Effects of Salt Field Waste-Generated Bio-Organic Fertilizer Application on Bacterial Community Structure in Tea Plantations Rhizosphere Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Determination of Total Polyphenols Content in Tea
2.3. Determination of Water Extract Content in Tea
- m0—mass of specimen (g).
- m1—Mass of tea residue after drying (g).
- w—Content of dry matter of specimen (%).
2.4. Determination of Caffeine Content in Tea
2.5. Determination of Free Amino Acid Content in Tea
2.6. Soil Sampling and Analysis
2.7. DNA Extraction and Library Construction
2.8. Data Analysis
2.9. Linear Discriminant Analysis Effect Size (LEfSe) Analysis
2.10. Statistical Analysis
3. Results
3.1. Effect of Salt Field Waste-Generated-BOF Treatment on the Quality of Tea Leaves
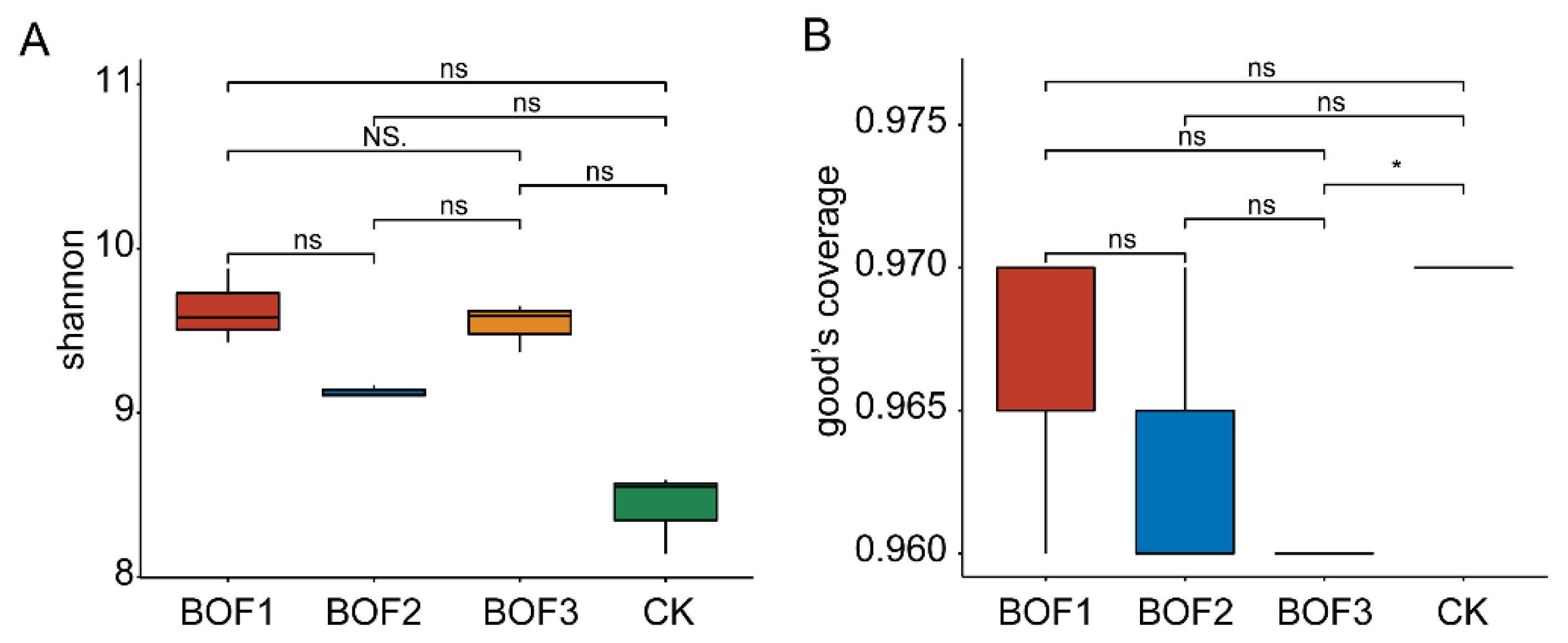
3.2. The α Diversity Analysis of Soil Bacterial Communities
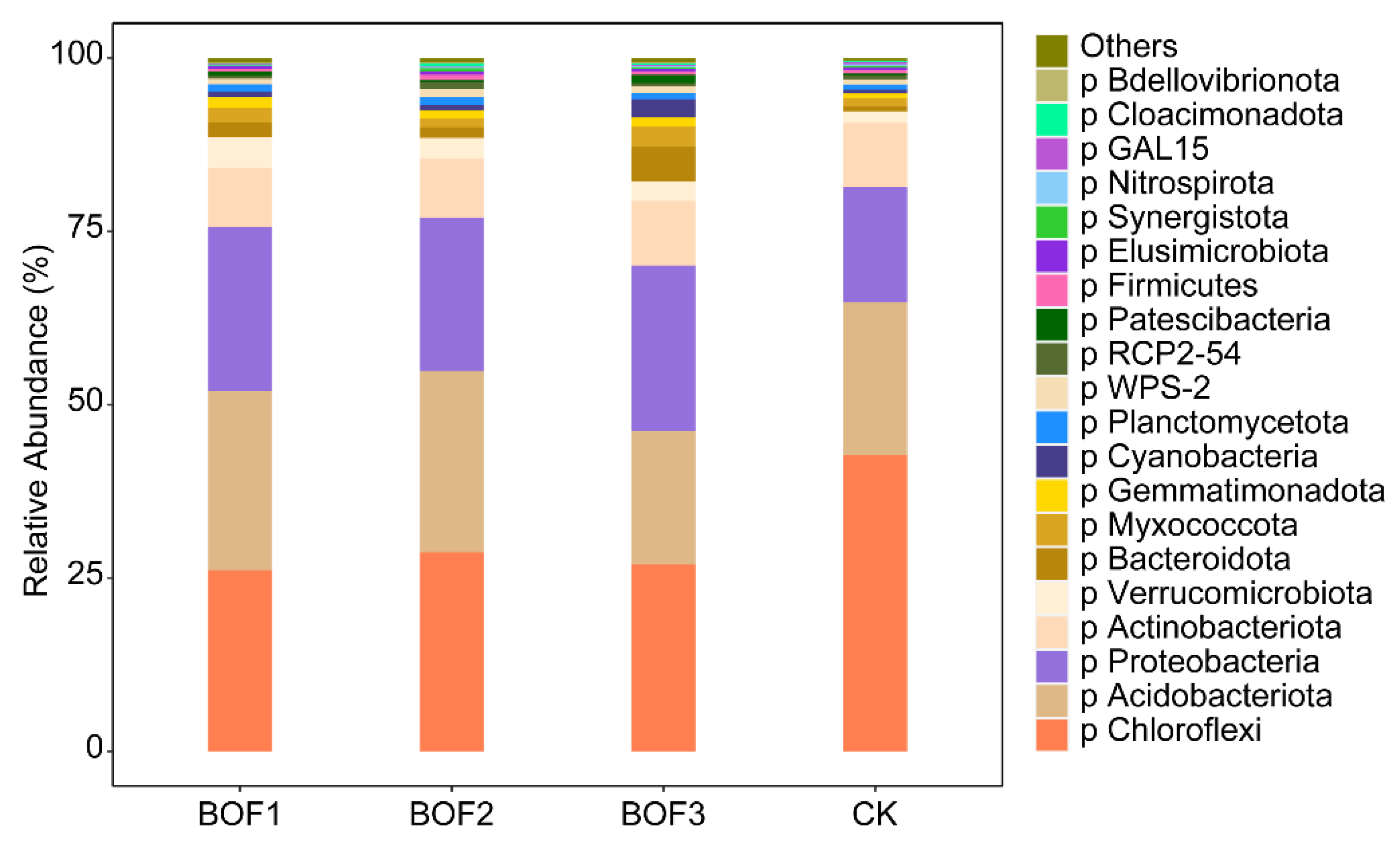
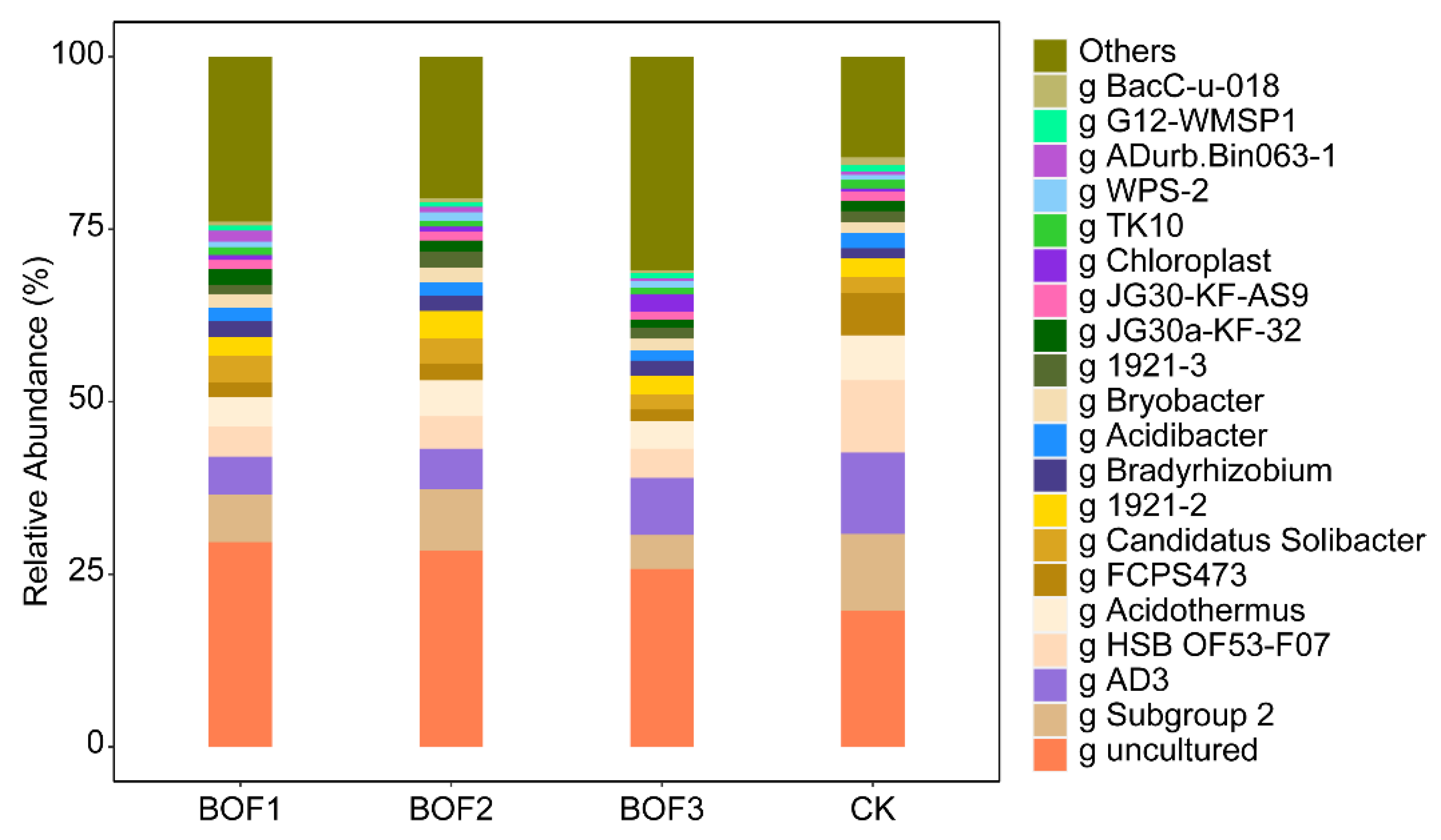
3.3. Compositional Analysis of Soil Bacterial Communities
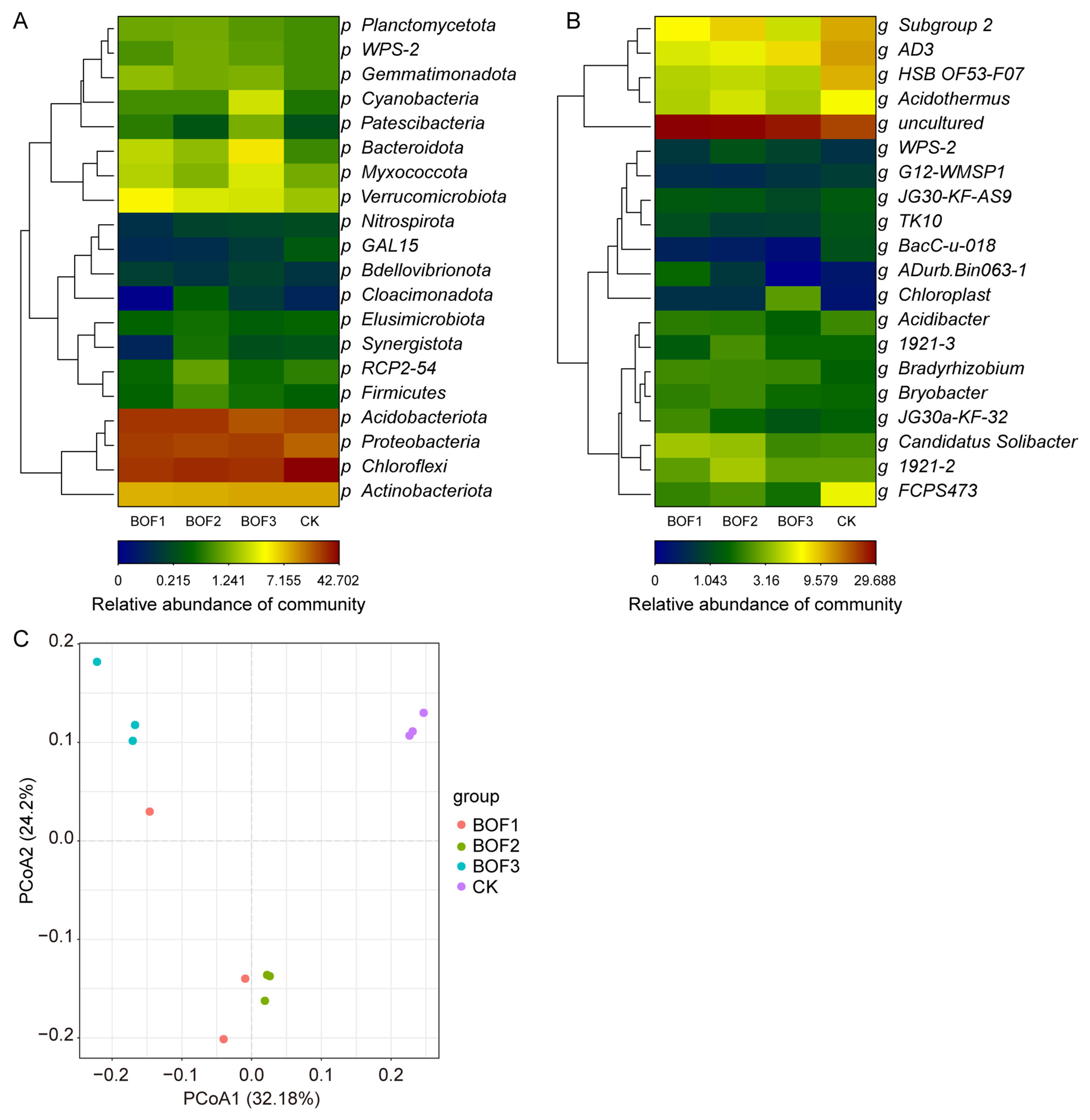
3.4. Heatmap, Clustering, and PCoA of Soil Bacteria in the Rhizosphere of Tea Tree
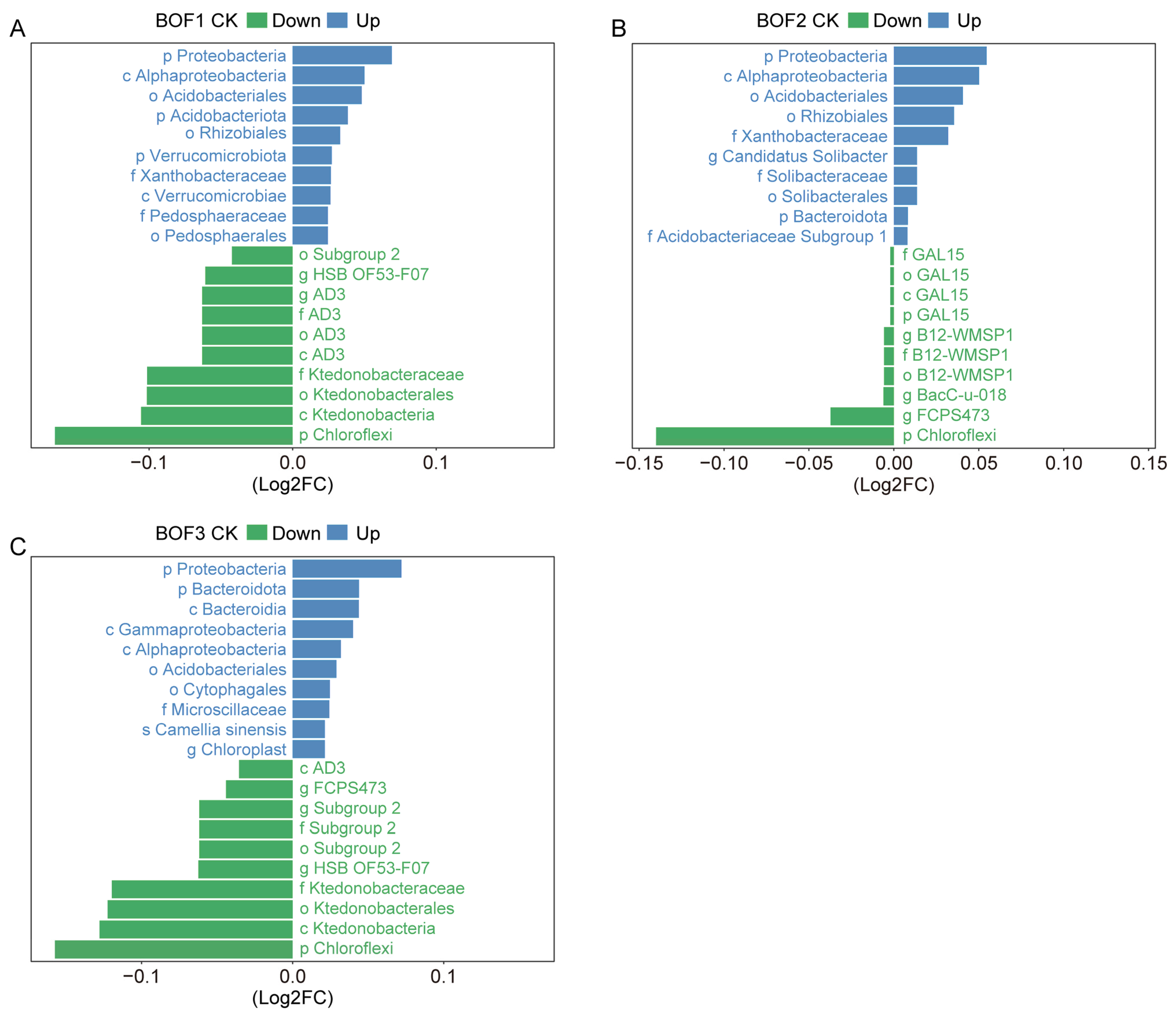
3.5. Comparison of Bacterial Community Composition Among the Different Treatments
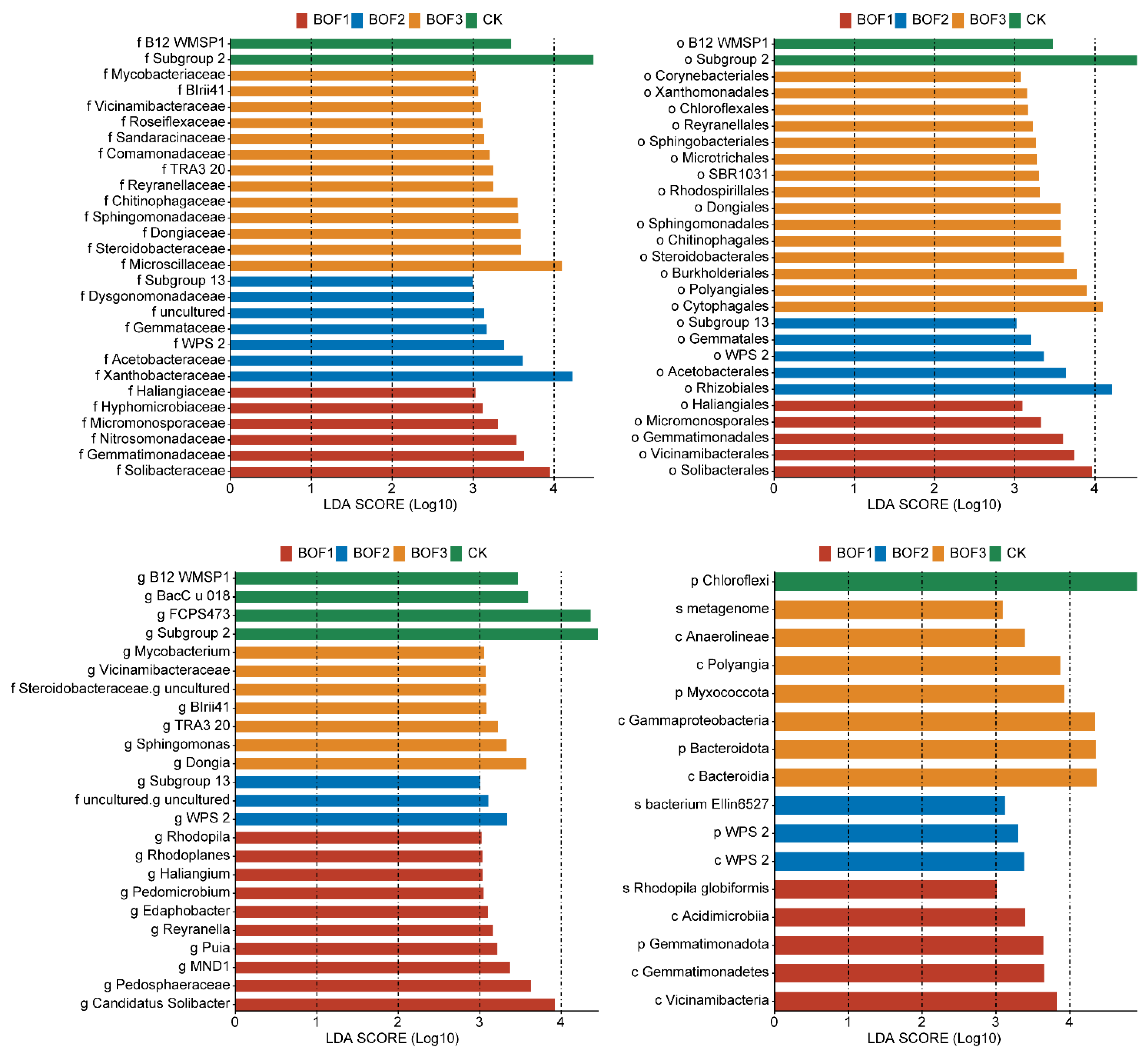
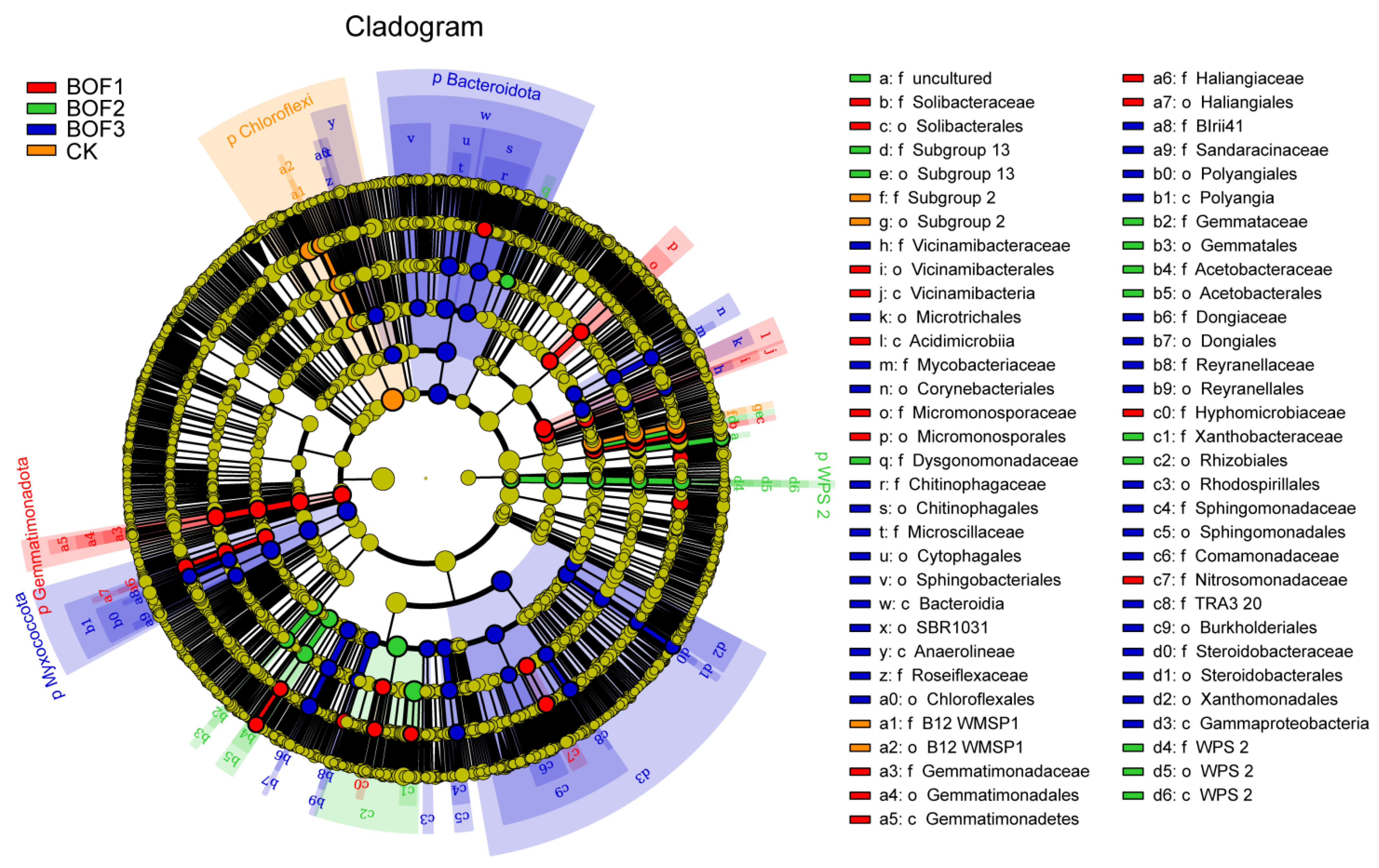
3.6. Taxonomic Biomarkers
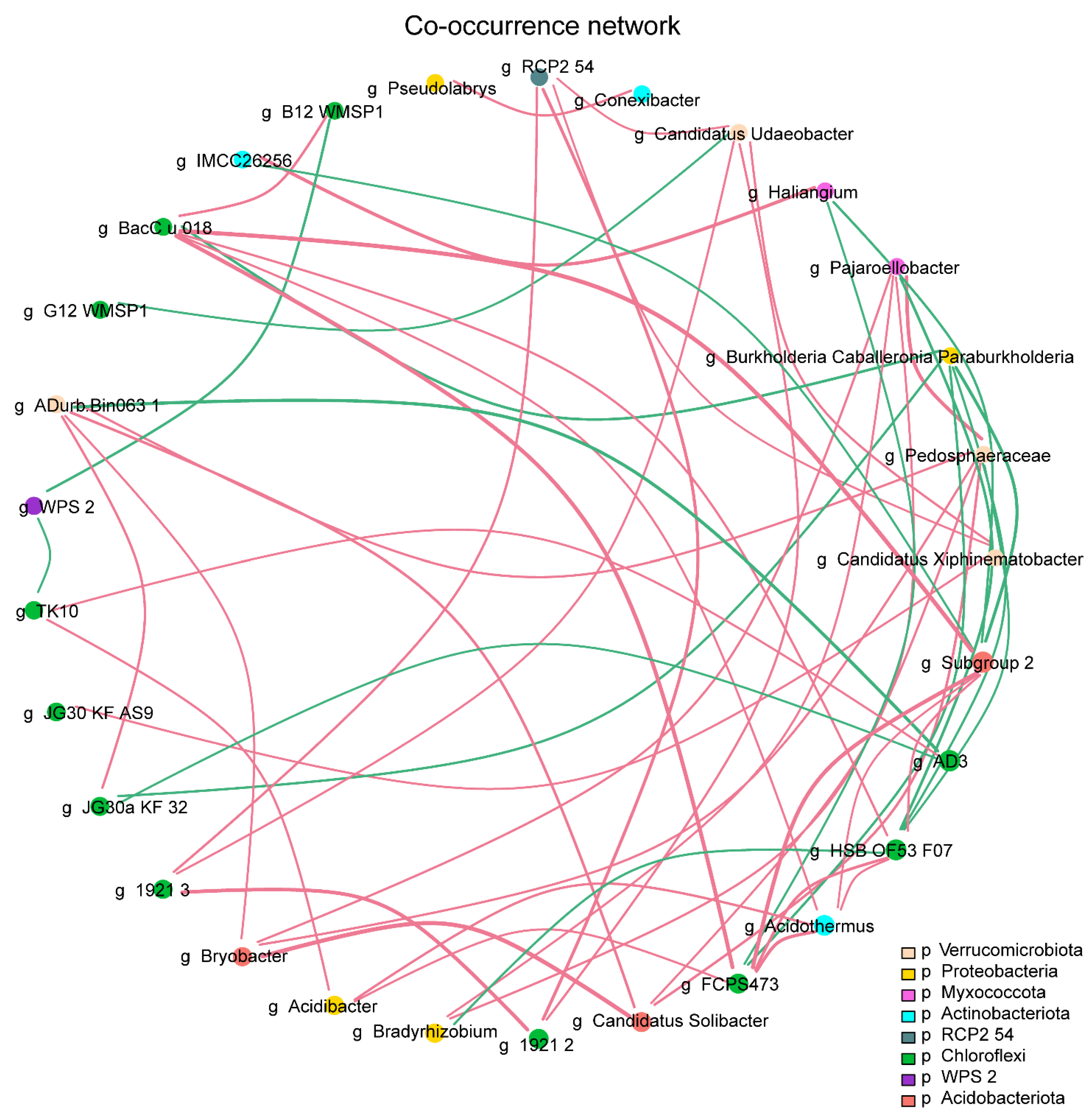
3.7. Network Associations Among Bacterial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukhopadhyay, M.; Mondal, T.K.; Chand, P.K. Biotechnological advances in tea (Camellia sinensis [L.] O. Kuntze): A review. Plant Cell Rep. 2016, 35, 255–287. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Tu, Y.; Zhang, G.; Xin, X.; Hu, H.; Qiu, W.; Ruan, D.; Zhao, Y. Mechanism of ultrasound and tea polyphenol assisted ultrasound modification of egg white protein gel. Ultrason. Sonochem. 2021, 81, 105857. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Shi, Y.; Yi, X.; Zhang, Q.; Fang, L.; Ma, L.; Ruan, J. Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agric. Ecosyst. Environ. 2018, 252, 74–82. [Google Scholar] [CrossRef]
