The Influence of the Farming System and Forecrop on the Yield and Chemical and Health-Promoting Composition of Spring Wheat Grain
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Field Management
- -
- sowing: sugar beet 19–23.04; spring barley 19–22.04; red clover 15–18.04; winter wheat 22–25.09; oat 12–15.04.
- -
- harvesting: sugar beet 17–20.10; spring barley 11–13.08; red clover 21–23.08; winter wheat 10–12.08; oat 19–21.08.
2.2. Plant Sampling, Measurement, and Chemical Analyses of Spring Wheat Grain
- -
- Nitrogen determinations were made in grains extracted from collected ear samples by the Kjeldahl procedure, while crude protein content was calculated using the factor N × 5.3. The sample is digested in sulfuric acid, using CuSO4/TiO2 as catalysts, converting N to NH3, which is distilled and titrated [40].
- -
- The amino acids were determined by HPLC using an automatic amino acid analyzer (AAA400; Ingos, Prague, Czech Republic) after previous acid hydrolysis with 6 M HCl for 24 h at 110 °C (method 994.12). Cysteine and methionine were determined after oxidative hydrolysis. Ion exchange chromatography was used to separation of amino acids using Tessek Ostion LG ANB (0.37 × 45 cm) column. Amino acid recognition was done by means of a photometric detector at a wavelength of 570 nm except for proline—440 nm [41,42].
- -
- For the determination of sulfur amino acids, the feed samples were oxidized (0 °C, 16 h) with formic acid and hydrogen peroxide (H2O2) (9:1/v:v) prior to HCl hydrolysis and then were separated using an Analysator AAA 400 Ingos (Prague, Czech Republic). For tryptophan content, the samples, after alkaline hydrolysis with lithium hydroxide (LiOH) (110 °C, 16 h) and 4-dimetylamino-benzaldehyde (DMAB), were examined colorimetrically at a wave length of 590 nm according to the Landry and Delhaye (1992) procedure [43].
- -
- Total dihydroxyphenol content was measured spectrophotometrically at a wavelength of λ = 725 nm (Shimadzu 1800 spectrophotometer, Shimadzu Corp. Kyoto, Japan) and expressed as caffeic acid equivalents. To make the measurement on the spectrophotometer, 50–500 µL of the extract (depending on the expected value of absorption of the tested sample) was transferred into a volumetric flask. A total of 2.0 mL methanol, 10 mL H2O, 2 mL Folin reagent, and 1.0 mL of a 10% solution of Na2CO3 were added. After half an hour samples were made up with deionized water up to the mark and measured on a spectrophotometer at a wavelength of λ = 725 nm in relation to the control sample [44].
- -
- The determination of total dietary fiber content was done by the enzymatic gravimetric method using a FOSS Fibertec 2010 system. The sample was digested with 3 enzymes: thermostable alpha-amylase, pepsin, and pancreatin. The undigested residue was weighed and then the supernatant of soluble dietary fiber was precipitated and weighed [45]. The mineral analysis of the isolate to determine Ca, Mg, Mn, Cu, Zn, Fe, Se (absorption—Varian lamp), and K (emission—without lamp) was performed by atomic absorption spectrometry in acetylene-air flame (Varian Spectra A 280 FS).
- -
- Essential (exogenous) Amino Acid Index (EAAI) was calculated as the geometric mean of all participating exogenous amino acids compared to the concentration of these amino acids in the egg reference protein using the following formula [46]:
2.3. Statistical Analyses
3. Results
4. Discussion
4.1. The Influence of the Farming System and Forecrop on the Yield of Spring Wheat
4.2. The Influence of the Farming System and Forecrop on the Quality of Spring Wheat Grain
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Munjal, R.; Dhanda, S.S. Assessment of drought resistance in Indian wheat cultivars for morpho-physiological traits. Ekin J. Crop Breed. Genet. 2016, 2, 74–81. [Google Scholar]
- Łaba, S.; Cacak-Pietrzak, G.; Łaba, R.; Sułek, A.; Szczepański, K. Food Losses in Consumer Cereal Production in Poland in the Context of Food Security and Environmental Impact. Agriculture 2022, 12, 665. [Google Scholar] [CrossRef]
- FAOSTAT. Agricultural Production Statistics 2000–2022. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 17 November 2024).
- Eurostat. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_crops#Cereals (accessed on 3 November 2024).
- Anon. How to Feed the World in 2050; High-level Experts Forum; FAO: Rome, Italy, 2009; p. 35. Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 5 November 2024).
- Porter, J.R.; Semenov, M.A. Crop responses to climatic variation. Phil. Transact. R. Soc. B-Biol. Sci. 2005, 360, 2021–2035. [Google Scholar] [CrossRef] [PubMed]
- Baublis, A.J.; Lu, C.; Clydesdale, F.M.; Decker, E.A. Potential of wheat-based cereals as a source of dietary antioxidants. J. Am. Coll. Nutr. 2000, 19, 308S–311S. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Swieca, M.; Dziki, D. Comparison of phenolic acids profile and antioxidant potential of six varieties of spelt (Triticum spelta L.). J. Agric. Food Chem. 2012, 60, 4603–4612. [Google Scholar] [CrossRef] [PubMed]
- Erenstein, O.; Poole, N.; Donovan, J. Role of staple cereals in human nutrition: Separating the wheat from the chaff in the infodemics age. Trends Food Sci. Technol. 2022, 119, 508–513. Available online: https://hdl.handle.net/10883/21937 (accessed on 5 November 2024). [CrossRef]
- Sułek, A.; Cacak-Pietrzak, G. The influence of production technology on yield selected quality parameters of spring wheat cultivars. Agric. Sci. Crop Sci. Anim. Sci. Res. Rural Dev. 2018, 2, 42–48. [Google Scholar] [CrossRef]
- Thorwarth, P.; Piepho, H.P.; Zhao, Y.; Ebmeyer, E.; Schacht, J.; Schachschneider, R.; Kazman, E.; Rief, J.C.; Würschum, T.; Longin, C.F.H. Higher grain yield and higher grain protein deviation underline the potential of hybrid wheat for a sustainable agriculture. Plant Breed. 2018, 137, 326–337. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B.L.; Fan, J.J.; Sun, M.; Yi, Y.; Guo, W.S.; Voldeng, H.D. Management of nitrogen fertilization to balance reducing lodging risk and increasing yield and protein content in spring wheat. Field Crops Res. 2019, 241, 107584. [Google Scholar] [CrossRef]
- Reeds, P.J. Dispensable and indispensable amino acids for humans. J. Nutri. 2000, 130, 1835–1840. [Google Scholar] [CrossRef]
- Chernova, E.; Bazhenova, I.; Bazhenova, T. Development of the composition of cereal dishes of higher biological value. BIO Web Conf. EDP Sci. 2021, 29, 01022. [Google Scholar] [CrossRef]
- FAO; WHO. Protein Quality Evaluation: Report of a Joint FAO-WHO Expert Consultation; FAO: Rome, Italy, 1991; Volume 51, Available online: https://blog.priceplow.com/wp-content/uploads/fao-who-protein-quality-evaluation-1991.pdf (accessed on 5 November 2024).
