Abstract
Phosphorus inputs play a critical role in modulating microbial dynamics in crop rhizosphere soils, yet their specific effects remain underexplored. This study investigated the impacts of P addition on soil respiration rates, enzyme activities, and microbial communities in maize and soybean rhizosphere soils under a 20-year corn-soybean rotation system. Results demonstrated that P inputs significantly elevated rhizosphere soil respiration rates. In maize, LP treatment yielded the highest initial rate (3.2 times CK on day 0) and maximum rate (1.45 times CK), peaking early (days 0–4). In soybean, HP treatment produced the peak rate, occurring on day 4. Glucosidase activity increased under P treatments, with HP in maize showing values up to 1.5–2 times CK before day 8, and HP in soybean peaking at 1.2 times CK on day 8. Acid and neutral phosphatase activities generally declined initially, reflecting feedback inhibition, while alkaline phosphatase rose early. Microbial community structure shifted markedly. Key taxa like Reyranella and Luteimonas increased with P concentration, while Gp1 decreased. Correlation analysis indicated strong associations; e.g., Proteobacteria positively correlated with acid phosphatase and negatively with neutral phosphatase. These findings underscore the crop-specific responses of rhizosphere microbiomes to P inputs, informing targeted fertilization strategies for enhanced nutrient efficiency and sustainable agriculture.
1. Introduction
Cultivated soil is considered to play a significant role in soil ecology and the global carbon cycle with the atmosphere. Relevant studies indicate that 5–20% of atmospheric CO2 originates from cultivated soil [1], with key influencing factors including soil temperature, moisture, soil organic matter content, fertilization practices, and tillage systems. It is generally believed that the primary drivers of soil respiration are soil temperature and moisture; however, recent research has found that different tillage systems and fertilization regimes can also alter soil respiration to some extent. For instance, Omonode et al. reported [2] higher CO2 fluxes under continuous maize cropping compared to rotation systems. In contrast, Agomoh et al. found [3] that the soil respiration rate in soybean rotation systems was twice that of continuous soybean cropping. Conversely, Norberg et al. showed [4] no significant effect of crop rotation on soil respiration. Thus, correlations between rotation systems and soil respiration exhibit notable regional differences.
Fertilization not only promotes higher crop yields but also intensifies the decomposition of organic matter by soil microorganisms, thereby enhancing soil respiration. Sainju et al. reported [5] that fertilized soil respiration was 14% higher than in unfertilized soil. Zhang et al. observed [6] a 4% increase in root respiration after NPK fertilization, while Zhai et al. found [7] no significant effect of fertilization on soil respiration. Additionally, Song et al. reported [8] that high-concentration fertilization had a negative impact on soil respiration. Pareja et al. suggested [9] that the effect of fertilization on soil respiration may be interrelated with tillage practices; however, compared to other factors, the impact of fertilization on soil respiration tends to be more pronounced [10].
Phosphorus supports plants’ basic metabolic functions and structure, while also being a component of nucleic acids and phospholipids in biological membranes, and participating in energy conversion in biological systems [11,12]. Plants mainly absorb orthophosphates from soil systems [13]. Although the total phosphorus content in soil is high, it readily forms precipitates with various metals in soil, leading to reduced phosphorus utilization by plants [14]. Soil microorganisms show special potential in the process of phosphorus transformation for plants [15,16], especially certain specific bacterial species that have specific responses to dynamic changes of soil phosphorus, such as Bacillus and Pseudomonas [17,18]. These bacterial species influence the activity of relevant enzymes in soil after sensing changes in phosphorus levels [19], affecting the metabolic efficiency of nutrients by soil microbial communities. Therefore, to enhance the efficiency of bio-transformation and utilization of phosphorus in soil, it is necessary to further explore the response changes in soil microbial community structure and activity to exogenous phosphorus input.
Soil respiration is divided into autotrophic respiration and heterotrophic respiration. The respiration of plant roots present in the soil is classified as autotrophic respiration, while the decomposition of nutrients by soil microorganisms and their own respiration, as well as the respiration of soil animals, are considered heterotrophic respiration [20]. Heterotrophic respiration accounts for a higher proportion of soil respiration, with soil microbial respiration alone making up about 52.1% of the total soil respiration [21]. Soil respiration is a key process in the global carbon cycle, and sensitive to phosphorus availability [22]. Phosphorus input can alter microbial community composition and metabolic activity, subsequently affecting soil organic matter decomposition and CO2 emission [23]. However, in agricultural systems, the mechanisms linking P input, microbial community shifts, and soil respiration remain elusive. Maize and soybean exhibit distinct rhizosphere traits related to P acquisition. Maize, a cereal crop, tends to enhance proton and organic acid release, facilitating the mobilization of inorganic P fractions [24]. In contrast, soybean, a legume, may acidify the rhizosphere during biological nitrogen fixation, benefiting neighboring maize but often at the cost of its own growth under P deficiency [25].
The crop rhizosphere is considered a hotspot for microbial activity, providing unique ecological niches for soil microorganisms [26]. Soil microorganisms in the plant rhizosphere zone have important functions in supporting plant growth and health [27]. This is because the living environment of rhizosphere microorganisms has more easily degradable carbon sources compared to non-rhizosphere microorganisms [28], resulting in significant differences in community composition diversity and functional diversity compared to microorganisms in non-rhizosphere soil [29]. During the crop growth stage, the number of pseudomonas, copiotrophs and oligotrophs in the non-rhizosphere region of the soil is ten times greater than that of the same bacterial species in the rhizosphere region [30], which also shows significant differences in microbial activity. At the same time, plants can also stimulate the extracellular enzyme activity of rhizosphere microorganisms through metabolites, affecting microbial biomass [31]. Therefore, for plants, the dynamic changes of rhizosphere microbial communities characterize, to some extent, the efficiency of plant growth and the transformation efficiency of nutrients in their root soil. Meanwhile, this also means that rhizosphere soil microorganisms better reflect the connection between crops and soil ecology than non-rhizosphere soil microorganisms, but there are relatively few studies on the dynamic response of rhizosphere microorganisms to phosphorus nutrient input.
The extracellular enzymes released by soil microorganisms are the basis for their manifestation of life activities. At the same time, enzyme secretion is influenced by multiple factors, including soil temperature, humidity, nutrient environment, and soil microbial community structure. Soil enzymes can, to some extent, characterize the transformation efficiency of substances in soil and soil nutrient intensity [32]. Soil respiration is the process by which microorganisms decompose nutrients in soil for life activities and subsequently release CO2 into the atmosphere [33], which is also one of the important indicators characterizing soil biological activity. Soil microbial communities are considered to be the most diverse and abundant communities in terrestrial ecosystems, occupying an important position in soil material decomposition and cycling. When soil microorganisms decompose soil nutrients for their own respiration, they require the participation of various extracellular enzymes. The dynamic changes in specific enzyme activities can effectively characterize the intensity and patterns of soil microbial life activities and significantly influence soil microbial community structure [34,35]. Therefore, a comprehensive analysis of the dynamic changes of soil respiration, soil enzymes, and soil microbial communities is significant for evaluating soil activity.
