Abstract
The study aimed to evaluate the cold tolerance of various peach cultivars under diverse low-temperature conditions (−5, −10, −15, −20, −25, −30, and −35 °C). A comprehensive assessment of their responses to cold was performed by integrating LT50 values with membership functions and evaluating local adaptability among the selected peach cultivars. The findings revealed that as temperatures dropped, electrical conductivity (REC), malondialdehyde (MDA), and hydrogen peroxide (H2O2) levels initially rose, then fell, and subsequently increased once more. Soluble sugar (SS) and soluble protein (SP) concentrations peaked at −25 °C and showed a significant negative correlation with semi-lethal temperature (LT50). The expression of free proline varied among different samples. Combining physiological analyses with field adaptation correlation assessments, it was found that ‘Ziyan Ruiyang’ exhibited a relatively low LT50 value of −29.67 °C and a membership function degree of 0.76, suggesting robust field adaptation abilities. At the same time, ‘Ganlu Shumi’ demonstrated stable trends in H2O2 and MDA levels, maintaining them at relatively low concentrations; it also had the lowest LT50 value, the highest membership function score, and the highest survival rate. Consequently, this cultivar could be a valuable resource for enhancing cold resistance under low-temperature stress. In summary, by correlating LT50 values with membership functions and observing local adaptability in these peach cultivars, we have established reliable data that can serve as a basis for identifying potential cross-breeding parents to develop new cold-resistant varieties.
1. Introduction
Peach (Prunus persica) is a highly esteemed fruit, and China serves as a major producer of this crop. According to data from the United Nations Food and Agriculture Organization (FAO), the area allocated for peach cultivation in China reached 825,000 hectares in 2021, resulting in a production volume of approximately 16.0165 million tonnes (https://www.abeedata.com/home/article/detail/id/20990, accessed on 23 October 2024). The abundance of solar and thermal resources constitutes one of the key factors contributing to the production of high-quality peaches. Gansu Province, situated in northwest China, benefits from rich solar and thermal resources along with substantial temperature variations between day and night. These conditions provide natural advantages for producing premium-quality peaches. However, low winter temperatures represent one of the primary challenges hindering the development of the peach industry in this region. Therefore, selecting cold-resistant peach varieties emerges as one of the most effective strategies to mitigate freezing damage [1].
Gansu Province is one of the origins of peach cultivation, boasting abundant peach resources and a diverse array of varieties. It serves as a crucial area for the preservation, evolution, and cultivation of peach germplasm resources in China. However, in the Hexi Corridor area of Gansu Province, its average absolute minimum temperature can easily cause frost damage to the cultivated species introduced every year [2]. Through long-term natural selection and artificial cultivation, this region has developed a group of local peach cultivars characterized by strong cold resistance, which represent valuable germplasm material for improving peach varieties and enhancing resistance breeding [3,4]. In this study, seven outstanding peach varieties from the Hexi Corridor in Gansu Province were selected as experimental materials to observe physiological and biochemical indices as well as adaptability. Evaluating their cold resistance holds significant importance for the utilization of germplasm resources and the breeding of superior cold-resistant varieties.
The cold resistance of fruit trees induced by low-temperature stress is a complex physiological and biochemical process reflected through various physiological and biochemical indices [5]. The conductivity method is a conventional technique employed to assess the freezing resistance of in vitro tissues. This method offers several advantages, including good fitting accuracy and simplicity, making it widely applicable across different plant species to effectively quantify their cold resistance [6,7]. Typically, the Logistic equation is an essential formula that links relative conductivity and semi-lethal temperature (LT50). The Logistic equation determines the LT50 of plants by fitting the relationship between relative conductivity and temperature. Thus, measures can be adopted to ensure the safety of plants during winter [8]. Hydrogen peroxide (H2O2) is one of the reactive oxygen species (ROS) generated during the metabolic processes within plant cells [9]. As a signaling molecule, H2O2 plays a crucial role in regulating plant growth and development while also aiding in stress responses [10]. Excessive accumulation of H2O2 can lead to damage to biological macromolecules as it reacts with intracellular lipids, proteins, and DNA, resulting in oxidative damage and disruptions in cellular metabolism that may ultimately harm plants [11]. At the same time, H2O2 enhances plants’ osmoregulatory capacity by regulating levels of osmoregulatory substances such as proline and soluble sugars; this mechanism contributes significantly to improving their tolerance against low-temperature stress [12]. MDA serves as an indicator of lipid peroxidation within plant cell membranes, and its concentration can indirectly reflect the extent of membrane system damage and the stress resistance of plants. Under stressful conditions, the antioxidant mechanisms in plants become activated to eliminate ROS present in their systems [13]. When these antioxidant defenses are insufficient to maintain homeostasis, an accumulation of excessive ROS occurs, leading to membrane lipid peroxidation and subsequent production of malondialdehyde (MDA). Numerous studies have demonstrated that the cold tolerance of plants can be assessed by measuring MDA activity, which is regarded as a final product of lipid peroxidation [14,15]. Under low-temperature stress, plant roots sustain damage that impairs water absorption capacity, resulting in reduced water status, stomatal conductance, and photosynthetic efficiency [16]. Through osmotic regulation processes, plants actively accumulate various organic or inorganic solutes such as soluble sugars, soluble proteins, and proline. This accumulation enhances cellular fluid concentration while lowering osmotic potential, consequently improving water uptake ability and bolstering cold resistance, thereby facilitating survival in low-temperature environments [17]. For instance, under conditions of low-temperature stress, exogenous application of salicylic acid has been shown to enhance the activity of antioxidant enzymes within seed embryos. This treatment mitigates damage induced by cold stress through increased levels of reduced glutathione along with osmoregulatory substances, including proline, soluble proteins, and soluble sugars [18]. However, plant cold resistance is a quantitative trait influenced by numerous factors. Therefore, evaluating multiple cold resistance indices can mitigate the errors associated with relying on a single index. The average membership function degree [19] was calculated using a specific formula to assess the differences in cold resistance among plants, thereby reducing the original correlations among the indices and enhancing the reliability of the evaluation metrics. This comprehensive identification method provides a more thorough and accurate reflection of plants’ actual cold resistance capabilities.
