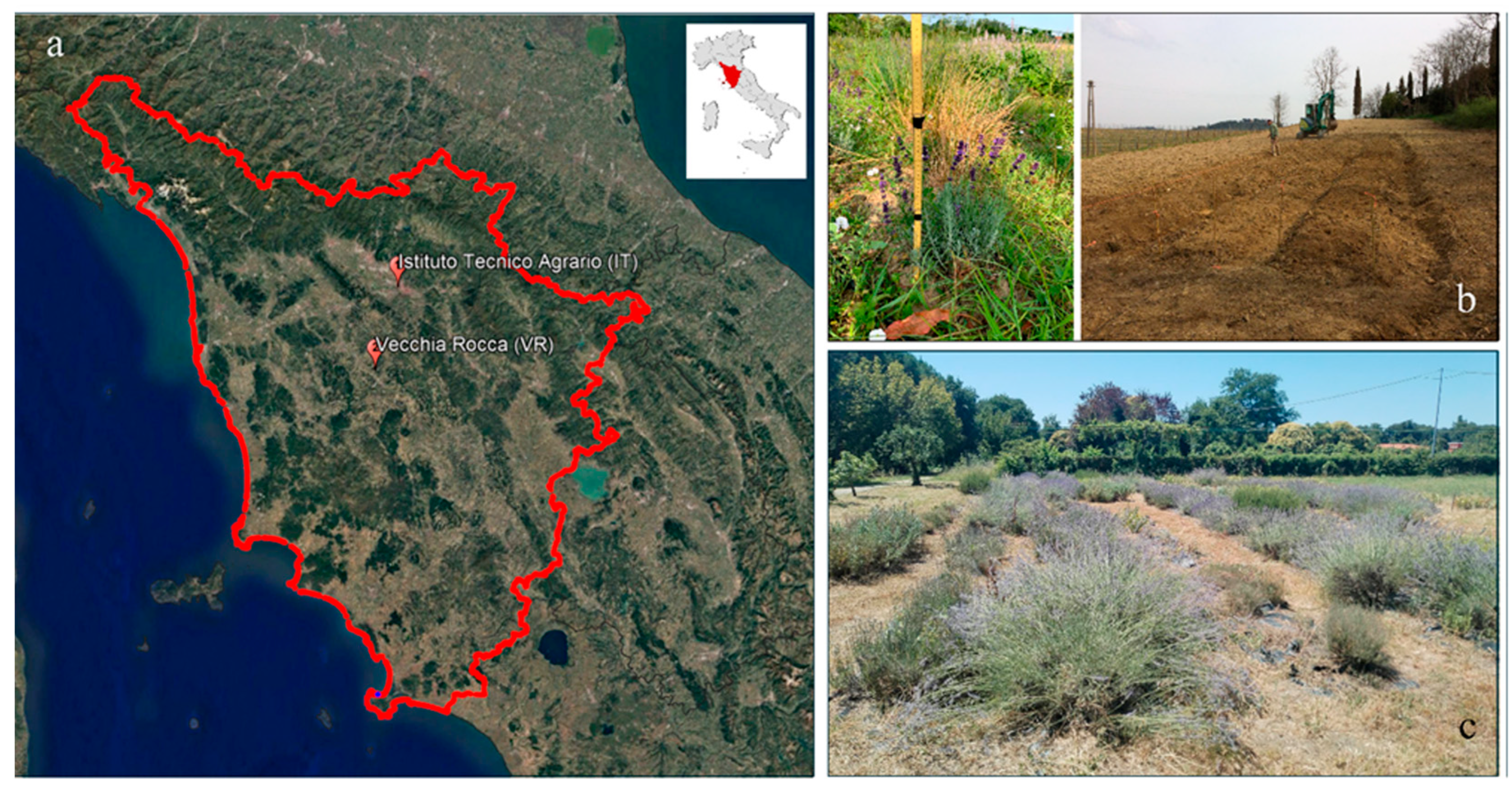
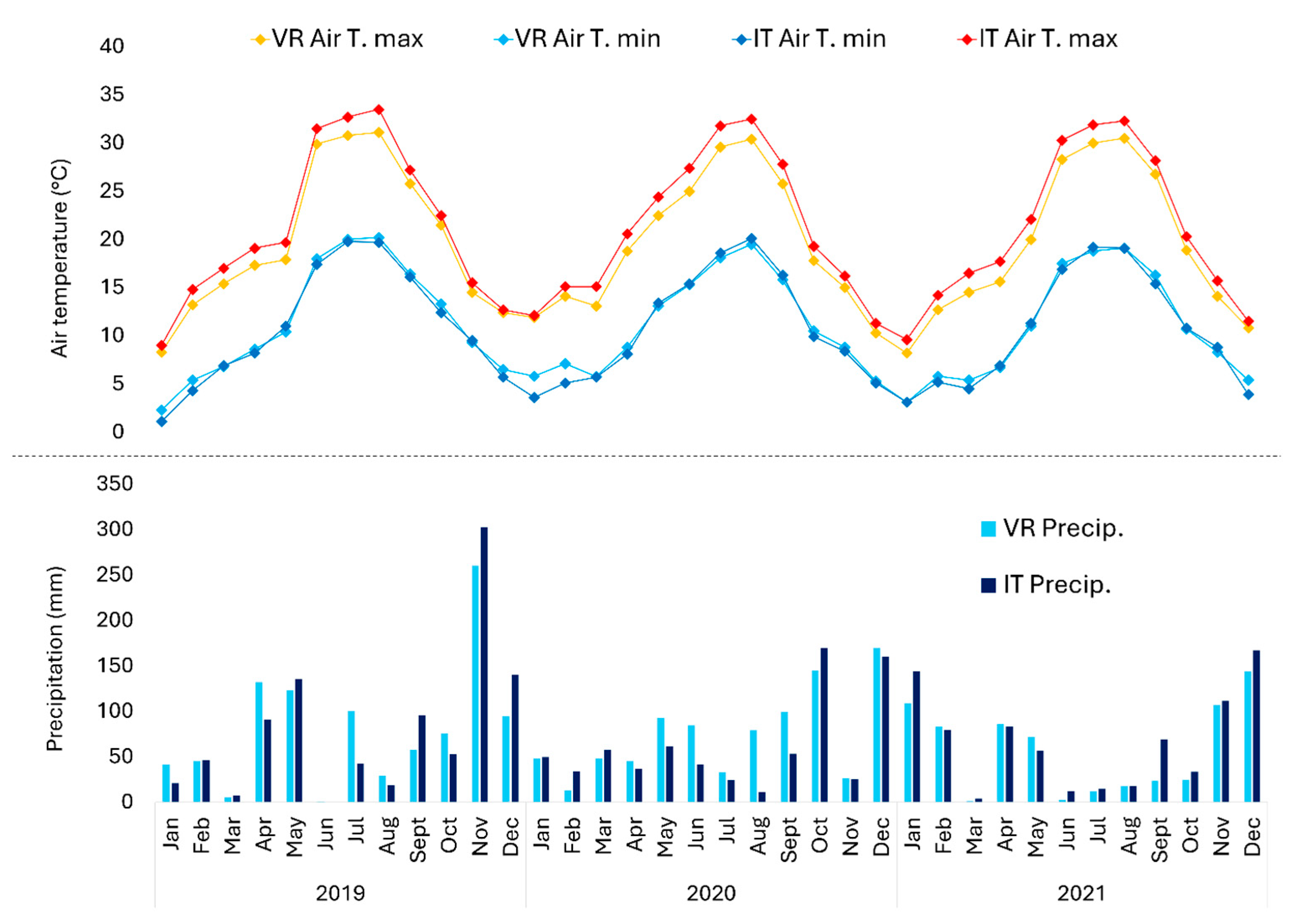
2.1. Study Areas
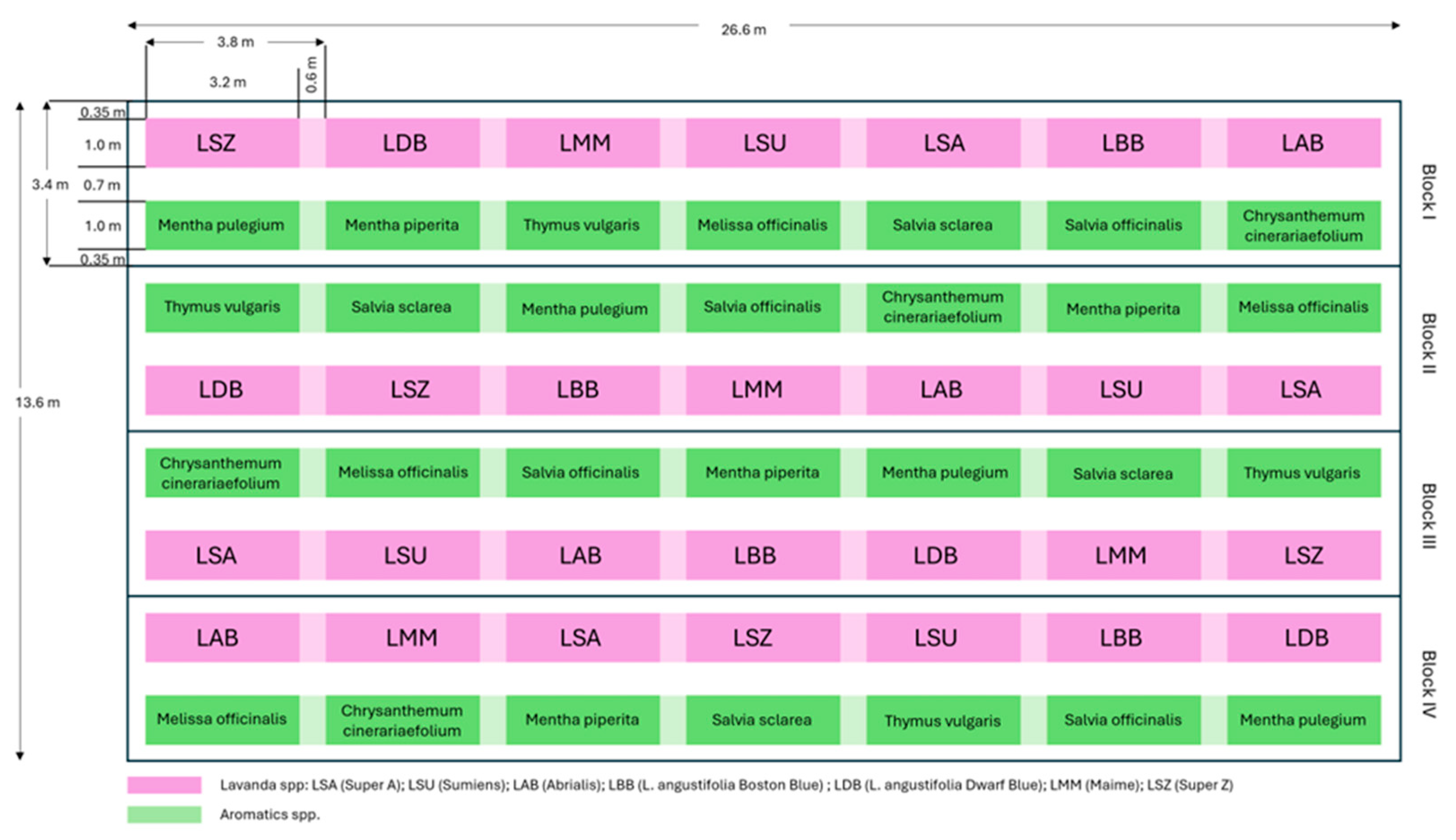
2.2. Experimental Design
2.3. Morphological Data Collection and Analysis of Secondary Metabolites
2.4. Statistical Analysis
3. Results
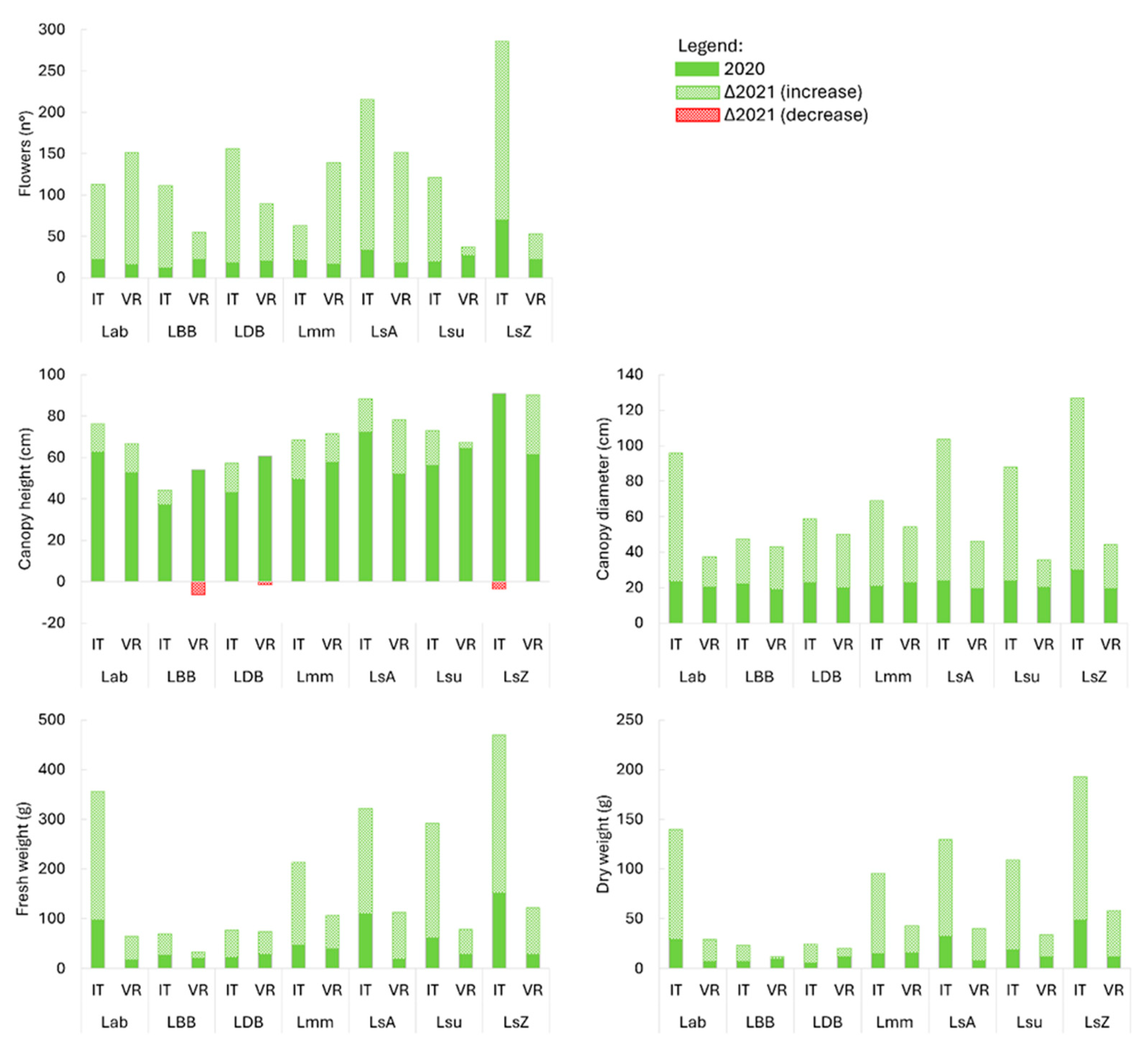
3.1. Biomass and Morphological Traits
3.2. Essential Oil Production
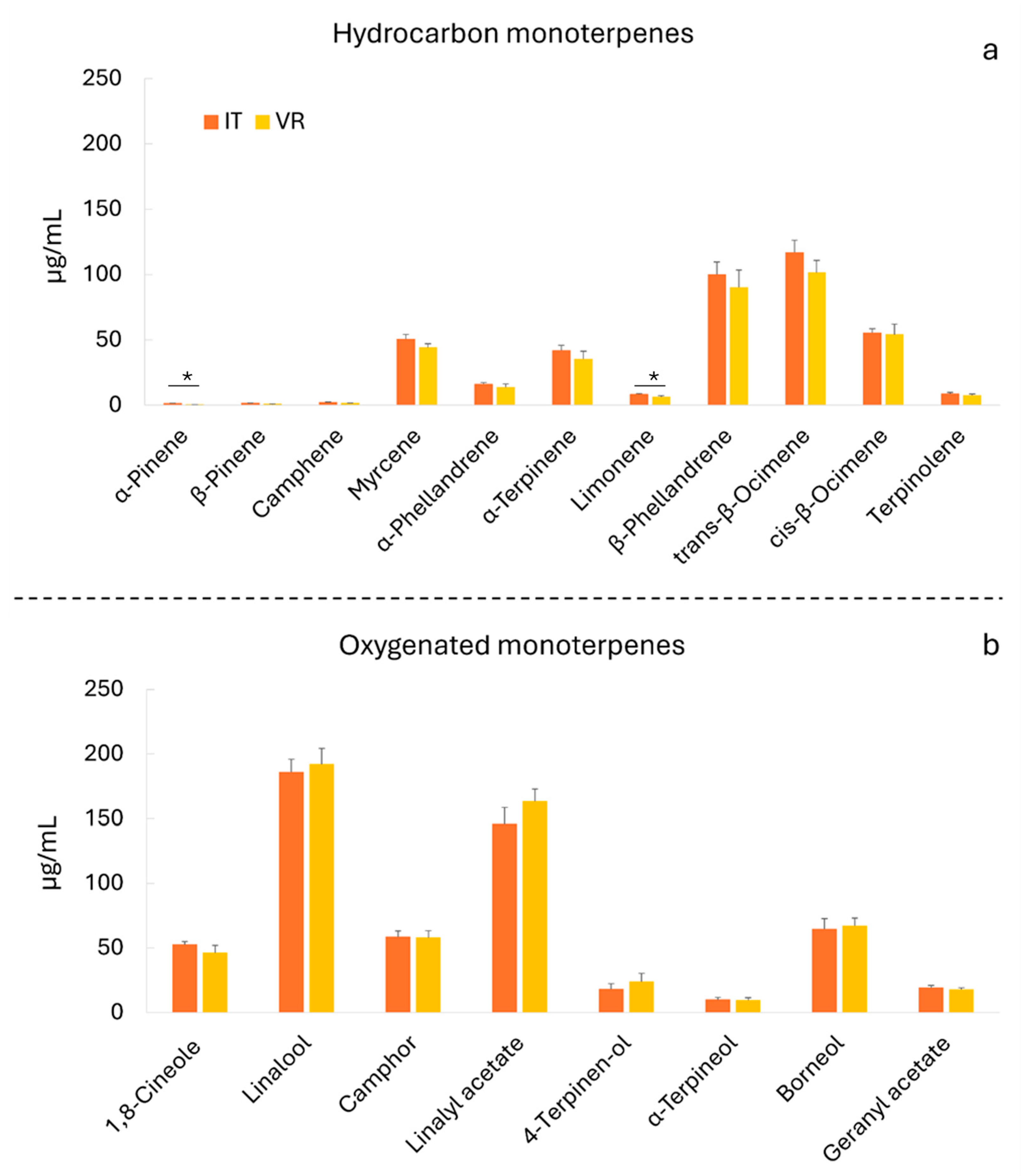
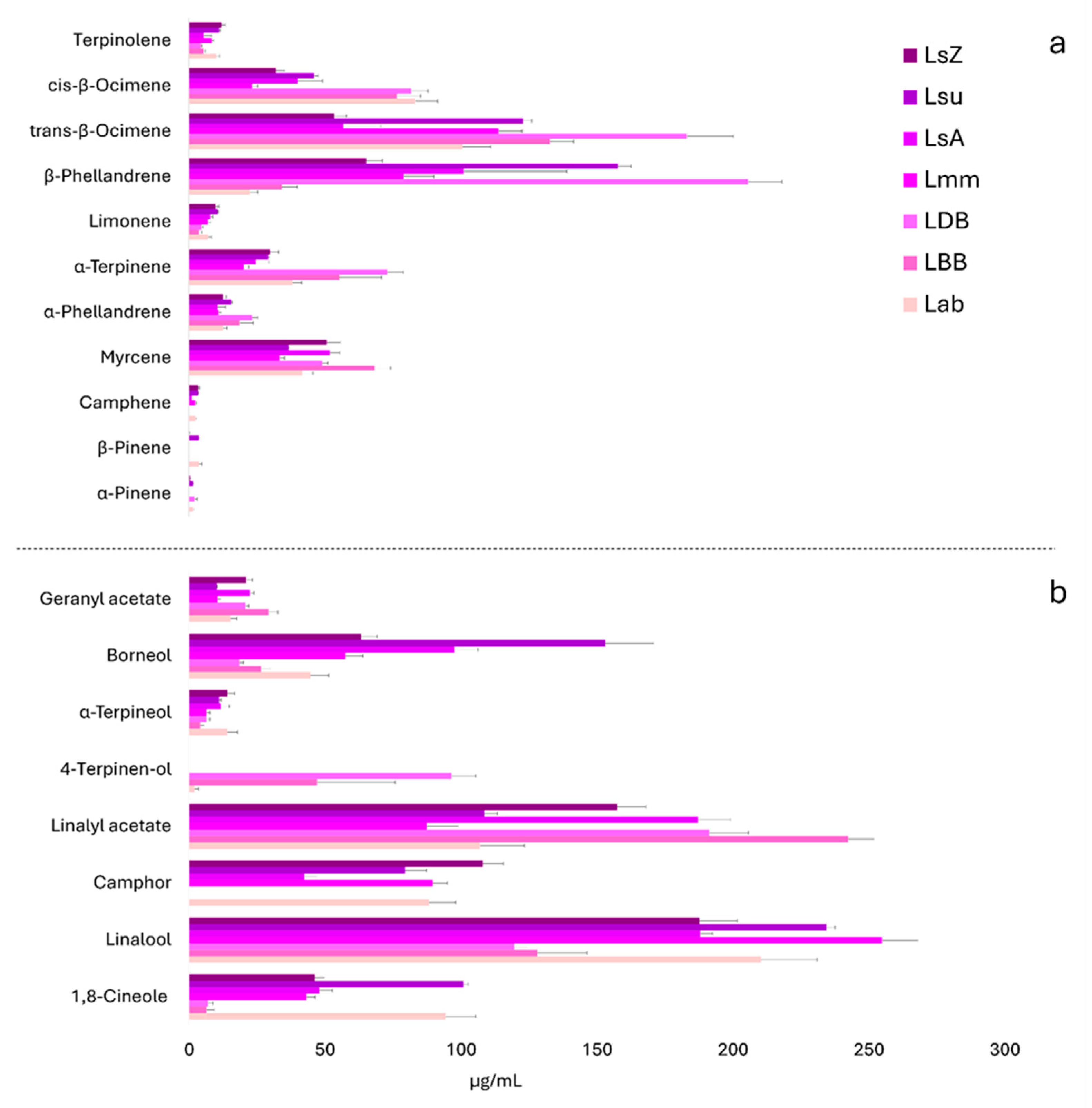
3.3. Essential Oil Composition
4. Discussion
4.1. Biomass and Morphological Parameters
4.2. Extracted and Essential Oil Content
4.3. Essential Oil Composition of L. angustifolia
4.4. Essential Oil Composition of L. × intermedia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EO | essential oil |
| ISO | International Organization for Standardization |
| IT | Istituto Tecnico Agrario di Firenze |
| VR | Fattoria Vecchia Rocca |
| LBB | L. Boston Blue |