Although rotation systems have been shown to enhance soil P availability [36], the effects of exogenous P input on the coupling between rhizosphere enzyme activities and microbial community assembly in maize-soybean systems remain poorly understood. We hypothesized that inorganic P addition would suppress phosphatase activity and shift the community composition toward Proteobacteria, with crop-specific optima. We analyzed the corresponding changes in enzyme activity and soil respiration rates by, and analyzed the correlation between various factors and microbial communities through amplicon methods, to measure the impact of different phosphorus treatments on rhizosphere soil biological activity, preliminarily analyzing the dynamic response of rhizosphere soil microorganisms to exogenous phosphorus input, with the hope of providing corresponding theoretical basis for efficient soil nutrient management and sustainable agricultural development.
2. Materials and Methods
2.1. Rhizosphere Soil Sample Collection
Soil samples were collected from the experimental fields in Gongzhuling (longitude 124°48′34″, latitude 43°30′23″), Jilin Academy of Agricultural Sciences, where a corn-soybean rotation system has been implemented continuously for 20 years. Using the random sampling method, plant root samples were collected from six areas in July. The excess soil was shaken off, and soil within 2 mm of the root surface was collected with a brush and defined as rhizosphere soil. A 2 mm aperture sieve was used to remove plant residues and other impurities from the soil. The rhizosphere soil of different crops was mixed separately and then re-evenly divided, and stored at 4 °C. The corn soil properties are as follows: pH 8.06, alkali-hydrolysable nitrogen 69.37 mg kg−1, available phosphorus 14.17 mg kg−1, available potassium 112.04 mg kg−1, and organic matter 26.51 g kg−1. The soybean soil properties are as follows: pH 7.63, alkali-hydrolysable nitrogen 86.21 mg kg−1, available phosphorus 16.02 mg kg−1, available potassium 153.97 mg kg−1, and organic matter 16.29 g kg−1.
Ten grams of rhizosphere soil was taken and placed in a Petri dish. The gravimetric method was used to control the soil moisture content to 60% then pre-cultured in a dark room at 25 °C for 5 days after activation with the addition of phosphorus solution, with the CO2 of the incubation room set at 500 ppm, temperature 25 °C, humidity 50%, and no light. Phosphorus nutrient solution was prepared according to the methods in references [37,38], To minimize the influence of phosphorus compounds on pH, a mixed solution of NaH2PO4 and Na2HPO4·2H2O at a ratio of 0.9:1 was used as the phosphorus nutrient solution. Based on local fertilization standards, the application rate of pure phosphorus was 75 kg hm−2, depth of tillage 20 cm. This was converted to a phosphorus addition of approximately 0.1 mmol per 10 g of soil. Additionally, a treatment with 10 times the field application rate (1 mmol) was set up as an excessive phosphorus treatment. Therefore, the experiment consisted of the following treatments: CK (deionized water control), LP (0.1 mmol P), and HP (1 mmol P). Three biological replicates were set up for each treatment.
2.2. Soil Respiration and Enzyme Activity Measurement
Referring to the method of the literature [39], we set up a separate gas environment (volume of 3 L, PVC material) for CO2 determination for each treatment, and the chamber was open during the incubation phase and sealed only during the determination phase. GMP343 portable soil respiration system (VAISALA, Helsinki, Finland) was used to measure the respiration rate of rhizosphere soil samples at days 0, 4, 8, 12, and 16. The total measurement time was set at 180 min, with data recorded every 5 min.
Soil acid, alkaline, and neutral phosphatase were measured using the p-nitrophenyl phosphate disodium colorimetric method [40,41]. Soil sucrase was measured using the 3,5-dinitrosalicylic acid colorimetric method. Soil N-acetyl-β-D-glucosaminidase was measured using the S-NAG Activity Detection Kit (Solarbio, BC4000, Beijing, China), following the instructions provided with the kit [31]. The above reagents are all of analytical grade (Macklin Biochemical Co., Ltd., Shanghai, China).
2.3. DNA Extraction and High-Throughput Sequencing
At the end of all treatments, total DNA from each soil sample was extracted using the specific operation method provided in the E.Z.N.A soil kit (Omega Bio-tek, Norcross, GA, USA), and DNA degradation and potential contamination were monitored using 1% agarose gel electrophoresis. The extracted DNA was diluted to 1 ng/μL for subsequent PCR amplification. The V3-V4 region of the 16S rRNA gene was amplified using the primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR procedure included an initial denaturation at 95 °C for 3 min, followed by denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 30 s, and a final extension at 72 °C for 10 min. The PCR products were purified using the Qiagen Gel Extraction Kit (Thermo Fisher Scientific, Waltham, MA, USA), and sequencing libraries were prepared according to the manufacturer’s instructions using the TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, USA). Purified amplicons were sequenced on an Illumina MiSeq platform. FLASH (v 1.2.11) was used to merge and assemble the sequences. The resulting spliced data were quality controlled using the QIIME (v 1.9.1) quality control process to filter out sequences with high N content or low quality, and chimeric sequences were removed to obtain valid data. UPARSE software (v 8.1.1861) was used for OTU clustering, with unique reads having ≥97% similarity being assigned to the same OTU. Corresponding operational taxonomic unit (OTU) enrichments were determined.
2.4. Data Analysis
A bioinformatics analysis was performed using the Majorbio cloud platform. The abundance at each taxonomic level was analyzed by QIIME (version 1.9.1), and the β-diversity distance was calculated. Uparse v. 7.0 and USEARCH v. 7.0 were used to cluster the operational taxonomic units (OTUs) and perform statistical analysis. An α-diversity analysis was conducted using Mothur v. 1.30. In the R 4.2.1 environment, differences between treatments in gene copy abundances and the microbial α-diversity indices were tested using ANOVAs followed by Tukey’s honestly significant difference test (p-value ≤ 0.05) using the agricolae package version 1.3-5. We performed principal coordinates analysis based on the Weighted Unifrac distance matrix to detect changes in the microbial community structure and a Permutational multivariate analysis of variance from to detect significant differences between treatments using the adonis function of the vegan package version 2.5-7. The Wilcox rank-sum test was used to analyze the significance difference between paired groups, and the method of FDR was used to test and correct the p value. At the OTU level, the threshold FDR < 0.01 and the p value < 0.05 determine that there is a significant difference in the relative abundance of bacteria between the two groups of samples.