- Yan, P.; Wu, L.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.; Zhang, L.; Fan, L.; Wenyan, H. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef]
- Ji, L.; Wu, Z.; You, Z.; Yi, X.; Ni, K.; Guo, S.; Ruan, J. Effects of organic substitution for synthetic N fertilizer on soil bacterial diversity and community composition: A 10-year field trial in a tea plantation. Agric. Ecosyst. Environ. 2018, 268, 124–132. [Google Scholar] [CrossRef]
- Liu, T.; Wang, S.; Chen, Y.; Luo, J.; Hao, B.; Zhang, Z.; Yang, B.; Guo, W. Bio-organic fertilizer promoted phytoremediation using native plant leymus chinensis in heavy Metal(loid)s contaminated saline soil. Environ. Pollut. 2023, 327, 121599. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Ma, D.; Wang, L.; Zhang, X.; Ding, Y.; Fan, K.; Xu, Z.; Yuan, C.; Jia, H.; et al. Differential responses of the rhizosphere microbiome structure and soil metabolites in tea (Camellia sinensis) upon application of cow manure. BMC Microbiol. 2022, 22, 55. [Google Scholar] [CrossRef]
- Hu, Z.; Ji, L.; Wan, Q.; Li, H.; Li, R.; Yang, Y. Short-Term Effects of Bio-Organic Fertilizer on Soil Fertility and Bacterial Community Composition in Tea Plantation Soils. Agronomy 2022, 12, 2168. [Google Scholar] [CrossRef]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, X.; Sui, M.; Jiang, G.; Xiao, J.; Wang, W.; Ning, Y. Situation and prospect of studying tea microorganisms. J. Huazhong Agric. Univ. 2022, 41, 24–32. [Google Scholar]
- Wang, Z.; Yang, T.; Mei, X.; Wang, N.; Li, X.; Yang, Q.; Dong, C.; Jiang, G.; Lin, J.; Xu, Y.; et al. Bio-Organic Fertilizer Promotes Pear Yield by Shaping the Rhizosphere Microbiome Composition and Functions. Microbiol. Spectr. 2022, 10, e0357222. [Google Scholar] [CrossRef]
