Responses of Soilless-Cultivated Golden Thistle to the Total Salt and Nitrogen Concentrations in the Nutrient Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. The Cultivation Practices
2.2. Experimental Setup
2.3. Sampling and Plant Growth Measurements
2.4. Laboratory Analysis
2.5. Statistical Analysis
3. Results
3.1. Yield, Plant Growth and Water Uptake
3.2. Electrical Conductivity and Macronutrient Concentrations in the Drainage Solution
3.3. Micronutrient Concentrations in the Drainage Solution
3.4. Plant Tissue Nutrient Status
Macronutrient Concentrations
3.5. Micronutrient Concentrations
3.6. Water Productivity and Agronomic Efficiency of Nutrients
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Papadimitriou, D.M.; Daliakopoulos, I.N.; Kontaxakis, E.; Sabathianakis, M.; Manios, T.; Savvas, D. Effect of Moderate Salinity on Golden Thistle (Scolymus hispanicus L.) Grown in a Soilless Cropping System. Sci. Hortic. 2022, 303, 111182. [Google Scholar] [CrossRef]
- Paschoalinotto, B.H.; Polyzos, N.; Compocholi, M.; Rouphael, Y.; Alexopoulos, A.; Dias, M.I.; Barros, L.; Petropoulos, S.A. Domestication of Wild Edible Species: The Response of Scolymus Hispanicus Plants to Different Fertigation Regimes. Horticulturae 2023, 9, 103. [Google Scholar] [CrossRef]
- Papadimitriou, D.; Kontaxakis, E.; Daliakopoulos, I.; Manios, T.; Savvas, D. Effect of N:K Ratio and Electrical Conductivity of Nutrient Solution on Growth and Yield of Hydroponically Grown Golden Thistle (Scolymus hispanicus L.). In Proceedings of the TERRAenVISION 2019, Barcelona, Spain, 2–7 September 2019; MDPI: Basel, Switzerland, 2020; p. 87. [Google Scholar]
- Papadimitriou, D.M.; Daliakopoulos, I.N.; Lydakis-Simantiris, N.; Cheiladaki, I.; Manios, T.; Savvas, D. Nitrogen Source and Supply Level Impact Water Uptake, Yield, and Nutrient Status of Golden Thistle in a Soilless Culture. Sci. Hortic. 2024, 336, 113384. [Google Scholar] [CrossRef]
- Pardo-de-Santayana, M.; Pieroni, A.; Puri, R.K. Ethnobotany in the New Europe: People, Health and Wild Plant Resources; Berghahn Books: New York, NY, USA, 2010; ISBN 9781845454562. [Google Scholar]
- Peduruhewa, P.S.; Jayathunge, K.G.L.R.; Liyanage, R. Potential of Underutilized Wild Edible Plants as the Food for the Future—A Review. J. Food Sec. 2021, 9, 136–147. [Google Scholar] [CrossRef]
- European Commission. EU Agricultural Outlook for Markets and Income, 2019–2030; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Romojaro, A.; Botella, M.Á.; Obón, C.; Pretel, M.T. Nutritional and Antioxidant Properties of Wild Edible Plants and Their Use as Potential Ingredients in the Modern Diet. Int. J. Food Sci. Nutr. 2013, 64, 944–952. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Cabrera Loera, R.D.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Díez Marqués, C.; Pardo-de-Santayana, M.; Tardío, J. Wild Vegetables of the Mediterranean Area as Valuable Sources of Bioactive Compounds. Genet. Resour. Crop Evol. 2012, 59, 431–443. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A Review on Antioxidants, Prooxidants and Related Controversy: Natural and Synthetic Compounds, Screening and Analysis Methodologies and Future Perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzortzakis, N. Optimising Fertigation of Hydroponically Grown Sowthistle (Sonchus oleraceus L.): The Impact of the Nitrogen Source and Supply Concentration. Agric. Water Manag. 2023, 289, 108528. [Google Scholar] [CrossRef]
- García-Herrera, P.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Díez-Marqués, C.; Molina, M.; Tardío, J. Nutrient Composition of Six Wild Edible Mediterranean Asteraceae Plants of Dietary Interest. J. Food Comp. Anal. 2014, 34, 163–170. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Majchrzak, D.; Wagner, K.-H.; Elmadfa, I.; Kafatos, A. The Antioxidant and Phylloquinone Content of Wildly Grown Greens in Crete. Food Chem. 2006, 99, 813–821. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Majchrzak, D.; Wagner, K.H.; Elmadfa, I.; Kafatos, A. Lipid Concentrations of Wild Edible Greens in Crete. Food Chem. 2006, 99, 822–834. [Google Scholar] [CrossRef]
- Bonasia, A.; Lazzizera, C.; Elia, A.; Conversa, G. Nutritional, Biophysical and Physiological Characteristics of Wild Rocket Genotypes As Affected by Soilless Cultivation System, Salinity Level of Nutrient Solution and Growing Period. Front. Plant Sci. 2017, 8, 300. [Google Scholar] [CrossRef]
