Abstract
Speed breeding, where environmental conditions such as photoperiod, temperature, and nutrient availability are manipulated to accelerate plant development and reduce breeding cycle length, can be especially beneficial in crops such as barley. Speed breeding combined with early harvest shortens the development cycle in some species, but it has not been successfully developed for barley. This study aimed to investigate whether a speed breeding system (SBS) combined with early harvest could further shorten the development cycle of barley for more efficient utilization in breeding programs. Eleven genotypes were evaluated under two systems: an SBS (photoperiod: 22 h; temperatures: 22 °C in the day, 16 °C at night) and normal breeding system (NBS, photoperiod: 16 h; temperatures: 22 °C in the day, 12 °C at night). On average, flowering occurred 15 days earlier in the SBS compared to the NBS. In the SBS, harvest at 21 days after flowering (DAF) achieved high germination rates and enabled a reduction in the cycle by 20%. When utilizing speed breeding with early harvest, the breeding cycle was complete in 88 days (21 DAF—SBS) compared to 110 days (28 DAF—NBS), which in turn will facilitate the faster development of targeted barley varieties and cultivars in the northern United States. This study is the first report of early-harvest success in barley when speed breeding conditions are used.
1. Introduction
Barley (Hordeum vulgare L.) is ranked fourth in terms of worldwide grain consumption, following wheat, maize, and rice [1]. It is primarily used as animal feed, malt for brewing and distilling, and food for human consumption [2,3]. It is anticipated that worldwide food demand will increase by 56% by 2050 [4,5]; therefore, crop production must be increased to meet this demand. Breeding new cultivars with higher yields and resistance to biotic and abiotic stresses is fundamental to maintaining and increasing overall productivity [6,7]. An increase in demand for organic small grains [8], driven by the need for sustainable cropping systems, has been observed in the last decade [9,10]. Specifically, breeding for multi-purpose barley that can serve food, feed, and malting markets has gained importance [3]. Genotype-by-environment interactions, including genotype-by-environment by management, are common in plant breeding, creating the need to breed for targeted environments [11,12,13]. For example, to breed for organic systems, germplasm must be evaluated under organic management. A traditional plant breeding cycle of selection, evaluation, and release of varieties takes 10–15 years [13,14]. Different strategies have been proposed to reduce the generational time and the length of the breeding cycle using methods such as shuttle breeding, anther culture, microspore culture, double haploid or embryo rescue [15,16,17,18,19,20], single-seed descent in greenhouses [21,22], and speed breeding [23]. Speed breeding is an easy and inexpensive method to accelerate line development in inbred crops when compared to other techniques [23,24,25,26]. It utilizes the manipulation of photoperiod, temperature, and nutrient availability, inducing physiological stress to accelerate plant development and reduce the length of growth cycles [23,24,25,26,27,28,29,30]. In addition, it is cost- and labor-effective and has the potential to reduce the length of the breeding cycle by shortening the time between generations.
Several studies have shown the effectiveness of using speed breeding in cereals and other species, such as barley [23], wheat [23], and oats [30]. Using this technique, it is possible to deliver up to six generations per year in spring wheat, spring barley, garden pea, and chickpea [23]; four generations in canola [23]; and five generations in oats [30]. On the other hand, only two to three generations per year are obtained in spring cereals under normal greenhouse conditions [23,30].
The transition from vegetative to reproductive stages in cereals is mainly controlled by the effect of day length and temperature [31,32]. Barley is a long-day crop, with the transition to the reproductive stage triggered as a response to long photoperiods (more than 16 h) [33,34,35] and higher temperatures between 18 and 25 °C [35,36]. Although optimum temperatures for wheat, for example, are 10.6, 21.0, and 20.7 °C at the terminal spikelet, anthesis, and grain filling stages, respectively, long-day cereals generally respond to higher temperatures by shortening their cycle duration [25]. Barley germplasm, adapted to high latitudes, has reduced photoperiod sensitivity [37,38]. Using speed breeding, it is possible to reduce the length of the barley cycle from seed to seed by modifying the photoperiod and temperature [23,27]. Furthermore, the time required to complete a full cycle could be shortened by performing early harvest (i.e., collecting spikes before they reach physiological maturity) as the embryo can be fully developed as soon as 14 days after flowering [39]. Therefore, speed breeding could potentially be combined with early harvest to further reduce the breeding cycle length [23,30]. Watson et al. (2018) [23] found high germination rates in spring wheat under speed breeding harvested two weeks after flowering, while González-Barrios et al. (2021) [30] demonstrated that it is possible to obtain viable seeds in oats with high confidence when they are harvested early. In contrast, the combination of speed breeding and early harvest was not successful in barley, where researchers were not able to obtain viable seeds when spikes were harvested two weeks after flowering [23,40]. While Watson et al. (2018) [23] were able to obtain a shorter growth cycle for barley with speed breeding, their experiment was restricted to germplasm grown in Australia and the UK, which are sensitive to photoperiod [41,42,43]. This research aimed to evaluate the effect of combining early harvest with speed breeding in barley germplasm with reduced photoperiod sensitivity, medium-to-late maturing cycles, and good adaptation to the northern region of the United States [44,45]. Specifically, the goals were to evaluate the effect of speed breeding on growth cycle length and, through sample spikes at different times after flowering, the effect of early harvest on this germplasm.
2. Materials and Methods
2.1. Plant Material
To study the effect of speed breeding in barley, eleven spring barley genotypes from both two- and six-row types were used (Table S1).