3. Results and Analysis
3.1. Effects of Phosphorus Input on Rhizosphere Soil Respiration Rate
Figure 1A shows the effect of phosphate fertilizer input on the respiration rate of maize rhizosphere soil. After the application of two concentrations of phosphate fertilizer solution, the respiration rate of maize rhizosphere soil was significantly higher than that of the control group. For example, on day 0, the respiration rate of the low-concentration (0.1 mol PN) treatment was 3.2 times that of the control group, while the high-concentration (1 mol PN) treatment was 2.5 times that of the control group. The respiration rate of the maize control group reached its maximum on day 12, which was 2.2 times higher than that on day 0. At other time points, the respiration rate was relatively low, showing a downward trend by day 16. The soil respiration rate of the low-concentration phosphate fertilizer treatment group on days 0 and 4 was higher than that at all other treatment time points for maize. Subsequently, the respiration rate significantly decreased by 40% on day 8 compared to day 4 and dropped to its lowest on day 16, though it remained higher than the values of the control and high-concentration treatment groups in the earlier stages. The high-concentration phosphate nutrient solution treatment group had a relatively high respiration rate on day 0, which was lower than that of the low-concentration treatment group but higher than that of the control group. Over time, the respiration rate gradually decreased, peaked on day 4 (1.12 times higher than day 0), and then dropped to its lowest on day 16, though it remained higher than the respiration rate of the control group’s rhizosphere soil.
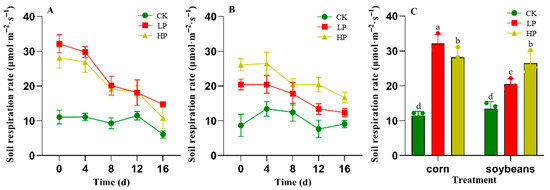
Figure 1.
Rhizosphere Soil Respiration Rate After Phosphorus Addition. Note: (A) is for corn, (B) is for soybean, and (C) shows the maximum respiration rates under different rotation modes. CK (deionized water control), LP (0.1 mmol P), and HP (1 mmol P). Different lowercase letters indicate significant differences between treatments (p < 0.05). The point in time when phosphorus was added to the soil for immediate measurement was defined as day 0. Error bars represent means ± SD (n = 3).
The changes in soybean respiration rate are shown in Figure 1B. The respiration rate of the soybean control group showed an initial increase followed by a decrease, reaching its maximum on day 4, which was approximately 3.5 times that of day 0. On day 8, the respiration rate remained relatively high, but by day 12, it significantly decreased by 50% compared to day 8. The respiration rate of the soybean high-concentration treatment group was higher than that of the low-concentration treatment group and the control group throughout the entire period, reaching its maximum on day 4, which was 1.33 times higher than the rate on day 0. Over time, the respiration rate gradually decreased, reaching its lowest value on day 16. The respiration rate of the soybean low-concentration treatment group reached its highest value on day 4, which was approximately 1.83 times that of day 0. At this time, the respiration rate was higher than that of the control group but lower than that of the high-concentration treatment group.
Figure 1C shows the effect of phosphate addition on the maximum respiration rate of rhizosphere soil under different crop rotation patterns during the entire treatment period. For maize, the respiration rate of the low-concentration phosphate treatment was approximately 1.45 times higher than that of the control group. For soybeans, the respiration rate of the high-concentration treatment was significantly higher than that of the low-concentration treatment and the control group, being approximately 1.14 times higher than that of the control group.
3.2. Effects of Phosphorus Input on Rhizosphere Soil Enzyme Activity
Figure 2 shows the phosphatase activity of rhizosphere soil under different treatments. The acid phosphatase activity (A) in corn rhizosphere soil showed a decreasing trend in the short term after adding phosphorus nutrient solution, and gradually increased with time. The enzyme activity of high-concentration treatment was lower than that of low-concentration treatment. The acid phosphatase activity of the corn control group showed a significant upward trend in the short term and gradually decreased with time. In the neutral phosphatase activity (B) of corn rhizosphere soil, the enzyme activity of the control group was higher than the two treatment groups, and the enzyme activity of the low-concentration treatment was greater than that of the high-concentration treatment. Overall, the enzyme activity changes gradually increased with time. In the alkaline phosphatase activity (C) of corn rhizosphere soil, the enzyme activity of the treatment groups was higher than that of the control group before day 4, and the high-concentration treatment group was significantly higher than the low-concentration treatment. After day 4, the enzyme activity of all three groups showed a decreasing trend.
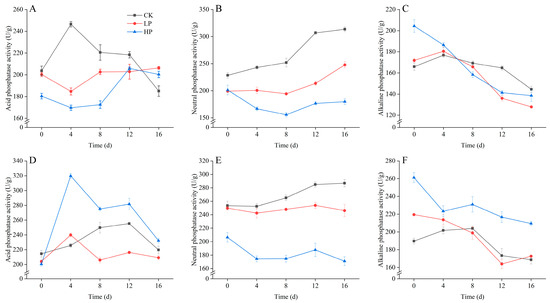
Figure 2.
Changes in Phosphatase Activity in Corn and Soybean Rhizosphere Soil. (A) is Corn acid phosphatase activity; (B) is Corn neutral phosphatase activity; (C) is Corn alkaline phosphatase activity; (D) is Soybean acid phosphatase activity; (E) is Soybean neutral phosphatase activity; (F) is Soybean alkaline phosphatase activity. CK (deionized water control), LP (0.1 mmol P), and HP (1 mmol P). The point in time when phosphorus was added to the soil for immediate measurement was defined as day 0. Error bars represent means ± SD (n = 3).
In the changes in acid phosphatase activity (D) of soybean rhizosphere soil, the high-concentration phosphorus treatment showed significantly increased activity on day 4, gradually decreasing in the late stage of the experiment. The enzyme activity of low-concentration treatment gradually increased with time, but was less than the high-concentration treatment and greater than the control group. The enzyme activity change of the control group was relatively small. Changes in neutral phosphatase in soybean rhizosphere soil are shown in (E). The enzyme activity of high-concentration treatment was significantly lower than the control group and low-concentration treatment group, and gradually decreased with time. The enzyme activity of the control group was greater than the treatment groups and gradually increased with time. In the alkaline phosphatase activity (F) of soybean rhizosphere soil, the enzyme activity of the treatment groups gradually decreased with time, and the high-concentration treatment group was significantly higher than the low-concentration treatment group. The enzyme activity of the control group showed an upward trend before day 8 and gradually decreased after day 8.
