Abstract
Polyamines (PAs) are low-molecular-weight compounds that contain amino groups. PAs are present in a variety of organisms, including plants, animals and microorganisms. In plants, the main PAs are putrescine (PUT), spermidine (SPD) and spermine (SPM). They play crucial physiological roles in plant development, including flowering, fruit set, growth, ripening and metabolic processes. In addition, PAs are components of the diet and have a role in health and disease. Furthermore, PAs have been demonstrated to help overcome the negative effects of adverse environmental factors of both biotic and abiotic stresses. Thus, the main objective of this review was to examine the recent literature regarding the mentioned effects of PAs apart from the impact of preharvest PAs treatments, applied at different stages of fruit development, on fresh fruit crop yield and fruit quality properties at harvest, and in their maintenance during storage, with a special emphasis on the fruit content in bioactive compounds with antioxidant activity. Moreover, this review addressed the impact of PAs on other physiological processes affecting crop yield such as flowering and fruit set.
1. Introduction
Polyamines (PAs) are polycationic molecules that are present in a variety of organisms, including plants, animals, and microorganisms [1,2]. In the former, they act as phytohormones, playing pivotal roles in plant development and metabolism. However, some scientists have questioned this assertion due to the fact that these compounds are found in high concentrations in plant tissues, which is contrary to the notion that they act as hormones, substances that, by definition, are active at very low doses. It may therefore be the case that they act merely as secondary messengers or as plant growth regulators (PGRS) [3]. PAs affect cellular activity and are involved in several plant physiological processes, including embryogenesis, photosynthesis, maintenance of membrane structures, flowering and fruit growth and ripening. Additionally, PAs have been demonstrated to alleviate the effects of both biotic and abiotic stress. Due to their cationic nature in their chemical structure, they can bind and form complexes with different molecules, such as certain proteins, phospholipids, pectins, DNA and RNA, among others.
The best known PAs are putrescine (PUT), spermidine (SPD), and spermine (SPM), which are the most commonly occurring in plant cells, while cadaverine is only found in legumes. PAs can exist in either free form or as conjugated molecules. Conjugated forms usually constitute up to 90% of the total PAs in plant cells. It is of great significance that the equilibrium between free and conjugated PAs forms is of paramount importance for the diverse roles they play in the physiological processes of the plant. PAs are primarily accumulated in and are associated with the cell wall, although they can also be found in vacuoles, chloroplasts and mitochondria. They are typically produced in elevated quantities when plants are subjected to stress conditions, including drought, nutritional deficiencies, salinity, and extreme temperatures, among other factors [4,5]. Thus, the main objective of the present review is providing to evidence regarding the impact of PAs in increasing crop yields and fruit quality properties at harvest and during postharvest storage. In addition, the impact of PA treatments in overcoming the negative impacts of stressful factors in crop production under the actual scenario of climate change are also addressed, as well as the effects of dietary PAs on human health.
2. History of Polyamines Discovery
From a historical perspective, PAs have a long and distinguished history in plant physiology [2,6]. The discovery of PAs preceded that of nucleic acids by two centuries. In 1674 and 1678, Antoine van Leeuwenhoek designed a primitive microscope and first described the presence of crystals in seminal fluid, respectively. These crystals were only observed in stored samples, not in fresh ones. Vauquelin reported in 1791 that the crystals were phosphate derivatives of an unknown new compound named ‘spermine phosphate’. A hundred and fifty years later, in 1878, Schreiner identified a new inorganic compound as an organic base derived from spermine phosphate, which he designated SPM after hydrolysis. In 1898, von Poehl advocated the use of spermine incurring various diseases because of its inhibition capacity for bacterial growth. The first evidence of PAs in plants was provided in 1911 in the Ravenna area (Italy) in Datura stramonium, a poisonous flowering plant that contained PUT. Finally, Rosenheim established the correct structure of PAs and verified these structures by chemical synthesis. Regarding Pas’ pathway-related enzymes, Zeller discovered diamine oxidase (DAO), also called histaminase, in 1938, and Canellakis and his group (1978) described the properties of antizyme, a molecule which specifically binds to ornithine decarboxylase (ODC) and neutralised it. Consequently, numerous authors have posited that PUT, SPD, SPM, and agmatine are ubiquitous polycationic molecules in higher plants. A summary of the timeline of the discovery of PAs is presented in Figure 1.
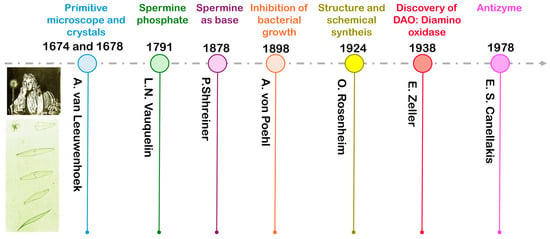
Figure 1.
History of the most important facts in the discovery of polyamines (PAs).
3. Polyamine Biosynthesis
The pathway for PA biosynthesis is shown in Figure 2. PUT is a key metabolic product as it is the substrate for the biosynthesis of the other PAs. PUT is synthesised from the amino acid arginine, which is converted to ornithine or agmatine via reactions catalysed by arginase or arginine decarboxylase (ADC), respectively, although the ADC pathway is preferred in plants, where PUT is formed in two steps, catalysed by agmatine iminohydrolase (AIH) and N-carbamoylputrescine amidohydrolase (NCPAH). Then, an aminopropyl group of decarboxylated S-adenosylmethionine (dcSAM), which is derived from S-adenosylmethionine (SAM), in a reaction catalysed by SAM decarboxylase (SAMDC), is transferred to PUT to form SPD by the action of spermidine synthase (SPDS). Finally, SPD is converted to SPM through the catalysis of spermine synthase (SPMS) by the addition of another aminopropyl group from dcSAM [7,8]. In addition, SAM is used to form 1-aminociclopropane-1-carboxylic acid (ACC) mediated by ACC synthase, which is further converted into ethylene by ACC-oxidase [9]. Thus, PAs and ethylene biosynthesis pathways are interconnected by their common precursor SAM, and usually a competence for SAM between both pathways has been claimed [7,9,10].
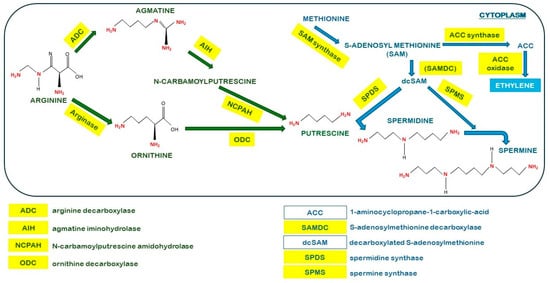
Figure 2.
Detailed pathway of polyamine (PA) biosynthesis.