- Sarrou, E.; Siomos, A.S.; Riccadona, S.; Aktsoglou, D.-C.; Tsouvaltzis, P.; Angeli, A.; Franceschi, P.; Chatzopoulou, P.; Vrhovsek, U.; Martens, S. Improvement of Sea Fennel (Crithmum maritimum L.) Nutritional Value through Iodine Biofortification in a Hydroponic Floating System. Food Chem. 2019, 296, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gerendas, J.; Sattelmacher, B. Effects of replacing of nitrate with urea or chloride on the growth and nitrate accumulation in pak-choi in the hydroponics. In Plant Nutrition for Sustainable Food Production and Environment; Developments in Plant and Soil Sciences, Ando, T., Fujita, K., Mae, T., Matsumoto, H., Mori, S., Sekiya, J., Eds.; Springer: Dordrecht, The Netherlands, 1997; Volume 78. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Hajisolomou, E.; Xylia, P.; Tzortzakis, N. Ammonium to Total Nitrogen Ratio Affects the Purslane (Portulaca oleracea L.) Growth, Nutritional, and Antioxidant Status. Heliyon 2023, 9, 11. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Qin, K.; Hazard, C.; Gatard, E.; Gastaldo, T.B.; Housley, M.J.; Nieters, C.E.; Mesquita, M. Airflow, Fertilizer Solution Recipes, and Calcium Concentrations Influence Lettuce and Spinach Growth in an Indoor Vertical Farm. Sci. Hortic. 2024, 328, 112948. [Google Scholar] [CrossRef]
- Voutsinos-Frantzis, O.; Karavidas, I.; Savvas, D.; Ntanasi, T.; Kaimpalis, V.; Consentino, B.B.; Aliferis, K.A.; Karkanis, A.; Sabatino, L.; Ntatsi, G. Impact of Nitrogen Limitation, Irrigation Levels, and Nitrogen-Rich Biostimulant Application on Agronomical and Chemical Traits of Hydroponically Grown Cichorium spinosum L. Horticulturae 2024, 10, 1063. [Google Scholar] [CrossRef]
- Mndi, O.; Sogoni, A.; Jimoh, M.O.; Wilmot, C.M.; Rautenbach, F.; Laubscher, C.P. Interactive Effects of Salinity Stress and Irrigation Intervals on Plant Growth, Nutritional Value, and Phytochemical Content in Mesembryanthemum crystallinum L. Agriculture 2023, 13, 1026. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 135–189. [Google Scholar]
- Voogt, W.; Bar-Yosef, B. Water and Nutrient Management and Crops Response to Nutrient Solution Recycling in Soilless Growing Systems in Greenhouses. In Soilless Culture: Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–507. ISBN 9780444636966. [Google Scholar]
- Boschiero, B.N.; Mariano, E.; Azevedo, R.A.; Ocheuze Trivelin, P.C. Influence of Nitrate—Ammonium Ratio on the Growth, Nutrition, and Metabolism of Sugarcane. Plant Physiol. Biochem. 2019, 139, 246–255. [Google Scholar] [CrossRef]
- Liu, X.; Hu, B.; Chu, C. Nitrogen Assimilation in Plants: Current Status and Future Prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2002; ISBN 0878938230. [Google Scholar]
- Sonneveld, C.; Voogt, W. Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009; ISBN 9789048125326. [Google Scholar]
- Rouphael, Y.; Kyriacou, M.C. Enhancing Quality of Fresh Vegetables through Salinity Eustress and Biofortification Applications Facilitated by Soilless Cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Cela, F.; Carmassi, G.; Najar, B.; Taglieri, I.; Sanmartin, C.; Cialli, S.; Ceccanti, C.; Guidi, L.; Venturi, F.; Incrocci, L. Salinity Impact on Yield, Quality and Sensory Profile of ‘Pisanello’ Tuscan Local Tomato (Solanum lycopersicum L.) in Closed Soilless Cultivation. Horticulturae 2024, 10, 570. [Google Scholar] [CrossRef]
- Giannothanasis, E.; Spanoudaki, E.; Kinnas, S.; Ntatsi, G.; Voogt, W.; Savvas, D. Development and Validation of an Innovative Algorithm for Sodium Accumulation Management in Closed-Loop Soilless Culture Systems. Agric. Water Manag. 2024, 301, 108968. [Google Scholar] [CrossRef]
- Blok, C.; Voogt, W.; Barbagli, T. Reducing Nutrient Imbalance in Recirculating Drainage Solution of Stone Wool Grown Tomato. Agric. Water Manag. 2023, 285, 108360. [Google Scholar] [CrossRef]
- Savvas, D.; Giannothanasis, E.; Ntanasi, T.; Karavidas, I.; Ntatsi, G. State of the Art and New Technologies to Recycle the Fertigation Effluents in Closed Soilless Cropping Systems Aiming to Maximise Water and Nutrient Use Efficiency in Greenhouse Crops. Agronomy 2024, 14, 61. [Google Scholar] [CrossRef]
- Savvas, D.; Giannothanasis, E.; Ntanasi, T.; Karavidas, I.; Drakatos, S.; Panagiotakis, I.; Neocleous, D.; Ntatsi, G. Improvement and Validation of a Decision Support System to Maintain Optimal Nutrient Levels in Crops Grown in Closed-Loop Soilless Systems. Agric. Water Manag. 2023, 285, 108373. [Google Scholar] [CrossRef]
- Fernández, J.E.; Alcon, F.; Diaz-Espejo, A.; Hernandez-Santana, V.; Cuevas, M.V. Water Use Indicators and Economic Analysis for On-Farm Irrigation Decision: A Case Study of a Super High Density Olive Tree Orchard. Agric. Water Manag. 2020, 237, 106074. [Google Scholar] [CrossRef]