Figure 3 shows the changes in sucrase and glucosidase activities in corn and soybean rhizosphere soil. In the sucrase activity (A) of corn rhizosphere soil, the low-concentration treatment had the highest enzyme activity on day 12, followed by a gradual decrease. The enzyme activity of high-concentration treatment was lower than that of the control group and low-concentration treatment group in the first 8 days, significantly increased after day 12, and was higher than the low-concentration and control groups on day 16. In the glucosidase activity (B) of corn rhizosphere soil, the enzyme activity of all treatments decreased with time. Before day 8, the enzyme activity of high-concentration treatment was significantly higher than that of low-concentration treatment and control group. After day 8, the difference in enzyme activity between high-concentration and low-concentration treatments gradually decreased, but both were higher than the enzyme activity of the control group.
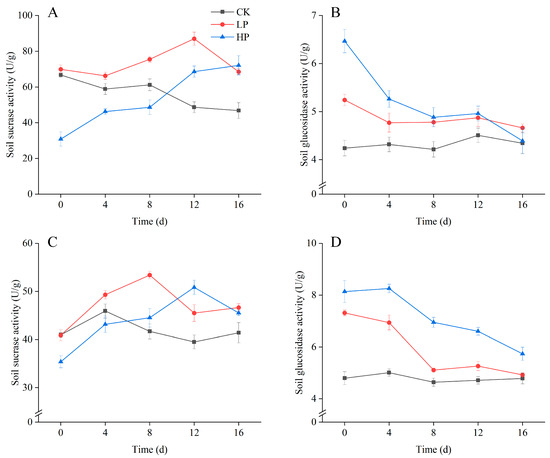
Figure 3.
Changes in Sucrase and Glucosidase Activities in Corn and Soybean Rhizosphere Soil. (A) is Corn soil sucrase activity; (B) is Corn soil glucosidase activity; (C) is Soybean soil sucrase activity; (D) is Soybean soil glucosidase activity. CK (deionized water control), LP (0.1 mmol P), and HP (1 mmol P). The point in time when phosphorus was added to the soil for immediate measurement was defined as day 0. Error bars represent means ± SD (n = 3).
In the sucrase activity (C) of soybean rhizosphere soil, the low-concentration treatment group had the highest enzyme activity on day 8, and the activity gradually decreased with time. The activity of the high-concentration treatment group was lower than that of the low-concentration treatment group and control group before day 8, and the enzyme activity was greater than the other two groups on day 12. In the glucosidase activity (D) of soybean rhizosphere soil, the enzyme activity of high-concentration treatment was significantly greater than the other two groups, with a peak value on day 8. The enzyme activity of the low-concentration treatment group showed a decreasing trend with time, but was always higher than the enzyme activity of the control group.
3.3. Effects of Phosphorus Input on Rhizosphere Soil Microbial Community
Figure 4A shows the phylogenetic tree of corn and soybean rhizosphere soil microorganisms and the heat map of microbial community structure under different treatments. After phosphorus addition treatment, the abundance of Reyranella and Luteimonas increased with treatment concentration, which was relatively significant in corn rhizosphere soil. KBS_96 showed higher abundance under high-concentration phosphorus treatment, and the abundance expression in corn soil was significantly higher than that in soybean rhizosphere soil. At the same time, Terracidiphilus also showed higher abundance in corn soil, and the abundance increased with the increase of treatment concentration. In addition, the abundance of Gp1, Achromobacter and Bradyrhizobium under the phylum Proteobacteria, and AG11 under the phylum Gemmatimonadota in corn rhizosphere soil decreased significantly after phosphorus addition treatment. In soybean rhizosphere soil, the abundance of Terracidiphilus, OLB17_426821, KBS_96, Luteimonas, Sphingomicrobium, Bradyrhizobium, and Reyranella increased to varying degrees after phosphorus treatment. The abundance of Gp1 decreased significantly as in corn rhizosphere soil, and the abundance of Nitrospira, VBCG01, and AG11 also showed varying degrees of decrease.
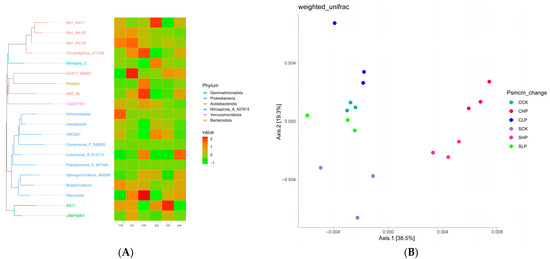
Figure 4.
Microbial Abundance Heat Map (A) and PCoA Analysis (B) of Rhizosphere Soil. CCK (Corn deionized water control), CLP (Corn 0.1 mmol P), and CHP (Corn 1 mmol P). SCK (Soybean deionized water control), SLP (Soybean 0.1 mmol P), and SHP (Soybean 1 mmol P). The statistical significance of the grouping effect was assessed by PERMANOVA with 999 permutations, revealing a significant global difference (pseudo-F = [8.34], R2 = 0.171, p = 0.001).
Figure 4B PCoA ordination of corn and soybean rhizosphere soil microbial communities after phosphorus treatment showed significant community differences between each treatment group and the control group. The community changes of high-concentration phosphorus treatment in corn and high-concentration phosphorus treatment in soybean were relatively significant compared with the control group. At the same time, there were significant differences between low-concentration phosphorus treatment and high-concentration phosphorus treatment in corn and soybean rhizosphere soil.
Volcano plot visualization (Figure 5) highlights significantly different species across treatments with criteria of (FC) ≥ 2.0 and FDR < 0.05. Compared with the control group, there were 100 species with significant changes in corn low-concentration phosphorus treatment (A), 45 species with increased abundance, with SHUZ01 showing the largest increase, and 55 species with decreased abundance, with Noviherbaspirillum_A_568106 showing the largest decrease. In corn high-concentration phosphorus treatment, 122 species showed significant changes, 59 species increased in abundance, with Sphingopyxis showing the largest increase, and 63 species decreased in abundance, with Noviherbaspirillum_A_568106 showing the largest decrease. Compared with low-concentration phosphorus treatment, 84 species in corn high-concentration treatment (C) showed significant changes, 54 species significantly increased in abundance compared with low concentration, among which Niabella showed the largest increase in abundance, and 30 species decreased in abundance, among which SHUZ01 showed the largest decrease. In soybean low-concentration phosphorus treatment (A), 91 species showed significant changes, 41 species increased in abundance, with UBA7657 showing the largest increase, and 50 species decreased in abundance, with Novosphingobium_485350 showing the largest decrease. In soybean high-concentration phosphorus treatment, 112 species showed significant changes, 55 species increased in abundance, with Rhodanobacter_613036 showing the largest increase, and 57 species decreased in abundance, with Nitrosocosmicus showing the largest decrease. Compared with low-concentration phosphorus treatment, 96 species in soybean high-concentration treatment (C) showed significant changes, 57 species significantly increased in abundance compared with low concentration, among which Thermosporothrix showed the largest increase in abundance, and 33 species decreased in abundance, among which 2_12_FULL_35_15 showed the largest decrease.
