Ornamental Traits and Sensory Analysis of ‘Biquinho Vermelha’ Pepper Treated with Paclobutrazol
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Crop Management
2.2. Experimental Design
2.3. Ornamental Quality Traits
2.4. Sensory Analysis
2.5. Statistical Analysis
3. Results
3.1. Ornamental Quality Traits
3.2. Sensory Analysis
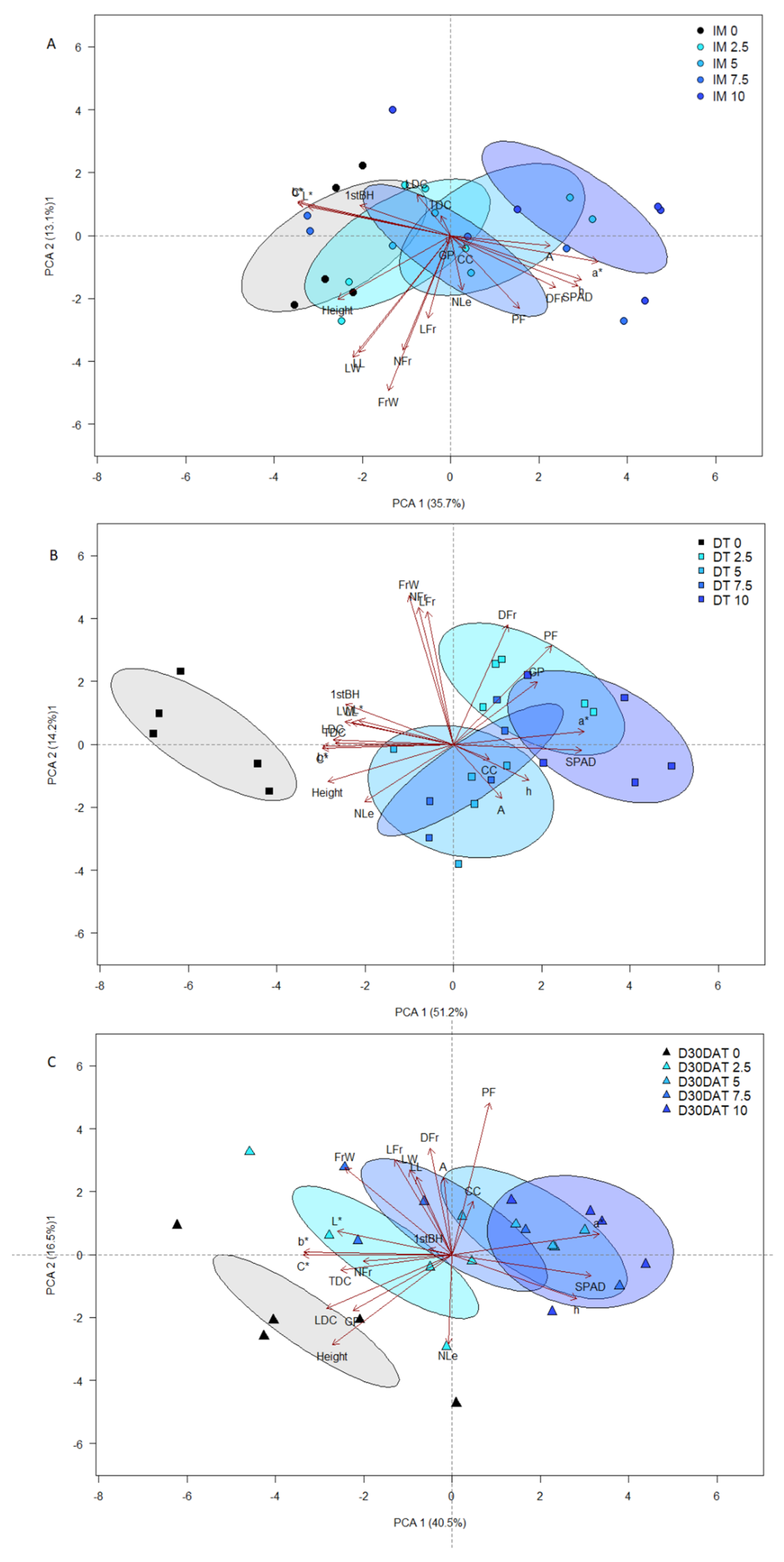
3.3. Principal Components Analysis (PCA)
4. Discussion
4.1. Ornamental Growth Traits
4.2. Ornamental Color Traits
4.3. Sensory Analysis
4.4. Interplay Between Ornamental Traits and Sensory Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Data Bridge Market Research. Global Flowers and Ornamental Plants Market—Industry Trends and Forecast to 2029. 2022. Available online: https://www.databridgemarketresearch.com/reports/global-flowers-and-ornamental-plants-market (accessed on 21 July 2024).
