Effect of Synthetic and Biological Plant Growth Stimulants and Soil Amendments on the Development of Maize in Various Soil Moisture Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Greenhouse Research
2.2. Field Research
2.3. Statistical Analysis
- In the first stage of research—two-way analysis of variance with interaction—to study the impact of two factors, drought stress and preparations on the following variables: chlorophyll fluorescence parameters (F0, Fm/v), gas exchange between the soil and the atmosphere (Ce—Soil respiration, Wflux—Net H2O Exchange Rate and NCER—Net CO2 Exchange Rate ) and plant growth parameters (height, fresh and dry weight).The first factor, i.e., the occurrence of drought stress, was considered at two levels:
- −
- Optimal soil moisture level; drought.
The second factor, i.e., preparations, was considered at five levels:- −
- Control—combination without the addition of any substance or microorganism; R. irregularis—Rhizophagus irregularis; P.p.—potassium polyacrylate; β-c—β-cyclocitral; R. irregularis+ P.p. + β-c—Rhizophagus irregularis + potassium polyacrylate + β-cyclocitral.
A two-way ANOVA model with interaction was used:where:yij = μ+αi + βj + (αβ)ij + eij- −
- yij—the estimated value of variables (chlorophyll fluorescence parameters, gas exchange parameters between the soil and the atmosphere and plant growth parameters) in the presence or absence of drought stress (i = 1, 2) and using the selected preparation (j = 1, 2,…, 5); μ—overall average; αi—effect of the occurrence or absence of stress (i = 1, 2); βj—effect of using the jth preparation (j = 1, 2,…, 5); (αβ)ij—interaction effect of drought stress and treatment and eij—random error.