In fruit tissues, PA levels depend on different processes, such as biosynthesis, catabolism, and transport. There is increasing evidence that PA catabolism is also involved in alleviating the symptoms of the various stresses by enhancing the activities of two enzymes: copper-containing amine oxidases (CuAOs) and flavin-containing PA oxidases (PAOs), both of which are found in many plant species [10,11,12].
4. Dietary Polyamines and Health
Numerous studies in humans have demonstrated the role of PAs on health and disease, as they are essential for cell growth, the maintenance of the gastrointestinal tract, and stimulation of the immune system, among others [13,14,15,16]. For instance, the highest concentrations of SPM and SPD in breast milk are found during the first week of lactation, demonstrating the importance of these substances in neonatal nutrition [14]. In addition, Eisenberg et al. [17] showed that SPM increases longevity in several experimental models and human lymphocyte cells by inducing autophagy. Other findings have led to the proposal of dietary supplementation with PAs due to their positive effects on preventing age-related diseases, cardiovascular and cognitive alterations and their anti-tumour activity [18,19,20,21]. However, more robust clinical studies should be conducted to provide more scientific evidence on the role of PAs in human health.
The estimation of the daily intake of PAs through a dietary survey and the preparation of tables of their content in foods showed a total intake of 250–400 μmol/day in control subjects, depending on the type of the diet, e.g., Mediterranean, vegetarian, vegan, etc. [14]. However, there are currently no official recommendations for the daily intake of PAs, and it is estimated that a healthy diet high in fruit and vegetables would provide less than half of the daily recommended intake of PAs. Some foods and their PA contents are shown in Table 1.

Table 1.
Levels of putrescine (PUT), spermidine (SPD) and spermine (SPM) (nmol/g) in different food categories.
5. Effects of Preharvest Application of Polyamines on Fruit Crop Performance
5.1. Effects of Polyamines on Overcoming Stressful Environmental Factors
There is a general consensus that food production is one of the most important economic activities worldwide, but also negatively impacted by climate change. In recent years, adverse climatic events have increased in frequency and intensity. Therefore, they negatively affect food availability, access, stability and use, as well as livelihood assets and opportunities in both rural and urban areas. There are many factors that contribute to climate change, including the combustion of gas, oil and coal on enormous scales, the large-scale production system of some foods, deforestation for livestock, etc. All these activities release millions of tonnes of CO2 into the atmosphere daily. For the Food and Agriculture Organization of the United Nations [26], “the growing threat of climate change to the global food supply and the challenges it poses to food security require urgent concerted policy action”.
The impacts of climate change on food chain production are various, but the most visible are the following: reduction in water availability, increased incidence of pests and diseases, loss of arable land, especially due to desertification, and changes in fruit growth patterns, all of which lead to reduced crop yields. For the food industry, combating the direct or indirect effects of climate change is a priority to avoid food security problems in the coming years. In this sense, the portfolio of the new agriculture also includes the use of elicitors, which induce any type of defence in the plant against biotic and abiotic stressors, so that the plant becomes more resistant to subsequent stresses. In addition, elicitors have been shown to have positive effects in terms of increasing crop yield, so that the research about their use has increased in recent years [27,28,29]. Pas, which are considered as elicitors, have also been demonstrated to play key roles in plant adaptation to various environmental stresses. However, most of the examples regarding the effects of PAs on increasing crop yield under different biotic and abiotic stresses (including wounds, insects, herbivores, extreme temperatures, salinity, heavy metals and metabolites produced by microorganisms, among others) are limited to rice and tomato as model plants [5,30,31,32,33,34]. For instance, SPM levels have been shown to improve grain yield in rice [32]. PAs interact with negatively charged phospholipids in cell membranes to enhance the membrane permeability and stability, thus, indirectly modulating the activity of membrane-associated enzymes and exerting a protective function against stress [3]. Moreover, PAs stimulate the expression of key antioxidant enzymes, including NADPH oxidase (NOX), superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR), which are essential for ROS detoxification. These mechanisms underscore the multifaceted role of PAs in maintaining cellular integrity and enhancing stress tolerance, leading to a better crop growth and improved yields [32,33,34]. On the other hand, PAs regulate plant defence responses via crosstalk with other plant hormones. For instance, postharvest loquat fruit pretreated with methyl jasmonate (10 µmol L−1) enhanced our resistance against anthracnose (Colletotrichum acutatum) due to the increased PUT, SPM, and SPD synthesis. Similarly, SA signalling affected both PA biosynthesis and the expression of GhPAO, which converts SPM back to SPD. In addition, a crosstalk between PAs and ABA has been discovered, wherein ABA triggers several genes in the PA biosynthesis pathway. Finally, a connection among ethylene and PAs has been also reported in peaches infected by Monilinia spp. [35,36]. Accordingly, the exogenous application of PAs will modulate their endogenous content, and thus confer plant resilience [37].
Apart from the above-mentioned effects of PAs as PGRS involved in plant resistance to both abiotic and biotic stresses, PAs are involved in fruit set, growth and ripening, as well as in the regulation of fruit quality-related traits [38,39]. Nowadays, PAs are gaining considerable interest to be used as substitutes of synthetic plant growth regulators to enhance plant growth and fruit set, yield and quality under both normal and stressful conditions [40,41]. In this sense, SPD and SPM treatments increased crop yield in wheat, due to enhanced grain size, under high-temperature stress, whilst PUT treatment increased yield in lettuce plants under salinity stress, which was attributed to increased net photosynthesis rate [40].
5.2. Flowering and Fruit Set
Flowering is claimed to be the most important plant physiological process leading to ensure plant genetic continuity, variability and ultimately food security. Fruit development begins with flowering, which is divided into several phenological stages starting with the conversion of the vegetative meristem of the shoot into the floral meristem, which is influenced by several environmental stimuli, such as photoperiod, ambient temperature, and seasonality [42]. From an agronomic point of view, knowledge of the effects of climatic factors on flowering and fruit set will serve as a basis for interpreting the plant responses to bioclimatic factors and conversely, once these are known, the responses of the plant can be predicted. Finally, from an economic point of view, these data are of great importance because, if properly treated, they could also be used to predict the appearance of a pest, the need to apply a specific fertiliser, the use of a hormonal product, etc. [43].
In two apricot cultivars, the application of 10 mM PUT at the flowering stage increased the endogenous concentration of PUT, SPD, and SPM and the percentage of functional ovules [44]. These authors postulated that free PA concentrations (PUT, SPD, and SPM) could be considered as markers of the ovary developmental stage, as did De Dios et al. [45] in damson plum. PAs have also been reported to have effects on stimulating pollen tube growth in several fruit species, such as pear, tomato, or plum, leading to enhanced fertilisation and fruit production [46]. On the other hand, SPD exogenous application to Malus domestica accelerated the flowering due to the upregulation of genes encoding for floral activator factors [47]. Moreover, SPD (at 0.1 and 0.5 mM) applied during vegetative growth, by foliar spray, accelerated flowering initiation and flower induction, and increased fruit set and the number of fruits harvested per plant in two eggplant cultivars [48]. Similarly, the increased fruit set and number of fruit harvested per tree, without a reduction in fruit size, have also been observed in lemon after tree treatment with PUT, which was attributed to accelerated pollen germination and pollen tube growth, as well as to the prolonged ovule viability [49].