- Carvalho, S.I.C.; Bianchetti, L.B.; Ribeiro, C.S.C.; Lopes, C.A. Pimentas do Gênero Capsicum No Brasil, 1st ed.; EMBRAPA: Brasília, Brazil, 2006. [Google Scholar]
- Costa, L.; Ribeiro, W.; Pinto, C.; Silva, F.; Finger, F. Quality of ornamental pepper grown in different substrates. Acta Hortic. 2015, 1060, 243–248. [Google Scholar] [CrossRef]
- EMBRAPA. Cultivares da Embrapa Hortaliças (1981–2013), 1st ed.; EMBRAPA: Brasília, Brazil, 2014; p. 179. [Google Scholar]
- França, C.d.F.M.; Ribeiro, W.S.; Santos, M.N.S.; Petrucci, K.P.d.O.S.; Rêgo, E.R.D.; Finger, F.L. Growth and quality of potted ornamental peppers treated with paclobutrazol. Pesqui. Agropecu. Bras. 2018, 53, 316–322. [Google Scholar] [CrossRef]
- Rademacher, W. Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 501–531. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, C.D.S.; de Faria, R.T.; Ventura, M.U.; Vendrame, W. The effect of plant growth regulators on height control in potted Arundina graminifolia orchids (Growth regulators in Arundina graminifolia). Acta Sci. Agron. 2014, 36, 489. [Google Scholar] [CrossRef]
- Brito, C.L.; Matsumoto, S.N.; Santos, J.L.; Gonçalves, D.N.; Ribeiro, A.F. Effect of paclobutrazol in the development of ornamental sunflower. Rev. Ciênc. Agrár. 2016, 39, 153–160. [Google Scholar] [CrossRef]
- Carvalho-Zanão, M.P.; Júnior, L.A.Z.; Grossi, J.A.S.; Pereira, N. Potted rose cultivars with paclobutrazol drench applications. Cienc. Rural 2018, 48, e20161002. [Google Scholar] [CrossRef]
- Ribeiro, W.S.; Carneiro, C.d.S.; França, C.d.F.M.; Pinto, C.M.F.; Lima, P.C.C.; Finger, F.L.; da Costa, F.B. Paclobutrazol application in potted ornamental pepper. Hortic. Bras. 2019, 37, 464–468. [Google Scholar] [CrossRef]
- Sabino, J.H.F.; Grossi, J.A.S.; da Silva, T.I.; Verly, O.M.; Martins Filho, S.; Barbosa, J.G. Potted platycodon production in response to paclobutrazol. Pesqui. Agropecu. Trop. 2021, 51, e68949. [Google Scholar] [CrossRef]
- Cruz, R.R.P.; Pires, R.R.; Guimarães, M.E.D.S.; Dias, M.G.; Pereira, A.M.; da Silva, T.I.; Ribeiro, W.S.; Grossi, J.A.S. Initial growth of Calendula officinalis L. plants treated with paclobutrazol. Comun. Sci. 2022, 13, e3924. [Google Scholar] [CrossRef]
- Park, J.; Faust, J.E. Fertilization and Paclobutrazol Application for Sustainable Production and Post-production Performance of Petunia. HortTechnology 2023, 33, 225–232. [Google Scholar] [CrossRef]
- Currey, C.J.; Lopez, R.G. Applying Plant Growth Retardants for Height Control. 2009. Available online: https://www.extension.purdue.edu/extmedia/ho/ho-248-w.pdf (accessed on 5 June 2024).