| LDB | L. Dwarf Blue |
| Lab | L. Abrialis |
| LsZ | L. Super Z |
| Lmm | L. Maime |
| Lsu | L. Sumiens |
References
- Moja, S.; Guitton, Y.; Nicolè, F.; Legendre, L.; Pasquier, B.; Upson, T.; Jullien, F. Genome size and plastid trnK-matK markers give new insights into the evolutionary history of the genus Lavandula L. Plant Biosyst. 2016, 150, 1216–1224. [Google Scholar] [CrossRef]
- Passalacqua, N.G.; Tundis, R.; Upson, T.M. A new species of Lavandula sect. Lavandula (Lamiaceae) and review of species boundaries in Lavandula angustifolia. Phytotaxa 2017, 292, 161–170. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef]
- Elmakaoui, A.; Bourais, I.; Oubihi, A.; Nassif, A.; Bezhinar, T.; Shariati, M.A.; Blinov, A.V.; Hleba, L.; El Hajjaji, S. Chemical composition and antibacterial activity of essential oil of Lavandula multifida. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e7559. [Google Scholar] [CrossRef]
- Lis-Balchin, M. Lavender: The Genus Lavandula (Medicinal and Aromatic Plants-Industrial Profiles); CRC Press: London, UK, 2002; p. 296. ISBN 9780429218590. [Google Scholar] [CrossRef]
- Muntean, L.S.; Tamas, M.; Muntean, S.; Muntean, L.; Duda, M.; Vârban, D.; Florian, S. Treatise of Cultivated and Spontaneous Medicinal Plants, 2nd ed.; Risoprint publishing House: Cluj-Napoca, Romania, 2016; p. 1078. ISBN 978-973-53-1873-4. [Google Scholar]
- Cáceres-Cevallos, G.J.; Quílez, M.; Ortiz de Elguea-Culebras, G.; Melero-Bravo, E.; Sánchez-Vioque, R.; Jordán, M.J. Agronomic Evaluation and Chemical Characterization of Lavandula latifolia Medik. under the Semiarid Conditions of the Spanish Southeast. Plants 2023, 12, 1986. [Google Scholar] [CrossRef] [PubMed]
- Crișan, I.; Ona, A.; Vârban, D.; Muntean, L.; Vârban, R.; Stoie, A.; Mihăiescu, T.; Morea, A. Current Trends for Lavender (Lavandula angustifolia Mill.) Crops and Products with Emphasis on Essential Oil Quality. Plants 2023, 12, 357. [Google Scholar] [CrossRef]
- de Bona, C.M.; Biasi, L.A. Influence of leaf retention on cutting propagation of Lavandula dentata L. Rev. Ceres 2010, 57, 526–529. [Google Scholar] [CrossRef]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef]
- Wells, R.; Truong, F.; Adal, A.M.; Sarker, L.S.; Mahmoud, S.S. Lavandula essential oils: A current review of applications in medicinal, food, and cosmetic industries of lavender. Nat. Prod. Commun. 2018, 13, 1403–1417. [Google Scholar] [CrossRef]
- Giray, F.H. An Analysis of World Lavender Oil Markets and Lessons for Turkey. J. Essent. Oil Bear. Plants 2018, 21, 1612–1623. [Google Scholar] [CrossRef]
- Persistence Market Research. Lavender Oil Market Size, Share & Trends Analysis (2025–2032). Report. 2025. Available online: https://www.persistencemarketresearch.com/market-research/lavender-oil-market.asp (accessed on 20 August 2025).