- Li, S.; Fan, W.; Xu, G.; Cao, Y.; Zhao, X.; Hao, S.; Deng, B.; Ren, S.; Hu, S. Bio-organic fertilizers improve Dendrocalamus farinosus growth by remolding the soil microbiome and metabolome. Front. Microbiol. 2023, 14, 1117355. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Herrera, R.M.; Virgen-Calleros, G.; Ruiz-López, M.; Zañudo-Hernández, J.; Délano-Frier, J.P.; Sánchez-Hernández, C. Extracts from green and brown seaweeds protect tomato (Solanum lycopersicum) against the necrotrophic fungus Alternaria solani. J. Appl. Phycol. 2014, 26, 1607–1614. [Google Scholar] [CrossRef]
- Jayaraman, J.; Norrie, J.; Punja, Z.K. Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J. Appl. Phycol. 2011, 23, 353–361. [Google Scholar] [CrossRef]
- Najafi Vafa, Z.; Sohrabi, Y.; Mirzaghaderi, G.; Heidari, G. Soil Microorganisms and Seaweed Application With Supplementary Irrigation Improved Physiological Traits and Yield of Two Dryland Wheat Cultivars. Front. Plant Sci. 2022, 13, 855090. [Google Scholar] [CrossRef]
- Tursun, A.O. Effect of foliar application of seaweed (organic fertilizer) on yield, essential oil and chemical composition of coriander. PLoS ONE 2022, 17, e0269067. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS ONE 2019, 14, e0216710. [Google Scholar] [CrossRef]
- Goni, O.; Langowski, L.; Feeney, E.; Quille, P.; O’Connell, S. Reducing Nitrogen Input in Barley Crops While Maintaining Yields Using an Engineered Biostimulant Derived From Ascophyllum nodosum to Enhance Nitrogen Use Efficiency. Front. Plant Sci. 2021, 12, 664682. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glockner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; McGinnis, S.; Madden, T.L. BLAST: Improvements for better sequence analysis. Nucleic Acids Res. 2006, 34, W6–W9. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- van der Heijden, M.G.