2.2. Experimental Design
A randomized complete block design with a factorial treatment arrangement of 11 genotypes and two systems (i.e., normal breeding (NBS) and speed breeding (SBS)) was used. Four replications were used, with each plot formed by four pots (subsamples per plot). Early harvest was evaluated in a split-plot design of the original experiment, where each pot of an experimental unit was harvested at either 14, 21, or 28 days after flowering (DAF), following González-Barrios et al.’s method (2021) [30].
2.3. Growing Conditions and Planting
The experiment was conducted under controlled conditions in the Walnut Street Greenhouse facilities at the University of Wisconsin at Madison. Two separate greenhouses were set up under either the NBS or SBS. TotalGrow high-intensity top-light 330 W white lamps (TotalGrow Lights, Holland, MI, USA) were used to provide lighting. The NBS consisted of 16 h of light at 22 °C, between 5 AM and 9 PM, and 8 h of dark at 12 °C; the SBS consisted of 22 h of light at 22 °C, between 5 AM and 3 AM, with 2 h of dark at 16 °C. During the experiment, the light intensity was maintained between 450 and 500 μmol m−2 s−1 in both greenhouses.
Three seeds per pot were planted in PRO-MIX® planting media (Premier Tech Growers & Consumers, Rivière-du-Loup, QC, Canada) on 25 January 2023. At the three-leaf stage, thinning was conducted, leaving one plant per pot. Each plot was fertilized with 25 g of Osmocote Smart-Release Plant Food Plus (Scotts Miracle-Gro Company, Marysville, OH, USA) applied at the four-leaf stage. The fertilizer composition was 15% nitrogen, 9% available phosphate, 12% soluble potash, 6% sulfur, 1.3% magnesium, 0.46% iron, 0.05% manganese, 0.05% zinc, 0.02% boron, and 0.02% molybdenum. Both experimental set-ups were watered daily, and stakes were used to guide plants and avoid lodging.
2.4. Phenological and Phenotypic Evaluation
Zadok’s growth scale (ZGS) [46] was used to score the most important phenological stages of barley. The date on which the main tiller reached each stage in each pot was recorded as three leaves (ZGS 13), four leaves (ZGS 14), first tiller (ZGS 21), first node (ZGS 31), booting (ZGS 43), heading (ZGS 59), and flowering (ZGS 69). Plant height was also scored at ZGS 14, ZGS 21, ZGS 31, ZGS 43, and ZGS 59. The spike corresponding to the main tiller was harvested at either 14, 21, or 28 days after it reached ZGS 69. Spikes were dried in an air-tight container with silica gel at 15 °C for 5 days, hand-threshed, and then the seed number and seed weight per spike (g) were recorded. Threshed seeds were stored at 4 °C for 4 days to homogenize any possible dormancy effects. For each harvest date, a germination test was performed. All the seeds from a spike (7 to 50 seeds) were placed on 20 by 30 cm (50 g m−2) filter creped germination paper moistened with sterile water, rolled, and placed inside sealed bags. The bags were kept at 15 °C, maintaining the moisture of the paper, and after seven days, germination was evaluated. A seed was marked as germinated if the radicle had emerged from the caryopsis (ZGS 05). The germination percentage was calculated as the percentage of germinated seeds out of the total number of seeds.
2.5. Statistical Models and Analysis
2.5.1. Phenology and Plant Height Traits
Best linear unbiased estimates (BLUEs) for genotype, system, and genotype-by-system interactions were estimated for days after emergence and plant height at ZGS 14 (four leaves), ZGS 21 (first tiller), ZGS 31 (first node), ZGS 43 (booting), and ZGS 59 (heading) using the following linear mixed model:
where yijkl is the response variable of the i-th genotype, j-th growing system, k-th block, and l-th pot; μ is the overall mean; Gi is the main effect of the genotype; Sj is the main effect of the system; βk is the main effect of the k-th block nested in the j-th system; GSij is the interaction effect between the i-th genotype and the j-th system; εijk is the experimental error at the plot level; and Pl(ijk) is the subsampling error at the pot level. Additionally, εijk ∼ N (0, σ2e) and Pl(ijk) ∼ N (0, σ2s), where σ2e is the variance associated with the plot and σ2s is the variance associated with the pot, with cov (εijk, Pl(ijk)) = 0.
yijkl = μ + Gi + Sj + βk(j) + GSij + εijk + Pl(ijk)
2.5.2. Yield Components and Germination Traits
The BLUEs for genotype, system, and genotype-by-system interactions were estimated for each harvest day for seed number per spike and germination percentage using the following linear mixed model:
where yjkl, Gi, Sj, βk, and GSij are similar to (1).
yijk = μ+ Gi + Sj + βk(j) + GSij + εijk
After performing the statistical modeling using (1) and (2), an analysis of variance was conducted to evaluate the effect of genotype, system, and genotype-by-system interaction for each trait. When genotype by system had a significant effect, contrasts were used to compare each genotype across systems. On the other hand, when no such effect was found, a Tukey test was conducted for the main effects. All statistical analyses were performed on R (version 4.3.1) [47]. The linear mixed-effect models were built using the lme4 package [48], BLUEs were estimated using the emmeans package [49], and Tukey’s pairwise multiple comparison tests were performed using the multcomp package [50].
To assess the effect of early harvest on seed viability, the binomial probability function was used to estimate the probability of obtaining viable seeds. For each harvest date (14, 21, and 28 DAF) and system, the probability of obtaining at least 5 germinated seeds (viability) was calculated using the binomial distribution as follows:
where the germination proportion is the success probability parameter (p), the number of trials (n) is the number of seeds harvested per spike, and the total number of successes (X) is 5 or more seeds.