If the null hypotheses about the lack of influence of the analyzed factors or their interactions were rejected, the Tukey procedure was used for multiple comparisons. - In the second stage of research—one-way analysis of variance—to test the effect of preparations on the following variables: plant height, yield, weight of 1000 grains, hectoliter weight and the content of protein, oil and starch in the grain.The considered factor, i.e., preparations, existed at five levels:
- −
- Control—combination without the addition of any substance or microorganism; R. irregularis—Rhizophagus irregularis; P.p.—potassium polyacrylate; β-c—β-cyclocitral; R. irregularis + P.p. + β-c—Rhizophagus irregularis + potassium polyacrylate + β-cyclocitral.
3. Results
3.1. Greenhouse Research
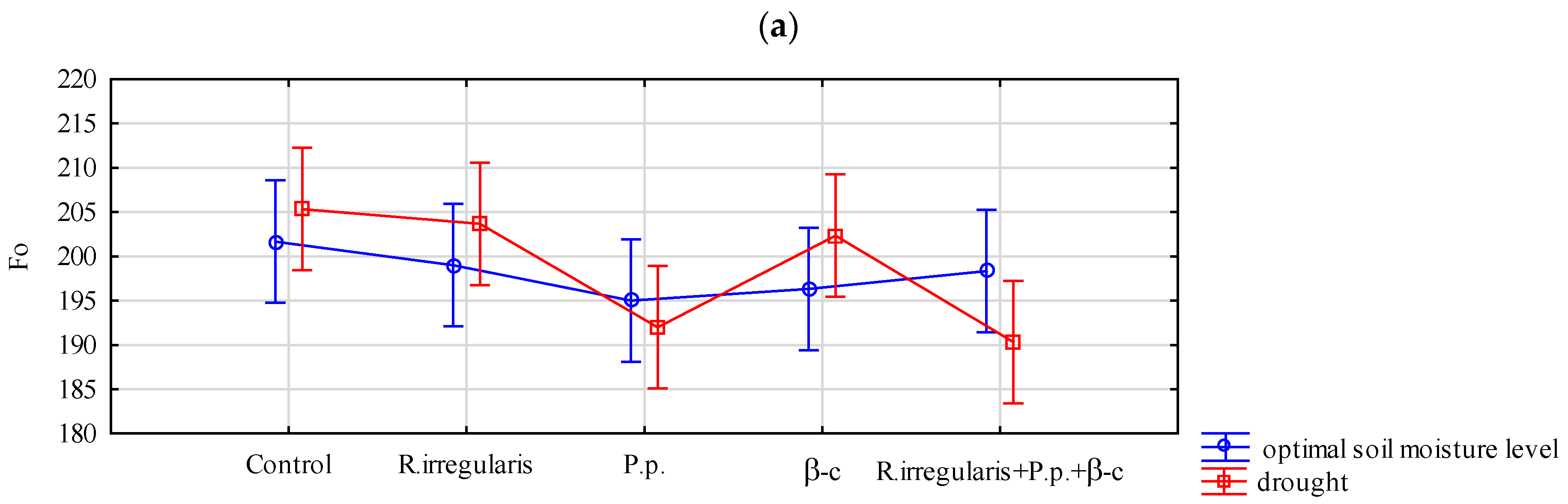
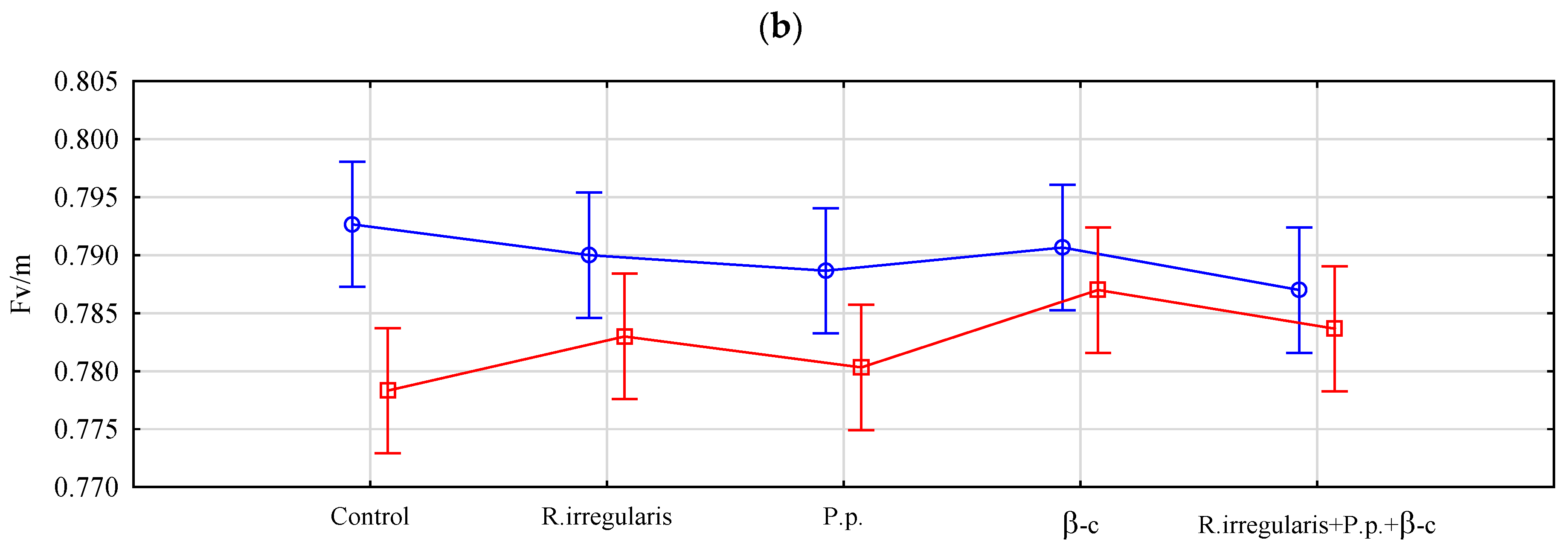
3.1.1. Plant Chlorophyll Fluorescence
3.1.2. Height and Weight of Maize in Greenhouse Conditions
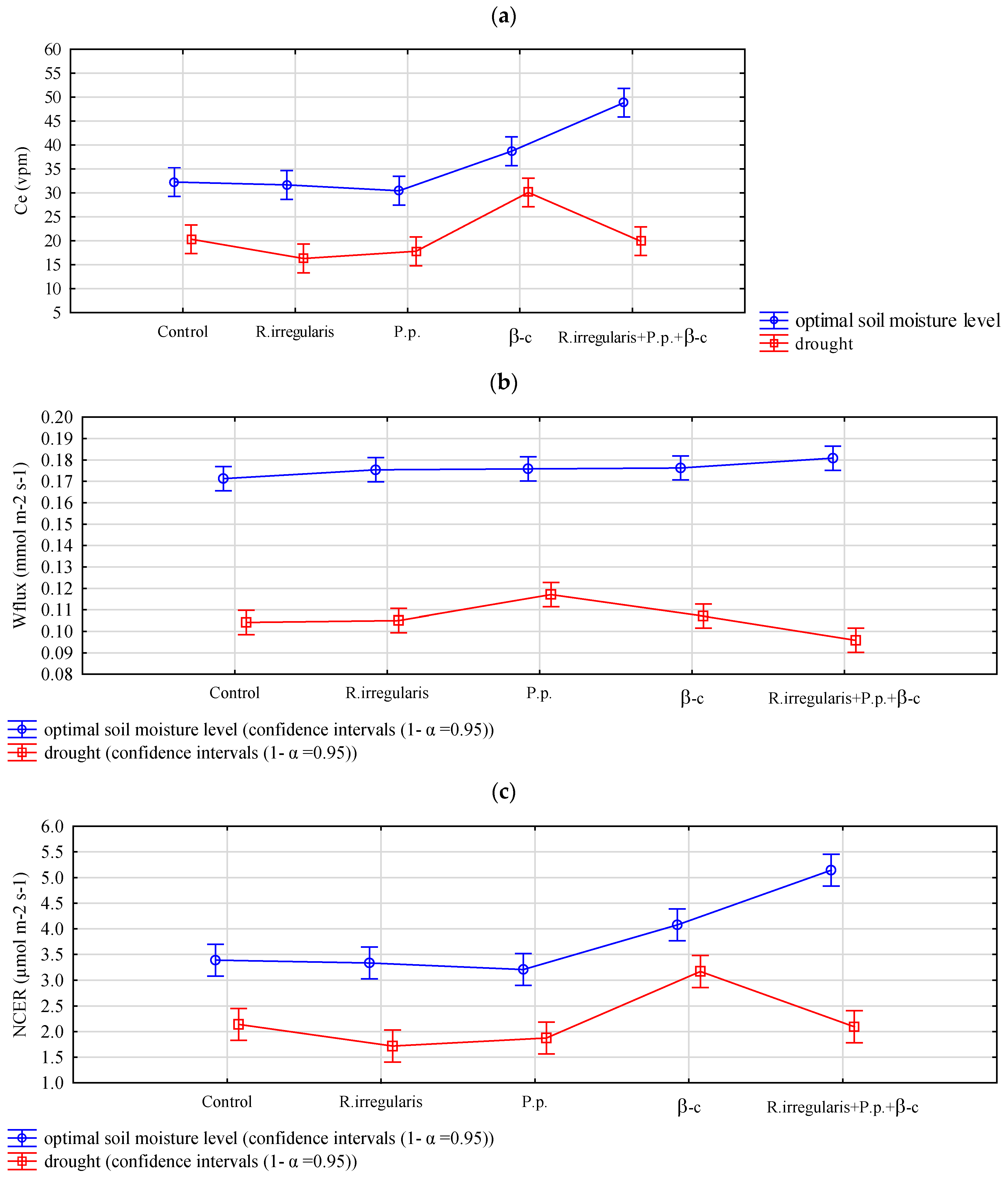
3.1.3. Gas Exchange Between the Soil and the Atmosphere
3.2. Field Research
3.2.1. Meteorological Conditions During the Research
3.2.2. Plant Height in Field Conditions
3.2.3. Plant Yield Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H. Drought stress in plants: An overview. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; p. 477. [Google Scholar]
- Sheoran, S.; Kaur, Y.; Kumar, S.; Shukla, S.; Rakshit, S.; Kumar, R. Recent advances for drought stress tolerance in maize (Zea mays L.): Present status and future prospects. Front. Plant Sci. 2022, 13, 872566. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Sec. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Bodner, G.; Nakhforoosh, A.; Kaul, H.P. Management of crop water under drought: A review. Agron. Sust. Devel. 2015, 35, 401–442. [Google Scholar] [CrossRef]
- Rai, N.; Rai, S.P.; Sarma, B.K. Prospects for abiotic stress tolerance in crops utilizing phyto- and bio-stimulants. Front Sustain Food Syst. 2021, 5, 754853. [Google Scholar] [CrossRef]
- Rosa, L.; Chiarelli, D.D.; Rulli, M.C.; Dell’Angelo, J.; D’Odorico, P. Global agricultural economic water scarcity. Sci. Adv. 2020, 6, eaaz6031. [Google Scholar] [CrossRef]