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Insam, H.; Gómez-Brandón, M.; Ascher, J. Manure-based biogas fermentation residues—Friend or foe of soil fertility? Soil Biol. Biochem. 2015, 84, 1–14. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, L.; Carswell, A.; Misselbrook, T.; Shen, J.; Han, J. Changes in soil organic carbon status and microbial community structure following biogas slurry application in a wheat-rice rotation. Sci. Total Environ. 2021, 757, 143786. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Wu, H.; Yan, S.; Guo, D.; Wang, G.; Ma, Y. Soil chemical and microbial responses to biogas slurry amendment and its effect on Fusarium wilt suppression. Appl. Soil Ecol. 2016, 107, 116–123. [Google Scholar] [CrossRef]
- Chao, Y.; Fu, G.; Yan, X.; Hang, Z.; Yang, Q.; Wang, H.; Pan, H.; Lou, Y.; Zhu, Y. Effects of Organic Fertilizer on Crop Quality, Soil Fertility and Environment: Research Progress. Chin. Agric. Sci. Bull. 2022, 38, 103–107. [Google Scholar] [CrossRef]
- Li, G.; Zhu, S.; Long, J.; Mao, H.; Dong, Y.; Hou, Y. Differences in microbial community structure and metabolic activity among tea plantation soils under different management strategies. Front. Microbiol. 2023, 14, 1219491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Sun, L.; Qiu, C.; Ding, Y.; Gu, H.; Wang, L.; Wang, Z.; Ding, Z. Organic mulching positively regulates the soil microbial communities and ecosystem functions in tea plantation. BMC Microbiol. 2020, 20, 103. [Google Scholar] [CrossRef]
- Mengesha, W.K.; Powell, S.M.; Evans, K.J.; Barry, K.M. Diverse microbial communities in non-aerated compost teas suppress bacterial wilt. World J. Microbiol. Biotechnol. 2017, 33, 49. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Liu, B.; Ni, K.; Ma, L.; Shi, Y.; Leng, Y.; Zheng, S.; Gao, S.; Yang, X.; Ruan, J. Rhizosphere Microbial Community Shows a Greater Response Than Soil Properties to Tea (Camellia sinensis L.) Cultivars. Agronomy 2023, 13, 221. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, W.; Xiao, H.; Zhai, Y.; Luo, Y.; Wang, Y.; Liu, Z.; Li, Q.; Huang, J. A Review on Rhizosphere Microbiota of Tea Plant (Camellia sinensis L): Recent Insights and Future Perspectives. J. Agric. Food Chem. 2023, 71, 19165–19188. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Z.; Wang, Y.; Li, J.; Wang, G.; Ye, J.; Wang, H.; He, H. Soil metagenomic analysis on changes of functional genes and microorganisms involved in nitrogen-cycle processes of acidified tea soils. Front. Plant Sci. 2022, 13, 998178. [Google Scholar] [CrossRef] [PubMed]
- Spieck, E.; Sass, K.; Keuter, S.; Hirschmann, S.; Spohn, M.; Indenbirken, D.; Kop, L.F.M.; Lucker, S.; Giaveno, A. Defining Culture Conditions for the Hidden Nitrite-Oxidizing Bacterium Nitrolancea. Front. Microbiol. 2020, 11, 1522. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Zheng, Z.; Chen, H.Y.H. Soil aggregate-associated bacterial metabolic activity and community structure in different aged tea plantations. Sci. Total Environ. 2019, 654, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, S.; Fan, L.C.; Guo, S.; Hu, Q.; Ahammed, G.J.; Yan, P.; Zhang, L.P.; Li, Z.Z.; Zhang, J.Y.; et al. Diversity in rhizospheric microbial communities in tea varieties at different locations and tapping potential beneficial microorganisms. Front. Microbiol. 2022, 13, 1027444. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, M.; Li, F.; Bai, Y.; Hou, J.; Li, X.; Cao, R.; Deng, Y.; Jiang, Y.; Wang, H.; et al. Changes in soil bacterial communities and functional groups beneath coarse woody debris across a subalpine forest successional series. Glob. Ecol. Conserv. 2023, 43, e02436. [Google Scholar] [CrossRef]
- Jibola-Shittu, M.Y.; Heng, Z.; Keyhani, N.O.; Dang, Y.; Chen, R.; Liu, S.; Lin, Y.; Lai, P.; Chen, J.; Yang, C.; et al. Understanding and exploring the diversity of soil microorganisms in tea (Camellia sinensis) gardens: Toward sustainable tea production. Front. Microbiol. 2024, 15, 1379879. [Google Scholar] [CrossRef]