3. Results
3.1. Growing Stages and Plant Height Comparison
Although there was a significant genotype-by-system interaction in terms of the number of days to reach all phenological stages, there were no crossover interactions (Figure 1). On average, ZGS 69 (flowering) was reached 15 days earlier in the SBS (i.e., a mean of 58.3 days) than in the NBS (i.e., a mean of 73.3 days) (Table S2). All genotypes reached ZGS 69 earlier in the SBS than in the NBS (i.e., between 7.1 and 23.8 days), except for ‘Purple Prince’ and ‘10.0662’, where no significant difference among systems was found in terms of days to reach flowering (Figure 1 and Table S2). Meanwhile, differences between genotypes across the SBS and NBS were not so evident for the early growth stages, i.e., ZGS 13 (three leaves), ZGS 14 (four leaves), and ZGS 21 (first tiller) (Figure 1 and Table S2).
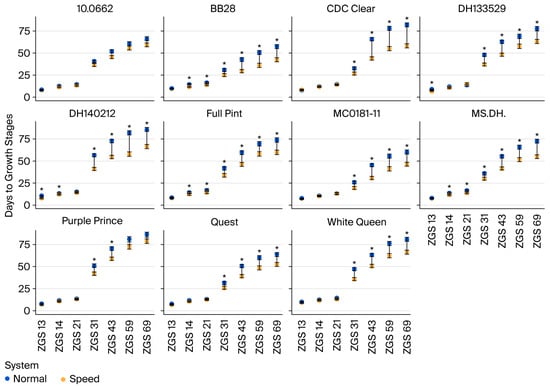
Figure 1.
Best linear unbiased estimates (BLUEs) for the number of days since emergence to reach ZGS 13, ZGS 14, ZGS 21, ZGS 31, ZGS 43, ZGS 59, and ZGS 69 in eleven barley genotypes evaluated under a normal breeding system (blue) and speed breeding system (orange); the connecting line (black) represents the difference between BLUEs in the two systems. The bars (black) show standard error (SE), indicating the upper and lower limits of each estimate. The * represent a 5% significant difference between systems for a genotype in a contrast test.
Variable results were observed for plant height at different growth stages. In the early stages, some genotypes were taller in the SBS than in the NBS, while at ZGS 59 (heading), four were taller in the NBS than in the SBS; however, the rest of the genotypes did not show statistical differences between systems regarding plant height (Table S3).
3.2. Effect of Early Harvest on Seed Number
Seed number per spike showed a significant genotypic effect but no system or genotype-by-system effect for harvest at 14 or 21 DAF (Table S4). Meanwhile, a significant genotype-by-system effect was found at 28 DAF with the genotypes ‘DH140212’ and ‘MS. × DH.’, with fewer seeds per spike in the SBS than in the NBS; no differences were found for the rest of the genotypes (Table S4). The genotypes ‘BB28’ and ‘MC0181-11’ were not included in the post-harvest analysis due to an accident not related to the treatments.
3.3. Effect of Early Harvest on Seed Germination and Viability
Germination percentage at 14 DAF showed a significant genotype-by-system cross-over interaction (Table S4), while only a significant genotypic effect was observed at 21 and 28 DAF. All the genotypes showed probabilities higher than 0.95 of obtaining five or more viable seeds at 14, 21, and 28 DAF in the NBS and 21 and 28 in the SBS (Table S4 and Figure 2). However, one genotype (‘10.0662’) had a probability lower than 0.95 at 14 DAF in the SBS (Table S4 and Figure 2).
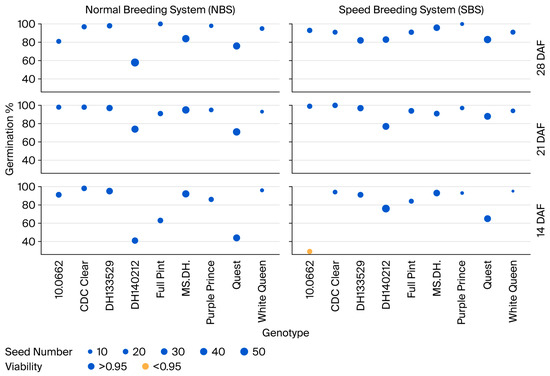
Figure 2.
Percentage of seed germination, seed number per spike, and seed viability at different times of early spike harvest for the normal breeding system (NBS) and speed breeding system (SBS). The size of the dots represents the seed number for a treatment, while the color shows the seed viability as a binary response of higher (blue) or lower (orange) than 0.95.
4. Discussion
Speed breeding has been studied by several researchers; however, its combination with early harvest has been understudied in barley. Our experiment showed that this combination, with harvest at 21 days after flowering, significantly reduced the length of the breeding cycle of a barley germplasm adapted to the northern United States.
4.1. Cycle Length Under Speed Breeding
Phenological development was accelerated in the SBS compared to the NBS, which was an expected response to extended photoperiods [51]. On average, the difference in cycle length between the SBS and NBS resulted in a reduction of 15 (7–24) days to reach ZGS 69 (flowering) (Table S2). These results are consistent with previously reported responses to speed breeding in barley [52]. The significant genotype-by-system interaction is consistent with results reported by González-Barrios et al. (2021) [30], where a diverse group of oat genotypes was studied. The differences in this interaction were attributed to changes in the number of days to reach growth stages by the genotypes, but no crossover interactions were observed (Figure 1). The diversity in our barley germplasm sample allowed us to characterize the effect of an SBS over a wide range of growing cycles (i.e., early, medium, late). Using a diverse population validated the effectiveness of combining this system with early harvest to shorten the breeding cycle across different lengths to maturity in our germplasm. We aimed to represent the diversity expected in a segregating population.