5.3. Fruit Growth
After flowering and fruit set, the next stage is fruit growth. In a broad sense, fruits are flowers or parts of flowers (with auxiliary organs) or inflorescences that have developed their tissues to contain the seeds until they mature, protecting them and facilitating their dispersion. In general, fruit growth follows two different patterns: (a) single sigmoid curve; and (b) double sigmoid curve, as it can be observed in Figure 3.
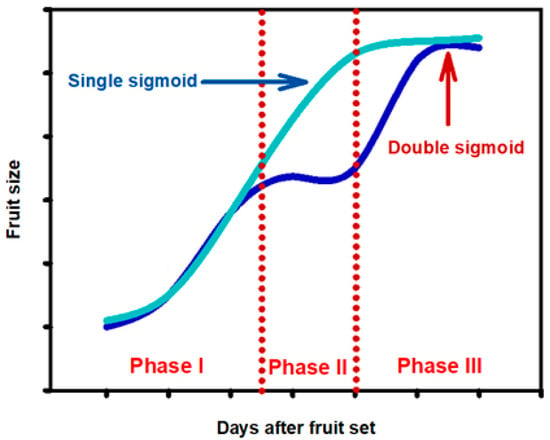
Figure 3.
Single- and double-sigmoid growth patterns of fruit species during on-tree growth and ripening processes. Figure drawn by the authors.
Fruits, such as citrus, pomegranate, table grape, apple and pear exhibit a single-sigmoid growth curve, while stone fruits, such as peach, sweet cherry, apricot, nectarine, or mango follow a double-sigmoid growth pattern. In the single-sigmoid curve, the initial period is characterised by cell proliferation (phase I), followed by a period of cell thickening (phase II), and a final period in which the fruit practically ceases growing and matures (phase III). In fruits with a hard endocarp, phase I is similar to the previous ones, followed by phase II, which is characterised by an intermediate stage in which the growth of the fruit stops and the endocarp lignifies. Then, a new period of linear growth occurs (phase III), in which the fruit grows rapidly due to a fast cell enlargement, culminating in a progressive slowing of fruit growth, which finally ceases and the fruit changes colour and ripens [50].
Fruit growth during phase II in fruit species that follow a sigmoid pattern is not uniform. In fact, they grow only at night, so that the apparently linear growth follows a broken line with a positive slope. During the day, transpiration reduces the water potential of the xylem, which reaches its lowest values, due to the low water supply from the branches to the fruit through the xylem stream and thus reducing cell fruit expansion. As the xylem water potential recovers, water transport to the fruit increases during the afternoon and night, and the fruit recovers its size and even increases its volume [51]. Several reports have shown that PA treatments applied during the on-tree fruit growth phases resulted in an increase in fruit size. For instance, in date palm, preharvest application of 1 mM PUT [alone or combined with 100 mg L−1 of gibberellic acid (GA3) and/or salicylic acid (SA)], enhanced fruit weight, length and volume, leading to increased productivity in ‘Zaghloul’ dates [52]. Accordingly, SPD treatment increased fruit size and yield in eggplant [48]. Similarly, a preharvest foliar spray treatment of loquat trees with 2 mM SPD induced a high percentage of fruit within the ‘Extra’ (weight over 40 g) category [53]. It is worth noting that, under optimal conditions, plants tend to produce a higher number of fruits. When this occurs, they are unable to fully satisfy all the fruit development processes simultaneously and an inverse relationship between the number of fruits and their size is usually found, which is attributed to the competition, especially for carbohydrates, that exists between the fruits. It is therefore important that treatments applied to increase a fruit set do not have any detrimental effect on fruit size. Then, the applied treatments should also have an impact on increasing plant photosynthesis to supply enough photoasimilates to the growing fruit.
5.4. Fruit Yield
From an agricultural point of view, farmers are looking for yield efficiency and producing fruit with the highest standard of quality. In fruit crops, yield is positively related to the percentage of fruit set, although the final yield depends on the post-flowering and preharvest drop. Several reports have provided evidences on the role of PA treatments in improving crop yield. For instance, the 0.25 mM SPD foliar treatments of apple tree increased crop yield due to enhanced fruit set and fruit growth [54]. Similarly, increases in crop yield have been reported for date palm, peach, and apricot following PUT or SPD treatments due to increased fruit size and weight [55,56,57]. Crop yield increases of up to 15% due to enhanced fruit size have also been reported in mango after foliar spray treatments with PUT at 25, 50, and 100 mg L−1, which were attributed to increased shoot number, length, and thickness, as well as to enhanced leaf area and chlorophyll content as compared with control trees [58].
In addition, the PUT foliar spraying of pepper plants, starting after the first flowers emerge and applied at 10-day intervals, increased the number of fruits per plant and fruit length, resulting in increased total yield per plant, both under control and deficit irrigation conditions, due to increased leaf chlorophyll content and water use efficiency [59]. Similarly, yield increases in strawberry were reported by [60] after 0.2 mM PUT spray treatments from 20 to 120 days after transplanting under both normal and deficit fertigation conditions. Accordingly, PUT treatments (at 15 and 30 ppm) of ‘Picual’ olive trees at full blossom and fruit set increased crop yield under saline irrigation water (4.40 ds m−1) conditions [61]. Thus, PAs could be an important tool to overcome the negative effects of current and future climate change events. In fact, Chen et al. [4] reported that the exogenous application of PUT and SPM induced crop tolerance and enhanced fruit yield in a wide range of fruit crops subjected to several types of stress (salinity, temperature, and water stress, among others). Thus, the exogenous applications of PAs alleviate plant stress symptoms, although the treatment efficacy depends on fruit species, the used polyamine and concentration and time of application [62,63]. In this sense, no beneficial effects on loquat tree yield were observed after SPD foliar treatment at fruit colour breaker stage, applied alone or in combination with acetylsalicylic acid (ASA) [53]. However, treatments of ‘Skeena’ sweet cherry trees with PUT or SPD at 0.01, 0.1, and 1 mM, applied by foliar spray at three key points of on-tree fruit development (pit hardening, colour change and seven days before harvest) increased crop yield by 13–52%, depending on the applied polyamine and concentration. Thus, the highest effect in terms of increasing crop yield was found for PUT at 0.1 mM, from 21 kg per tree to 32 kg per tree in the control and 0.1 mM PUT treated trees, respectively, followed by SPD at 0.1 mM that rendered 26 kg per tree, which was 24% more than controls (Figure 4A). The increase in crop yield was due to an enhanced number of fruits per tree and fruit weight. The highest increase in the number of fruits per tree was observed for 0.1 mM PUT treatment, up to 25% with respect to control tress, followed by 0.1 mM SPD, with up to 22.5% (Figure 4B). For fruit weight, the highest increase, ca. 14%, was found for the 0.1 mM PUT treatment, with a weight of 10 g, while fruit from the control trees weighed 8.8 g (Figure 4C). Thus, the most effective treatment for increasing the sweet cherry yield was PUT at 0.1 mM.