- Najar, B.; Pistelli, L.; Fratini, F. Exploitation of marginal hilly land in Tuscany through the cultivation of Lavandula angustifolia Mill.: Characterization of its essential oil and antibacterial activity. Molecules 2022, 27, 3216. [Google Scholar] [CrossRef]
- Istituto Nazionale di Statistica (ISTAT). Superfici e Produzioni Agricole–Lavanda, Anno 2021; ISTAT: Roma, Italy, 2021; Available online: https://www.istat.it (accessed on 20 August 2025).
- Najar, B.; Demasi, S.; Caser, M.; Gaino, W.; Cioni, P.L.; Pistelli, L.; Scariot, V. Cultivation substrate composition influences morphology, volatilome and essential oil of Lavandula angustifolia Mill. Agronomy 2019, 9, 411. [Google Scholar] [CrossRef]
- Vijulie, I.; Lequeux-Dincă, A.-I.; Preda, M.; Mareci, A.; Matei, E. Could Lavender Farming Go from a Niche Crop to a Suitable Solution for Romanian Small Farms? Land 2022, 11, 662. [Google Scholar] [CrossRef]
- Gül, M.; Kart, M.C.O.; Sirikci, B.S. Determining Costs and Profitability of Lavender Farms in Isparta Province of Turkey. J. Essent. Oil Bear. Plants 2016, 19, 686–692. [Google Scholar] [CrossRef]
- Giray, F.H.; Kadakoğlu, B.; Çetin, F.; Bamoi, A.G.A. Rural tourism marketing: Lavender tourism in Turkey. Cienc. Rural. 2019, 49, e20180651. [Google Scholar] [CrossRef]
- Ramírez-García, S.; Gago-García, C.; Serrano-Cabronero, M.M.; Babinger, F.; Santander-Del-Amo, F. Lavender fields in Spain. Tourism articulation of imaginaries from a Provençal Mediterranean oneiric. Rev. Tur. Desenvolv. 2023, 43, 77–98. [Google Scholar] [CrossRef]
- Martinelli, E.; Tagliazucchi, G. Improving the Small Farmers’ Agribusiness Orientation in the Lavender Industry: An Empirical Study in the Emilia-Romagna Apennines. In Agribusiness Innovation and Contextual Evolution, Volume I; Galati, A., Fiore, M., Thrassou, A., Vrontis, D., Eds.; Palgrave Intersections of Business and the Sciences, in association with Gnosis Mediterranean Institute for Management Science; Palgrave Macmillan: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Hassiotis, C.N.; Vlachonasios, K.E. How biological and environmental factors affect the quality of lavender essential oils. Physiologia 2025, 5, 11. [Google Scholar] [CrossRef]
- Matysiak, B.; Nogowska, A. Impact of fertilization strategies on the growth of lavender and nitrates leaching to the environment. Hortic. Sci. 2016, 43, 76–83. [Google Scholar] [CrossRef]
- Perović, A.B.; Karabegović, I.T.; Krstić, M.S.; Veličković, A.V.; Avramović, J.M.; Danilović, B.R.; Veljković, V.B. Novel hydrodistillation and steam distillation methods of essential oil recovery from lavender: A comprehensive review. Ind. Crops Prod. 2024, 211, 118244. [Google Scholar] [CrossRef]
- Hassiotis, C.N.; Ntana, F.; Lazari, D.M.; Poulios, S.; Vlachonasios, K.E. Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during the flowering period. Ind. Crops Prod. 2014, 62, 359–366. [Google Scholar] [CrossRef]
- Mavandi, P.; Abbaszadeh, B.; Emami Bistgani, Z.; Barker, A.V.; Hashemi, M. Biomass, nutrient concentration and the essential oil composition of lavender (Lavandula angustifolia Mill.) grown with organic fertilizers. J. Plant Nutr. 2021, 44, 3061–3071. [Google Scholar] [CrossRef]
- Eldeghedy, H.I.; El Gendy, A.E.N.G.; Nassrallah, A.A.; Aboul Enein, A.M.; Omer, E.A. Comparative chemical profiles of Lavandula species essential oils grown in Egypt and others from France and Australia: Evidence from chemometric analysis. J. Essent. Oil Bear. Plants 2022, 25, 52–63. [Google Scholar] [CrossRef]
- Détár, E.; Németh, É.Z.; Gosztola, B.; Demján, I.; Pluhár, Z. Effects of variety and growth year on the essential oil properties of lavender (Lavandula angustifolia Mill.) and lavandin (Lavandula × intermedia Emeric ex Loisel.). Biochem. Syst. Ecol. 2020, 90, 104020. [Google Scholar] [CrossRef]
- Pistelli, L.; Najar, B.; Giovanelli, S.; Lorenzini, L.; Tavarini, S.; Angelini, L.G. Agronomic and phytochemical evaluation of lavandin and lavender cultivars cultivated in the Tyrrhenian area of Tuscany (Italy). Ind. Crops Prod. 2017, 109, 37–44. [Google Scholar] [CrossRef]
- Wainer, J.; Thomas, A.; Chimhau, T.; Harding, K.G. Extraction of essential oils from Lavandula × intermedia ‘Margaret Roberts’ using steam distillation, hydrodistillation, and cellulase-assisted hydrodistillation: Experimentation and cost analysis. Plants 2022, 11, 3479. [Google Scholar] [CrossRef]
- Kırkıncı, S.; Gercek, Y.C.; Baştürk, F.N.; Bayram, N.E.; Gıdık, B. Evaluation of lavender essential oils and by-products using microwave hydrodistillation and conventional hydrodistillation. Sci. Rep. 2024, 14, 20922. [Google Scholar] [CrossRef]