- Mabvongwe, O.; Manenji, B.T.; Gwazane, M.; Chandiposha, M. The effect of paclobutrazol application time and variety on growth, yield, and quality of potato (Solanum tuberosum L.). Adv. Agric. 2016, 2016, 1585463. [Google Scholar] [CrossRef]
- França, C.d.F.M.; Da Costa, L.C.; Ribeiro, W.S.; Mendes, T.D.C.; Santos, M.N.D.S.; Finger, F.L. Evaluation of paclobutrazol application method on quality characteristics of ornamental pepper. Ornam. Hortic. 2017, 23, 307–310. [Google Scholar] [CrossRef]
- Grossi, J.S.; de Moraes, P.; Tinoco, S.d.A.; Barbosa, J.; Finger, F.; Cecon, P. Effects of paclobutrazol on growth and fruiting characteristics of ’Pitanga’ ornamental pepper. Acta Hortic. 2005, 683, 333–336. [Google Scholar] [CrossRef]
- Ferreira, T.d.S.; Pêgo, R.G.; Silva, K.A.L.; Xavier, M.C.G.; Carmo, M.G.F.D. Efeitos do Paclobutrazol na produção e qualidade de pimenteiras de vaso com potencial ornamental. DELOS Desarro. Local Sosten. 2023, 16, 1382–1401. [Google Scholar] [CrossRef]
- Furlani, P. Hydroponic vegetable production in Brazil. Acta Hortic. 1999, 481, 777–778. [Google Scholar] [CrossRef]
- Minolta Corp. Precise Color Communication: Color Control from Perception to Instrumentation. Konica Minolta Sensing, Inc. 2007. Available online: https://www.konicaminolta.com/instruments/knowledge/color/pdf/color_communication.pdf (accessed on 30 June 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 11 December 2023).
- Murdoch, D.J.; Chow, E.D. A graphical display of large correlation matrices. Am. Stat. 1996, 50, 178–180. [Google Scholar] [CrossRef]
- Bosch, E.; Cuquel, F.L.; Tognon, G.B. Physalis size reduction for potted ornamental plant use. Ciênc. Agrotec. 2016, 40, 555–564. [Google Scholar] [CrossRef]
- Santos Filho, F.B.; Silva, T.I.; Dias, M.G.; Alves, A.C.L.; Grossi, J.A.S. Paclobutrazol reduces growth and increases chlorophyll indices and gas exchanges of basil (Ocimum basilicum). Braz. J. Biol. 2022, 82, e262364. [Google Scholar] [CrossRef]
- Bañón, D.; Ortuño, M.F.; Sánchez-Blanco, M.J.; Pagán, B.L.; Bañón, S. Effects of Paclobutrazol and Mepiquat Chloride on the Physiological, Nutritional, and Morphological Behavior of Potted Icterina Sage under Greenhouse Conditions. Agronomy 2023, 13, 2161. [Google Scholar] [CrossRef]
- Kurniawati, A.; Krisantini, K.; Firdausa, N.P.; Suketi, K. Effect of growth regulator paclobutrazol on size fitting of basil as a potted plant. Ornam. Hortic. 2023, 29, 7–13. [Google Scholar] [CrossRef]
- Tellez, H.O.; Bonfim, G.V.D.; de Carvalho, A.C.P.P.; de Azevedo, B.M. Use of paclobutrazol and ethylene in the potted production of ornamental pineapple. Ornam. Hortic. 2023, 29, 48–56. [Google Scholar] [CrossRef]