- Pereira, L.S.; Cordery, I.; Iacovides, I. Improved Indicators of Water Use Performance and Productivity for Sustainable Water Conservation and Saving. Agric. Water Manag. 2012, 108, 39–51. [Google Scholar] [CrossRef]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; van Kessel, C. Efficiency of Fertilizer Nitrogen in Cereal Production: Retrospects and Prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar]
- Campbell, C.R.; Plank, C.O. Preparation of Plant Tissue for Laboratory Analysis. In Handbook of Reference Methods for Plant Analysis; Kalra, Y., Ed.; CRC Press: Boca Raton, FL, USA, 1997; p. 320. ISBN 9780367802233. [Google Scholar]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of phosphate in Natural Waters. Anal. Chem. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Schnetger, B.; Lehners, C. Determination of Nitrate plus Nitrite in Small Volume Marine Water Samples Using Vanadium(III)Chloride as a Reduction Agent. Mar. Chem. 2014, 160, 91–98. [Google Scholar] [CrossRef]
- Zenki, M.; Nose, K.; Tôei, K. Spectrophotometric Determination of Boron with an Azomethine H Derivative Spektralphotometrische Borbestimmung Mit Einem Azomethin H-Derivat. Anal. Bioanal. Chem. 1989, 334, 238–241. [Google Scholar] [CrossRef]
- Adhikari, N.D.; Simko, I.; Mou, B. Phenomic and Physiological Analysis of Salinity Effects on Lettuce. Sensors 2019, 19, 4814. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Assimakopoulou, A.; Panagopoulos, P.; Bakea, M.; Vidalis, N.; Karapanos, I.C.; Petropoulos, S.A. Impact of Salinity on the Growth and Chemical Composition of Two Underutilized Wild Edible Greens: Taraxacum Officinale and Reichardia Picroides. Horticulturae 2021, 7, 160. [Google Scholar] [CrossRef]
- Vidalis, N.; Kourkouvela, M.; Argyris, D.-C.; Liakopoulos, G.; Alexopoulos, A.; Petropoulos, S.A.; Karapanos, I. The Impact of Salinity on Growth, Physio-Biochemical Characteristics, and Quality of Urospermum Picroides and Reichardia Picroides Plants in Varied Cultivation Regimes. Agriculture 2023, 13, 1852. [Google Scholar] [CrossRef]
- Ntanasi, T.; Karavidas, I.; Consentino, B.B.; Spyrou, G.P.; Giannothanasis, E.; Marka, S.; Gerakari, M.; Passa, K.; Gohari, G.; Bebeli, P.J.; et al. Innovative and Sustainable Management Practices and Tools for Enhanced Salinity Tolerance of Vegetable Crops. Horticulturae 2025, 11, 1004. [Google Scholar] [CrossRef]
- Neocleous, D.; Savvas, D.; Giannothanasis, E.; Ntatsi, G. Partial Substitution of Nitrate by Chloride in Fertigation Recipes Allows for Lower Nitrate Input in Hydroponic Lettuce Crops. Front. Plant Sci. 2024, 15, 1411572. [Google Scholar] [CrossRef] [PubMed]
- Cedeño, J.; Magán, J.J.; Thompson, R.B.; Fernández, M.D.; Gallardo, M. Reducing Nutrient Loss in Drainage from Tomato Grown in Free-Draining Substrate in Greenhouses Using Dynamic Nutrient Management. Agric. Water Manag. 2023, 287, 108418. [Google Scholar] [CrossRef]
- White, P.J. Ion Uptake Mechanisms of Individual Cells and Roots: Short-Distance Transport. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 7–48. [Google Scholar]
- Kathpalia, R.; Bhatla, S.C. Plant Mineral Nutrition. In Plant Physiology, Development and Metabolism; Springer Nature: Singapore, 2018; pp. 37–81. [Google Scholar]
- Chang, D.C.; Park, C.S.; Kim, S.Y.; Lee, Y.B. Growth and Tuberization of Hydroponically Grown Potatoes. Potato Res. 2012, 55, 69–81. [Google Scholar] [CrossRef]
- Manzocco, L.; Foschia, M.; Tomasi, N.; Maifreni, M.; Dalla Costa, L.; Marino, M.; Cortella, G.; Cesco, S. Influence of Hydroponic and Soil Cultivation on Quality and Shelf Life of Ready-to-Eat Lamb’s Lettuce (Valerianella locusta L. Laterr). J. Sci. Food Agric. 2011, 91, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Wang, Y.; Zhang, X.; Li, T.; Grundy, S.; Yang, Q.; Cheng, R. A Review of Environment Effects on Nitrate Accumulation in Leafy Vegetables Grown in Controlled Environments. Foods 2020, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.M.; Wang, Z.H.; Li, S.X.; Wang, G.X.; Song, H.X.; Wang, X.N. Effects of Nitrate Supply on Plant Growth, Nitrate Accumulation, Metabolic Nitrate Concentration and Nitrate Reductase Activity in Three Leafy Vegetables. Plant Sci. 2004, 167, 635–643. [Google Scholar] [CrossRef]
- Anjana; Umar, S.; Iqbal, M. Factors Responsible for Nitrate Accumulation: A Review. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 533–549. [Google Scholar]
- Jakobsen, S.T. Interaction between Plant Nutrients: III. Antagonism between Potassium, Magnesium and Calcium. Acta Agric. Scand. B Soil Plant Sci. 1993, 43, 1–5. [Google Scholar] [CrossRef]
- Vanitha, P.; Indirani, R. Synergistic and Antagonistic Interactions of Calcium with Other Nutrients in Soil and Plants. SSRN Electron. J. 2019. Available online: https://ssrn.com/abstract=3503225 (accessed on 20 August 2025).