- Situ, Y.; Yang, Y.; Huang, C.; Liang, S.; Mao, X.; Chen, X. Effects of several superabsorbent polymers on soil exchangeable cations and crop growth. Environ. Technol. Innov. 2023, 30, 103126. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, H.; Liu, B.; Wu, Y.; Song, J. Effects of super-absorbent polymers on the physical and chemical properties of soil following different wetting and drying cycles. Soil. Use Manag. 2010, 26, 253–260. [Google Scholar] [CrossRef]
- Kabiri, K.; Omidian, H.; Zohuriaan-Mehr, M.; Doroudiani, S. Superabsorbent hydrogel composites and nanocomposites: A review. Polym. Compos. 2011, 32, 277–289. [Google Scholar] [CrossRef]
- Kulikowski, Ł.; Kulikowski, E.; Matuszwski, A.; Kiepurski, J. Hydrożele w środowisku naturalnym—Historia i technologie/Hydrogels in the natural environment—History and technologies. Inżynieria Ekologiczna 2018, 19, 205–218. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Patra, S.K.; Poddar, R.; Brestic, M.; Acharjee, P.U.; Bhattacharya, P.; Sengupta, S.; Pal, P.; Bam, N.; Biswas, B.; Barek, V.; et al. Prospects of hydrogels in agriculture for enhancing crop and water productivity under water deficit condition. Int. J. Polym. Sci. 2022, 2022, 4914836. [Google Scholar] [CrossRef]
- Somé, Y.S.C.; Traoré, D.; Zoromé, M.; Ouoba, P.A.; Da, D.E.C. Assessment of the effectiveness of potassium polyacrylate on crop production. J. Agric. Chem. Environ. 2021, 10, 113–123. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef]
- Dickinson, A.J.; Lehner, K.; Mi, J.; Jia, K.-P.; Mijar, M.; Dinneny, J.; Al-Babili, S.; Benfey, P.N. β-Cyclocitral is a conserved root growth regulator. Proc. Natl. Acad. Sci. USA 2019, 116, 10563–10567. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Tonny, S.H.; Afzal, S.; Farooqui, Z.; Alam, P.; Ahmed, M.; Yu, F.; Hayat, S. β-cyclocitral: Emerging bioactive compound in plants. Molecules 2022, 27, 6845. [Google Scholar] [CrossRef]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Verzera, A. Bioactive volatiles in Sicilian (South Italy) saffron: Safranal and its related compounds. J. Essent. Oil Res. 2017, 29, 221–227. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Purkar, V.; Mitra, S. β-cyclocitral, a master regulator of multiple stress-responsive genes in Solanum lycopersicum L. plants. Plants 2021, 10, 2465. [Google Scholar] [CrossRef] [PubMed]
- Rini, M.V.; Susilowati, E.; Riniarti, M.; Lukman, I. Application of Glomus sp. and a mix of Glomus sp. with Gigaspora sp. in improving the Agarwood (Aquilaria malaccensis Lamk.) seedling growth in Ultisol soil. IOP Conf. Ser. Earth Environ. Sci. 2020, 449, 012004. [Google Scholar] [CrossRef]
- Öpik, M.; Moora, M.; Liira, J.; Zobel, M. Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J. Ecol. 2006, 94, 778–790. [Google Scholar] [CrossRef]
- Castiglione, A.M.; Mannino, G.; Contartese, V.; Bertea, C.M.; Ertani, A. Microbial biostimulants as response to modern agriculture needs: Composition, role and application of these innovative products. Plants 2021, 10, 1533. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Kaur, M.; Kaushik, P.; Alyemeni, M.N.; Alsahli, A.A.; Ahmad, P. Arbuscular mycorrhiza in combating abiotic stresses in vegetables: An eco-friendly approach. Saudi. J. Biol. Sci. 2021, 28, 1465. [Google Scholar] [CrossRef] [PubMed]
- Pons, S.; Fournier, S.; Chervin, C.; Be’card, G.; Rochange, S.; Frei Dit Frey, N.; Puech Pagès, V. Phytohormone production by the arbuscular mycorrhizal fungus Rhizophagus irregularis. PLoS ONE 2020, 15, e0240886. [Google Scholar] [CrossRef]
- Aljawasim, B.D.; Khaeim, H.M.; Manshood, M.A. Assessment of arbuscular mycorrhizal fungi (Glomus spp.) as potential biocontrol agents against damping-off disease Rhizoctonia solani on cucumber. J. Crop. Prot. 2020, 9, 141–147. [Google Scholar]