- Ribeiro, C.S.C.; Lopes, C.A.; Carvalho, S.I.C.; Henz, G.P.; Reifschneider, F.J.B. Pimentas Capsicum, 1st ed.; Embrapa Hortaliças: Brasília, Brazil, 2008; p. 202. [Google Scholar]
- Ahmad, I.; Dole, J.M.; Whipker, B.E. Paclobutrazol or uniconazole effects on ethylene sensitivity of potted ornamental plants and plugs. Sci. Hortic. 2015, 192, 350–356. [Google Scholar] [CrossRef]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Chursi, O.; Kozai, N.; Ogata, T.; Higuchi, H.; Yonemoto, Y. Application of Paclobutrazol for Flowering and Fruit Pro-duction of ‘Irwin’ Mango (Mangifera indica L.) in Okinawa. Trop. Agric. Dev. 2008, 52, 69–73. [Google Scholar] [CrossRef]
- Burondkar, M.M.; Rajan, S.; Upreti, K.K.; Reddy, Y.T.N.; Singh, V.K.; Sabale, S.N.; Naik, M.M.; Nigade, P.M.; Saxena, P. Advancing Alphonso mango harvest season in lateritic rocky soils of Konkan region through manipulation in time of paclo-butrazol application. J. Appl. Hortic. 2013, 15, 178–182. [Google Scholar] [CrossRef]
- Upreti, K.K.; Reddy, Y.; Prasad, S.S.; Bindu, G.; Jayaram, H.; Rajan, S. Hormonal changes in response to paclobutrazol induced early flowering in mango cv. Totapuri. Sci. Hortic. 2013, 150, 414–418. [Google Scholar] [CrossRef]
- Silva, L.d.S.; Cavalcante, H.L.; da Cunha, J.G.; Lobo, J.T.; Carreiro, D.A.; Neto, V.B.d.P. Organic acids allied with paclobutrazol modify mango tree ‘Keitt’ flowering. Rev. Bras. Frutic. 2022, 44, e003. [Google Scholar] [CrossRef]
- Rahman, H.; Rahman, H.; Halder, B.C.; Ahmed, M.; Nishi, N.J. Applying Paclobutrazol and Flower Bud Pruning Modify the Fruiting Time and Fruit Quality of ‘Amrapali’ Mango (Mangifera indica L.). Hortic. J. 2023, 92, 255–260. [Google Scholar] [CrossRef]
- Huang, S.; Luo, H.; Ashraf, U.; Abrar, M.; He, L.; Zheng, A.; Wang, Z.; Zhang, T.; Tang, X. Seed treatment with paclobutrazol affects early growth, photosynthesis, chlorophyll fluorescence and physiology of rice. Appl. Ecol. Environ. Res. 2019, 17, 999–1012. [Google Scholar] [CrossRef]
- Neitzke, R.S.; Fischer, S.Z.; Vasconcelos, C.S.; Barbieri, R.L.; Treptow, R.O. Pimentas ornamentais: Recepção e comportamento do público consumidor. Hortic. Bras. 2016, 34, 102–109. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Amarante, C.V.T.D.; Steffens, C.A.; Mafra, L.; Albuquerque, J.A. Yield and fruit quality of apple orchards under conventional and organic production systems. Pesqui. Agropecu. Bras. 2008, 43, 333–340. [Google Scholar] [CrossRef]

| Ornamental Quality Traits | PBZ | AP | PBZ X AP |
|---|---|---|---|
| Plant height | * | * | * |