- Rozbicki, J.; Ceglińska, A.; Gozdowski, D.; Jakubczyk, M.; Cacak-Pietrzak, G.; Mądry, W.; Golba, J.; Piechociński, M.; Sobczyński, G.; Stadnicki, M.; et al. Influence of the cultivar, environment and management on the grain yield and bread-making quality in winter wheat. J. Cer. Sci. 2015, 61, 126–132. [Google Scholar] [CrossRef]
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Dupont, F.M.; Altenbach, S.B. Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis. J. Cereal Sci. 2003, 38, 133–146. [Google Scholar] [CrossRef]
- Gooding, M.J.; Ellis, R.H.; Shewry, P.R.; Schofield, J.D. Effects of restricted water availability and increased temperature on the grain filling, drying and quality of winter wheat. J. Cereal Sci. 2003, 37, 295–309. [Google Scholar] [CrossRef]
- Hoffman, B.; Burcus, Z. Adaptation of wheat (Triticum aestivum) genotypes and related species to water deficiency. Cereal Res. Commun. 2005, 33, 681–686. [Google Scholar] [CrossRef]
- Kaur, V.; Behl, R.K.B. Grain Yield in Wheat as Affected by Short Periods of High Temperature, Drought and their Interaction during Pre- and Post-anthesis Stages. Cereal Res. Commun. 2010, 38, 514–520. [Google Scholar] [CrossRef]
- Sieling, K.; Stahl, C.; Winkelmann, C.; Christen, O. Growth and yield of winter wheat in the first 3 years of a monoculture under varying N fertilization in NW Germany. Eur. J. Agron. 2005, 22, 71–84. [Google Scholar] [CrossRef]
- Bailey, K.L.; Gossen, B.D.; Lafond, G.P.; Watson, P.R.; Derksen, D.A. Effect of tillage and crop rotation on root and foliar diseases of wheat and pea in Saskatchewan from 1991 to 1998: Univariate and multivariate analyses. Can. J. Plant Sci. 2001, 81, 789–803. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Ghorbani, M.; Krexner, T.; Hörtenhuber, S.J.; Bernas, J.; Neugschwandtner, R.W.; Konvalina, P.; Moudrý, J. Life cycle assessment of biochar and cattle manure application in sugar beet cultivation—Insights into root yields, white sugar quality, environmental aspects in field and factory phases. J. Clean. Prod. 2024, 476, 143772. [Google Scholar] [CrossRef]
- Kulig, B.; Lepiarczyk, A.; Oleksy, A.; Kołodziejczyk, M. The effect of tillage system and forecrop on the yield and values of LAI and SPAD indices of spring wheat. Eur. J. Agron. 2010, 33, 43–51. [Google Scholar] [CrossRef]
- Wanic, M.; Treder, K.; Denert, M. Effect of forecrops on the yield and quality of common wheat and spelt wheat grain. J. Elem. 2019, 24, 369–383. [Google Scholar] [CrossRef]
- Jaroszewska, A.; Stankowski, S.; Tomaszewicz, T.; Gibczyńska, M. Analysis of the multi-annual effect of tillage systems and forecrops on texture and physical properties of soil. Agron. Sci. 2024, 79, 83–93. [Google Scholar] [CrossRef]
- Krejčířová, L.; Capouchova, I.; Petr, J.; Bicanová, E.; Faměra, O. The effect of organic and conventional growing systems on quality and storage protein composition of winter wheat. Plant Soil. Environ. 2007, 53, 499–505. [Google Scholar] [CrossRef]
- Tomczyńska-Mleko, M.; Kwiatkowski, C.A.; Harasim, E.; Leśniowska-Nowak, J.; Mleko, S.; Terpiłowski, K.; Pérez-Huertas, S.; Klikocka-Wiśniewska, O. Influence of Farming System and Forecrops of Spring Wheat on Protein Content in the Grain and the Physicochemical Properties of Unsonicated and Sonicated Gluten. Molecules 2022, 27, 3926. [Google Scholar] [CrossRef]
- Mitura, K.; Cacak-Pietrzak, G.; Feledyn-Szewczyk, B.; Szablewski, T.; Studnicki, M. Yield and Grain Quality of Common Wheat (Triticum aestivum L.) Depending on the Different Farming Systems (Organic vs. Integrated vs. Conventional). Plants 2023, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Ciołek, A.; Makarska, E.; Wesołowski, M.; Cierpiała, R. Content of selected nutrients in wheat, barley and oat grain from organic and conventional farming. J. Elem. 2012, 2, 181–189. [Google Scholar] [CrossRef]
- Wilbois, K.-P.; Schmidt, J.E. Reframing the Debate Surrounding the Yield Gap between Organic and Conventional Farming. Agronomy 2019, 9, 82. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E.; Pawłowski, L.; Pawłowski, A.; Pawłowska, M.; Kołodziej, B. Organic Versus Conventional Farming: Nutritional Value and Health Safety of Food Products, 1st ed.; Routledge: London, UK, 2023. [Google Scholar] [CrossRef]
- Kuś, J.; Jończyk, K.; Stalenga, J.; Feledyn-Szewczyk, B.; Mróz, A. Yielding of selected spring wheat varieties in organic and integrated cultivation. J. Res. Appl. Agric. Eng. 2011, 56, 18–23, (In Polish, Abstract In English). [Google Scholar]
- Feledyn-Szewczyk, B.; Kuś, J.; Jończyk, K.; Stalenga, J. The suitability of different winter and spring wheat varieties for cultivation in organic farming. In Organic Agriculture Towards Sustainability; Pilipavicius, V., Ed.; InTech: Rijeka, Croatia, 2014; Volume 9, pp. 197–225. [Google Scholar] [CrossRef]
- Mäder, P.; Hahn, D.; Dubois, D.; Gunst, L.; Alföldi, T.; Bergmann, H.; Oehme, M.; Amadó, R.; Schneider, H.; Graf, U.; et al. Wheat quality in organic and conventional farming: Results of 21 year field experiment. J. Sci. Food Agric. 2007, 87, 1826–1835. [Google Scholar] [CrossRef]
- Żuchowski, J.; Jończyk, K.; Pecio, Ł.; Oleszek, W. Phenolic acid concentrations in organically and conventionally cultivated spring and winter wheat. J. Sci. Food Agric. 2011, 91, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Mazzoncini, M.; Antichi, D.; Tavarini, S.; Silvestri, N.; Lazzeri, L.; D’Avino, L. Effect of defatted oilseed meals applied as organic fertilizers on vegetable crop production and environmental impact. Ind. Crops Prod. 2015, 75 Pt A, 54–64. [Google Scholar] [CrossRef]