- Lewis, J.D. Mycorrhizal fungi, evolution and diversification of. In Encyclopedia of Evolutionary Biology; Kliman, R.M., Ed.; Academic Press: Oxford, UK, 2016. [Google Scholar] [CrossRef]
- Jhala, A.J.; Ramirez, A.H.M.; Knezevic, S.Z.; Van Damme, P.; Singh, M. Herbicide tank mixtures for broad-spectrum weed control in Florida citrus. Weed Technol. 2013, 27, 129–137. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Martin, O.; Scholze, M.; Ermler, S.; McPhie, J.; Bopp, S.K.; Kienzler, A.; Parissis, N.; Kortenkamp, A. Ten years of research on synergisms and antagonisms in chemical mixtures: A systematic review and quantitative reappraisal of mixture studies. Environ. Int. 2021, 146, 106206. [Google Scholar] [CrossRef]
- Feizene, D.; Povilaitis, V.; Kadžienė, G. Springtime soil surface respiration and soil vapour flux in different long-term agroecosystems. Ekologija. 2008, 54, 216–225. [Google Scholar] [CrossRef]
- Skowera, B.; Puła, J. Skrajne warunki pluwiotermiczne w okresie wiosennym na obszarze Polski w latach 1971–2000. (Pluviometric extreme conditions in spring season in Poland in the years 1971–2000). Acta Agrophys. 2004, 3, 171–177. [Google Scholar]
- Biostimulants Market Size, Share & Trends Analysis Report By Active Ingredients (Acid Based, Microbial), By Crop Type, By Application (Foliar, Soil Treatment); By Region, And Segment Forecasts, 2023–2030. Report ID: GVR-2-68038-346-1: San Francisco, CA, USA. Available online: https://www.grandviewresearch.com/industry-analysis/biostimulants-market (accessed on 29 December 2024).
- Soil Amendments Market Share, Size, Trends, Industry Analysis Report, By Product (Organic, Inorganic); By Soil Type (Clay, Sand, Loam, Silt); By Application; By Region; Segment Forecast, 2022–2030. Report ID: PM2546: San Francisco, CA, USA. Available online: https://www.polarismarketresearch.com/industry-analysis/soil-amendments-market (accessed on 29 December 2024).