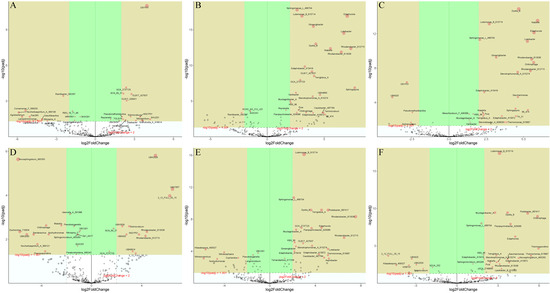
Figure 5.
Differentially Expressed Species in Corn and Soybean Rhizosphere Soil Microorganisms After Phosphorus Treatment. Note: Volcano plots of corn low-concentration phosphorus treatment vs. control group (A), corn high-concentration treatment vs. control group (B), corn low-concentration treatment vs. high-concentration treatment (C), soybean low-concentration phosphorus treatment vs. control group (D), soybean high-concentration treatment vs. control group (E), and soybean low-concentration treatment vs. high-concentration treatment (F). Volcano plots were constructed using fold change values and p-values. Vertical lines correspond to 2.0-fold up- and down-regulation between normal samples and AD samples (N vs. D), horizontal lines represent p-values (<0.05), and red areas represent differentially expressed species with statistical significance.
Different microbial communities showed varying degrees of correlation with environmental factors in all treatments Figure 6A. Proteobacteria, Bacteroidota, and Firmicutes_D showed relatively high positive correlation with acid phosphatase activity, while Eisenbacteria, Gemmatimonadota, Sumerlaeota, Elusimicrobiota, and Bdellovibrionota_E showed significant negative correlation with acid phosphatase activity. Neutral phosphatase showed significant positive correlation with Eisenbacteria, Gemmatimonadota, Planctomycetota, Elusimicrobiota, and Bdellovibrionota_E, and significant negative correlation with Proteobacteria, Bacteroidota, and Firmicutes_D. Chloroflexota, Dormibacterota, and Fusobacteriota showed significant positive correlation with alkaline phosphatase, while Proteobacteria showed relatively high negative correlation. Fibrobacterota showed significant positive correlation with sucrase activity, while Actinobacteriota showed negative correlation with sucrase. Methylomirabilota showed significant negative correlation with glucosidase activity, while Omnitrophota, Fibrobacterota, and Spirochaetota showed relatively high positive correlation with glucosidase activity. Spirochaetota and Cyanobacteria showed significant positive correlation with soil respiration rate, while Fusobacteriota and Chloroflexota showed significant negative correlation with soil respiration rate.
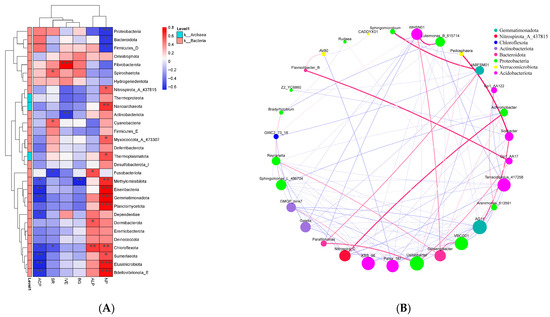
Figure 6.
Correlation Heat Map Between Microbial Communities and Environmental Factors (A) and Inter-Community Network Analysis (B). Spearman’s r > 0.6 FDR < 0.05. The size of each node is proportional to the relative abundance of the operational taxonomic units (OTUs). A red edge indicates a positive correlation, and a blue edge indicates a negative correlation. * p < 0.05, ** p < 0.01 and *** p < 0.001, based on Wilcoxon rank sum tests.
Figure 6B Correlation network analysis showed that the five microorganisms with the highest Degree were AG11 under the phylum Gemmatimonadota, Usitatibacter and VBCG01, and Terracidiphilus_417258 and KBS_96. These species played a relatively important role in all treatment groups.
4. Discussion
Phosphorus addition significantly influenced rhizosphere soil respiration rates and enzyme activities in a long-term corn-soybean rotation system. Specifically, low phosphorus addition resulted in the highest respiration rates in corn rhizosphere, peaking early (days 0–4) and reflecting heightened microbial activity likely driven by phosphorus limitation stress that stimulates adaptive responses in the microbial community. In contrast, high phosphorus addition yielded the maximum respiration rates in soybean rhizosphere, peaking at day 4, possibly due to enhanced nutrient availability supporting more robust microbial metabolism in a legume-specific context. These mechanisms align with prior studies on positive phosphorus effects on soil respiration [42,43,44], emphasizing the need for tailored phosphorus management to sustain rhizosphere ecology and crop productivity.
Different phosphatase enzyme activities exhibited contrasting responses to phosphorus treatment, reflecting complex regulatory mechanisms tied to soil pH, microbial adaptation, and P bioavailability. Acid phosphatase activity, which predominates in acidic soils and is secreted by both plants and microorganisms, initially decreased following P addition due to negative feedback inhibition—where elevated inorganic P levels suppress enzyme synthesis to conserve energy, as microbes and roots perceive sufficient P availability without needing to mineralize organic forms [45,46]. Over time, however, acid phosphatase activity increased as exogenous P reserves depleted, triggering induction of the enzyme to hydrolyze organic P compounds and sustain nutrient cycling. In contrast, alkaline phosphatase activity, primarily microbial-derived and optimal in neutral to alkaline conditions, showed higher initial levels under early P treatment, potentially due to stimulated microbial proliferation [47,48]. This activity subsequently declined as prolonged P exposure led to downregulation, aligning with resource allocation theories where microbes reduce investment in P-acquisition enzymes under replete conditions [49]. These patterns reflect the reduction of phosphatase production by rhizosphere microorganisms when inorganic P reserves are abundant, followed by the induction of these enzymes to obtain organic P supply when exogenous P reserves are depleted [50].
Analysis of microbial community composition revealed that phosphorus inputs induced significant shifts in corn and soybean rhizosphere microbiomes, with distinct taxa responding to varying P concentrations in a crop-dependent manner. For instance, Proteobacteria genera such as Reyranella and Luteimonas exhibited increased abundance under higher P levels, potentially due to their enhanced competitive advantage in P-enriched environments where they can efficiently utilize available inorganic P for rapid growth and biofilm formation, thereby outcompeting oligotrophic taxa [51,52,53]. Gp1 and Terracidiphilus showed decreased abundance in corn variable increases in soybean under HP. The observed correlations between microbial groups and phosphatase activities further illuminate their roles in phosphorus cycling: Proteobacteria and Bacteroidota displayed positive correlations with acid phosphatase, likely owing to their secretion of extracellular enzymes that facilitate organic P mineralization in acidic microzones, thereby linking microbial metabolism to enhanced P bioavailability for plant uptake [54,55,56].