- Pyun, S.-I. Thermodynamic Aspects of Energy Conversion Systems with Focus on Osmotic Membrane and Selectively Permeable Membrane (Donnan) Systems Including Two Applications of the Donnan Potential. ChemTexts 2021, 7, 20. [Google Scholar] [CrossRef]
- Kochian, L.V. Mechanisms of micronutrient uptake and translocation in plants. In Micronutrients in Agriculture; Mortvedt, J.J., Cox, F.R., Shuman, L.M., Welch, R.M., Eds.; Soil Science Society of America: Madison, WI, USA, 1991; pp. 229–296. [Google Scholar] [CrossRef]

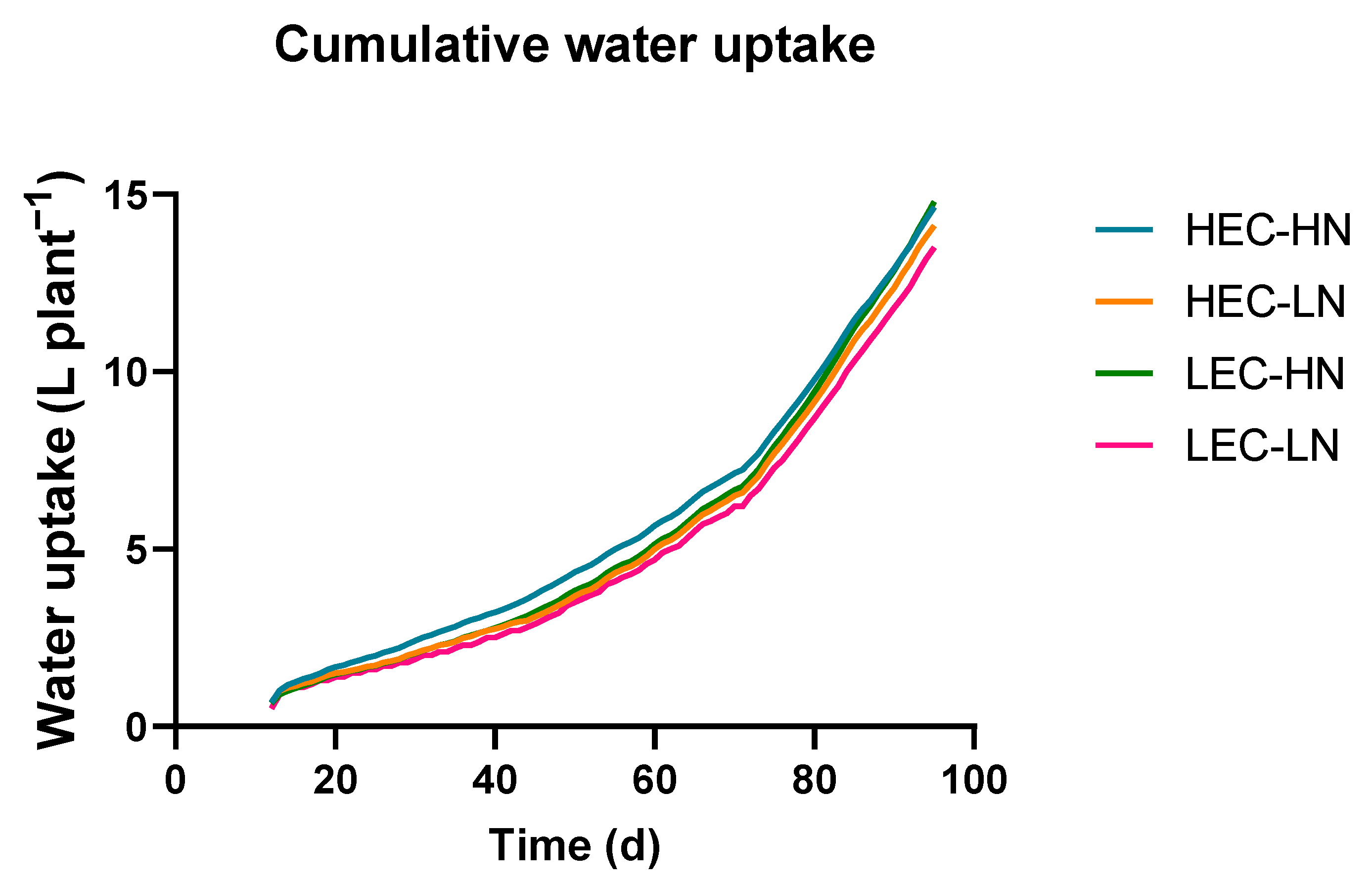
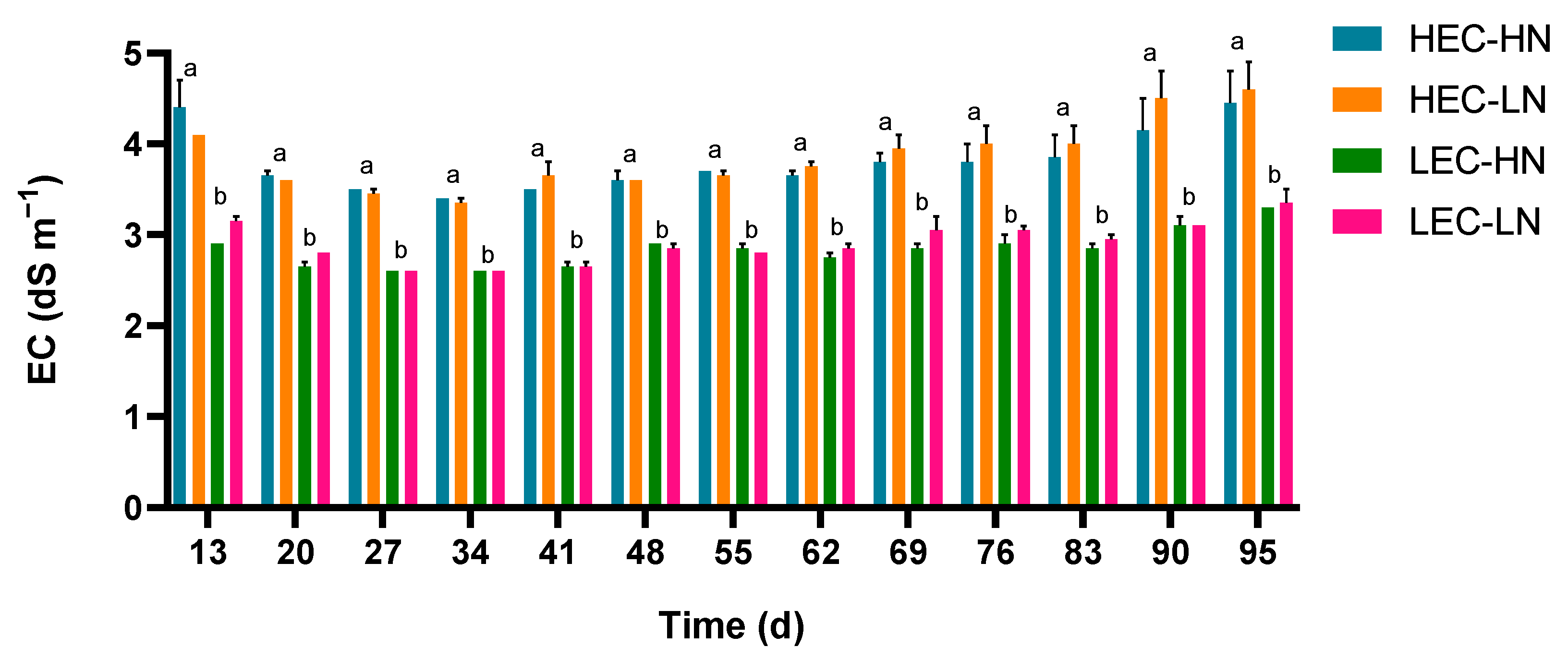
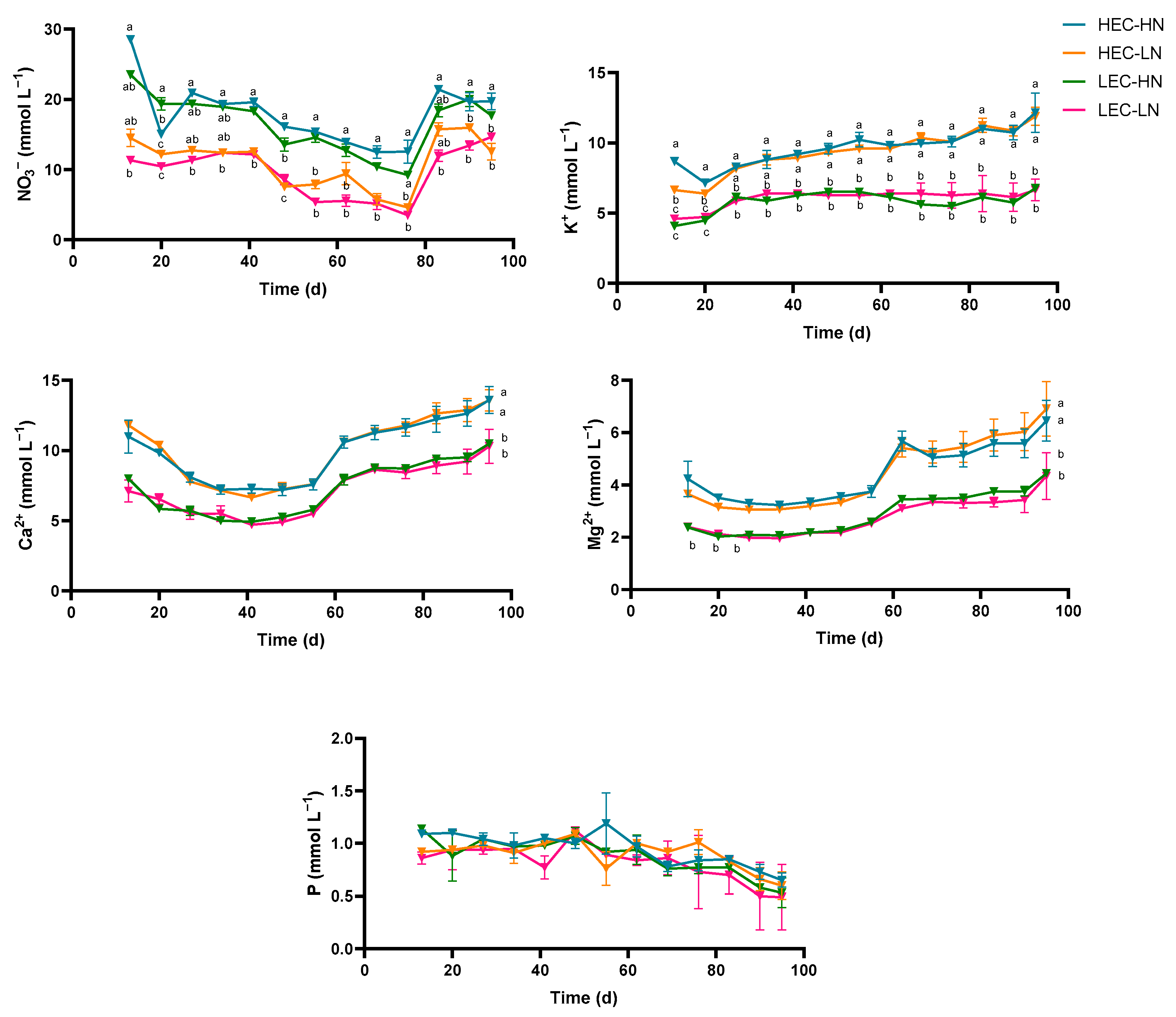
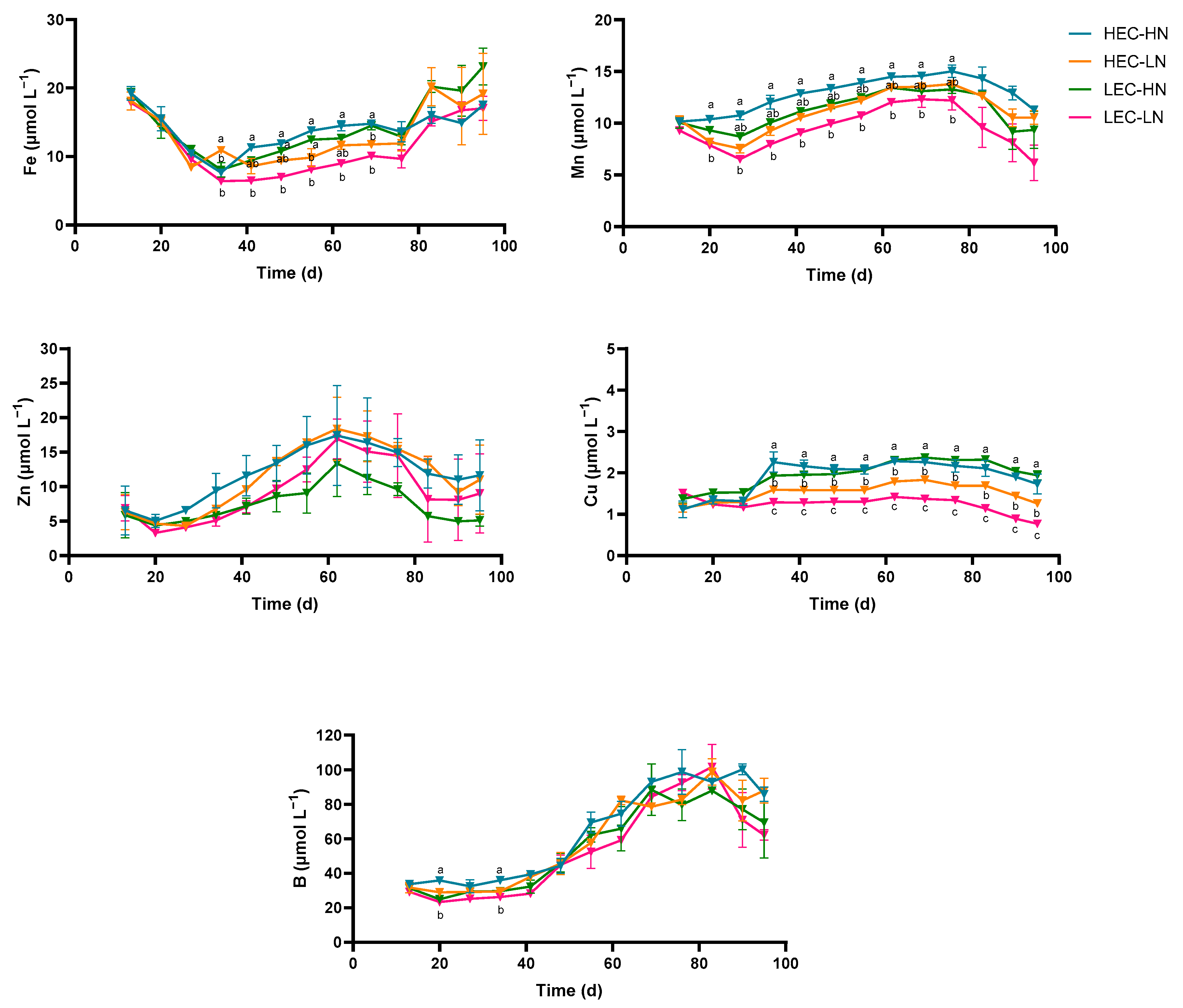
| Parameter | HEC-HN | HEC-LN | LEC-HN | LEC-LN |
|---|---|---|---|---|
| EC (dS m−1) | 2.80 | 2.80 | 2.20 | 2.20 |
| pH | 5.60 | 5.60 | 5.60 | 5.60 |
| K+ (mmol L−1) | 9.30 | 9.30 | 7.00 | 7.00 |
| Ca2+ (mmol L−1) | 5.00 | 5.00 | 3.75 | 3.75 |
| Mg2+ (mmol L−1) | 2.50 | 2.50 | 1.90 | 1.90 |
| NH4+ (mmol L−1) | 1.30 | 1.30 | 1.30 | 1.30 |
| SO42− (mmol L−1) | 4.01 | 4.01 | 1.01 | 1.01 |
| NO3− (mmol L−1) | 16.00 | 12.00 | 16.00 | 12.00 |
| P (mmol L−1) | 1.20 | 1.20 | 1.20 | 1.20 |
| Fe (μmol L−1) | 20.00 | 20.00 | 20.00 | 20.00 |
| Mn (μmol L−1) | 10.00 | 10.00 | 10.00 | 10.00 |
| Zn (μmol L−1) | 6.00 | 6.00 | 6.00 | 6.00 |
| Cu (μmol L−1) | 0.80 | 0.80 | 0.80 | 0.80 |
| B (μmol L−1) | 30.00 | 30.00 | 30.00 | 30.00 |
| Mo (μmol L−1) | 0.50 | 0.50 | 0.50 | 0.50 |
| Si (mmol L−1) | 0.00 | 0.00 | 0.00 | 0.00 |
| Cl− (mmol L−1) | 0.40 | 4.40 | 0.40 | 4.40 |
| Na+ (mmol L−1) | 0.40 | 0.40 | 0.40 | 0.40 |
| HCO3− (mmol L−1) | 0.39 | 0.39 | 0.39 | 0.39 |
| K:(K + Ca + Mg) mol:mol | 0.55 | 0.55 | 0.55 | 0.55 |
| Ca:(K + Ca + Mg) mol:mol | 0.30 | 0.30 | 0.30 | 0.30 |