| First bifurcation height | * | * | * |
| Canopy longitudinal diameter | * | * | * |
| Canopy transverse diameter | NS | * | * |
| Canopy compactness | * | * | NS |
| Fruit number | * | * | NS |
| Leave number | NS | * | NS |
| Fruit diameter | NS | * | NS |
| Fruit length | * | * | NS |
| Shoot fresh weight | * | * | NS |
| SPAD Index | * | * | * |
| Plant fullness | * | * | * |
| Leaf length | * | * | * |
| Leaf width | * | * | * |
| Days to anthesis | * | * | NS |
| Lightness (L*) | * | NS | NS |
| Red/green coordinate (a*) | * | * | * |
| Yellow/blue coordinate (b*) | * | * | * |
| Chroma (C*) | * | * | * |
| Hue angle (h°) | * | NS | NS |
| Ornamental Quality Traits | Application Protocol | PBZ Concentrations (mg L−1) | CV (%) | ||||
|---|---|---|---|---|---|---|---|
| 0 | 2.5 | 5.0 | 7.5 | 10.0 | |||
| Plant height (cm) | IM | 34.8 Ba | 32.8 Aab | 29.9 Aab | 30.7 Aab | 26.3 Ab | |
| DT | 44.4 Aa | 20.6 Bbc | 25.5 ABb | 26.5 ABb | 14.3 Bc | 16.2 | |
| D30DAT | 31.4 Ba | 25.1 Bab | 19.0 Bb | 20.3 Bb | 17.7 Bb | ||
| First bifurcation height (cm) | IM | 8.1 Aa | 7.8 ABa | 6.8 ABa | 7.6 ABa | 6.1 Ba | |
| DT | 9.2 Aa | 6.4 Bb | 5.2 Bb | 5.7 Bb | 4.1 Bb | 19.2 | |
| D30DAT | 8.5 Aa | 8.9 Aa | 8.0 Aa | 8.0 Aa | 8.4 Aa | ||
| SPAD chlorophyll Index | IM | 52.6 Aa | 56.6 Ba | 57.2 Aa | 55.6 Ba | 58.8 Ba | |
| DT | 51.3 Ac | 67.0 Aab | 62.8 Ab | 65.8 Aab | 72.1 Aa | 6.9 | |
| D30DAT | 53.6 Ab | 53.9 Bab | 60.0 Aab | 56.9 Bab | 60.9 Ba | ||
| Plant fullness (g·cm−1) | IM | 3.3 Aa | 3.5 Ba | 3.6 ABa | 3.7 ABa | 3.7 Ba | |
| DT | 2.2 Ab | 5.0 Aa | 3.0 Bb | 3.1 Bb | 6.0 Aa | 20.4 | |
| D30DAT | 2.9 Ab | 4.0 ABab | 4.7 Aa | 4.8 Aa | 4.5 Ba | ||
| Canopy longitudinal diameter (cm) | IM | 43.6 Aa | 38.6 Aa | 39.0 Aa | 43.1 Aa | 36.2 Aa | |
| DT | 45.3 Aa | 30.2 Bb | 30.5 Bb | 30.4 Bb | 22.6 Bb | 14.7 | |
| D30DAT | 34.10 Ba | 29.3 Bab | 25.4 Bb | 25.2 Bb | 24.4 Bb | ||
| Canopy transverse diameter (cm) | IM | 24.9 Ba | 30.3 Aa | 29.4 Aa | 35.0 Aa | 29.1 Aa | 19.3 |
| DT | 34.4 Aa | 22.0 Bab | 26.4 Ab | 25.3 Bb | 20.8 Bb | ||
| D30DAT | 28.4 ABa | 23.7 ABa | 22.7 Aa | 22.4 Ba | 21.7 ABa | ||
| Leaf lenght (mm) | IM | 59.0 Aa | 57.2 Aab | 53.1 ABab | 55.4 ABab | 50.7 Bb | 6.9 |
| DT | 56.0 Aa | 48.6 Bb | 48.2 Bb | 50.4 Bab | 47.2 Bb | ||
| D30DAT | 55.6 Aa | 61.6 Aa | 57.9 Aa | 56.5 Aa | 57.4 Aa | ||
| Leaf width (mm) | IM | 39.0 Aa | 35.5 Bab | 33.3 Bb | 36.3 Aab | 32.1 Bb | 7.9 |
| DT | 36.1 Aa | 31.0 Cb | 30.4 Bb | 31.6 Bab | 29.1 Bb | ||
| D30DAT | 38.3 Aa | 41.1 Aa | 39.1 Aa | 39.1 Aa | 39.7 Aa | ||
| Chroma (C*) | IM | 19.4 ABa | 19.3 Aa | 17.2 Aa | 17.8 Aa | 16.4 Aa | 11.0 |
| DT | 21.5 Aa | 13.0 Bbc | 15.4 ABb | 15.4 Ab | 11.6 Bc | ||