The germplasm in our speed breeding system took on average 58.3 days to reach ZGS 69 (flowering), with a 15-day difference between systems in the range of 7 to 24 days (Table S2); this is longer than that in Watson et al.’s (2018) [23] study, which took 38 days to flower. We attribute the differences in cycle length between studies to variation in maturity and photoperiod response between our northern United States barley germplasm and the Australian and UK germplasms used by Watson et al. (2018) [23]; the germplasms used by those authors has photoperiod sensitivity due to the presence of a dominant copy of the PPD-H1 (Photoperiod-H1) gene, making them early-maturing or short-cycle cultivars [41,42,43]. To avoid heat stress during the grain filling period, barley cultivars with early flowering and shorter growth cycles are preferred in regions like Australia, which experience more extreme heat in summer compared to the northern United States [45]. In the latter region, barley is planted during the spring and harvested at the end of summer; hence, cultivars tend to be insensitive to photoperiod [41,53,54], and the material used in this study corresponds to medium–late cycle genotypes (Table S1). Another difference when compared to Watson et al. (2018) [23] is that we compared speed breeding to our normal growing method, which includes supplementary light and temperature (due to the lack of light and below-zero temperatures during winter); our normal breeding protocol was already faster than barley grown in field conditions (Tables S1 and S2) [44]. The reduction in the breeding cycle from the normal breeding system (NBS) to the speed breeding system (SBS)—through a combination of temperature and photoperiod [25,51]—corresponds to a 20% decrease, indicating a transition from two to three generations per growing season. Since temperature and photoperiod are not always independent factors, achieving faster growth cycles requires both warmer temperatures and longer photoperiods [55,56]. The mechanisms underlying this growth acceleration occur throughout the plant’s development. During the vegetative stage, leaf emergence speeds up, and fewer leaves develop before heading [51]. In the reproductive stage, faster transitions between developmental phases lead to earlier flowering [57]. Additionally, during grain filling, increased metabolic activity shortens the time to seed maturity [58]. These processes are likely upregulated by increased sugar and amino acid availability under long photoperiods, accelerating growth and development rates [57,59]. The diversity of mechanisms acting over the different stages of development induced by speed breeding explains the diversity in response observed in our experiment. Even though each genotype will respond slightly differently to the SBS in each development stage, the sum of their development cycle will be shortened overall.
4.2. Early Harvest for Enhanced Efficiency
Early harvest offers a valuable strategy to accelerate generational advancement. Our study found viable seeds at early harvest in speed breeding, while other studies were unsuccessful. Our study differs from previous barley studies as it included nine diverse genotypes (Table S4), allowing us to refine a combination of harvest timing and system to better account for and understand genotype-by-system interactions. Additionally, a wider range of after-flowering harvest times, including 14, 21, and 28 DAF, was evaluated compared to previous studies that only evaluated early harvest at 14 DAF [23,40]. One of our genotypes (i.e., ‘10.0662’) had a low probability of delivering viable seeds at 14 DAF in the SBS, making harvest at this time impractical in speed breeding, which is in agreement with the results reported by Watson et al. (2018) [23]. Overall, we were able to find a successful strategy for early harvest (at 21 DAF) in speed breeding in our barley germplasm. Our results, therefore, suggest that 21 DAF in the SBS is the best option to obtain viable seeds (i.e., more than five viable seeds with a probability > 0.95) in the shortest time, similar to results previously reported for other crops [30].
4.3. Germination and Seed Viability at Premature Harvest
Our research was not able to recover five viable seeds for all genotypes at 14 DAF in SBS. We recovered some viable seed but not enough to have secure propagation of the genotype, aligning with previous reports using the combinations of early harvest and speed breeding in barley [23,40]. Our experiment showed a 0.95 or higher probability of recovering at least five seeds for all genotypes when 21 DAF in the SBS was used. Seeds harvested at this time are not expected to have reached physiological maturity [60]. A high probability of obtaining at least five viable seeds in all genotypes would ensure the propagation of all lines in a single-seed descent strategy in speed breeding. On average, the length of the growing cycle using 21 DAF in the SBS was 88 days (i.e., 58 for flowering, 21 DAF for harvest, 5 days for drying, and 4 days for cold stratification). In the NBS, with harvest at 28 DAF, the cycle could last over 110 days (73 days for flowering, 28 DAF for harvest, 5 days for drying, and 4 days for cold stratification). The 14 DAF–NBS combination showed a high probability of recovering at least five viable seeds. If the NBS is combined with early harvest, the length of the cycle significantly reduces to 96 days (73 days for flowering, 14 DAF for harvest, 5 days for drying, and 4 days for cold stratification). When the SBS cannot be used due to space or resource limitations, early harvest under the NBS is a simple technique that can lead to a 14-day reduction in cycle length. Nevertheless, 21 DAF with the SBS is the most efficient option for generational advancement, delivering 4.2 generations per year versus 3.3 (28 DAF–NBS) or 3.8 (14 DAF–NBS). The use of the SBS with early harvest would allow us to obtain three generations in the period between growing seasons, harvesting F3 seeds from the field, and advancing them to F6 seeds to be planted in the field in the next growing season. Alternatively, using the NBS, we would instead plant F5 seeds.
Breeding populations are diverse by design [61], including in terms of days to maturity. To facilitate the incorporation of speed breeding in plant breeding schemes, the process needs to be optimized for diverse breeding populations. Modern breeding programs have a diverse set of selection tools, such as genomics [62], phenomics [63], and methods to improve selection intensity, accuracy, and efficiency [30,64,65,66]. Recently, in barley, the development of environmental modeling and genomics applied to breeding methods has proven to be an excellent selection strategy [67,68,69,70]. The next step is to shorten the length of the breeding cycle, which has been highlighted as the principal constraint for increasing genetic gain per unit of time [25,71,72]. Based on our results, speed breeding with early harvest can be combined with genomic selection strategies to decrease the duration between cross-development and line testing, which will allow an increase in genetic gain per unit of time [73]. A fast-track breeding cycle will allow us to meet future food demand [4,5] and respond faster to challenges imposed by changing environments [74]. The relevance of our work lies in developing a scheme that ensures fast generational advancement in barley populations with diverse maturity dates.