| Mg:(K + Ca + Mg) mol:mol | 0.15 | 0.15 | 0.15 | 0.15 |
| NH4: Total-N mol:mol | 0.08 | 0.10 | 0.08 | 0.10 |
| Treatment | Number of Leaves | Rosette Diameter (cm) | Shoot Fresh Weight (g) | Roots Fresh Weight (g) | Shoot Dry Weight (g) | Roots Dry Weight (g) |
|---|---|---|---|---|---|---|
| HEC-HN | 43 | 50.8 | 177.9 | 117.0 | 18.9 | 17.8 |
| HEC-LN | 41 | 51.7 | 193.9 | 132.1 | 14.8 | 17.0 |
| LEC-HN | 48 | 52.6 | 231.8 | 125.1 | 24.6 | 21.4 |
| LEC-LN | 47 | 49.8 | 241.5 | 122.5 | 22.1 | 21.6 |
| Main effects | ||||||
| LEC | 48 a | 51.2 | 236.6 a | 123.8 | 23.3 a | 21.5 |
| HEC | 42 b | 51.2 | 185.9 b | 124.5 | 16.8 b | 17.4 |
| LN | 44 | 50.7 | 217.7 | 127.3 | 18.4 | 19.3 |
| HN | 46 | 51.7 | 204.8 | 121.0 | 21.7 | 19.6 |
| Statistical significance | ||||||
| EC | * | NS | ** | NS | ** | NS |
| N | NS | NS | NS | NS | NS | NS |
| EC × N | NS | NS | NS | NS | NS | NS |
| Treatment | Total-N (mg g−1) | P (mg g−1) | K (mg g−1) | Ca (mg g−1) | Mg (mg g−1) | NO3− (mg Kg−1 FW) |
|---|---|---|---|---|---|---|
| 48 DAT | ||||||
| Main effects | ||||||
| LEC | 46.2 | 9.41 | 65.6 b | 2.62 | 4.09 | 2450 |
| HEC | 44.6 | 9.24 | 74.8 a | 2.04 | 3.71 | 2664 |
| LN | 44.5 | 8.69 | 71.4 | 2.58 | 4.03 | 1821 b |
| HN | 46.3 | 9.96 | 69.0 | 2.08 | 3.77 | 3293 a |
| Statistical significance | ||||||
| EC | NS | NS | * | NS | NS | NS |
| N | NS | NS | NS | NS | NS | *** |
| EC × N | NS | NS | NS | NS | NS | NS |
| 96 DAT | ||||||
| Main effects | ||||||
| LEC | 32.3 b | 9.01 | 74.4 b | 6.44 a | 5.36 | 2711 |
| HEC | 37.2 a | 8.64 | 81.1 a | 4.64 b | 5.64 | 3081 |
| LN | 32.9 b | 8.84 | 78.8 | 5.20 | 5.55 | 2391 b |
| HN | 36.6 a | 8.82 | 76.8 | 5.88 | 5.45 | 3601 a |
| Statistical significance | ||||||
| EC | * | NS | * | * | NS | NS |
| N | * | NS | NS | NS | NS | * |
| EC × N | NS | NS | NS | NS | NS | NS |