- WRB IUSS Working Group. World Reference Base for Soil Resources 2014. Update 2015. International Soil Classification SystemforNaming Soils and Creating Legends for Soil Maps. In World Soil Resources Reports; FAO: Rome, Italy, 2015. [Google Scholar]
- ISO 5983-1:2005; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content. Part 1: Kjeldahl Method. International Organization for Standarization: Geneva, Switzerland, 2005.
- Bütikofer, U.; Fuchs, D.; Bosset, J.O.; Gmür, W. Automated HPLC-amino acid determination of protein hydrolysates by precolumn derivatization with OPA and FMOC and comparison with classical ion exchange chromatography. Chromatographia 1991, 31, 441–447. [Google Scholar] [CrossRef]
- Csomós, E.; Simon-Sarkadi, L. Characterisation of Tokaj wines based on free amino acids and biogenic amines using ion-exchange chromatography. Chromatographia 2002, 56, S185–S188. [Google Scholar] [CrossRef]
- Landry, J.; Delhaye, S. Determination of tryptophan in feedstuffs—Comparison of two methods of hydrolysis prior to HPLC analysis. J. Sci. Food Agric. 1992, 58, 438–441. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. Available online: http://www.ajevonline.org/content/16/3/144.full.pdf+html (accessed on 7 October 2024). [CrossRef]
- McCleary, B.; Devries, J.W.; Rader, J.; Cohen, G.; Prosky, L. Determination of total dietary fiber (CODEX definition) by Enzymatic-Gravimetric method and liquid chromatography. J. AOAC Int. 2010, 93, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Oser, B.L. Method for integrating essential amino acid content in the nutritional evaluation of protein. J. Am. Diet. Assoc. 1951, 27, 396. [Google Scholar] [CrossRef] [PubMed]
- Cox, W.; Cherney, J.; Sorrells, M. Organic Compared with Conventional Wheat Had Competitive Yields during the Early Years of Organic Production in the Northeast USA. Agronomy 2019, 9, 380. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Jończyk, K.; Stalenga, J. The Effect of Crop Production Systems and Cultivars on Spring Wheat (Triticum aestivum L.) Yield in a Long-Term Experiment. Agriculture 2024, 14, 625. [Google Scholar] [CrossRef]
- Verdi, L.; Dalla Marta, A.; Falconi, F.; Orlandini, S.; Mancini, M. Comparison between organic and conventional farming systems using Life Cycle Assessment (LCA): A case study with an ancient wheat variety. Eur. J. Agron. 2022, 141, 1266. [Google Scholar] [CrossRef]
- van Stappen, F.; Loriers, A.; Mathot, M.; Planchon, V.; Stilmant, D.; Debode, F. Organic Versus Conventional Farming: The Case of wheat Production in Wallonia (Belgium). Agric. Agric. Sci. Procedia 2015, 7, 272–279. [Google Scholar] [CrossRef]
- Döring, T.F.; Neuhoff, D. Upper limits to sustainable organic wheat yields. Sci. Rep. 2021, 11, 12729. [Google Scholar] [CrossRef]
- Paunescu, R.A.; Bonciu, E.; Rosculete, E.; Paunescu, G.; Rosculete, C.A. The Effect of Different Cropping Systems on Yield, Quality, Productivity Elements, and Morphological Characters in Wheat (Triticum aestivum). Plants 2023, 12, 2802. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, G.; Wang, C.; Lu, H.; Li, S.; Xie, Y.; Ma, D.; Zhu, Y.; Guo, T. Effect of irrigation and nitrogen application on grain amino acid composition and protein quality in winter wheat. PLoS ONE 2017, 12, e0178494. [Google Scholar] [CrossRef] [PubMed]
- Haliniarz, M.; Nowak, A.; Woźniak, A.; Sekutowski, T.R.; Kwiatkowski, C.A. Production and Economic Effects of Environmentally Friendly Spring Wheat Production Technology. Pol. J. Environ. Stud. 2018, 27, 1523–1532. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Buczek, J.; Kwiatkowski, C.A.; Harasim, E. The Course of Physiological Processes, Yielding, and Grain Quality of Hybrid and Population Wheat as Affected by Integrated and Conventional Cropping Systems. Agronomy 2022, 12, 1345. [Google Scholar] [CrossRef]
- Kubar, M.S.; Zhang, Q.; Feng, M.; Wang, C.; Yang, W.; Kubar, K.A.; Riaz, S.; Gul, H.; Samoon, H.A.; Sun, H.; et al. Growth, Yield and Photosynthetic Performance of Winter Wheat as Affected by Co-Application of Nitrogen Fertilizer and Organic Manures. Life 2022, 12, 1000. [Google Scholar] [CrossRef] [PubMed]
- Katamadze, A.; Vergara-Díaz, O.; Uberegui, E.; Yoldi-Achalandabaso, A.; Araus, J.L.; Vicente, R. Evolution of wheat architecture, physiology, and metabolism during domestication and further cultivation: Lessons for crop improvement. Crop J. 2023, 11, 1080–1096. [Google Scholar] [CrossRef]
- Thomsen, I.; Schweinzer, A.; Friedel, J.; Samson, M.-F.; Carcea, M.; Narducci, V.; Turfani, V.; Askegaard, M.; Surböck, A.; Freyer, B.; et al. Management effects on quality of organically grown winter wheat. J. Sustain. Agric. 2012, 37, 172–192. [Google Scholar]
- Vinogradov, D.V.; Vysotskaya, E.A. The role of an agricultural forecrop in increasing the yield of winter wheat. IOP Conf. Ser. Earth Environ. Sci. 2022, 996, 012021. [Google Scholar] [CrossRef]
- Szymańska, G.; Faligowska, A.; Panasiewicz, K.; Szukała, J.; Ratajczak, K.; Sulewska, H. The long-term effect of legumes as forecrops on the productivity of rotation winter triticale–winter rape with nitrogen fertilisation. Plant Soil Environ. 2019, 70, 128–134. [Google Scholar] [CrossRef]