- Mazen, A.M.; Radwan, D.E.M.; Ahmed, A.F. Growth responses of maize plants cultivated in sandy soil amended by different superabsorbant hydrogels. J. Plant Nutr. 2015, 38, 325–337. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef]
- Ramírez-Flores, M.R.; Bello-Bello, E.; Rellán-Álvarez, R.; Sawers, R.J.H.; Olalde-Portugal, V. Inoculation with the mycorrhizal fungus Rhizophagus irregularis modulates the relationship between root growth and nutrient content in maize (Zea mays ssp. Mays L.). Plant Direct. 2019, 3, e00192. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Carvalho, M.F.; Magalhães, C.; Janoušková, M.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating with inocula of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria for nutritional enhancement of maize under different fertilization regimes. Arch. Agron. Soil. Sci. 2019, 65, 31–43. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Briantais, J.-M.; Dacosta, J.; Goulas, Y.; Ducruet, J.-M.; Moya, I. Heat stress induces in leaves an increase of the minimum level of chlorophyll fluorescence, Fo: A time-resolved analysis. Photosynth. Res. 1996, 48, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Gholamin, R.; Khayatnezhad, M. The effect of end season drought stress on the chlorophyll content, chlorophyll fluorescence parameters and yield in maize cultivars. Sci. Res. Essays. 2011, 6, 5351–5357. [Google Scholar]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.C.; Silverio, J.M.; Scalon, S.P.Q.; Vieira, M.C. Hydrogel and water regimes in the chlorophyll-a fluorescence and growth of Campomanesia xanthocarpa seedlings. Eng. Agric. 2021, 3, 330–337. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Radzikowska-Kujawska, D.; John, P.; Piechota, T.; Nowicki, M.; Kowalczewski, P.Ł. Response of winter wheat (Triticum aestivum L.) to selected biostimulants under drought conditions. Agriculture 2022, 13, 121. [Google Scholar] [CrossRef]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.-K. Chlorophyll fluorescence imaging for early detection of drought and heat stress in strawberry plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef]
- Koncz, P.; Balogh, J.; Papp, M.; Hidy, D.; Pinter, K.; Fóti, S.; Klumpp, K.; Nagy, Z. Higher soil respiration under mowing than under grazing explained by biomass differences. Nutr. Cycl. Agroecosyst. 2015, 103, 201–215. [Google Scholar] [CrossRef]
- Kou, X.; Han, W.; Kang, J. Responses of root system architecture to water stress at multiple levels: A meta-analysis of trials under controlled conditions. Front. Plant Sci. 2022, 13, 1085409. [Google Scholar] [CrossRef]
- Rogers, E.D.; Benfey, P.N. Regulation of plant root system architecture: Implications for crop advancement. Curr. Opin. Biotechnol. 2015, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Van Gerrewey, T.; Geelen, D.A. Meta-Analysis of Biostimulant Yield Effectiveness in Field Trials. Front. Plant Sci. 2022, 13, 836702. [Google Scholar] [CrossRef]

| K-Index Classes | Values |
|---|---|
| Extremely dry | k ≤ 0.4 |
| Very dry | 0.4 < k ≤ 0.7 |
| Dry | 0.7 < k ≤ 1.0 |
| Slightly dry | 1.0 < k ≤ 1.3 |
| Optimum | 1.3 < k ≤ 1.6 |
| Slightly humid | 1.6 < k ≤ 2.0 |
| Humid | 2.0 < k ≤ 2.5 |
| Very humid | 2.5 < k ≤ 3.0 |