| Treatment | Total-N (mg g−1) | P (mg g−1) | K (mg g−1) | Ca (mg g−1) | Mg (mg g−1) |
|---|---|---|---|---|---|
| 48 DAT | |||||
| HEC-HN | 24.9 b | 8.60 | 32.5 | 0.33 | 1.09 |
| HEC-LN | 24.4 b | 8.07 | 38.0 | 0.34 | 1.00 |
| LEC-HN | 29.3 a | 8.29 | 29.5 | 0.32 | 1.10 |
| LEC-LN | 22.1 c | 9.93 | 23.5 | 0.34 | 1.38 |
| Main effects | |||||
| LEC | 25.7 | 9.11 | 26.5 b | 0.33 | 1.24 |
| HEC | 24.6 | 8.33 | 35.3 a | 0.34 | 1.04 |
| LN | 23.2 b | 9.00 | 30.8 | 0.34 | 1.19 |
| HN | 27.1 a | 8.44 | 31.0 | 0.33 | 1.09 |
| Statistical significance | |||||
| EC | NS | NS | * | NS | NS |
| N | ** | NS | NS | NS | NS |
| EC × N | ** | NS | NS | NS | NS |
| 96 DAT | |||||
| 29.3 | 8.21 | 24.25 | 0.34 | 1.17 | |
| HEC-LN | 25.8 | 8.64 | 20.00 | 0.28 | 0.98 |
| LEC-HN | 26.9 | 8.60 | 20.63 | 0.31 | 1.10 |
| LEC-LN | 25.1 | 9.37 | 17.00 | 0.29 | 1.00 |
| Main effects | |||||
| LEC | 26.0 | 8.99 | 18.8 b | 0.30 | 1.05 |
| HEC | 27.6 | 8.42 | 22.1 a | 0.31 | 1.08 |
| LN | 25.5 b | 9.00 | 18.5 b | 0.28 | 0.99 |
| HN | 28.1 a | 8.41 | 22.4 a | 0.32 | 1.13 |
| Statistical significance | |||||
| EC | NS | NS | * | NS | NS |
| N | ** | NS | * | NS | NS |
| EC × N | NS | NS | NS | NS | NS |
| Treatment | Fe (mg Kg−1) | Mn (mg Kg−1) | Zn (mg Kg−1) | Cu (mg Kg−1) | B (mg Kg−1) |
|---|---|---|---|---|---|
| 48 DAT | |||||
| HEC-HN | 92.5 | 55.9 | 37.8 | 8.32 | 35.9 b |
| HEC-LN | 75.5 | 54.0 | 40.7 | 8.20 | 41.0 ab |
| LEC-HN | 63.4 | 47.5 | 40.4 | 6.82 | 31.5 b |
| LEC-LN | 58.5 | 40.3 | 36.2 | 5.26 | 59.6 a |
| Main effects | |||||
| LEC | 60.9 b | 43.9 b | 38.3 | 6.04 b | 45.6 |
| HEC | 84.0 a | 55.0 a | 39.2 | 8.26 a | 38.4 |
| LN | 67.0 | 47.1 | 38.5 | 6.73 | 50.3 a |
| HN | 77.9 | 51.7 | 39.1 | 7.57 | 33.7 b |
| Statistical significance | |||||
| EC | ** | ** | NS | * | NS |