5. Conclusions
The combination of speed breeding and early harvest significantly reduced the time necessary to complete one generation of barley. We recovered at least five viable seeds with a 0.95 probability or higher, which is enough viable seeds to advance the next generation in a single-seed descent strategy under speed breeding. Also, our study demonstrated that early harvest in normal growing conditions recovers viable seeds with high probability, with a reduction of 13% in the length of the growing cycle. The most effective approach was 21 DAF in the SBS, where the length of the cycle was reduced by 20%; this demonstrates that speed breeding is a viable alternative to accelerate generational advance and enhance genetic gain per unit of time in barley breeding programs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15102275/s1, Table S1: Characterization of barley genotypes used in this study in terms of grain yield (GY), plant height (PH), and heading date (HD) based on historical information and the environment; Table S2: Best linear unbiased estimates (BLUEs) and standard errors (in parentheses) for number of days since emergence to reach ZGSs 13, 14, 21, 31, 43, 59, and 69 in eleven barley genotypes evaluated under normal breeding systems (NBSs) and speed breeding systems (SBSs); Table S3: Best linear unbiased estimates (BLUEs) and standard errors (in parentheses) for plant height (cm) at ZGSs 14, 21, 30, 43, and 59 in eleven different barley genotypes evaluated under NBSs and SBSs; Table S4: Best linear unbiased estimates (BLUEs) and standard errors (in parentheses) for seed number per spike (Seed #), germination % (Germ), and viability (Viab) evaluated as the probability (prob) of having at least 5 viable seeds when harvested at 3 DAF for nine barley genotypes under NBSs and SBSs.
Author Contributions
Formal analysis, investigation, visualization, writing—original draft: G.G.; Writing—review and editing: B.M.; Writing—review and editing: P.S.; Conceptualization, investigation, project administration, resources, supervision, writing—original draft: L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by WARF and WCIA and the OREI Naked Barley Project, project award no. USDA OREI 2020-51300-32179, from the U.S. Department of Agriculture’s National Institute of Food and Agriculture.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
This work was supported by a grant from the WARF and WCIA and the OREI Naked Barley Project, project award no. USDA OREI 2020-51300-32179, from the U.S. Department of Agriculture’s National Institute of Food and Agriculture. We would like to thank three anonymous reviewers for their helpful comments.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
BLUE: best linear unbiased estimate, DAE: days after emergence, DAF: days after flowering, DAP: days after planting, NBS: normal breeding system, SBS: speed breeding system, ZGS: Zadok’s growth scale.
References
- FAOSTAT. Cereals Balance Data 2023. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 29 August 2025).
- Geng, L.; Li, M.; Zhang, G.; Ye, L. Barley: A Potential Cereal for Producing Healthy and Functional Foods. Food Qual. Saf. 2022, 6, fyac012. [Google Scholar] [CrossRef]
- Meints, B.; Hayes, P.M. Breeding Naked Barley for Food, Feed, and Malt. In Plant Breeding Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 95–119. ISBN 978-1-119-61680-1. [Google Scholar]
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Lenaerts, B.; Collard, B.C.Y.; Demont, M. Review: Improving Global Food Security through Accelerated Plant Breeding. Plant Sci. 2019, 287, 110207. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A. Plant Abiotic Stress Challenges from the Changing Environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef] [PubMed]
- Jorasch, P. The Global Need for Plant Breeding Innovation. Transgenic Res. 2019, 28, 81–86. [Google Scholar] [CrossRef]
- Baker, B.P.; Meints, B.M.; Hayes, P.M. Organic Barley Producers’ Desired Qualities for Crop Improvement. Org. Agric. 2020, 10, 35–42. [Google Scholar] [CrossRef]
- Muller, A.; Schader, C.; El-Hage Scialabba, N.; Brüggemann, J.; Isensee, A.; Erb, K.-H.; Smith, P.; Klocke, P.; Leiber, F.; Stolze, M.; et al. Strategies for Feeding the World More Sustainably with Organic Agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef]
- Gamage, A.; Gangahagedara, R.; Gamage, J.; Jayasinghe, N.; Kodikara, N.; Suraweera, P.; Merah, O. Role of Organic Farming for Achieving Sustainability in Agriculture. Farming Syst. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Cooper, M.; Voss-Fels, K.P.; Messina, C.D.; Tang, T.; Hammer, G.L. Tackling G × E × M Interactions to Close On-Farm Yield-Gaps: Creating Novel Pathways for Crop Improvement by Predicting Contributions of Genetics and Management to Crop Productivity. Theor. Appl. Genet. 2021, 134, 1625–1644. [Google Scholar] [CrossRef]