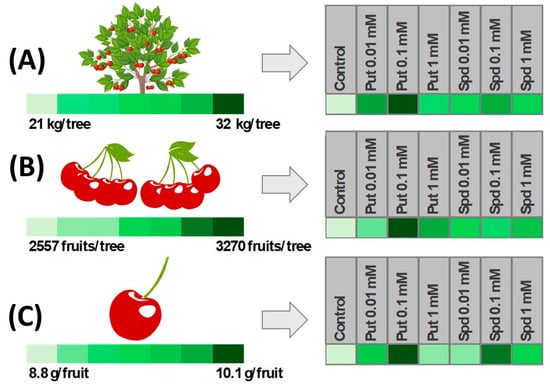
Figure 4.
Effect of preharvest treatments with putrescine (PUT) or spermidine (SPD) on sweet cherry yield (A), the number of fruits per tree (B) and fruit weight (C). Experiments were conducted in 2022 with nine trees per treatment. Deeper green colour in the heatmaps indicates a greater increase induced by the treatment. Original data from the authors.
5.5. Fruit Ripening and Quality
Fruit ripening occurs at the end of the phase III of fruit growth and classically, fruits are divided into two groups, climacteric and non-climacteric, based on their ripening pattern. Climacteric fruits, such as tomato, banana, apple, kiwifruit, avocado or papaya show a sharp increase in respiration rate and ethylene production at the beginning of ripening, and this hormone is a crucial signal for initiating and coordinating the ripening process, inducing colour changes, softening, loss of acidity and increase in sugars and aroma compounds [64,65]. On the contrary, ethylene does not seem to have a significant effect on the onset of ripening in non-climacteric fruits, such as strawberry, orange, lemon, blueberry or cucumber, with abscisic acid (ABA) being the primary hormone controlling their ripening process [9,66]. Thus, the concentration of abscisic acid (ABA) increases at the end of the fruit growth period or during ripening in non-climacteric fruits [67]. In addition, ABA and ethylene play a role in plant responses to drought, salt and temperature stresses, affecting the molecular mechanisms controlling ripening under environmental stress conditions. Thus, the genetic manipulation of ABA and ethylene biosynthesis and signalling offers potential strategies aiming to increase the resilience of fleshy fruits under present and future climate change events [68]. Additionally, ABA levels increase in plant tissues under osmotic stress induced by salinity and fruits ripen earlier [69]. On the other hand, other plant hormones, including auxin, jasmonic acid, gibberellins, brassinosteroids, salicylic acid and melatonin also play a key role in climacteric and non-climacteric fruit ripening [70].
Strawberry (Fragaria ananassa) has been used as a model of non-climacteric fruit, and in this plant species, the application of exogenous PUT delayed ripening, while exogenous SPM and SPD promoted ripening [71]. On the other hand, a progressive increase in the ABA content is coupled with a decrease in indoleacetic acid (IAA) in developing strawberry fruit, suggesting that the ABA/IAA ratio serves as a signal to trigger ripening. Then, PAs would inhibit the above signal by decreasing the ABA levels. In citrus, PAs has also been reported to play a crucial role in controlling fruit ripening and quality (Killiny and Nehela, 2020) [72]. The role of PAs is via interactions with ABA and ethylene, although the crosstalk between PAs and other phytohormones should deserve further investigation. PAs have also been reported to play a role in fruit ripening, as they are usually found at higher levels in the early stages of fruit development, but further show a continuing decrease, reaching the minimum concentration at the ripening stage (Figure 5), in both climacteric and non-climacteric fruit [9,50]. Postharvest treatments with PAs have shown beneficial effects in delaying fruit ripening, and thus, leading to the maintenance of fruit quality traits for a longer period. For instance, in apple fruit, the application of PAs delayed fruit softening and reduced chilling damage, due to the protection of cell walls and membranes. Similar effects of postharvest SPD and PUT treatments on retarding postharvest ripening and maintaining fruit quality parameters have been reported in a wide range of fruit species, including orange, pomegranate, lemon, plum, peach and apricot, among others [37,41,73,74]. However, the effects of PA treatments on the tree ripening process and fruit quality have been less studied.
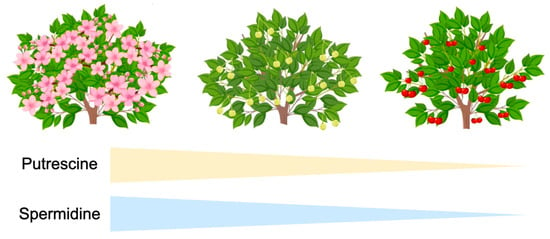
Figure 5.
Changes in polyamine (PA) levels in relation to fruit growth and ripening [9,50]. Figure from the authors.
From a commercial point of view, quality can be defined as the set of characteristics that a product possesses that enable it to meet standard criteria previously stipulated by the consumer. However, this concept varies from product to product and means different things to different people. In the case of fruit, colour, firmness, total sugars and acidity are the main characteristics that can be objectively considered as the determining factors of quality and related to consumers’ acceptance. Thus, cropping systems and preharvest treatments aim to improve fruit quality at harvest, and good results have been obtained by applying plant elicitors, such as methyl jasmonate, salicylic acid, acetyl salicylic acid, methyl salicylate and oxalic acid, with additional effects on increasing plant tolerance to abiotic stresses [75]. In this sense, PAs also play an important role in improving fruit quality traits. In fact, our research group performed an experiment in order to increase the peel and flesh colour in blood orange fruit (Citrus sinensis L. Osbeck), cv. ‘Sanguinelli’, by applying three PUT and SPD treatments, by foliar spraying 2 L per tree of 0.01, 0.1 or 1 mM of PUT or SPD, at monthly intervals, from October to 4 days before harvest. The best results in terms of increased peel and skin colour were observed for SPD, as can be seen in Figure 6A,B. Thus, the colour hue angle in peel was 55.2 and 44.6 in fruit from control and 0.01 SPD treated-trees, respectively, and 72.8 and 61.9 for flesh. It is important noting that lower hue angle values indicate a redder coloration, and in turn, fruit from the treated trees were redder than those from control trees, either in peel tissues as in flesh ones. Similar treatments with PUT and SPD also resulted in an increase in red colour at harvest in ‘Sunburst’ sweet cherries, as indicated by the lower hue angle values in fruit from treated-trees than in those from the controls (Figure 6C,D).