Phosphorus inputs influenced rhizosphere soil dynamics in a long-term corn-soybean rotation system, LP maximizing rates in corn via early microbial activation. Enzyme activities, including acid, neutral, and alkaline phosphatases, exhibited temporal shifts reflecting feedback inhibition and induction mechanisms tied to P bioavailability, while glucosidase increased to support carbon cycling. Microbial communities underwent significant restructuring, with Proteobacteria (e.g., Reyranella, Luteimonas) proliferating under higher P and Gp1 declining.
Furthermore, in addition to the aforementioned changes in bacterial communities, the improvement of soil activity due to phosphorus input may also involve the role of arbuscular mycorrhizal fungal (AMF) networks. AMF form mutualistic symbioses with most crops, and their extensive mycelial networks significantly enhance the host plant’s ability to acquire sparsely mobile phosphorus in the soil, as well as facilitate nutrient redistribution among plants [57,58]. Although this study did not specifically analyze AMF, the overall changes in the microbial community indicate that phosphorus input shaped distinct soil microecological conditions. Previous studies have shown that maize-soybean rotation generally promotes the development of AMF diversity and networks [59]. We speculate that a more developed mycorrhizal network may contribute to the efficient utilization of phosphorus in rotation systems (especially RC0), thereby supporting crop growth even with reduced fertilizer inputs.
These findings highlight the pivotal role of rhizosphere microbiomes in P-mediated nutrient transformations [60], advocating for tailored fertilization strategies to optimize soil biological activity, P utilization efficiency, and sustainable crop productivity.
5. Conclusions
This study indicates that phosphorus input significantly influences rhizosphere processes in maize-soybean systems by altering microbial community structure, regulating soil enzyme activities, and stimulating soil respiration. Crop species dictates the response pattern: maize exhibits highest microbial activity under low phosphorus, whereas soybean shows stronger responses under high phosphorus. Therefore, agricultural management should adopt crop-specific phosphorus strategies and focus on rhizosphere microbiome functionality. Future work should explore underlying molecular mechanisms and field applications to enhance phosphorus use efficiency and promote sustainable agricultural development.
Author Contributions
Conceptualization, W.Z., D.R. and Y.W.; Methodology, D.R., Y.W., F.M., T.C., X.Y., W.Z. and H.Z.; Software, D.R., J.Z., Q.Q. and X.Y.; Validation, F.M., T.C., J.Z., Q.Q. and X.Y.; Formal analysis, F.M., D.Y., J.Z., Q.Q., W.Z. and H.Z.; Investigation, Y.W., D.Y., J.Z., W.Z. and H.Z.; Resources, D.R., F.M., T.C., D.Y., Q.Q., X.Y. and W.Z.; Writing—original draft preparation, D.R., Y.W. and W.Z.; Writing—review and editing, D.R., Y.W. and W.Z.; Visualization, D.R., Q.Q. and X.Y.; Supervision, Y.W., F.M. and T.C.; Project administration, W.Z. and H.Z.; Funding acquisition, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Project of the National Key Research and Development Program (2024YFD2300101); Agricultural Science and Technology Innovation Project of Jilin Province (CXGC2022RCB010, CXGC2022RCB002); National Modern Agricultural Industry Technology System Construction Project (CARS-04-PS15); Soybean Industry Technology System Project of Jilin Province (JARS-2025).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to express our gratitude to all editors and reviewers for their patience and help.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, X.; Wang, S.; Zhuang, Q.; Jin, X.; Bian, Z.; Zhou, M.; Meng, Z.; Han, C.; Guo, X.; Jin, W.; et al. A Review on Carbon Source and Sink in Arable Land Ecosystems. Land 2022, 11, 580. [Google Scholar] [CrossRef]
- Omonode, R.A.; Vyn, T.J.; Smith, D.R.; Hegymegi, P.; Gál, A. Soil Carbon Dioxide and Methane Fluxes from Long-Term Tillage Systems in Continuous Corn and Corn–Soybean Rotations. Soil Tillage Res. 2007, 95, 182–195. [Google Scholar] [CrossRef]
- Agomoh, I.V.; Drury, C.F.; Yang, X.; Phillips, L.A.; Reynolds, W.D. Crop Rotation Enhances Soybean Yields and Soil Health Indicators. Soil Sci. Soc. Am. J. 2021, 85, 1185–1195. [Google Scholar] [CrossRef]
- Norberg, L.; Berglund, Ö.; Berglund, K. Seasonal CO2 Emission under Different Cropping Systems on Histosols in Southern Sweden. Geoderma Reg. 2016, 7, 338–345. [Google Scholar] [CrossRef]
- Sainju, U.M.; Jabro, J.D.; Stevens, W.B. Soil Carbon Dioxide Emission and Carbon Content as Affected by Irrigation, Tillage, Cropping System, and Nitrogen Fertilization. J. Environ. Qual. 2008, 37, 98–106. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, L.; Sun, N.; Ding, X.; Li, J.; Wang, B.; Li, D. Soil CO2 and N2O Emissions in Maize Growing Season under Different Fertilizer Regimes in an Upland Red Soil Region of South China. J. Integr. Agric. 2014, 13, 604–614. [Google Scholar] [CrossRef]
- Zhai, L.; Liu, H.; Zhang, J.; Huang, J.; Wang, B. Long-Term Application of Organic Manure and Mineral Fertilizer on N2O and CO2 Emissions in a Red Soil from Cultivated Maize-Wheat Rotation in China. Agric. Sci. China 2011, 10, 1748–1757. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J. Effects of Soil Moisture, Temperature, and Nitrogen Fertilization on Soil Respiration and Nitrous Oxide Emission during Maize Growth Period in Northeast China. Acta Agric. Scand. B Plant Soil Sci. 2009, 59, 97–106. [Google Scholar] [CrossRef]
- Pareja-Sánchez, E.; Cantero-Martínez, C.; Álvaro-Fuentes, J.; Plaza-Bonilla, D. Tillage and Nitrogen Fertilization in Irrigated Maize: Key Practices to Reduce Soil CO2 and CH4 Emissions. Soil Tillage Res. 2019, 191, 29–36. [Google Scholar] [CrossRef]