- Cooper, M.; Messina, C.D.; Tang, T.; Gho, C.; Powell, O.M.; Podlich, D.W.; Technow, F.; Hammer, G.L. Predicting Genotype × Environment × Management (G × E × M) Interactions for the Design of Crop Improvement Strategies. In Plant Breeding Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 467–585. ISBN 978-1-119-87415-7. [Google Scholar]
- Priyadarshan, P.M. Breeding Self-Pollinated Crops. In PLANT BREEDING: Classical to Modern; Priyadarshan, P.M., Ed.; Springer: Singapore, 2019; pp. 223–241. ISBN 978-981-13-7095-3. [Google Scholar]
- Acquaah, G. Principles of Plant Genetics and Breeding, 2nd ed.; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-0470664759. [Google Scholar]
- Ohnoutkova, L.; Vlcko, T.; Ayalew, M. Barley Anther Culture. In Barley: Methods and Protocols; Harwood, W.A., Ed.; Springer: New York, NY, USA, 2019; pp. 37–52. ISBN 978-1-4939-8944-7. [Google Scholar]
- Castillo, A.M.; Vallés, M.P.; Cistué, L. Comparison of Anther and Isolated Microspore Cultures in Barley. Effects of Culture Density and Regeneration Medium. Euphytica 2000, 113, 1–8. [Google Scholar] [CrossRef]
- Humphreys, D.G.; Knox, R.E. Doubled Haploid Breeding in Cereals. In Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 241–290. ISBN 978-3-319-22521-0. [Google Scholar]
- Zheng, Z.; Wang, H.B.; Chen, G.D.; Yan, G.J.; Liu, C.J. A Procedure Allowing up to Eight Generations of Wheat and Nine Generations of Barley per Annum. Euphytica 2013, 191, 311–316. [Google Scholar] [CrossRef]
- Shen, X.; Gmitter, F.G.; Grosser, J.W. Immature Embryo Rescue and Culture. In Plant Embryo Culture; Thorpe, T.A., Yeung, E.C., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 710, pp. 75–92. ISBN 978-1-61737-987-1. [Google Scholar]
- Rogo, U.; Fambrini, M.; Pugliesi, C. Embryo Rescue in Plant Breeding. Plants 2023, 12, 3106. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.; Caligari, P.D.S.; Thomas, W.T.B. Comparison of Spring Barley Lines Produced by Single Seed Descent, Pedigree Inbreeding and Doubled Haploidy. Plant Breed. 1986, 97, 138–146. [Google Scholar] [CrossRef]
- Surma, M.; Adamski, T.; Kaczmarek, Z.; Czajka, S. Phenotypic Distribution of Barley SSD Lines and Doubled Haploids Derived from F1 and F2 Hybrids. Euphytica 2006, 149, 19–25. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed Breeding Is a Powerful Tool to Accelerate Crop Research and Breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Hickey, L.T.; Dieters, M.J.; DeLacy, I.H.; Kravchuk, O.Y.; Mares, D.J.; Banks, P.M. Grain Dormancy in Fixed Lines of White-Grained Wheat (Triticum aestivum L.) Grown under Controlled Environmental Conditions. Euphytica 2009, 168, 303–310. [Google Scholar] [CrossRef]
- Bhatta, M.; Sandro, P.; Smith, M.R.; Delaney, O.; Voss-Fels, K.P.; Gutierrez, L.; Hickey, L.T. Need for Speed: Manipulating Plant Growth to Accelerate Breeding Cycles. Curr. Opin. Plant Biol. 2021, 60, 101986. [Google Scholar] [CrossRef]
- Samantara, K.; Bohra, A.; Mohapatra, S.R.; Prihatini, R.; Asibe, F.; Singh, L.; Reyes, V.P.; Tiwari, A.; Maurya, A.K.; Croser, J.S.; et al. Breeding More Crops in Less Time: A Perspective on Speed Breeding. Biology 2022, 11, 275. [Google Scholar] [CrossRef]
- Marenkova, A.G.; Blinkov, A.O.; Radzeniece, S.; Kocheshkova, A.A.; Karlov, G.I.; Lavygina, V.A.; Patrushev, M.V.; Divashuk, M.G. Testing and Modification of the Protocol for Accelerated Growth of Malting Barley under Speed Breeding Conditions. Nanotechnol. Russ. 2024, 19, 808–814. [Google Scholar] [CrossRef]
- Gaoua, O.; Arslan, M.; Obedgiu, S. Speed Breeding Advancements in Safflower (Carthamus tinctorius L.): A Simplified and Efficient Approach for Accelerating Breeding Programs. Mol. Breed. 2025, 45, 13. [Google Scholar] [CrossRef]
- Wang, G.; Sun, Z.; Yang, J.; Ma, Q.; Wang, X.; Ke, H.; Huang, X.; Zhang, L.; Wang, G.; Gu, Q.; et al. The Speed Breeding Technology of Five Generations per Year in Cotton. Theor. Appl. Genet. 2025, 138, 79. [Google Scholar] [CrossRef]
- González-Barrios, P.; Bhatta, M.; Halley, M.; Sandro, P.; Gutiérrez, L. Speed Breeding and Early Panicle Harvest Accelerates Oat (Avena sativa L.) Breeding Cycles. Crop Sci. 2021, 61, 320–330. [Google Scholar] [CrossRef]
- Hill, C.B.; Li, C. Genetic Architecture of Flowering Phenology in Cereals and Opportunities for Crop Improvement. Front. Plant Sci. 2016, 7, 1906. [Google Scholar] [CrossRef] [PubMed]