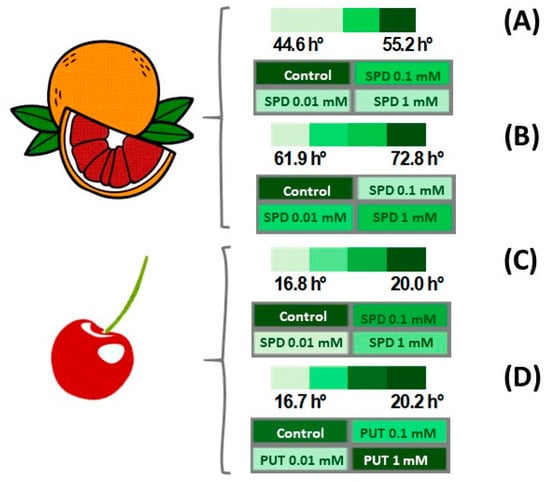
Figure 6.
Effect of the preharvest application of SPD on colour, expressed as the hue angle (h°), of skin (A) and flesh (B) at harvest in ‘Sanguinelli’ blood orange. The effect of the preharvest application of SPD (C) and PUT (D) on colour, expressed as hue angle (h°), in ‘Sunburst’ sweet cherry. The heatmaps show that a deeper green colour indicates higher hue angle values. Original data from the authors.
Blood oranges are characterised by a red-pigmented skin and flesh with low acidity and a balanced taste in sweetness and bitterness. This citrus cultivar, apart from accumulating carotenoids, synthesises anthocyanins, which makes it more attractive to consumers due to its red colour. ‘Sanguinelli’ is a late orange cultivar, which is harvested in Spain from mid-January to early April, and requires winters with low temperatures to develop its red colour. However, because of climate change, night-time temperatures are rising, leading to a reduction in anthocyanin biosynthesis and, in turn, less red pigmented fruit, which is detrimental to the fruit’s market quality. In this sense, preharvest treatments with PAs would lead to an increase in anthocyanin biosynthesis, even under these unfavourable temperatures.
Other research has reported that fruit juice acidity, which is an important fruit quality parameter for lemons, was increased by up to 2% by treating lemon trees with PUT at the beginning of the bud formation stage [49]. Similarly, overall quality traits, such as firmness, TSS and TSS/TA ratio and even bioactive compounds with antioxidant properties, such as phenolics and ascorbic acid, were increased in peach, mango and strawberry at harvest as a result of PUT trees or plant treatments [57,58,60], as well as in apricot and pear fruits after foliar spray treatments with PUT or SPD [56,76]. Accordingly, foliar spray treatments with PUT (1.5 or 5 mmol L−1) resulted in melon fruits with enhanced glucose, fructose and especially sucrose content, as well as with increased antioxidant activity with respect to controls [77]. Moreover, 150 ppm PUT spraying on papaya trees increased the fruit weight, flesh thickness, flesh/peel ratio and total reducing and non-reducing sugars, while decreasing fruit acidity, and these quality traits were maintained during storage, resulting in extended fruit shelf-life [78]. Fruit quality properties were also maintained during storage in grapes as a consequence of the 1 and 2 mM PUT or SPD preharvest foliar spraying of grapevines, since reduced berry weight loss, softening and fungal decay, and higher phenolic and anthocyanin contents and antioxidant activity were observed in fruit from treated plants as compared to berries from control grapevines [79]. Similarly, foliar spray treatments of 1, 2 and 3 mM PUT, at 14 days before harvest led to significantly reduced losses of fruit weight, total acidity and firmness, as well as to lower decay incidence [80]. The maintenance of firmness was attributed to the reduced activity of the cell-wall-degrading enzymes, such as pectin methylesterase (PME) and cellulase.
5.6. Fruit Disorders
Physiological disorders first appear in the external part of the fruit because of an stressful environment (nutrient deficiency, extreme temperatures or scarce water supply) during fruit development. As the fruit ripens, in some cases, physiological disorders appear in the skin and are considered a marketing defect. One of these disorders is creasing or albedo breakdown, which affects the peel of sweet oranges. This disorder was reduced by PUT (500–1000 μM) applied to a fruit set due to the enhanced albedo thickness and endogenous content of PUT, SPD and SPM [81].
Fruit growers must face the challenges of climate change, one of which is the unpredictability of the weather. In this sense, in southeastern Spain, excessive rainfall and heatwaves have occurred in recent years, affecting negatively crop yields. Excessive rainfall is responsible for others among the most severe fruit physiological disorders, namely cracking, which affects economically important fruit species, such as cherries, mandarins and pomegranates. Cherry cracking is characterised by the appearance of cracks in the cuticle. In the spring of 2023, southeastern Spain experienced a cold snap, which brought torrential rain and unsettled weather with many thunderstorms and flash floods. The results were devastating for sweet cherry, with a large number of fruits showing symptoms of cracking (Figure 7).

Figure 7.
Photograph of sweet cherry with cracking symptoms. Cold drop occurred on 24 May and harvest on 24 June 2024. Original figure from the authors.
However, PUT and SPD tree treatments were effective in reducing the number of cracked fruits which were measured after one week of heavy rainfall and at harvest (Table 2). It is worth nothing that 50% of the control cherries were not marketable, whereas the percentage of fruit with visual cracking was reduced to 30% on PUT- or SPD-treated trees. Cracking most commonly appears around the stem cavity where water can easily accumulate, but it can also occur anywhere else on the fruit. Cracking is therefore triggered by the difference in osmotic pressure between rainwater on the fruit epidermis and the concentrated solution of sugars and other substances in the cherry pulp, which causes water to enter the fruit to balance the osmotic difference. There are several theories that attempt to explain the mechanism of water absorption by cherries exposed to rain or high humidity. The different susceptibility of cherry cultivars to cracking is well known. It is believed that this phenomenon is related to the different permeability and elasticity of the cherry cuticle, which would allow more or less water to enter the interior of the cherry [82].

Table 2.
Effect of preharvest treatments with putrescine (PUT) and spermidine (SPD) on cracking (%) in ‘Skeena’ sweet cherries. Experiments were conducted in 2022 with nine trees per treatment *.
6. Conclusions and Future Prospects
PAs could be considered as a next generation of plant hormones, playing myriad roles in controlling many cellular functions, such as growth, development and stress tolerance. In this review, we report the latest information of PAs on their role in fruit development, from flowering to ripening as well as on crop yield and fruit quality properties. Upon flowering, PAs play important role, by increasing the percentage of functional ovules and fruit set, postulating that free PA concentrations (PUT, SPD and SPM) could be considered markers at this stage, since their content was high upon flowering. The enhancement of plant productivity, the improvement in agricultural sustainability, and enriched fruit quality, either at harvest or during storage, are important effects of the preharvest application of PAs. PAs have also been effective in enhancing stress tolerance and overcoming the stress negative impacts on crop yield. However, how PAs, alone or by cross-talk with other plant hormones, regulate the aforementioned processes, as well as the involved molecular mechanisms, remains unknown and deserving of further research.