- Zhang, N.; Tao, R.; Liu, P.; Hu, H.; Gao, L.; Guo, L.; Zhu, Z.; Ma, Y. Effects of organic fertilizer coupled with chemical fertilizer on growth and quality of tea and soil fertility. Acta Agric. Zhejiangensis 2023, 35, 1844–1852. [Google Scholar]
- Lin, S.; Zhuang, J.Q.; Chen, T.; Zhang, A.J.; Zhou, M.M.; Lin, W.X. Analysis of nutrient and microbial Biolog function diversity in teasoils with different planting years in Fujian Anxi. Chin. J. Eco-Agric. 2012, 20, 1471–1477. [Google Scholar] [CrossRef]
- Li, C.J.; Yang, W.H.; Zhou, B.Q.; Zhang, Y.; Lin, Y.; Xing, S.H. Effects of Biochar Based Fertilizer on Soil Nutrients, Tea Output and Quality in An Acidified Tea Field. Chin. J. Soil Sci. 2021, 52, 387–397. [Google Scholar] [CrossRef]
- Liu, W.; Cui, S.; Wu, L.; Qi, W.; Chen, J.; Ye, Z.; Ma, J.; Liu, D. Effects of Bio-organic Fertilizer on Soil Fertility, Yield, and Quality of Tea. J. Soil Sci. Plant Nutr. 2023, 23, 5109–5121. [Google Scholar] [CrossRef]
- Hug, L.A.; Castelle, C.J.; Wrighton, K.C.; Thomas, B.C.; Sharon, I.; Frischkorn, K.R.; Williams, K.H.; Tringe, S.G.; Banfield, J.F. Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling. Microbiome 2013, 1, 22. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Ye, J.; Li, Y.; Lin, Y.; Liu, C. Effects of different amount of biochar application on soil property and bacterial community structure in acidified tea garden. J. Plant Nutr. Fertil. 2020, 26, 1967–1977. [Google Scholar] [CrossRef]
- Chen, C.; Ai, J.; Chen, L.; Li, Y.; Tang, X.; Li, J. Nitrogen metabolism pathways and functional microorganisms in typical karst wetlands. Environ. Sci. Pollut. Res. Int. 2024, 31, 22494–22506. [Google Scholar] [CrossRef] [PubMed]