- Aiqing, S.; Somayanda, I.; Sebastian, S.V.; Singh, K.; Gill, K.; Prasad, P.V.V.; Jagadish, S.V.K. Heat Stress during Flowering Affects Time of Day of Flowering, Seed Set, and Grain Quality in Spring Wheat. Crop Sci. 2018, 58, 380–392. [Google Scholar] [CrossRef]
- Haas, M.; Schreiber, M.; Mascher, M. Domestication and Crop Evolution of Wheat and Barley: Genes, Genomics, and Future Directions. J. Integr. Plant Biol. 2019, 61, 204–225. [Google Scholar] [CrossRef]
- Kirby, E.J.M.; Eisenberg, B.E. Some Effects of Photoperiod on Barley. J. Exp. Bot. 1966, 17, 204–213. [Google Scholar] [CrossRef]
- Takahashi, R.; Yasuda, S. Varietal Differences in Responses to Photoperiod and Temperature in Barley. Berichte Des. Ohara Inst. Für Landwirtsch. Biol. 1960, 11, 365–384. [Google Scholar]
- Ochagavía, H.; Kiss, T.; Karsai, I.; Casas, A.M.; Igartua, E. Responses of Barley to High Ambient Temperature Are Modulated by Vernalization. Front. Plant Sci. 2022, 12, 776982. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.M.; Sharma, R.; Pasam, R.K.; Graner, A.; Kilian, B.; Schnurbusch, T. Genetic Dissection of Photoperiod Response Based on GWAS of Pre-Anthesis Phase Duration in Spring Barley. PLoS ONE 2014, 9, e113120. [Google Scholar] [CrossRef]
- Göransson, M.; Hallsson, J.H.; Lillemo, M.; Orabi, J.; Backes, G.; Jahoor, A.; Hermannsson, J.; Christerson, T.; Tuvesson, S.; Gertsson, B.; et al. Identification of Ideal Allele Combinations for the Adaptation of Spring Barley to Northern Latitudes. Front. Plant Sci. 2019, 10, 542. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Borisjuk, L.; Junker, B.H.; Mock, H.-P.; Rolletschek, H.; Seiffert, U.; Weschke, W.; Wobus, U. Barley Grain Development. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 281, pp. 49–89. ISBN 978-0-12-381258-2. [Google Scholar]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P.; et al. Speed Breeding in Growth Chambers and Glasshouses for Crop Breeding and Model Plant Research. Nat. Protoc. 2018, 13, 2944–2963. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Beales, J.; Faure, S.; Dunford, R.P.; Laurie, D.A. The Pseudo-Response Regulator Ppd-H1 Provides Adaptation to Photoperiod in Barley. Science 2005, 310, 1031–1034. [Google Scholar] [CrossRef]
- Maurer, A.; Draba, V.; Jiang, Y.; Schnaithmann, F.; Sharma, R.; Schumann, E.; Kilian, B.; Reif, J.C.; Pillen, K. Modelling the Genetic Architecture of Flowering Time Control in Barley through Nested Association Mapping. BMC Genom. 2015, 16, 290. [Google Scholar] [CrossRef]
- Walia, H.; Wilson, C.; Condamine, P.; Ismail, A.M.; Xu, J.; Cui, X.; Close, T.J. Array-Based Genotyping and Expression Analysis of Barley Cv. Maythorpe and Golden Promise. BMC Genom. 2007, 8, 87. [Google Scholar] [CrossRef]
- Massman, C.; Meints, B.; Hernandez, J.; Kunze, K.; Hayes, P.M.; Sorrells, M.E.; Smith, K.P.; Dawson, J.C.; Gutierrez, L. Genetic Characterization of Agronomic Traits and Grain Threshability for Organic Naked Barley in the Northern United States. Crop Sci. 2022, 62, 690–703. [Google Scholar] [CrossRef]
- Hu, H.; Wang, P.; Angessa, T.T.; Zhang, X.-Q.; Chalmers, K.J.; Zhou, G.; Hill, C.B.; Jia, Y.; Simpson, C.; Fuller, J.; et al. Genomic Signatures of Barley Breeding for Environmental Adaptation to the New Continents. Plant Biotechnol. J. 2023, 21, 1719–1721. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.E. Estimated Marginal Means, Aka Least-Square Means; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Parrado, J.D.; Savin, R.; Slafer, G.A. Photoperiod Sensitivity of Ppd-H1 and Ppd-H1 Isogenic Lines of a Spring Barley Cultivar: Exploring Extreme Photoperiods. J. Exp. Bot. 2023, 74, 6608–6618. [Google Scholar] [CrossRef]
- Rossi, N.; Powell, W.; Mackay, I.J.; Hickey, L.; Maurer, A.; Pillen, K.; Halliday, K.; Sharma, R. Investigating the Genetic Control of Plant Development in Spring Barley under Speed Breeding Conditions. Theor. Appl. Genet. 2024, 137, 115. [Google Scholar] [CrossRef]
- Cockram, J.; Jones, H.; Leigh, F.J.; O’Sullivan, D.; Powell, W.; Laurie, D.A.; Greenland, A.J. Control of Flowering Time in Temperate Cereals: Genes, Domestication, and Sustainable Productivity. J. Exp. Bot. 2007, 58, 1231–1244. [Google Scholar] [CrossRef]
- Jones, H.; Leigh, F.J.; Mackay, I.; Bower, M.A.; Smith, L.M.J.; Charles, M.P.; Jones, G.; Jones, M.K.; Brown, T.A.; Powell, W. Population-Based Resequencing Reveals That the Flowering Time Adaptation of Cultivated Barley Originated East of the Fertile Crescent. Mol. Biol. Evol. 2008, 25, 2211–2219. [Google Scholar] [CrossRef]
- Hemming, M.N.; Walford, S.A.; Fieg, S.; Dennis, E.S.; Trevaskis, B. Identification of High-Temperature-Responsive Genes in Cereals. Plant Physiol. 2012, 158, 1439–1450. [Google Scholar] [CrossRef]