| Extremely humid | k > 3.0 |
| Results of Two-Factor Analysis of Variance (p-Value) | ||
|---|---|---|
| Factor | F0 | Fv/m |
| Preparation | 0.026 * | 0.521 ns |
| Drought stress/optimal soil moisture conditions | 0.754 ns | 2.29 × 10−4 ** |
| Interaction effect | 0.209 ns | 0.244 ns |
| Results of Tukey’s test—Average feature values and homogeneous groups | ||
| Preparation | F0 | Fv/m |
| Control | 203.5 b | 0.786 |
| R. irregularis | 201.3 ab | 0.787 |
| P. p. | 193.5 a | 0.785 |
| β-c | 199.3 ab | 0.789 |
| R. irregularis +p. p.+ β-c | 194.3 ab | 0.785 |
| HSD | 9.910 | - |
| Drought stress/optimal soil moisture conditions | F0 | Fv/m |
| Optimal soil moisture level | 198.1 | 0.790 b |
| Drought | 198.7 | 0.782 a |
| HSD | - | 0.003 |
| Height and Weight of Maize in Greenhouse Conditions | |||
|---|---|---|---|
| Results of Two-Factor Analysis of Variance (p-Value) | |||
| Factor | Plant Height (cm) | Fresh Weight (g) | Dry Weight (g) |
| Preparation | 0.039 * | 1.46 × 10−11 ** | 2.69 × 10−10 ** |
| Drought stress/optimal soil moisture conditions | 0.003 ** | 1.21 × 10−20 ** | 1.24 × 10−9 ** |
| Interaction effect | 0.633 ns | 3.73 × 10−5 ** | 0.021 * |
| Results of Tukey’s test—Average feature values and homogeneous groups | |||
| Preparation | Plant height (cm) | Fresh weight (g) | Dry weight (g) |
| Control | 104.0 a | 138.0 ab | 14.8 b |
| R. irregularis | 107.1 a | 139.9 b | 14.6 b |
| P. p. | 108.7 a | 156.3 c | 15.7 b |
| β-c | 104.1 a | 130.5 a | 12.9 a |
| R. irregularis +p. p.+ β-c | 109.7 a | 174.5 d | 19.5 c |
| HSD | 6.335 | 8.810 | 1.435 |
| Drought stress/optimal soil moisture conditions | Plant height (cm) | Fresh weight (g) | Dry weight (g) |
| Optimal soil moisture level | 108.9 b | 185.4 b | 17.1 b |
| Drought | 104.5 a | 110.2 a | 13.9 a |
| HSD | 4.007 | 3.886 | 0.633 |
| Interaction | Plant height (cm) | Fresh weight (g) | Dry weight (g) |
| Control + om | 106.2 | 177.4 e | 17.3 b |
| R. irregularis + om | 111.5 | 177.9 e | 16.6 b |
| P. p. + om | 109.7 | 196.1 f | 17.3 b |
| β-c + om | 105.5 | 156.1 d | 13.6 a |
| R. irregularis + p.p. + β-c + om | 111.5 | 219.7 g | 20.8 c |
| Control + drought | 101.8 | 98.6 a | 12.4 a |
| R. irregularis + drought | 102.8 | 101.9 ab | 12.7 a |
| P. p. + drought | 107.7 | 116.5 bc | 14.2 a |
| β-c + drought | 102.6 | 104.8 ab | 12.2 a |
| R. irregularis + p. p. + β-c +drought | 107.8 | 129.2 c | 18.1 b |
| HSD | - | 14.757 | 2.403 |
| Results of Two-Factor Analysis of Variance (p-Value) | |||
|---|---|---|---|
| Factor | Ce (vpm) | Wflux (mmol m−2s−1) | NCER (µmol m−2s−1) |
| Preparation | 6.94 × 10−17 ** | 0.020 * | 2.14 × 10−17 ** |
| Drought stress/optimal soil moisture conditions | 1.66 × 10−39 ** | 8.61 × 10−103 ** | 2.01 × 10−40 ** |
| Interaction effect | 7.95 × 10−10 ** | 2.94 × 10−4 ** | 3.42 × 10−10 ** |
| Results of Tukey’s test—Average feature values and homogeneous groups | |||
| Preparation | Ce (vpm) | Wflux (mmol m−2s−1) | NCER (µmol m−2s−1) |
| Control | 26.3 a | 0.1377 a | 2.77 a |
| R. irregularis | 24.0 a | 0.1402 ab | 2.53 a |
| P. p. | 24.1 a | 0.1465 b | 2.54 a |
| β-c | 34.4 b | 0.1417 ab | 3.62 b |
| R. irregularis +p. p.+ β-c | 34.4 b | 0.1383 a | 3.62 b |
| HSD | 4.161 | 0.0078 | 0.43 |