- Sosulski, T.; Szymańska, M.; Szara, E.; Sulewski, P. Soil Respiration under 90 Year-Old Rye Monoculture and Crop Rotation in the Climate Conditions of Central Poland. Agronomy 2020, 11, 21. [Google Scholar] [CrossRef]
- Divjot, K.; Rana, K.L.; Tanvir, K.; Yadav, N.; Yadav, A.N.; Kumar, M.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Biodiversity, Current Developments and Potential Biotechnological Applications of Phosphorus-Solubilizing and-Mobilizing Microbes: A Review. Pedosphere 2021, 31, 43–75. [Google Scholar]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Bucher, M. Functional Biology of Plant Phosphate Uptake at Root and Mycorrhiza Interfaces. New Phytol. 2007, 173, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Igual, J.M.; Valverde Portal, Á.; Cervantes, E.; Velázquez Pérez, E. Phosphate-Solubilizing Bacteria as Inoculants for Agriculture: Use of Updated Molecular Techniques in Their Study. Agronomie 2001, 21, 561–568. [Google Scholar] [CrossRef]
- Djuuna, I.A.F.; Prabawardani, S.; Massora, M. Population Distribution of Phosphate-Solubilizing Microorganisms in Agricultural Soil. Microbes Environ. 2022, 37, ME21041. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Tian, J.; Lu, X.; Chen, Q.; Kuang, X.; Liang, C.; Deng, L.; Lin, D.; Cai, K.; Tian, J. Phosphorus Fertilization Affects Soybean Rhizosphere Phosphorus Dynamics and the Bacterial Community in Karst Soils. Plant Soil 2022, 475, 137–152. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C. Long-Term Nutrient Inputs Shift Soil Microbial Functional Profiles of Phosphorus Cycling in Diverse Agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Y.; Mou, Z.; Kuang, L.; Wu, W.; Zhang, J.; Wang, F.; Hui, D.; Peñuelas, J.; Sardans, J.; et al. Phosphorus Addition Decreases Microbial Residual Contribution to Soil Organic Carbon Pool in a Tropical Coastal Forest. Global Change Biol. 2021, 27, 454–466. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-Associated Increases in the Global Soil Respiration Record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y.; He, T.; Wang, Y. Aboveground and Belowground Litter Have Equal Contributions to Soil CO2 Emission: An Evidence from a 4-Year Measurement in a Subtropical Forest. Plant Soil 2017, 421, 7–17. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, B. A Global Meta-Analysis of Soil Respiration and Its Components in Response to Phosphorus Addition. Soil Biol. Biochem. 2019, 135, 38–47. [Google Scholar] [CrossRef]
- Johnston, E.R.; Kim, M.; Hatt, J.K.; Phillips, J.R.; Yao, Q.; Song, Y.; Hazen, T.C.; Mayes, M.A.; Konstantinidis, K.T. Phosphate Addition Increases Tropical Forest Soil Respiration Primarily by Deconstraining Microbial Population Growth. Soil Biol. Biochem. 2019, 130, 43–54. [Google Scholar] [CrossRef]
- Khan, A.; Yang, X.; Sun, B.; Zhang, S.; He, B. Responses of Crop and Soil Phosphorus Fractions to Long-Term Fertilization Regimes in a Loess Soil in Northwest China. Agronomy 2023, 13, 3072. [Google Scholar] [CrossRef]
- Jemo, M.; Abaidoo, R.C.; Nolte, C.; Tchienkoua, M.; Sanginga, N.; Horst, W.J. Phosphorus Benefits from Grain-Legume Crops to Subsequent Maize Grown on Acid Soils of Southern Cameroon. Plant Soil 2006, 284, 385–397. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A Review on the Plant Microbiome: Ecology, Functions, and Emerging Trends in Microbial Application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Lemanceau, P.; Blouin, M.; Muller, D.; Moënne-Loccoz, Y. Let the Core Microbiota Be Functional. Trends Plant Sci. 2017, 22, 583–595. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere Size and Shape: Temporal Dynamics and Spatial Stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Zhang, B.; Hong, J.; Zhang, Q.; Jin, D.; Gao, C. Contrast in Soil Microbial Metabolic Functional Diversity to Fertilization and Crop Rotation under Rhizosphere and Non-Rhizosphere in the Coal Gangue Landfill Reclamation Area of Loess Hills. PLoS ONE 2020, 15, e0229341. [Google Scholar] [CrossRef]
- Donn, S.; Kirkegaard, J.A.; Perera, G.; Richardson, A.E.; Watt, M. Evolution of Bacterial Communities in the Wheat Crop Rhizosphere. Environ. Microbiol. 2015, 17, 610–621. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Greco, A.; Drake, J.E.; Finzi, A.C. Root Carbon Inputs to the Rhizosphere Stimulate Extracellular Enzyme Activity and Increase Nitrogen Availability in Temperate Forest Soils. Biogeochemistry 2013, 115, 65–76. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, J.; Zhang, L.; Ren, C.; Han, X.; Yang, G.; Doughty, R.; Deng, J. Understory Plants Regulate Soil Respiration through Changes in Soil Enzyme Activity and Microbial C, N, and P Stoichiometry Following Afforestation. Forests 2018, 9, 436. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 Efflux from Soil and Review of Partitioning Methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Dick, W.A. Relationships between Enzyme Activities and Microbial Growth and Activity Indices in Soil. Soil Sci. Soc. Am. J. 1983, 47, 945–951. [Google Scholar] [CrossRef]
- Tomar, U.; Baishya, R. Seasonality and Moisture Regime Control Soil Respiration, Enzyme Activities, and Soil Microbial Biomass Carbon in a Semi-Arid Forest of Delhi, India. Ecol. Processes 2020, 9, 50. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, J.; Li, L.; Wang, X.; Li, X.; Qu, J. Effect of Soybean and Maize Rotation on Soil Microbial Community Structure. Agronomy 2019, 9, 42. [Google Scholar] [CrossRef]
- Liu, M.; Gan, B.; Li, Q.; Xiao, W.; Song, X. Effects of Nitrogen and Phosphorus Addition on Soil Extracellular Enzyme Activity and Stoichiometry in Chinese Fir (Cunninghamia lanceolata) Forests. Front. Plant Sci. 2022, 13, 834184. [Google Scholar] [CrossRef]
- Mehnaz, K.R.; Corneo, P.E.; Keitel, C.; Dijkstra, F.A. Carbon and Phosphorus Addition Effects on Microbial Carbon Use Efficiency, Soil Organic Matter Priming, Gross Nitrogen Mineralization and Nitrous Oxide Emission from Soil. Soil Biol. Biochem. 2019, 134, 175–186. [Google Scholar] [CrossRef]