| N | NS | NS | NS | NS | ** |
| EC × N | NS | NS | NS | NS | * |
| 96 DAT | |||||
| HEC-HN | 43.0 b | 100.1 | 37.5 | 7.08 | 62.0 |
| HEC-LN | 163.1 a | 125.3 | 44.0 | 8.27 | 84.4 |
| LEC-HN | 38.1 b | 103.8 | 32.8 | 7.63 | 49.2 |
| LEC-LN | 47.4 b | 108.7 | 45.6 | 9.05 | 54.1 |
| Main effects | |||||
| LEC | 42.7 | 106.3 | 39.2 | 8.34 | 51.6 b |
| HEC | 103.1 | 112.7 | 40.7 | 7.67 | 73.2 a |
| LN | 105.2 | 117.0 a | 44.8 | 8.66 a | 69.2 a |
| HN | 40.6 | 101.9 b | 35.1 | 7.36 b | 55.6 b |
| Statistical significance | |||||
| EC | ** | NS | NS | NS | *** |
| N | *** | * | NS | *** | ** |
| EC × N | ** | NS | NS | NS | NS |
| Treatment | Fe (mg Kg−1) | Mn (mg Kg−1) | Zn (mg Kg−1) | Cu (mg Kg−1) | B (mg Kg−1) |
|---|---|---|---|---|---|
| 48 DAT | |||||
| LEC | 41.6 | 20.3 | 61.8 | 7.27 | 34.2 a |
| HEC | 53.1 | 17.5 | 67.0 | 8.71 | 17.8 b |
| LN | 49.3 | 18.8 | 66.0 | 7.74 | 24.0 |
| HN | 45.4 | 18.9 | 62.8 | 8.25 | 28.1 |
| Statistical significance | |||||
| EC | NS | NS | NS | NS | * |
| N | NS | NS | NS | NS | NS |
| EC × N | NS | NS | NS | NS | NS |
| 96 DAT | |||||
| LEC | 30.0 b | 23.0 a | 64.8 | 8.67 | 18.7 |
| HEC | 48.7 a | 19.6 b | 73.4 | 8.18 | 21.1 |
| LN | 40.7 | 23.0 a | 66.8 | 7.80 | 15.3 b |
| HN | 38.1 | 19.7 b | 71.3 | 9.04 | 24.5 a |
| Statistical significance | |||||
| EC | * | * | NS | NS | NS |
| N | NS | * | NS | NS | * |
| EC × N | NS | NS | NS | NS | NS |
| Treatment | WP | AEN | AEK | AECa | AEMg | AES | AEP |
|---|---|---|---|---|---|---|---|
| HEC-HN | 3.92 | 16.20 | 10.82 | 19.62 | 64.59 | 30.58 | 105.48 |
| HEC-LN | 4.73 | 25.43 | 13.05 | 23.67 | 77.94 | 36.90 | 127.28 |
| LEC-HN | 4.51 | 18.60 | 16.50 | 30.04 | 97.59 | 139.41 | 121.12 |
| LEC-LN | 4.60 | 24.68 | 16.84 | 30.64 | 99.55 | 142.21 | 123.55 |
| Main effects | |||||||