- Knapp, S.; van der Heijden, M.G.A. A global meta-analysis of yield stability in organic and conservation agriculture. Nat. Commun. 2018, 9, 3632. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.; Wilkinson, J.N.; Shotton, P.; Sufar, E.K.; Hasanaliyeva, G.; Volakakis, N.; Cakmak, I.; Ozturk, L.; Bilsborrow, P.; Iversen, P.O.; et al. Improving Crop Health, Performance, and Quality in Organic Spring Wheat Production: The Need to Understand Interactions between Pedoclimatic Conditions, Variety, and Fertilization. Agronomy 2023, 13, 2349. [Google Scholar] [CrossRef]
- Rempelos, L.; Wang, J.; Sufar, E.K.; Almuayrifi, M.S.B.; Knutt, D.; Leifert, H.; Leifert, A.; Wilkinson, A.; Shotton, P.; Hasanaliyeva, G.; et al. Breeding Bread-Making Wheat Varieties for Organic Farming Systems: The Need to Target Productivity, Robustness, Resource Use Efficiency and Grain Quality Traits. Foods 2023, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-X.; Wang, Z.-H.; Stewart, B.A. Chapter Five—Responses of Crop Plants to Ammonium and Nitrate, N. Adv. Agron. 2013, 118, 205–397. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. Chapter Two—A Meta-Analysis and Review of Plant-Growth Response to Humic Substances: Practical Implications for Agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar] [CrossRef]
- Rathor, P.; Upadhyay, P.; Ullah, A.; Gorim, L.Y.; Thilakarathna, M.S. Humic acid improves wheat growth by modulating auxin and cytokinin biosynthesis pathways. AoB Plants 2024, 16, plae018. [Google Scholar] [CrossRef] [PubMed]
- El-Shabrawi, H.; Bakry, A.B.; Ahmed, M.; Abou-El-Lail, M. Humic and Oxalic Acid Stimulates Grain Yield and Induces Accumulation of Plastidial Carbohydrate Metabolism Enzymes in Wheat Grown under Sandy Soil Conditions. Agric. Sci. 2015, 6, 175–185. [Google Scholar] [CrossRef]
- Bera, B.; Bokado, K.; Barkha and Arambam, S. Effect of Humic Acid on Growth, Yield and Soil Properties in Rice: A Review. Int. J. Plant Soil Sci. 2024, 36, 26–35. [Google Scholar] [CrossRef]
- Shoup, F.K.; Pomeranz, Y.; Deyoe, C.W. Amino Acid Composition of Wheat Varieties and Flours Varying Widely in Bread-Making Potentialities. J. Food Sci. 2006, 31, 94–101. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E.; Feledyn-Szewczyk, B.; Joniec, J. The Antioxidant Potential of Grains in Selected Cereals Grown in an Organic and Conventional System. Agriculture 2022, 12, 1485. [Google Scholar] [CrossRef]
- Škrbić, B.; Onija, A. Multivariate analyses of microelement contents in wheat cultivated in Serbia (2002). Food Control 2007, 18, 338–345. [Google Scholar] [CrossRef]
- Zhao, F.J.; Su, Y.H.; Dunham, S.J.; Rakszegi, M.; Bedo, Z.; McGrath, S.P.; Shewry, P.R. Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J. Cereal Sci. 2009, 49, 290–295. [Google Scholar] [CrossRef]
- Suchowilska, E.; Wiwart, M.; Kandler, W.; Krska, R. A comparison of macro- and microelement concentrations in the whole grain of four Triticum species. Plant Soil. Environ. 2012, 58, 141–147. [Google Scholar] [CrossRef]
- Wiśniowska-Kielian, B.; Klima, K. Comparison of microelement contents in the winter wheat grain from organic and conventional farms. J. Res. Appl. Agric. Eng. 2007, 52, 100–103. (In Polish) [Google Scholar]
- Ekholm, P.; Reinivuo, H.; Mattila, P.; Pakkala, H.; Koponen, J.; Happonen, A.; Hellström, J.; Ovaskainen, M.L. Changes in the mineral and trace element contents of cereals, fruits and vegetables in Finland. J. Food Compos. Anal. 2007, 20, 487–495. [Google Scholar] [CrossRef]
- Gebruers, K.; Dornez, E.; Boros, D.; Fraś, A.; Dynkowska, W.; Bedo, Z.; Rakszegi, M.; Delcour, J.A.; Courtin, C.M. Variation in the content of dietary fiber and components thereof in wheats in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9740–9749. [Google Scholar] [CrossRef]
- Mie, A.; Andersen, H.R.; Gunnarsson, S.; Kahl, J.; Kesse-Guyot, E.; Rembiałkowska, E.; Quaglio, G.; Grandjean, P. Human health implications of organic food and organic agriculture: A comprehensive review. Environ. Health 2017, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Çakmakçı, S.; Çakmakçı, R. Quality and Nutritional Parameters of Food in Agri-Food Production Systems. Foods 2023, 12, 351. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A. Yield and chemical composition of spring triticale grain depending on cropping and tillage systems. Int. J. Plant Prod. 2016, 10, 45–52. [Google Scholar] [CrossRef]
- Woźniak, A.; Makarski, B. Content of minerals, total protein and wet gluten in grain of spring wheat depending on cropping systems. J. Elem. 2013, 18, 297–305. [Google Scholar] [CrossRef]
- Szuba-Trznadel, A.; Gałka, B.; Kamińska, J.; Jama-Rodzeńska, A.; Król, Z.; Jarki, D.; Fuchs, B. Diversity of chemical composition and nutritional value in grain from selected winter wheat cultivars grown in south-western Poland. Sci. Rep. 2024, 14, 2630. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Tian, J.; Hao, Z.; Zhang, W. Protein Content and Amino Acid Composition in Grains of Wheat-Related Species. Agric. Sci. China 2008, 7, 272–279. [Google Scholar] [CrossRef]
- Dogan, Y. Investigation of micro and macro element content of wheat varieties grown commonly in Turkey. Oxid. Commun. 2015, 38, 1265–1274. Available online: https://hdl.handle.net/20.500.12514/1174 (accessed on 5 November 2024).