- Denys, J.C.; Renaud, E.N.C.; Simon, J.E. Comparative study of essential oil quantity and composition from ten cultivars of organically grown lavender and lavandin. In Lavender: The Genus Lavandula. Medicinal and Aromatic Plants—Industrial Profiles, 1st ed.; Lis-Balchin, M., Ed.; Taylor & Francis Inc.: London, UK; New York, NY, USA, 2002; pp. 232–242. [Google Scholar]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Baydar, H.; Kineci, S. Scent composition of essential oil, concrete, absolute and hydrosol from lavandin (Lavandula × intermedia Emeric ex Loisel.). J. Essent. Oil Bear. Plants 2009, 12, 131–136. [Google Scholar] [CrossRef]
- Kotsiris, G.; Nektarios, P.A.; Paraskevopoulou, A.T. Lavandula angustifolia growth and physiology is affected by substrate type and depth when grown under Mediterranean semi intensive green roof conditions. HortScience 2012, 47, 311–317. [Google Scholar] [CrossRef]
- Pokajewicz, K.; Czarniecka-Wiera, M.; Krajewska, A.; Maciejczyk, E.; Wieczorek, P.P. Lavandula × intermedia—A Bastard Lavender or a Plant of Many Values? Part I. Biology and Chemical Composition of Lavandin. Molecules 2023, 28, 2943. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Manushkina, T.; Kachanova, T.; Samoilenko, M. The effect of plant growth regulators on productivity of lavender (Lavandula angustifolia Mill.) in the conditions of the Southern Steppe of Ukraine. Agron. Res. 2023, 21, 834–845. [Google Scholar] [CrossRef]
- Ramachandran, P.; Ramirez, A.; Dinneny, J.R. Rooting for survival: How plants tackle a challenging environment through a diversity of root forms and functions. Plant Physiol. 2024, 197, kiae586. [Google Scholar] [CrossRef] [PubMed]
- Saunier, A.; Ormeño, E.; Moja, S.; Fernandez, C.; Robert, E.; Dupouyet, S.; Despinasse, Y.; Baudino, S.; Nicolè, F.; Bousquet-Mélou, A. Lavender sensitivity to water stress: Comparison between eleven varieties across two phenological stages. Ind. Crops Prod. 2022, 182, 114531. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, D.; Hang, H.; Chen, S.; Liu, H.; Su, J.; Lv, H.; Jia, H.; Zhao, G. Effects of balancing exchangeable cations Ca, Mg, and K on the growth of tomato seedlings (Solanum lycopersicum L.) based on increased soil cation exchange capacity. Agronomy 2024, 14, 629. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Marques, R.R.; Carmeis Filho, A.C.A.; Soratto, R.P.; Costa, C.H.M.; Ferrari Neto, J.; Castro, G.S.A.; Pariz, C.M.; Castilhos, A.M.; Franzluebbers, A.J. Lime and gypsum combination improves crop and forage yields and estimated meat production and revenue in a variable charge tropical soil. Nutr. Cycl. Agroecosyst. 2019, 115, 347–372. [Google Scholar] [CrossRef]
- Bossolani, J.W.; Crusciol, C.A.C.; Merloti, L.F.; Moretti, L.G.; Costa, N.R.; Tsai, S.M.; Kuramae, E.E. Long-term lime and gypsum amendment increase nitrogen fixation and decrease nitrification and denitrification gene abundances in the rhizosphere and soil in a tropical no-till intercropping system. Geoderma 2020, 375, 114476. [Google Scholar] [CrossRef]
- Jaggard, K.W.; Qi, A.; Ober, E.S. Possible changes to arable crop yields by 2050. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2835–2851. [Google Scholar] [CrossRef]
- Despinasse, Y.; Moja, S.; Soler, C.; Jullien, F.; Pasquier, B.; Bessière, J.-M.; Noûs, C.; Baudino, S.; Nicolè, F. Structure of the Chemical and Genetic Diversity of the True Lavender over Its Natural Range. Plants 2020, 9, 1640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chalvin, C.; Drevensek, S.; Chollet, C.; Gilard, F.; Šolić, E.M.; Dron, M.; Bendahmane, A.; Boualem, A.; Cornille, A. Study of the genetic and phenotypic variation among wild and cultivated clary sages provides interesting avenues for breeding programs of a perfume, medicinal and aromatic plant. PLoS ONE 2021, 16, e0248954. [Google Scholar] [CrossRef]
- Bustamante, M.Á.; Michelozzi, M.; Barra Caracciolo, A.; Grenni, P.; Verbokkem, J.; Geerdink, P.; Safi, C.; Nogues, I. Effects of soil fertilization on terpenoids and other carbon-based secondary metabolites in Rosmarinus officinalis plants: A comparative study. Plants 2020, 9, 830. [Google Scholar] [CrossRef]
- Liao, Z.; Liu, L.; Rennenberg, H.; Du, B. Water deprivation modifies the metabolic profile of lavender (Lavandula angustifolia Mill.) leaves. Physiol. Plant. 2024, 176, e14365. [Google Scholar] [CrossRef]
- Seira, E.; Poulaki, S.; Hassiotis, C.; Poulios, S.; Vlachonasios, K.E. Gene expression of monoterpene synthases is affected rhythmically during the day in Lavandula angustifolia flowers. Physiologia 2023, 3, 433–441. [Google Scholar] [CrossRef]
- ISO 3515:2002/Cor 1:2004; Oil of Lavender (Lavandula angustifolia Mill.). International Organization for Standardization (ISO): Geneva, Switzerland, 2004.