| D30DAT | 18.4 Ba | 16.6 Bab | 14.0 Bb | 15.8 Aab | 13.6 Bb | ||
| PBZ (mg L−1) | L* | h° | CC | FrW (g) | A | NFr | LFr (mm) |
|---|---|---|---|---|---|---|---|
| 0 | 38.8 a | 114.8 b | 0.7 b | 101.2 ab | 38.1 c | 67.9 a | 22.5 ab |
| 2.5 | 37.1 b | 115.9 a | 0.8 ab | 103.3 a | 39.9 bc | 64.0 a | 23.5 a |
| 5.0 | 37.0 b | 116.1 a | 0.8 ab | 89.6 ab | 41.1 ab | 56.3 a | 21.8 ab |
| 7.5 | 37.7 ab | 115.5 ab | 0.9 a | 95.1 ab | 42.4 a | 64.7 a | 21.7 ab |
| 10.0 | 36.7 b | 116.3 a | 0.9 a | 86.1 b | 41.5 ab | 56.5 a | 21.0 b |
| CV (%) | 3.2 | 0.9 | 14.0 | 15.8 | 5.6 | 19.7 | 9.7 |
| Aplication Protocol | CC | A | NFr | LFr (mm) | DFr (mm) | NLe | FrW (g) |
|---|---|---|---|---|---|---|---|
| IM | 0.7 b | 39.6 b | 69.7 a | 22.7 a | 12.5 a | 118.5 ab | 108.1 a |
| DT | 0.8 a | 41.7 a | 53.7 b | 20.9 b | 13.0 a | 128.2 a | 85.4 b |
| D30DAT | 0.9 a | 41.0 ab | 62.2 a | 22.7 a | 11.2 b | 101.3 b | 91.6 b |
| CV (%) | 14.0 | 5.6 | 19.7 | 9.7 | 10.1 | 23.0 | 15.8 |
| Application Protocol | PBZ (mg L−1) | Global Preference |
|---|---|---|
| Immersion | 0 | 4.82 b |
| 2.5 | 5.18 a | |
| 5.0 | 5.54 a | |
| 7.5 | 4.89 b | |
| 10.0 | 4.70 c | |
| Drenching at transplanting | 0 | 4.02 d |
| 2.5 | 4.85 b | |
| 5.0 | 4.14 d | |
| 7.5 | 4.63 c | |
| 10.0 | 4.42 c | |
| Drenching at 30DAT | 0 | 5.42 a |
| 2.5 | 5.33 a | |
| 5.0 | 5.03 b | |
| 7.5 | 4.78 b | |
| 10.0 | 4.36 c | |
| CV (%) | 27.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, B.R.; Costa, L.C.; Verruma-Bernardi, M.R.; Rodrigues, J.; Sala, F.C.; Finger, F.L.; França, C.F.M. Ornamental Traits and Sensory Analysis of ‘Biquinho Vermelha’ Pepper Treated with Paclobutrazol. Agronomy 2025, 15, 75. https://doi.org/10.3390/agronomy15010075
Morales BR, Costa LC, Verruma-Bernardi MR, Rodrigues J, Sala FC, Finger FL, França CFM. Ornamental Traits and Sensory Analysis of ‘Biquinho Vermelha’ Pepper Treated with Paclobutrazol. Agronomy. 2025; 15(1):75. https://doi.org/10.3390/agronomy15010075
Chicago/Turabian StyleMorales, Beatriz R., Lucas C. Costa, Marta R. Verruma-Bernardi, Josiane Rodrigues, Fernando C. Sala, Fernando L. Finger, and Christiane F. M. França. 2025. "Ornamental Traits and Sensory Analysis of ‘Biquinho Vermelha’ Pepper Treated with Paclobutrazol" Agronomy 15, no. 1: 75. https://doi.org/10.3390/agronomy15010075
APA StyleMorales, B. R., Costa, L. C., Verruma-Bernardi, M. R., Rodrigues, J., Sala, F. C., Finger, F. L., & França, C. F. M. (2025). Ornamental Traits and Sensory Analysis of ‘Biquinho Vermelha’ Pepper Treated with Paclobutrazol. Agronomy, 15(1), 75. https://doi.org/10.3390/agronomy15010075