| HEC | 4.33 | 20.82 | 11.94 b | 21.65 b | 71.27 b | 33.74 b | 116.38 |
| LEC | 4.55 | 21.64 | 16.67 a | 30.34 a | 98.57 a | 140.81 a | 122.34 |
| HN | 4.21 | 17.40 b | 13.66 | 24.83 | 81.09 | 84.99 | 113.30 |
| LN | 4.67 | 25.06 a | 14.95 | 27.16 | 88.75 | 89.55 | 125.42 |
| Statistical significance | |||||||
| EC | NS | NS | *** | *** | *** | *** | NS |
| N | NS | *** | NS | NS | NS | NS | NS |
| EC × N | NS | NS | NS | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniou, F.; Papadimitriou, D.M.; Giannothanasis, E.; Ntanasi, T.; Kalozoumis, P.; Manios, T.; Ntatsi, G.; Savvas, D. Responses of Soilless-Cultivated Golden Thistle to the Total Salt and Nitrogen Concentrations in the Nutrient Solution. Agronomy 2025, 15, 2287. https://doi.org/10.3390/agronomy15102287
Maniou F, Papadimitriou DM, Giannothanasis E, Ntanasi T, Kalozoumis P, Manios T, Ntatsi G, Savvas D. Responses of Soilless-Cultivated Golden Thistle to the Total Salt and Nitrogen Concentrations in the Nutrient Solution. Agronomy. 2025; 15(10):2287. https://doi.org/10.3390/agronomy15102287
Chicago/Turabian StyleManiou, Filippa, Dimitrios M. Papadimitriou, Evangelos Giannothanasis, Theodora Ntanasi, Panagiotis Kalozoumis, Thrassyvoulos Manios, Georgia Ntatsi, and Dimitrios Savvas. 2025. "Responses of Soilless-Cultivated Golden Thistle to the Total Salt and Nitrogen Concentrations in the Nutrient Solution" Agronomy 15, no. 10: 2287. https://doi.org/10.3390/agronomy15102287
APA StyleManiou, F., Papadimitriou, D. M., Giannothanasis, E., Ntanasi, T., Kalozoumis, P., Manios, T., Ntatsi, G., & Savvas, D. (2025). Responses of Soilless-Cultivated Golden Thistle to the Total Salt and Nitrogen Concentrations in the Nutrient Solution. Agronomy, 15(10), 2287. https://doi.org/10.3390/agronomy15102287