- Caprari, C.; Fantasma, F.; Monaco, P.; Divino, F.; Iorizzi, M.; Ranalli, G.; Fasano, F.; Saviano, G. Chemical Profiles, In Vitro Antioxidant and Antifungal Activity of Four Different Lavandula angustifolia L. EOs. Molecules 2023, 28, 392. [Google Scholar] [CrossRef]
- Capetti, F.; Marengo, A.; Cagliero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Adulteration of Essential Oils: A Multitask Issue for Quality Control. Three Case Studies: Lavandula angustifolia Mill., Citrus limon (L.) Osbeck and Melaleuca alternifolia (Maiden & Betche) Cheel. Molecules 2021, 26, 5610. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078. [Google Scholar] [CrossRef]
- Maietti, S.; Rossi, D.; Guerrini, A.; Useli, C.; Romagnoli, C.; Poli, F.; Bruni, R.; Sacchetti, G. A multivariate analysis approach to the study of chemical and functional properties of chemo-diverse plant derivatives: Lavender essential oils. Flavour Fragr. J. 2013, 28, 144–154. [Google Scholar] [CrossRef]
- Germinara, G.S.; Di Stefano, M.G.; De Acutis, L.; Rotundo, G. Bioactivities of Lavandula angustifolia essential oil against the stored grain pest Sitophilus granarius. Bull. Insectology 2017, 70, 129–138. [Google Scholar]
- Shellie, R.; Mondello, L.; Marriott, P.; Dugo, G. Characterisation of lavender essential oils by using gas chromatography–mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2002, 970, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Danh, L.T.; Han, L.N.; Triet, N.D.A.; Zhao, J.; Mammucari, R.; Foster, N. Comparison of chemical composition, antioxidant and antimicrobial activity of lavender (Lavandula angustifolia L.) essential oils extracted by supercritical CO2, hexane and hydrodistillation. Food Bioprocess Technol. 2013, 6, 3481–3489. [Google Scholar] [CrossRef]
- Cong, Y.; Abulizi, P.; Zhi, L.; Wang, X. Chemical composition of the essential oil of Lavandula angustifolia from Xinjiang, China. Chem. Nat. Compd. 2008, 44, 810–815. [Google Scholar] [CrossRef]
- Turgut, A.C.; Emen, F.M.; Canbay, H.S.; Demirdöğen, R.E.; Çam, N.; Kılıç, D.; Yeşilkaynak, T. Chemical characterization of Lavandula angustifolia Mill. as a phytocosmetic species and investigation of its antimicrobial effect in cosmetic products. JOTCSA 2017, 4, 283–298. [Google Scholar] [CrossRef]
- Errafiy, N.; Ammar, E.; Soukri, A. Protective effect of some essential oils against oxidative and nitrosative stress on Tetrahymena thermophila growth. J. Essent. Oil Res. 2013, 25, 339–347. [Google Scholar] [CrossRef]
- Hassiotis, C.N.; Tarantilis, P.A.; Daferera, D.; Polissiou, M.G. Etherio, a new variety of Lavandula angustifolia with improved essential oil production and composition from naturally selected genotypes growing in Greece. Ind. Crops Prod. 2010, 32, 77–82. [Google Scholar] [CrossRef]
- Kiprovski, B.; Zeremski, T.; Varga, A.; Čabarkapa, I.; Filipović, J.; Lončar, B.; Aćimović, M. Essential Oil Quality of Lavender Grown Outside Its Native Distribution Range: A Study from Serbia. Horticulturae 2023, 9, 816. [Google Scholar] [CrossRef]
- Belhadj Mostefa, M.; Kabouche, A.; Abaza, I.; Aburjai, T.; Touzani, R.; Kabouche, Z. Chemotypes investigation of Lavandula essential oils growing at different North African soils. J. Mater. Environ. Sci. 2014, 5, 1896–1901. [Google Scholar]
- Djenane, D.; Lefsih, K.; Yangüela, Y.; Roncalès, P. Composition chimique et activité anti-Salmonella enteritidis CECT 4300 des huiles essentielles d’Eucalyptus globulus, de Lavandula angustifolia et de Satureja hortensis: Tests in vitro et efficacité sur les œufs entiers liquides conservés à 7 ± 1 °C. Phytotherapie 2011, 9, 343–353. [Google Scholar] [CrossRef]
- Mantovani, A.L.L.; Vieira, G.P.G.; Cunha, W.R.; Groppo, M.; Santos, R.A.; Rodrigues, V.; Magalhães, L.G.; Crotti, A.E.M. Chemical composition, antischistosomal and cytotoxic effects of the essential oil of Lavandula angustifolia grown in Southeastern Brazil. Rev. Bras. Farm. 2013, 23, 877–884. [Google Scholar] [CrossRef]