| Drought stress/optimal soil moisture conditions | Ce (vpm) | Wflux (mmol m−2s−1) | NCER (µmol m−2s−1) |
| Optimal soil moisture level | 36.4 b | 0.1759 b | 3.83 b |
| Drought | 20.9 a | 0.1058 a | 2.20 a |
| HSD | 1.889 | 0.0035 | 0.20 |
| Interaction | Ce (vpm) | Wflux (mmol m−2s−1) | NCER (µmol m−2s−1) |
| Control + om | 32.3 bc | 0.1713 c | 3.39 bc |
| R. irregularis + om | 31.7 b | 0.1754 c | 3.34 b |
| P. p. + om | 30.5 b | 0.1758 c | 3.21 b |
| β-c + om | 38.7 c | 0.1763 c | 4.08 c |
| R. irregularis + p.p. + β-c + om | 48.9 d | 0.1808 c | 5.14 d |
| Control + drought | 20.3 a | 0.1042 a | 2.14 a |
| R. irregularis + drought | 16.3 a | 0.1050 ab | 1.72 a |
| P. p. + drought | 17.8 a | 0.1171 b | 1.88 a |
| β-c + drought | 30.1 b | 0.1071 ab | 3.17 b |
| R. irregularis + p. p. + β-c +drought | 19.9 a | 0.0958 a | 2.09 a |
| HSD | 6.814 | 0.0128 | 0.70 |
| Months | Decade | Average for the Month | ||
|---|---|---|---|---|
| I | II | III | ||
| May | 1.7 | 1.0 | 0.6 | 1.0 |
| June | 0.0 | 0.3 | 1.4 | 0.6 |
| July | 0.4 | 0.5 | 2.0 | 1.0 |
| August | 5.6 | 0.9 | 2.2 | 2.7 |
| September | 0.0 | 0.3 | 0.6 | 0.2 |
| October | 1.6 | 1.4 | 5.3 | 2.8 |
| Maize Yield | ||||||
|---|---|---|---|---|---|---|
| Results of One-Way Analysis of Variance (p-Value) | ||||||
| Factor | Yield (t ha−1) | HLW (kg) | TKW (g) | Content in Grain (%) | ||
| Protein | Oil | Starch | ||||
| Preparation | 0.005 ** | 0.805 ns | 0.271 ns | 0.861 ns | 0.883 ns | 0.787 ns |
| Results of Tukey’s test—Average feature values and homogeneous groups | ||||||
| Preparation | Yield (t ha−1) | HLW (kg) | TKW (g) | Content in grain (%) | ||
| Protein | Oil | Starch | ||||
| Control | 13.2 a | 68.58 | 299.24 | 10.50 | 3.95 | 69.80 |
| R. irregularis | 14.5 b | 67.93 | 311.02 | 10.20 | 3.98 | 69.93 |
| P. p. | 13.8 ab | 67.85 | 320.39 | 10.38 | 3.88 | 70.03 |
| β-c | 14.5 b | 68.73 | 311.43 | 10.28 | 3.88 | 70.08 |
| R. irregularis +p. p.+ β-c | 14.2 ab | 68.45 | 313.64 | 10.45 | 3.93 | 69.85 |
| HSD | 1.041 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzanka, M.; Sobiech, Ł.; Głowicka-Wołoszyn, R.; Radzikowska-Kujawska, D. Effect of Synthetic and Biological Plant Growth Stimulants and Soil Amendments on the Development of Maize in Various Soil Moisture Conditions. Agronomy 2025, 15, 96. https://doi.org/10.3390/agronomy15010096
Grzanka M, Sobiech Ł, Głowicka-Wołoszyn R, Radzikowska-Kujawska D. Effect of Synthetic and Biological Plant Growth Stimulants and Soil Amendments on the Development of Maize in Various Soil Moisture Conditions. Agronomy. 2025; 15(1):96. https://doi.org/10.3390/agronomy15010096
Chicago/Turabian StyleGrzanka, Monika, Łukasz Sobiech, Romana Głowicka-Wołoszyn, and Dominika Radzikowska-Kujawska. 2025. "Effect of Synthetic and Biological Plant Growth Stimulants and Soil Amendments on the Development of Maize in Various Soil Moisture Conditions" Agronomy 15, no. 1: 96. https://doi.org/10.3390/agronomy15010096
APA StyleGrzanka, M., Sobiech, Ł., Głowicka-Wołoszyn, R., & Radzikowska-Kujawska, D. (2025). Effect of Synthetic and Biological Plant Growth Stimulants and Soil Amendments on the Development of Maize in Various Soil Moisture Conditions. Agronomy, 15(1), 96. https://doi.org/10.3390/agronomy15010096