- McFarlane, K.J.; Cusack, D.F.; Dietterich, L.H.; Hedgpeth, A.L.; Finstad, K.M.; Nottingham, A.T. Experimental Warming and Drying Increase Older Carbon Contributions to Soil Respiration in Lowland Tropical Forests. Nat. Commun. 2024, 15, 7084. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, H.; Wang, Y.; Xu, Y.; Wang, Y.; Guo, S.; Sun, J. Integrated Soil Improvement and Economic Benefits Evaluation of Vegetable—Rice Production Systems for Paddy Fields in Subtropical China. Plant Soil 2025, 507, 159–179. [Google Scholar] [CrossRef]
- Criquet, S.; Braud, A. Effects of Organic and Mineral Amendments on Available P and Phosphatase Activities in a Degraded Mediterranean Soil under Short-Term Incubation Experiment. Soil Tillage Res. 2008, 98, 164–174. [Google Scholar] [CrossRef]
- Lu, X.; Wen, L.; Sun, H.; Fei, T.; Liu, H.; Ha, S.; Tang, S.; Wang, L. Responses of Soil Respiration to Phosphorus Addition in Global Grasslands: A Meta-Analysis. J. Clean. Prod. 2022, 349, 131413. [Google Scholar] [CrossRef]
- Shi, J.; Gong, J.; Baoyin, T.; Luo, Q.; Zhai, Z.; Zhu, C.; Yang, B.; Wang, B.; Zhang, Z.; Li, X. Short-Term Phosphorus Addition Increases Soil Respiration by Promoting Gross Ecosystem Production and Litter Decomposition in a Typical Temperate Grassland in Northern China. Catena 2021, 197, 104952. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Z.; Yang, Q.; Jian, C.; Lai, S.; Chen, Y.; Xu, B. N and P Addition Increase Soil Respiration but Decrease Contribution of Heterotrophic Respiration in Semiarid Grassland. Agric. Ecosyst. Environ. 2021, 318, 107493. [Google Scholar] [CrossRef]
- Zheng, M.M.; Wang, C.; Li, W.X.; Guo, L.; Cai, Z.J.; Wang, B.R.; Chen, J.; Shen, R.F. Changes of Acid and Alkaline Phosphatase Activities in Long-Term Chemical Fertilization Are Driven by the Similar Soil Properties and Associated Microbial Community Composition in Acidic Soil. Eur. J. Soil Biol. 2021, 104, 103312. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Maspons, J.; Molowny-Horas, R.; Fernández-Martínez, M.; Janssens, I.A.; Richter, A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. The Effect of Global Change on Soil Phosphatase Activity. Global Change Biol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef]
- Neumann, G.; Kandeler, E. Rhizosphere Management for More Productive and More Resilient Soil Systems. In Biological Approaches to Regenerative Soil Systems; CRC Press: Boca Raton, FL, USA, 2023; pp. 333–344. [Google Scholar]
- Kumar, V.; Srivastava, A.K.; Suprasanna, P. Plant Nutrition and Food Security in the Era of Climate Change; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Janes-Bassett, V.; Blackwell, M.S.; Blair, G.; Davies, J.; Haygarth, P.M.; Mezeli, M.M.; Stewart, G. A Meta-Analysis of Phosphatase Activity in Agricultural Settings in Response to Phosphorus Deficiency. Soil Biol. Biochem. 2022, 165, 108537. [Google Scholar] [CrossRef]
- Charoenphun, N.; Lekjing, S.; Venkatachalam, K. Effect of Exogenous Melatonin Application on Maintaining Physicochemical Properties, Phytochemicals, and Enzymatic Activities of Mango Fruits during Cold Storage. Horticulturae 2025, 11, 222. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J.; Li, T.; Shao, P.; Ma, J.; Dong, K. Effects of Nitrogen and Phosphorus Imbalance Input on Rhizosphere and Bulk Soil Bacterial Community of Suaeda Salsa in the Yellow River Delta. Front. Mar. Sci. 2023, 10, 1131713. [Google Scholar] [CrossRef]
- Lv, H.; Ji, C.; Ding, J.; Yu, L.; Cai, H. High Levels of Zinc Affect Nitrogen and Phosphorus Transformation in Rice Rhizosphere Soil by Modifying Microbial Communities. Plants 2022, 11, 2271. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, H.; Chen, M.; Wang, D.; Cao, X.; Chu, B.; Xu, X. Response of Soil Bacterial Communities to Nitrogen and Phosphorus Additions in an Age-Sequence of Subtropical Forests. iForest Biogeosc. For. 2021, 14, 71. [Google Scholar] [CrossRef]
- Huo, D.; Malacrinò, A.; Lindsey, L.E.; Benitez, M.-S. Subtle Responses of Soil Bacterial Communities to Corn-Soybean-Wheat Rotation. Phytobiomes J. 2023, 7, 392–400. [Google Scholar] [CrossRef]
- Kodadinne Narayana, N.; Kingery, W.L.; Shankle, M.W.; Ganapathi Shanmugam, S. Differential Response of Soil Microbial Diversity and Community Composition Influenced by Cover Crops and Fertilizer Treatments in a Dryland Soybean Production System. Agronomy 2022, 12, 618. [Google Scholar] [CrossRef]
- Rao, D.; Meng, F.; Yan, X.; Zhang, M.; Yao, X.; Kim, K.S.; Zhao, J.; Qiu, Q.; Xie, F.; Zhang, W. Changes in Soil Microbial Activity, Bacterial Community Composition and Function in a Long-Term Continuous Soybean Cropping System after Corn Insertion and Fertilization. Front. Microbiol. 2021, 12, 638326. [Google Scholar] [CrossRef] [PubMed]
- Leake, J.; Johnson, D.; Donnelly, D.; Muckle, G.; Boddy, L.; Read, D. Networks of Power and Influence: The Role of Mycorrhizal Mycelium in Controlling Plant Communities and Agroecosystem Functioning. Can. J. Bot. 2004, 82, 1016–1045. [Google Scholar] [CrossRef]
- Perez-Lamarque, B.; Petrolli, R.; Strullu-Derrien, C.; Strasberg, D.; Morlon, H.; Selosse, M.; Martos, F. Fungal Sharing, Specialization, and Structural Distinctiveness in the Plant Root Microbiomes of Distantly Related Plant Lineages. Caractérisation Modélisation L’évolution Interact. Hôtes-Microbiotes 2021, 209, 209. [Google Scholar]
- Zhang, R.; Mu, Y.; Li, X.; Li, S.; Sang, P.; Wang, X.; Wu, H.; Xu, N. Response of the Arbuscular Mycorrhizal Fungi Diversity and Community in Maize and Soybean Rhizosphere Soil and Roots to Intercropping Systems with Different Nitrogen Application Rates. Sci. Total Environ. 2020, 740, 139810. [Google Scholar] [CrossRef]
- Abbasi, S. Plant–Microbe Interactions Ameliorate Phosphate-Mediated Responses in the Rhizosphere: A Review. Front. Plant Sci. 2023, 14, 1074279. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).