- Siddiqi, R.A.; Singh, T.P.; Rani, M.; Sogi, D.S.; Bhat, M.A. Diversity in Grain, Flour, Amino Acid Composition, Protein Profiling, and Proportion of Total Flour Proteins of Different Wheat Cultivars of North India. Front. Nutr. 2020, 7, 141. [Google Scholar] [CrossRef]
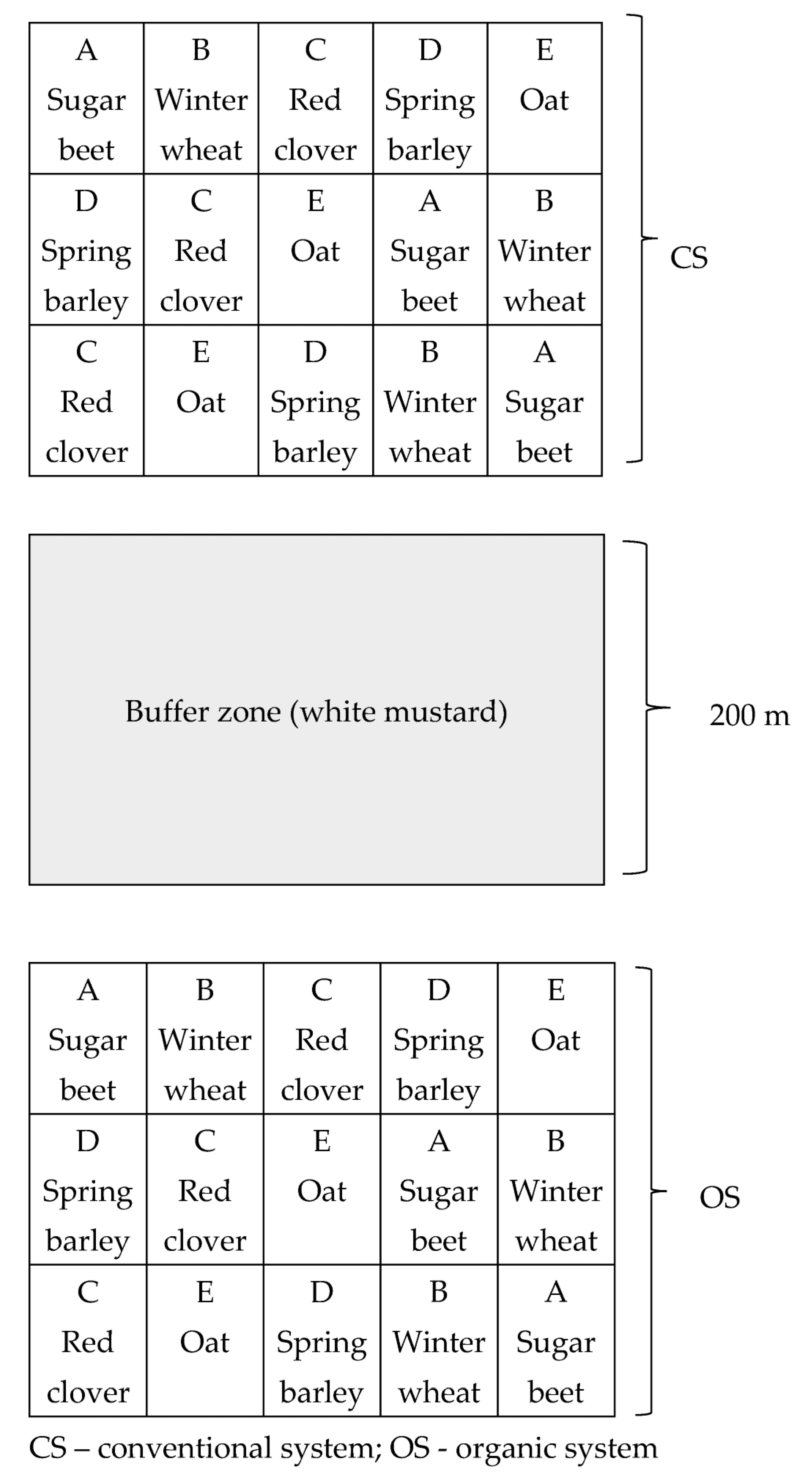

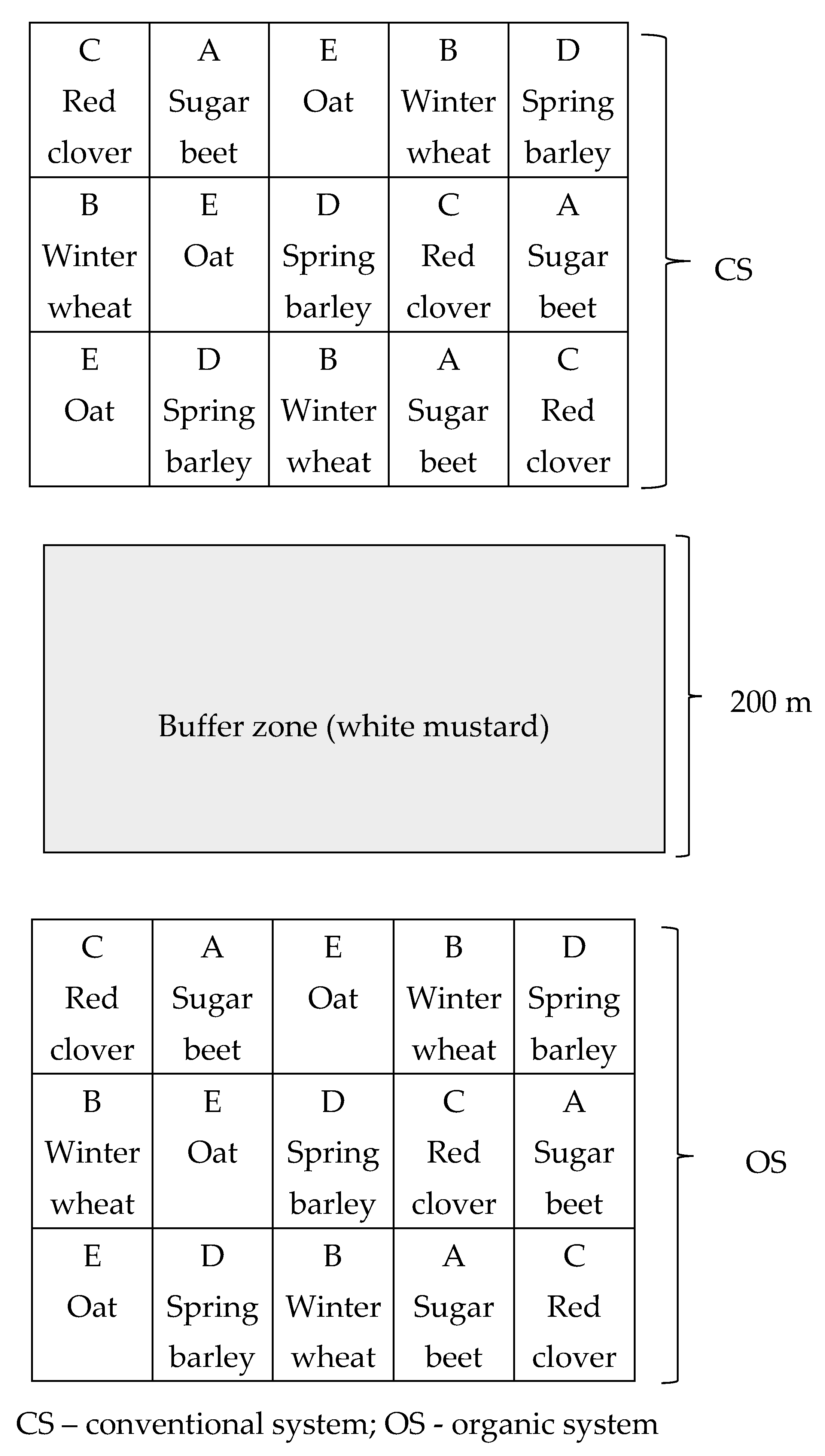
| Farming Treatment | Soil pH 1 M KCl | Nitrogen (N) (%) | Phosphorus (P) mg kg−1 | Potassium (K) mg kg−1 | Organic Carbon (C) (%) |
|---|---|---|---|---|---|
| 2020 | |||||
| Organic | 6.4 | 0.10 | 129 | 210 | 1.17 |
| Conventional | 6.3 | 0.14 | 134 | 218 | 1.25 |
| 2021 | |||||
| Organic | 6.4 | 0.11 | 126 | 207 | 1.16 |
| Conventional | 6.3 | 0.15 | 131 | 216 | 1.23 |
| 2022 | |||||
| Organic | 6.4 | 0.11 | 127 | 212 | 1.18 |
| Conventional | 6.3 | 0.14 | 135 | 220 | 1.27 |
| Technical Parameters | Content |
|---|---|
| Humic acid content | 62% |
| Carbon content of humic acid | up to 62% |
| Minerals in dry matter | |
| Nitrogen (N) | 10.3 g kg−1 |
| Phosphorus (P) | 1.05 g kg−1 |
| Sodium (Na) | 12.80 g kg−1 |
| Potassium (K) | 1.18 g kg−1 |
| Calcium (Ca) | 16.80 g kg−1 |
| Ferrum (Fe) | 14.50 g kg−1 |
| Zinc (Zn) | 64 mg kg−1 |
| Brom (Br) | 77 mg kg−1 |
| Copper (Cu) | 19 mg kg−1 |
| Selenium (Se) | 6 mg kg−1 |
| Moisture content | 20% |
| Fertilization | Component | Crop Plants | ||||
|---|---|---|---|---|---|---|
| Sugar Beet | Spring Barley | Red Clover | Winter Wheat | Oat | ||
| Mineral fertilization (kg ha−1) | N (before sowing) | 90 | 65 | 20 | 90 | 45 |
| P (before sowing) | 90 | 45 | 30 | 70 | 35 | |
| K (before sowing) | 120 | 85 | 45 | 110 | 55 | |
| Manure fertilization (t ha−1) | (autumn; before sowing) | 25 | - | - | - | - |
| Mineral Fertilization | Crop Plants | ||||
|---|---|---|---|---|---|
| Sugar Beet | Spring Barley | Red Clover | Winter Wheat | Oat | |
| Humac Agro; before sowing (kg ha−1) | 450 | 320 | 55 | 380 | 280 |
| Originating from organic livestock production; autumn; before sowing (t ha−1) | 25 | - | - | - | - |
| Wheat Forecrop (WF) | Farming System (FS) | Mean | |
|---|---|---|---|
| Organic | Conventional | ||
| Sugar beet | 5.13 (±0.08) c | 6.82 (±0.08) a | 5.97 a |
| Spring barley | 4.19 (±0.07) e | 5.23 (±0.04) c | 4.71 c |
| Red clover | 4.77 (±0.09) d | 6.31 (±0.07) b | 5.54 b |
| Oat | 4.81 (±0.05) d | 6.27 (±0.09) b | 5.54 b |
| Winter wheat | 4.07 (±0.06) e | 5.19 (±0.05) c | 4.63 c |
| Mean | 4.59 b | 5.96 a | - |