- Delmont, T.O.; Quince, C.; Shaiber, A.; Esen, O.C.; Lee, S.T.; Rappe, M.S.; McLellan, S.L.; Lucker, S.; Eren, A.M. Nitrogen-fixing populations of Planctomycetes and Proteobacteria are abundant in surface ocean metagenomes. Nat. Microbiol. 2018, 3, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, H.; Dai, H.; Xu, Z. Characterization of Plant-Growth-Promoting Rhizobacteria for Tea Plant (Camellia sinensis) Development and Soil Nutrient Enrichment. Plants 2024, 13, 2659. [Google Scholar] [CrossRef]
- Whitman, T.; Pepe-Ranney, C.; Enders, A.; Koechli, C.; Campbell, A.; Buckley, D.H.; Lehmann, J. Dynamics of microbial community composition and soil organic carbon mineralization in soil following addition of pyrogenic and fresh organic matter. ISME J. 2016, 10, 2918–2930. [Google Scholar] [CrossRef]
- Zhou, Z.; Tran, P.Q.; Kieft, K.; Anantharaman, K. Genome diversification in globally distributed novel marine Proteobacteria is linked to environmental adaptation. ISME J. 2020, 14, 2060–2077. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liang, J.; Shi, J.; Wang, S.; Lu, J. Effects of canopy damage on soil CO2 fixation bacterial community structure in Xiaokeng forest farm. Microbiol. China 2017, 44, 2297–2306. [Google Scholar]
- Zhang, R.; Fu, X.; Zhong, H.; Sui, X.; Liu, Y. Changes in Soil Bacterial Community and Function in Winter Following Long-Term Nitrogen (N) Deposition in Wetland Soil in Sanjiang Plain, China. Microorganisms 2023, 11, 2634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Rong, L.; Zhang, L. Soil nutrient variability mediates the effects of erosion on soil microbial communities: Results from a modified topsoil removal method in an agricultural field in Yunnan plateau, China. Environ. Sci. Pollut. Res. Int. 2022, 29, 3659–3671. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hu, F.; Abd Elbasit, M.A.M.; Zheng, F.; Liu, P.; Xiao, H.; Zhang, Q.; Zhang, J. Holocene erosion triggered by climate change in the central Loess Plateau of China. CATENA 2018, 160, 103–111. [Google Scholar] [CrossRef]