Author Contributions
Conceptualisation, M.S. and D.V.; methodology, F.G.-A., J.P.-M., and M.E.G.-P.; investigation, F.G.-A., J.P.-M., and M.E.G.-P.; writing—original draft preparation, F.G.-A., J.P.-M., and M.E.G.-P.; writing—review and editing, M.S. and D.V.; visualisation, all authors; supervision, M.S. and D.V.; project administration, M.S. and D.V.; funding acquisition, M.S. and D.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Direcció General de Ciència i Investigació of the Generalitat Valenciana, Spain, throughout the project PROMETEO/2021/089.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
Authors thank the Direcció General de Ciència i Investigació of the Generalitat Valenciana, Spain, for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Michael, A.J. Polyamine function in archaea and bacteria. J. Bio. Chem. 2018, 293, 18693–18701. [Google Scholar] [CrossRef]
- Pegg, A.E. Introduction to the Thematic Minireview Series: Sixty plus years of polyamine research. J. Biol. Chem. 2018, 293, 18681–18692. [Google Scholar] [CrossRef]
- Jangra, A.; Chaturvedi, S.; Kumar, N.; Singh, H.; Sharma, V.; Thakur, M.; Tiwari, S.; Chhokar, V. Polyamines: The gleam of next-generation plant growth regulators for growth, development, stress mitigation, and hormonal crosstalk in plants—A systematic review. J. Plant Growth Regul. 2023, 42, 5167–5191. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Blázquez, M.A. Polyamines: Their role in plant development and stress. Annu. Rev. Plant Biol. 2024, 75, 95–117. [Google Scholar] [CrossRef]
- Bachrach, U. The early history of polyamine research. Plant Physiol. Biochem. 2010, 48, 490–495. [Google Scholar] [CrossRef]
- Takahashi, T.; Tong, W. Regulation and diversity of polyamine biosynthesis in plants. In Polyamines, 2nd ed.; Kusano, T., Suzuki, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 27–44. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Biosynthetic pathways of hormones in plants. Metabolites 2023, 13, 884. [Google Scholar] [CrossRef]
- Fortes, A.M.; Agudelo-Romero, P. Polyamine metabolism in climacteric and non-climacteric fruit ripening. In Polyamines. Methods in Molecular Biology, 2nd ed.; Alcázar, R., Tiburcio, A., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1694, pp. 433–447. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Alcazar, R. Potential applications of polyamines in agriculture and plant biotechnology. In Polyamines. Methods and Protocols., 2nd ed.; Tiburcio, A.F., Alcazar, R., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1694, pp. 489–508. [Google Scholar]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotech. 2019, 48, 53–65. [Google Scholar] [CrossRef]
- Wang, W.; Paschalidis, K.; Feng, J.C.; Song, J.; Liu, J.H. Polyamine catabolism in plants: A universal process with diverse functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef]
- Kasahara, N.; Teratani, T.; Yokota, S.; Sakuma, Y.; Sasanuma, H.; Fujimoto, Y.; Ijichi, T.; Urahashi, T.; Yoshitomi, H.; Kitayama, J.; et al. Dietary polyamines promote intestinal adaptation in an experimental model of short bowel syndrome. Sci Rep. 2024, 14, 4605. [Google Scholar] [CrossRef]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Bardócz, S. Polyamines in food and their consequences for food quality and human health. Trends Food Sci. Technol. 1995, 6, 341–346. [Google Scholar] [CrossRef]
- Ruiz-Cano, D.; Pérez-Llamas, F.; Zamora, S. Polyamines, implications for infant health. Arch. Argent. Pediatr. 2012, 110, 244–250. [Google Scholar] [CrossRef]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Casero, R.A.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer. 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Hirano, R.; Shirasawa, H.; Kurihara, S. Health-promoting effects of dietary polyamines. Med. Sci. 2021, 9, 8. [Google Scholar] [CrossRef]
- Sánchez, M.; Suárez, L.; Banda, G.; Barreiro-Alonso, E.; Rodríguez-Uña, I.; Rubín, J.M.; Cantabrana, B. Age-associated polyamines in peripheral blood cells and plasma in 20 to 70 years of age subjects. Amino Acids 2023, 55, 789–798. [Google Scholar] [CrossRef]
- Holbert, C.E.; Casero, R.A.; Stewart, T.M. Polyamines: The pivotal amines in influencing the tumor microenvironment. Discov. Oncol. 2024, 15, 173. [Google Scholar] [CrossRef]
- Eliassen, K.A.; Ragnhild, R.; Risøen, U.; Rønning, H.F. Dietary polyamines. Food Chem. 2002, 78, 273–280. [Google Scholar] [CrossRef]
- Kalač, P.; Křıžek, M.; Pelikánová, T.; Langová, M.; Veškrna, O. Contents of polyamines in selected foods. Food Chem. 2005, 90, 561–564. [Google Scholar] [CrossRef]
- Moret, S.; Smela, D.; Populin, T.; Conte, L. A survey on free biogenic amine content of fresh and preserved vegetables. Food Chem. 2005, 89, 355–361. [Google Scholar] [CrossRef]
- Cipolla, B.G.; Havouis, R.; Moulinoux, J.P. Polyamine contents in current foods: A basis for polyamine reduced diet and a study of its long-term observance and tolerance in prostate carcinoma patients. Amino Acids 2007, 33, 203–212. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of Food and Agriculture: Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- Malik, N.A.A.; Kumar, I.S.; Nadarajah, K. Elicitor and receptor molecules: Orchestrators of plant defense and immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Mendoza, P.L.; Rico-Chávez, A.K.; Ferrusquía-Jimenez, N.I.; Carbajal-Valenzuela, I.A.; Villagómez-Aranda, A.L.; Torres-Pacheco, I.; Guevara-González, R.G. Science of the total environment plant hormesis: Revising of the concepts of biostimulation, elicitation and their application in a sustainable agricultural production. Sci. Total Environ. 2023, 894, 164883. [Google Scholar] [CrossRef] [PubMed]
- Melo-Sabogal, D.V.; Contreras-Medina, L.M. Elicitors and biostimulants to mitigate water stress in vegetables. Horticulturae 2024, 10, 837. [Google Scholar] [CrossRef]
- Kaur-Shawney, R.; Tiburcio, A.F.; Altabella, T.; Galston, A.W. Polyamines in plants: An overview. J. Cell Mol. Biol. 2003, 2, 1–12. [Google Scholar]