| Specification | Total Dietary Fiber Content (%) | O-Dihydroxyphenol Content (g 100 g−1) | Polyphenol Content (mg Catechin g−1 DM) |
|---|---|---|---|
| Farming system (FS) | |||
| Organic | 16.46 (±0.12) a | 0.183 (±0.02) a | 2.01 (±0.06) a |
| Conventional | 15.57 (±0.10) b | 0.149 (±0.02) b | 1.77 (±0.05) b |
| Wheat forecrop (WF) | |||
| Sugar beet | 16.59 (±0.09) a | 0.182 (±0.03) a | 2.05 (±0.05) a |
| Spring barley | 15.22 (±0.08) c | 0.157 (±0.01) c | 1.76 (±0.03) c |
| Red clover | 16.68 (±0.07) a | 0.176 (±0.02) a | 2.05 (±0.05) a |
| Oat | 16.22 (±0.09) b | 0.162 (±0.03) b | 1.86 (±0.05) b |
| Winter wheat | 15.37 (±0.06) c | 0.152 (±0.02) c | 1.74 (±0.04) c |
| Factor interaction | |||
| FS | ** | ** | ** |
| WF | ** | ** | ** |
| FS × WF | ns | ns | ns |
| Specification | Total Nitrogen (%) | Potassium (g kg−1) | Magnesium (mg kg−1) | Selenium (µg kg−1) |
|---|---|---|---|---|
| Farming system (FS) | ||||
| Organic | 1.39 (±0.12) b | 1.79 (±0.10) b | 705 (±6) a | 44.16 (±0.12) a |
| Conventional | 2.23 (±0.09) a | 2.37 (±0.13) a | 611 (±5) b | 22.54 (±0.12) b |
| Wheat forecrop (WF) | ||||
| Sugar beet | 1.97 (±0.11) a | 2.26 (±0.12) a | 716 (±7) a | 38.25 (±0.12) b |
| Spring barley | 1.72 (±0.08) c | 1.92 (±0.11) c | 610 (±4) d | 26.25 (±0.13) d |
| Red clover | 1.93 (±0.10) a | 2.15 (±0.10) ab | 698 (±5) b | 41.35 (±0.11) a |
| Oat | 1.62 (±0.07) d | 1.94 (±0.08) c | 642 (±6) c | 26.40 (±0.14) d |
| Winter wheat | 1.82 (±0.08) b | 2.07 (±0.09) b | 612 (±5) d | 34.50 (±0.10) c |
| Factor interaction | ||||
| FS | ** | ** | ** | ** |
| WF | ** | ** | ** | ** |
| FS × WF | ** | ** | ** | ** |
| Farming System (FS) | Wheat Forecrop (WF) | Total Nitrogen (%) | Potassium (g kg−1) | Magnesium (mg kg−1) | Selenium (µg kg−1) |
|---|---|---|---|---|---|
| Organic | Sugar beet | 1.53 (±0.09) c | 1.97 (±0.05) d | 766 (±7) a | 48.2 (±0.11) b |
| Spring barley | 1.42 (±0.07) c | 1.72 (±0.04) e | 673 (±4) c | 37.3 (±0.09) c | |
| Red clover | 1.50 (±0.08) c | 1.84 (±0.07) d | 732 (±6) b | 51.0 (±0.10) a | |
| Oat | 1.19 (±0.04) d | 1.65 (±0.07) e | 696 (±5) c | 47.2 (±0.13) b | |
| Winter wheat | 1.43 (±0.06) c | 1.79 (±0.06) e | 659 (±3) c | 38.1 (±0.10) c | |
| Conventional | Sugar beet | 2.41 (±0.05) a | 2.66 (±0.10) a | 688 (±6) c | 28.3 (±0.08) d |
| Spring barley | 2.12 (±0.06) b | 2.12 (±0.08) c | 548 (±3) e | 15.2 (±0.07) f | |
| Red clover | 2.37 (±0.04) a | 2.46 (±0.11) b | 665 (±4) c | 31.7 (±0.10) d | |