- Porker, K.; Coventry, S.; Fettell, N.; Cozzolino, D.; Eglinton, J. Using a Novel PLS Approach for Envirotyping of Barley Phenology and Adaptation. Field Crops Res. 2020, 246, 107697. [Google Scholar] [CrossRef]
- Gol, L.; Tomé, F.; von Korff, M. Floral Transitions in Wheat and Barley: Interactions between Photoperiod, Abiotic Stresses, and Nutrient Status. J. Exp. Bot. 2017, 68, 1399–1410. [Google Scholar] [CrossRef]
- García, G.A.; Serrago, R.A.; Dreccer, M.F.; Miralles, D.J. Post-Anthesis Warm Nights Reduce Grain Weight in Field-Grown Wheat and Barley. Field Crops Res. 2016, 195, 50–59. [Google Scholar] [CrossRef]
- Digel, B.; Pankin, A.; von Korff, M. Global Transcriptome Profiling of Developing Leaf and Shoot Apices Reveals Distinct Genetic and Environmental Control of Floral Transition and Inflorescence Development in Barley. Plant Cell 2015, 27, 2318–2334. [Google Scholar] [CrossRef]
- Wan, Y.; Poole, R.L.; Huttly, A.K.; Toscano-Underwood, C.; Feeney, K.; Welham, S.; Gooding, M.J.; Mills, C.; Edwards, K.J.; Shewry, P.R.; et al. Transcriptome Analysis of Grain Development in Hexaploid Wheat. BMC Genom. 2008, 9, 121. [Google Scholar] [CrossRef]
- Acquaah, G. Conventional Plant Breeding to Modern Plant Breeding: Evolution, Achievements, and Limitations. In Plant Molecular Breeding in Genomics Era: Concepts and Tools; Al-Khayri, J.M., Ingle, K.P., Jain, S.M., Penna, S., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 1–42. ISBN 978-3-031-68586-6. [Google Scholar]
- Varshney, R.K.; Roorkiwal, M.; Sorrells, M.E. (Eds.) Genomic Selection for Crop Improvement; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-63168-4. [Google Scholar]
- Crossa, J.; Fritsche-Neto, R.; Montesinos-Lopez, O.A.; Costa-Neto, G.; Dreisigacker, S.; Montesinos-Lopez, A.; Bentley, A.R. The Modern Plant Breeding Triangle: Optimizing the Use of Genomics, Phenomics, and Enviromics Data. Front. Plant Sci. 2021, 12, 651480. [Google Scholar] [CrossRef]
- Berro, I.; Lado, B.; Nalin, R.S.; Quincke, M.; Gutiérrez, L. Training Population Optimization for Genomic Selection. Plant Genome 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Berro, I.; Varela, J.; Gutiérrez, L. An Image-Based Methodology to Evaluate Oat Panicle Architecture. Crop Sci. 2023, 63, 648–661. [Google Scholar] [CrossRef]
- Hoefler, R.; González-Barrios, P.; Bhatta, M.; Nunes, J.A.R.; Berro, I.; Nalin, R.S.; Borges, A.; Covarrubias, E.; Diaz-Garcia, L.; Quincke, M.; et al. Do Spatial Designs Outperform Classic Experimental Designs? J. Agric. Biol. Environ. Stat. 2020, 25, 523–552. [Google Scholar] [CrossRef]
- Kunze, K.H.; Meints, B.; Massman, C.; Gutiérrez, L.; Hayes, P.M.; Smith, K.P.; Bergstrom, G.C.; Sorrells, M.E. Genome-Wide Association of an Organic Naked Barley Diversity Panel Identified Quantitative Trait Loci for Disease Resistance. Plant Genome 2024, 17, e20530. [Google Scholar] [CrossRef]
- Kunze, K.H.; Meints, B.; Massman, C.; Gutiérrez, L.; Hayes, P.M.; Smith, K.P.; Sorrells, M.E. Genotype × Environment Interactions of Organic Winter Naked Barley for Agronomic, Disease, and Grain Quality Traits. Crop Sci. 2024, 64, 678–696. [Google Scholar] [CrossRef]
- Massman, C.; Meints, B.; Hernandez, J.; Kunze, K.; Smith, K.P.; Sorrells, M.E.; Hayes, P.M.; Gutierrez, L. Genomic Prediction of Threshability in Naked Barley. Crop Sci. 2023, 63, 674–689. [Google Scholar] [CrossRef]
- Neyhart, J.; Silverstein, K.A.T.; Smith, K.P. Accurate Predictions of Barley Phenotypes Using Genomewide Markers and Environmental Covariates. Crop Sci. 2022, 62, 1821–1833. [Google Scholar] [CrossRef]
- Hickey, L.T.; Germán, S.E.; Pereyra, S.A.; Diaz, J.E.; Ziems, L.A.; Fowler, R.A.; Platz, G.J.; Franckowiak, J.D.; Dieters, M.J. Speed Breeding for Multiple Disease Resistance in Barley. Euphytica 2017, 213, 64. [Google Scholar] [CrossRef]
- Nannuru, V.K.R.; Dieseth, J.A.; Lillemo, M.; Meuwissen, T.H.E. Evaluating Genomic Selection and Speed Breeding for Fusarium Head Blight Resistance in Wheat Using Stochastic Simulations. Mol. Breed. 2025, 45, 14. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, M.; Gutierrez, L.; Cammarota, L.; Cardozo, F.; Germán, S.; Gómez-Guerrero, B.; Pardo, M.F.; Lanaro, V.; Sayas, M.; Castro, A.J. Multi-Trait Genomic Prediction Model Increased the Predictive Ability for Agronomic and Malting Quality Traits in Barley (Hordeum vulgare L.). G3 Genes Genomes Genet. 2020, 10, 1113–1124. [Google Scholar] [CrossRef]
- Xiong, W.; Reynolds, M.; Xu, Y. Climate Change Challenges Plant Breeding. Curr. Opin. Plant Biol. 2022, 70, 102308. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).