- Sequera-Mutiozabal, M.; Antoniou, C.; Tiburcio, A.F.; Alcázar, R.; Fotopoulos, V. Polyamines: Emerging hubs promoting drought and salt stress tolerance in plants. Curr. Mol. Biol. Rep. 2017, 3, 28–36. [Google Scholar] [CrossRef]
- Amiri, H.; Banakar, M.H.; Hassan Gavyar, P.H. Polyamines: New plant growth regulators promoting salt stress tolerance in plants. J. Plant Growth Regul. 2024, 43, 4923–4940. [Google Scholar] [CrossRef]
- Roy, T.; Pal, N.; Das, N. Regulation of the polyamine pool in plants: Metabolic implications and stress mitigation, with emphasis on microbial influence. Physiol. Mol. Plant Pathol. 2024, 132, 102317. [Google Scholar] [CrossRef]
- Yang, H.; Fang, Y.; Liang, Z.; Qin, T.; Liu, J.H.; Liu, T. Polyamines: Pleiotropic molecules regulating plant development and enhancing crop yield and quality. Plant Biotechnol. J. 2024, 22, 3194–3201. [Google Scholar] [CrossRef]
- Asija, S.; Seth, T.; Umar, S.; Gupta, R. Polyamines and their crosstalk with phytohormones in the regulation of plant defense responses. J. Plant Growth Regul. 2023, 42, 5224–5246. [Google Scholar] [CrossRef]
- Liu, X.D.; Zeng, Y.Y.; Zhang, X.Y.; Tian, X.Q.; Hasan, M.M.; Yao, G.Q.; Fang, X.Z. Polyamines inhibit abscisic acid-induced stomatal closure by scavenging hydrogen peroxide. Physiol. Plant. 2023, 175, e13903. [Google Scholar] [CrossRef] [PubMed]
- Navakoudis, E.; Kotzabasis, K. Polyamines: A bioenergetic smart switch for plant protection and development. J. Plant Physiol. 2022, 270, 153618. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pareek, S.; Sagar, N.A.; Valero, D.; Serrano, M. Modulatory effects of exogenously applied polyamines on postharvest physiology, antioxidant system and shelf life of fruits: A review. Int. J. Mol. Sci. 2017, 18, 1789. [Google Scholar] [CrossRef]
- Fortes, A.M.; Agudelo-Romero, P.; Pimentel, D.; Alkan, N. Transcriptional modulation of polyamine metabolism in fruit species under abiotic and biotic stress. Front. Plant Sci. 2019, 10, 816. [Google Scholar] [CrossRef]
- Kaur, Y.; Das, N. Roles of polyamines in growth and development of the solanaceous crops under normal and stressful conditions. J. Plant Growth Regul. 2023, 42, 4989–5010. [Google Scholar] [CrossRef]
- Zahid, G.; Iftikhar, S.; Shimira, F.; Ahmad, H.M.; Aka Kaçar, Y. An overview and recent progress of plant growth regulators (PGRs) in the mitigation of abiotic stresses in fruits: A review. Sci. Hortic. 2023, 309, 111621. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chaudhury, R.; Dutta, S.; Basak, M.; Dey, S.; Schäffner, A.R.; Das, M. Role of metabolites in flower development and discovery of compounds controlling flowering time. Plant Physiol. Biochem. 2022, 190, 109–118. [Google Scholar] [CrossRef]
- Agustí, M. Preharvest factors affecting postharvest quality of citrus fruit. In Postharvest Diseases and Disorders Control of Citrus Fruit, 2nd ed.; Schirra, M., Ed.; Research Singpost: Trivandrum, India, 1999; pp. 1–34. [Google Scholar]
- Alburquerque, N.; Egea, J.; Burgos, L.; Martínez-Romero, D.; Valero, D.; Serrano, M. The influence of polyamines on apricot ovary development and fruit set. Ann. Appl. Biol. 2006, 149, 27–33. [Google Scholar] [CrossRef]
- De Dios, P.; Matilla, A.J.; Gallardo, M. Flower fertilization and fruit development prompt changes in free polyamines and ethylene in damson plum (Prunus insititia L.). J. Plant Physiol. 2006, 163, 86–97. [Google Scholar] [CrossRef]
- Gupta, S.; Novák, O.; Kulkarni, M.G.; Doležalova, I.; Van Staden, J.; Doležal, K. Unleashing the potential of biostimulants in stimulating pollen germination and tube growth. J. Plant Growth Regul. 2024, 43, 3392–3423. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, X.; Yan, J.; Fan, L.; Rong, C.; Mo, C.; Zhang, M. Effect of exogenous spermidine on floral induction, endogenous polyamine and hormone production, and expression of related genes in ‘Fuji’ apple (Malus domestica Borkh.). Sci. Rep. 2019, 9, 12777. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, Z.; Haghighi, M.; Kappel, N. The effect of spermidine and methionine application thorough two biosynthetic paths on flowering of early and late flowering genotypes of eggplant (Solanum melongena L.). Sci. Hortic. 2022, 306, 111459. [Google Scholar] [CrossRef]
- Karabiyik, Ş. Putrescine affects fruit yield and quality by promoting effective pollination period in Citrus limon. Appl. Fruit Sci. 2024, 66, 559–567. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality, 1st ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2010; p. 287. [Google Scholar] [CrossRef]
- Bons, H.K.; Kaur, M. Role of plant growth regulators in improving fruit set, quality and yield of fruit crops: A review. J. Hortic. Sci. Biotechnol. 2019, 95, 137–146. [Google Scholar] [CrossRef]
- Talaat, N.B.; Nesiem, M.R.A.; Gadalla, E.G.; Ali, S.F. Putrescine, in combination with gibberellic acid and salicylic acid, improves date palm fruit quality via triggering protein and carbohydrate accumulation and enhancing mineral, amino acid, sugar, and phytohormone acquisition. J. Plant Growth Regul. 2023, 1–17. [Google Scholar] [CrossRef]
- Hadjipieri, M.; Georgiadou, E.C.; Drogoudi, P.; Fotopoulos, V.; Manganaris, G.A. The efficacy of acetylsalicylic acid, spermidine and calcium preharvest foliar spray applications on yield efficiency, incidence of physiological disorders and shelf-life performance of loquat fruit. Sci. Hortic. 2021, 289, 110439. [Google Scholar] [CrossRef]
- Sayyad-Amin, P.; Davarynejad, G.H.; Abedy, B. The effect of polyamines and SICS on the compatibility, fertility and yield indices of apple cv. Golden Delicious. Adv. Hortic. Sci. 2018, 32, 213–219. [Google Scholar] [CrossRef]
- Tavakoli, K.; Rahemi, M. Effect of polyamines, 2, 4-D, isopropyl ester and naphthalene acetamide on improving fruit yield and quality of date (Phoenix dactylifera L.). Int. J. Hortic. Sci. Technol. 2014, 1, 163–169. [Google Scholar] [CrossRef]
- Ali, E.A.; Sarrwy, S.M.A.; Hassan, H.S.A. Improving Canino apricot trees productivity by foliar spraying with polyamines. J. Appl. Sci. Res. 2010, 6, 1359–1365. [Google Scholar]