| Oat | 2.06 (±0.05) b | 2.24 (±0.09) c | 589 (±7) d | 22.8 (±0.08) e | |
| Winter wheat | 2.22 (±0.06) b | 2.35 (±0.08) b | 566 (±6) e | 14.7 (±0.09) f |
| Specification | Calcium (mg kg−1) | Copper (mg kg−1) | Manganese (mg kg−1) | Iron (mg kg−1) | Zinc (mg kg−1) |
|---|---|---|---|---|---|
| Farming system (FS) | |||||
| Organic | 347 (±4) a | 4.35 (±0.6) a | 40.5 (±0.9) a | 39.3 (±0.4) a | 30.6 (±0.3) a |
| Conventional | 290 (±3) b | 3.64 (±0.7) b | 36.4 (±1.0) b | 36.1 (±0.3) b | 28.0 (±0.2) b |
| Wheat forecrop (WF) | |||||
| Sugar beet | 338 (±2) a | 4.25 (±0.8) a | 40.3 (±1.1) a | 39.7 (±0.5) a | 31.6 (±0.3) a |
| Spring barley | 308 (±4) c | 3.75 (±0.7) c | 37.4 (±0.8) c | 36.2 (±0.6) bc | 27.5 (±0.2) b |
| Red clover | 335 (±5) a | 4.15 (±0.6) a | 39.6 (±1.2) ab | 39.9 (±0.2) a | 32.0 (±0.3) a |
| Oat | 313 (±4) b | 4.02 (±0.4) b | 38.2 (±0.9) b | 37.4 (±0.4) b | 28.3 (±0.4) b |
| Winter wheat | 296 (±3) c | 3.83 (±0.5) c | 36.7 (±1.3) c | 35.5 (±0.3) c | 27.3 (±0.1) b |
| Factor interaction | |||||
| FS | ** | ** | ** | ** | ** |
| WF | ** | ** | ** | ** | ** |
| FS × WF | ns | ns | ns | ns | ns |
| Amin. | Sugar Beet | FI | Spring Barley | FI | Red Clover | FI | Oat | FI | Winter Wheat | FI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conv. | Org. | Conv. | Org. | Conv. | Org. | Conv. | Org. | Conv. | Org. | ||||||
| Asp * | 6.31 a | 5.94 b | ** | 5.97 a | 5.33 b | ** | 6.15 a | 5.88 b | ** | 5.82 a | 5.21 b | ** | 5.79 a | 5.13 b | ** |
| Thr | 3.66 a | 3.51 a | ns | 3.44 a | 3.31 a | ns | 3.72 a | 3.64 a | ns | 3.52 a | 3.54 a | ns | 3.24 a | 3.34 a | ns |
| Ser | 6.24 a | 5.52 b | ** | 5.95 a | 5.15 b | ** | 6.32 a | 5.47 b | ** | 5.99 a | 5.23 b | ** | 5.84 a | 5.09 b | ** |
| Glu | 35.8 b | 40.0 a | ** | 35.7 a | 33.1 b | ** | 36.2 b | 38.9 a | ** | 34.5 a | 35.7 a | ns | 34.1 a | 33.8 a | ns |
| Pro | 12.2 b | 13.1 a | ** | 12.7 a | 11.2 b | ** | 11.8 b | 12.9 a | ** | 11.3 b | 12.5 a | ** | 11.6 a | 10.9 a | ns |
| Gly | 4.86 b | 5.22 a | ** | 5.06 a | 4.78 b | ** | 4.91 b | 5.12 a | ** | 5.00 a | 4.65 b | ** | 4.88 a | 4.72 a | ns |
| Ala | 4.65 a | 4.11 b | ** | 4.23 a | 4.18 a | ns | 4.75 a | 4.70 a | ns | 4.38 a | 4.43 a | ns | 4.19 a | 4.02 a | ns |
| Cys-A | 3.05 a | 2.08 b | ** | 2.94 a | 2.00 b | ** | 3.22 a | 2.43 b | ** | 2.99 a | 2.80 a | ns | 2.85 a | 1.93 b | ** |
| Val | 4.19 b | 5.22 a | ** | 3.95 b | 4.77 a | ** | 4.02 b | 5.19 a | ** | 4.01 b | 5.16 a | ** | 3.88 b | 4.67 a | ** |