- Hancock, G.R.; Kunkel, V.; Wells, T.; Martinez, C. Soil organic carbon and soil erosion—Understanding change at the large catchment scale. Geoderma 2019, 343, 60–71. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.; Li, Y.; Wang, G.; Liu, J.; Liu, J.; Liu, X.; Jin, J. Microbial association with the dynamics of particulate organic carbon in response to the amendment of elevated CO(2)-derived wheat residue into a Mollisol. Sci. Total Environ. 2017, 607–608, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Qian, S.Y.; Pan, X.C.; Chen, Y.L.; Bai, S.B.; Xu, F. Effects of Simulated Acid Rain and Nitrogen Deposition on Soil Bacterial Community Structure and Diversity in the Masson Pine Forest. Huan Jing Ke Xue=Huanjing Kexue 2023, 44, 2315–2324. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Zhou, L.; Huang, X.; You, X.; Lin, J.; Han, H.; Huang, X. Effects of Salt Field Waste-Generated Bio-Organic Fertilizer Application on Bacterial Community Structure in Tea Plantations Rhizosphere Soil. Agronomy 2025, 15, 87. https://doi.org/10.3390/agronomy15010087
Yu C, Zhou L, Huang X, You X, Lin J, Han H, Huang X. Effects of Salt Field Waste-Generated Bio-Organic Fertilizer Application on Bacterial Community Structure in Tea Plantations Rhizosphere Soil. Agronomy. 2025; 15(1):87. https://doi.org/10.3390/agronomy15010087
Chicago/Turabian StyleYu, Chengran, Liuting Zhou, Xiaoyun Huang, Xiaofeng You, Jiali Lin, Haidong Han, and Xiusheng Huang. 2025. "Effects of Salt Field Waste-Generated Bio-Organic Fertilizer Application on Bacterial Community Structure in Tea Plantations Rhizosphere Soil" Agronomy 15, no. 1: 87. https://doi.org/10.3390/agronomy15010087
APA StyleYu, C., Zhou, L., Huang, X., You, X., Lin, J., Han, H., & Huang, X. (2025). Effects of Salt Field Waste-Generated Bio-Organic Fertilizer Application on Bacterial Community Structure in Tea Plantations Rhizosphere Soil. Agronomy, 15(1), 87. https://doi.org/10.3390/agronomy15010087