- Ali, I.; Abbasi, N.A.; Hafiz, I.A. Physiological response and quality attributes of peach fruit cv. Flordaking as affected by different treatments of calcium chloride, putrescine and salicylic acid. Pak. J. Agric. 2014, 51, 33–39. [Google Scholar]
- Almutairi, K.F.; Górnik, K.; Ayoub, A.; Abada, H.S.; Mosa, W.F.A. Performance of mango trees under the spraying of some biostimulants. Sustainability 2023, 15, 15543. [Google Scholar] [CrossRef]
- Ghahremani, Z.; Alizadeh, B.; Barzegar, T.; Nikbakth, J.; Ranjbar, M.E.; Nezamdoost, D. The mechanism of enhancing drought tolerance threshold of pepper plant treated with putrescine and salicylic acid. Plant Stress 2023, 9, 100199. [Google Scholar] [CrossRef]
- Asghari, M.; Ahmadi, F.; Hajitagilou, R. Mitigating the adverse effects of deficit fertigation on strawberry yield, quality and phytochemical compounds by salicylic acid and putrescine treatments. J. Berry Res. 2021, 11, 119–132. [Google Scholar] [CrossRef]
- Hagagg, L.F.; Abd-Alhamid, N.; Hassan, H.S.; Hassan, A.M.; Geanidy, E.A. Influence of foliar application with putrescine, salicylic, and ascorbic acid on the productivity and physical and chemical fruit properties of picual olive trees. Bull. Nat. Res. Cent. 2020, 44, 87. [Google Scholar] [CrossRef]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A.; et al. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 4, 765068. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene control of fruit ripening: Revisiting the complex network of transcriptional regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; López-Miranda, S.; Pérez-López, A.J.; Acosta-Motos, J.R. Strategies to delay ethylene-mediated ripening in climacteric fruits: Implications for shelflife extension and postharvest quality. Horticulturae 2024, 10, 840. [Google Scholar] [CrossRef]
- Perotti, M.F.; Posé, D.; Martin-Pizarro, C. Non-climacteric fruit development and ripening regulation. The Phytohormones Show. J. Exp. Bot. 2023, 74, 6237–6253. [Google Scholar] [CrossRef]
- Gao, F.; Li, J.; Li, W.; Shi, S.; Song, S.; Shen, Y.; Guo, J. Abscisic acid and polyamines coordinately regulate strawberry drought responses. Plant Stress 2024, 11, 100387. [Google Scholar] [CrossRef]
- Bianchetti, R.; Ali, A.; Gururani, M. Abscisic acid and ethylene coordinating fruit ripening under abiotic stress. Plant Sci. 2024, 349, 112243. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Singh, R.; Kaur, H.; Kumar, A.; Vashishth, A.; Shadwan, M.; Sing Tuli, H. Plant growth regulator-mediated response under abiotic stress: A review. J. App. Biol. Biotechnol. 2024, 12, 13–21. [Google Scholar] [CrossRef]
- Kou, X.; Feng, Y.; Yuan, S.; Zhao, X.; Wu, C.; Wang, C.; Xue, Z. Different regulatory mechanisms of plant hormones in the ripening of climacteric and non-climacteric fruits: A review. Plant Mol. Biol. 2021, 107, 477–497. [Google Scholar] [CrossRef]
- Guo, J.; Wang, S.; Yu, X.; Dong, R.; Li, Y.; Mei, X.; Shen, Y. Polyamines regulate strawberry fruit ripening by abscisic acid, auxin, and ethylene. Plant Physiol. 2018, 177, 339–351. [Google Scholar] [CrossRef]
- Killiny, N.; Nehela, Y. Citrus polyamines: Structure, biosynthesis, and physiological functions. Plants 2020, 9, 426. [Google Scholar] [CrossRef]
- Serrano, M.; Zapata, P.J.; Martínez-Romero, D.; Díaz-Mula, H.M.; Valero, D. Polyamines as an ecofriendly postharvest tool to maintain fruit quality. In Eco-Friendly Technology for Postharvest Produce Quality; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 219–242. [Google Scholar] [CrossRef]
- Serrano, M.; Valero, D. Application of polyamines to maintain functional properties in stored fruits. In Polyamines. Methods in Molecular Biology, 2nd ed.; Alcázar, R., Tiburcio, A., Eds.; Humana Press: New York, NY, USA, 2018; pp. 449–458. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Garrido-Auñón, F.; Puente-Moreno, J.; Díaz-Mula, M.H.; Serrano, M.; Valero, D. The effects of preharvest treatments on the postharvest storage quality of different horticultural products. In Sustainable Postharvest Technologies for Fruits and Vegetables, 2nd ed.; Ali, S., Mir, S.A., Dar, B.N., Ejaz, S., Eds.; CRC Press: Oxford, UK, 2024; Volume 29, pp. 442–456. [Google Scholar]
- Sayyad-Amin, P.; Davarynejad, G.; Abedi, B.; Ameri, A. The effects of putrescine and salicylic acid on postharvest traits of pear (Pyrus communis L. ‘Williams’). Erwerbs-Obstbau 2022, 64, 753–757. [Google Scholar] [CrossRef]
- Piñero, M.C.; Otálora, G.; Collado, J.; López-Marín, J.; Del Amor, F.M. Foliar application of putrescine before a short-term heat stress improves the quality of melon fruits (Cucumis melo L.). J. Sci. Food Agric. 2021, 101, 1428–1435. [Google Scholar] [CrossRef]
- Rao, G.S.K.; Krishna, V.S.; Srinivasulu, B.; Sivaram, G.T.; Padmaja, V.; Arunodhayam, K. Effect of foliar sprays of different chemicals and plant growth regulators on quality attributes of papaya (Carica papaya L.) cv. Arka Surya. J. Pharm. Innov. 2023, 12, 4201–4208. [Google Scholar]
- Mirdehghan, S.H.; Rahimi, S. Pre-harvest application of polyamines enhances antioxidants and table grape (Vitis vinifera L.) quality during postharvest period. Food Chem. 2016, 196, 1040–1047. [Google Scholar] [CrossRef]
- Singh, V.; Jawandha, S.K.; Gill, P.P.S.; Singh, D. Preharvest putrescine application extends the shelf life and maintains the pear fruit quality. Int. J. Fruit Sci. 2022, 22, 514–524. [Google Scholar] [CrossRef]
- Hussain, Z.; Singh, Z. Involvement of polyamines in creasing of sweet orange Citrus sinensis (L.) Osbeck fruit. Sci. Hortic. 2015, 190, 203–210. [Google Scholar] [CrossRef]
- Ruiz-Aracil, M.C.; Valverde, J.M.; Beltrá, A.; Carrión-Antolí, A.; Lorente-Mento, J.M.; Nicolás-Almansa, M.; Guillén, F. Putrescine increases frost tolerance and effectively mitigates sweet cherry (Prunus avium L.) cracking: A study of four different growing cycles. Agronomy 2024, 14, 23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).