| Met | 1.16 b | 2.12 a | ** | 1.07 b | 2.02 a | ** | 1.75 b | 3.77 a | ** | 1.55 b | 2.97 a | ** | 1.21 b | 2.19 a | ** |
| Ile | 3.95 a | 3.67 b | ** | 3.62 a | 3.41 a | ns | 3.98 a | 3.79 a | ns | 3.58 a | 3.50 a | ns | 3.38 a | 3.29 a | ns |
| Leu | 8.36 a | 7.91 b | ** | 7.94 a | 7.21 b | ** | 8.41 a | 8.22 a | ns | 8.16 a | 8.08 a | ns | 7.77 a | 7.56 a | ns |
| Tyr | 2.85 a | 2.36 b | ** | 2.73 a | 2.21 b | ** | 2.90 a | 2.77 b | ** | 2.81 a | 2.70 a | ns | 2.69 a | 2.59 a | ns |
| Phe | 5.52 a | 4.86 b | ** | 5.02 a | 4.46 b | ** | 5.60 a | 5.39 a | ns | 5.13 a | 5.02 a | ns | 4.78 a | 4.68 a | ns |
| His | 2.71 a | 2.58 a | ns | 2.64 a | 2.39 b | ** | 2.82 a | 2.70 a | ns | 2.63 a | 2.51 a | ns | 2.47 a | 2.43 a | ns |
| Lys | 2.44 b | 3.43 a | ** | 2.23 b | 3.11 a | ** | 2.49 b | 3.61 a | ** | 2.17 b | 3.25 a | ** | 2.19 b | 3.09 a | ** |
| Arg | 4.72 b | 5.35 a | ** | 5.01 a | 4.63 b | ** | 4.25 b | 4.98 a | ** | 4.19 b | 4.87 a | ** | 4.97 a | 4.15 b | ** |
| Trp | 0.29 b | 0.87 a | ** | 0.15 b | 0.45 a | ** | 0.33 b | 0.82 a | ** | 0.41 b | 0.65 a | ** | 0.21 b | 0.38 a | ** |
| Total Amino Acids | 112.96 a | 117.85 a | ns | 110.35 a | 103.71 b | ** | 113.62 a | 120.28 a | ns | 108.14 a | 112.77 a | ns | 106.04 a | 103.96 a | ns |
| EAAI ** | 73.9 b | 81.2 a | ** | 66.7 a | 69.3 a | ns | 75.8 b | 85.4 a | ** | 71.8 b | 79.2 a | ** | 64.1 a | 66.3 a | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harasim, E.; Kwiatkowski, C.A.; Buczek, J. The Influence of the Farming System and Forecrop on the Yield and Chemical and Health-Promoting Composition of Spring Wheat Grain. Agronomy 2025, 15, 39. https://doi.org/10.3390/agronomy15010039
Harasim E, Kwiatkowski CA, Buczek J. The Influence of the Farming System and Forecrop on the Yield and Chemical and Health-Promoting Composition of Spring Wheat Grain. Agronomy. 2025; 15(1):39. https://doi.org/10.3390/agronomy15010039
Chicago/Turabian StyleHarasim, Elżbieta, Cezary A. Kwiatkowski, and Jan Buczek. 2025. "The Influence of the Farming System and Forecrop on the Yield and Chemical and Health-Promoting Composition of Spring Wheat Grain" Agronomy 15, no. 1: 39. https://doi.org/10.3390/agronomy15010039
APA StyleHarasim, E., Kwiatkowski, C. A., & Buczek, J. (2025). The Influence of the Farming System and Forecrop on the Yield and Chemical and Health-Promoting Composition of Spring Wheat Grain. Agronomy, 15(1), 39. https://doi.org/10.3390/agronomy15010039