- ISO 8902:2009; Essential oil of Lavandin (Lavandula × intermedia Emeric ex Loisel.)—Specifications. International Organization for Standardization: Geneva, Switzerland, 2009.
- Steltenkamp, R.J.; Casazza, W.T. Composition of the essential oil of lavandin. J. Agric. Food Chem. 1967, 15, 1063–1069. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Laquale, S.; Argentieri, M.P.; Bellardi, M.G.; Avato, P. Nematicidal Activity of Essential Oil from Lavandin (Lavandula × intermedia Emeric ex Loisel.) as Related to Chemical Profile. Molecules 2021, 26, 6448. [Google Scholar] [CrossRef] [PubMed]
- Tardugno, R.; Serio, A.; Pellati, F.; D’Amato, S.; Chaves López, C.; Bellardi, M.G.; Di Vito, M.; Savini, V.; Paparella, A.; Benvenuti, S. Lavandula × intermedia and Lavandula angustifolia essential oils: Phytochemical composition and antimicrobial activity against foodborne pathogens. Nat. Prod. Res. 2019, 33, 3330–3335. [Google Scholar] [CrossRef] [PubMed]
- Radi, M.; Eddardar, Z.; Drioiche, A.; Remok, F.; Hosen, M.E.; Zibouh, K.; Ed-Damsyry, B.; Bouatkiout, A.; Amine, S.; Touijer, H.; et al. Comparative study of the chemical composition, antioxidant, and antimicrobial activity of the essential oils extracted from Lavandula abrialis and Lavandula stoechas: In vitro and in silico analysis. Front. Chem. 2024, 12, 1353385. [Google Scholar] [CrossRef]
- Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandin (Lavandula × intermedia Emeric ex Loiseleur) essential oil from Spain: Determination of aromatic profile by gas chromatography–mass spectrometry, antioxidant and lipoxygenase inhibitory bioactivities. Nat. Prod. Res. 2016, 30, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Renaud, E.N.C.; Charles, D.J.; Simon, J.E. Essential oil quantity and composition from 10 cultivars of organically grown lavender and lavandin. J. Essent. Oil Res. 2001, 13, 269–273. [Google Scholar] [CrossRef]
- Kara, N.; Baydar, H. Determination of lavender and lavandin cultivars (Lavandula sp.) containing high quality essential oil in Isparta, Turkey. Turk. J. Field Crops 2013, 18, 58–65. [Google Scholar]
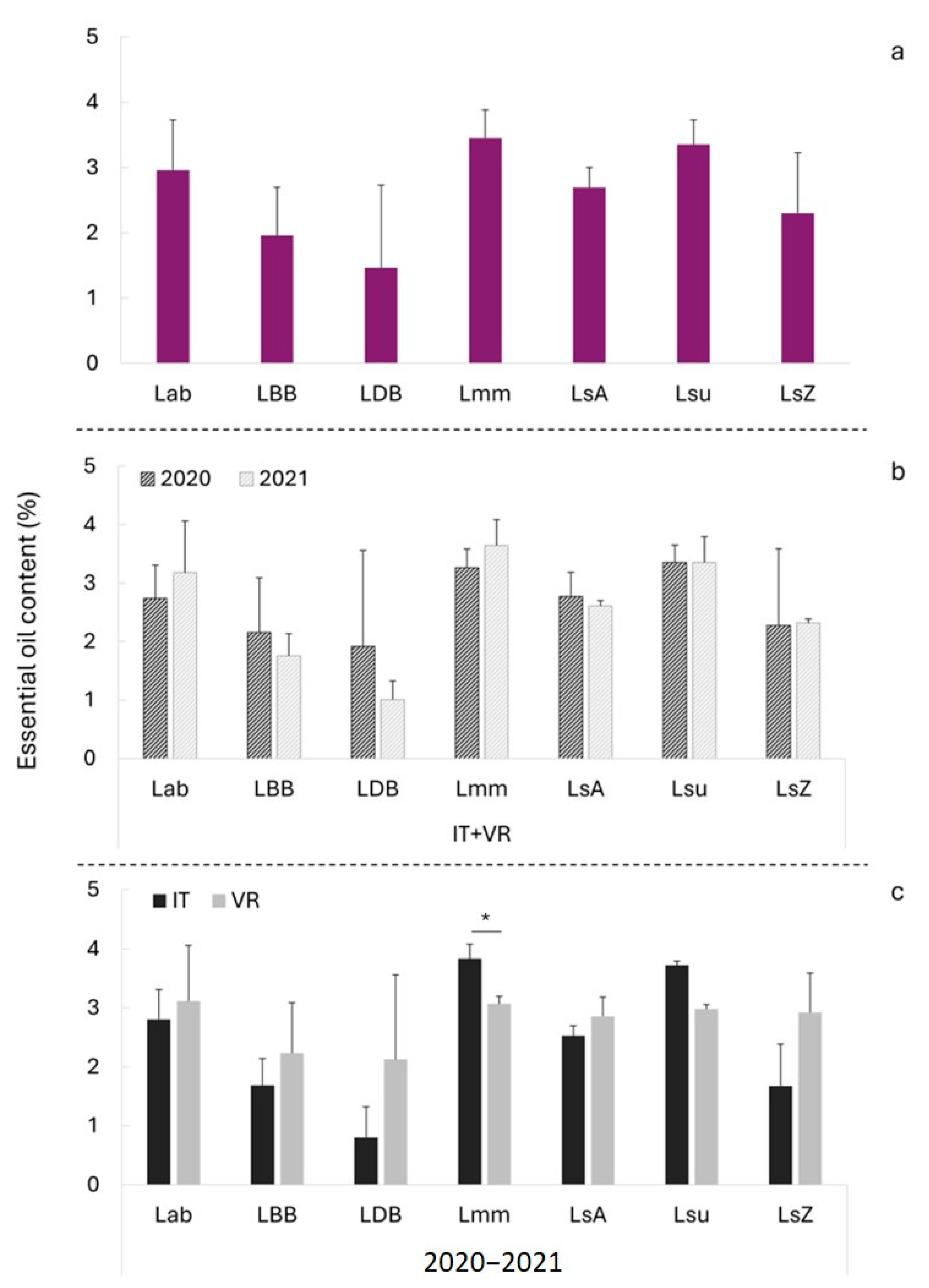
| Site | Cultivar | Oil Content (%) | Δ% | |
|---|---|---|---|---|
| Name | Name | 2020 | 2021 | 2020–2021 |
| IT | Lab | 3.3 | 2.3 | −30.5 |
| LBB | 1.2 | 2.1 | 73.9 | |
| LDB | 0.3 | 1.3 | 393 | |
| Lmm | 3.6 | 4.1 | 14.1 | |
| LsA | 2.3 | 2.7 | 14.9 | |
| Lsu | 3.6 | 3.8 | 4.4 | |
| LsZ | 1.3 | 2.4 | 146.1 | |
| VR | Lab | 2.2 | 4.1 | 87.3 |
| LBB | 3.1 | 1.4 | −55.5 | |
| LDB | 3.6 | 0.7 | −80.6 | |
| Lmm | 2.9 | 3.2 | 8.7 | |
| LsA | 3.2 | 2.5 | −20.7 | |
| Lsu | 3.1 | 2.9 | −5 | |
| LsZ | 3.6 | 2.3 | −37.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretta, M.; Brilli, L.; Leolini, L.; Rossi, R.; Palchetti, E. Analysis of Morphological Traits, Essential Oil Yield, and Secondary Metabolites in Seven Lavandins and Lavenders Grown in Two Pedoclimatic Areas in Tuscany (Italy). Agronomy 2025, 15, 2310. https://doi.org/10.3390/agronomy15102310
Moretta M, Brilli L, Leolini L, Rossi R, Palchetti E. Analysis of Morphological Traits, Essential Oil Yield, and Secondary Metabolites in Seven Lavandins and Lavenders Grown in Two Pedoclimatic Areas in Tuscany (Italy). Agronomy. 2025; 15(10):2310. https://doi.org/10.3390/agronomy15102310
Chicago/Turabian StyleMoretta, Michele, Lorenzo Brilli, Luisa Leolini, Riccardo Rossi, and Enrico Palchetti. 2025. "Analysis of Morphological Traits, Essential Oil Yield, and Secondary Metabolites in Seven Lavandins and Lavenders Grown in Two Pedoclimatic Areas in Tuscany (Italy)" Agronomy 15, no. 10: 2310. https://doi.org/10.3390/agronomy15102310
APA StyleMoretta, M., Brilli, L., Leolini, L., Rossi, R., & Palchetti, E. (2025). Analysis of Morphological Traits, Essential Oil Yield, and Secondary Metabolites in Seven Lavandins and Lavenders Grown in Two Pedoclimatic Areas in Tuscany (Italy). Agronomy, 15(10), 2310. https://doi.org/10.3390/agronomy15102310