When plants overwinter, they are susceptible to frost damage under natural conditions. The field identification method assesses the cold resistance of plants by evaluating, comparing, and classifying the organs and tissues of frozen specimens according to specific standards [20]. Generally, the cold resistance of deciduous fruit trees is evaluated based on the degree of freezing damage observed in their branches. For instance, Cui [21] et al. employed this method to select apricot germplasm resources exhibiting strong cold resistance. Similarly, Gao [22] et al. utilized it to compare the cold resistance among five grape rootstocks introduced into the Shihezi area. The field identification method is both straightforward and objective, allowing for an evaluation of cold resistance across large-scale and extensive groups. Although it is a traditional technique, varieties selected through this method are considered highly accurate and reliable when tested within a natural environment. At present, studies on peach’s low-temperature stress mostly focus on the determination of physiological indicators and then screening cold-resistant varieties by membership function or semi-lethal temperature. There are still few studies on the comprehensive analysis of plant cold resistance by combining physiological and biochemical data (laboratory data) with field test data.
In this study, we measured the relative electrical conductivity (REC), the concentration of osmotic regulating substances (including soluble sugars, soluble proteins, and proline), as well as the concentrations of malondialdehyde and hydrogen peroxide in seven peach varieties subjected to different temperature gradients. The cold resistance of these varieties in the laboratory was evaluated comprehensively by the membership function method. Additionally, their adaptability to cold resistance in field environments was assessed through cultivation in open fields located in cold regions. Then, combined with laboratory data and field data, the best cold-resistant peach varieties were screened. This comprehensive analysis not only focuses on the theoretical mechanism but also emphasizes the application of cold resistance in practical agricultural production and provides a theoretical basis for cultivating new cold-resistant varieties and selecting suitable cultivars.
2. Material and Methods
2.1. Overview of the Experimental Site
The field test site is situated within the experimental demonstration base for new peach varieties in Tuanjie Village, Wenshu Town, Jiayuguan City (East longitude: 98°24′, North latitude: 39°40′). The altitude of the site is 1650 m, with an annual average sunshine duration of 2634 h. The average annual temperature is recorded at 6.9 °C, while the extreme minimum temperature can drop to −31.6 °C. The annual effective accumulated temperature of ≥10 °C totals 3064 °C (The sum of the daily effective cumulative temperature we selected, daily effective accumulated temperature = the average temperature of the day (more than 15 °C) −10 °C), and the frost-free period lasts for approximately 130 days [23]. Annual precipitation averages between 82 and 86 mm, and irrigation facilities are available to support cultivation. The soil organic matter content is 0.92%, and the pH value is 8.7.
2.2. Plant Materials
Six peach varieties, ‘Ziyan Ruiyu’, ‘Ziyan Ruiyang’, ‘Ziyan Ruiqiu’, ‘Ganlu Shumi’, ‘Ganlu Qiumi’, and ‘Ganlu Shuangmi’, were selected as the experimental materials, which are Gansu local cold-resistant resources offspring. The control variety used in this study was ‘Qingpi Liguang’ (Gansu local resources). The branches were sampled from the Gansu Academy of Agricultural Sciences Peach Germplasm Repository, located in Gansu province, China. All the resources’ rootstocks consisted of Honghua Shantao (P. davidiana Franch). On 15 January 2022 (Figure 1), a total of 80 branches of equal length (25–30 cm) and diameter (0.8–1.0 cm) were collected from all sides of five-year-old trees. The branches were cleaned using distilled water, and their shoot scissors were sealed with paraffin wax. Each branch was divided into eight groups, and each group was carefully wrapped with gauze and plastic bags for protection during subsequent treatments. The groups were randomly assigned to seven adjustable temperature incubators, set to temperatures of −5 °C (control), −10 °C, −15 °C, −20 °C, −25 °C, −30 °C and −35 °C. The cold treatment lasted for 12 h, and the cooling or heating rate was 4 °C/h. After cold exposure, the middle parts of the branches (excluding flower and leaf buds) were sampled promptly and frozen in liquid nitrogen before being stored at −80 °C until further use (electrolyte leakage measurements excluded freezing effects). Branch materials were obtained from three different trees as independent biological replicates.

Figure 1.
Growth status of experimental plants. (A) Images of the peach blossom period, taken on 27 April 2024. (B) Pictures of the dormant period, taken on 25 November 2024.
2.3. Test Methods
Physical and Chemical analysis: REC is representative of membrane integrity and is determined with a conductometer [24]. In the Logistic equation: y = k/(1 + ae−bt), y is the relative conductivity, t represents the treatment temperature, k is the saturation capacity of the relative conductivity, and a and b are the equation parameters. In order to determine the values of a and b, the equation is linearized, and the values of a and b and the correlation coefficient r are obtained by linear regression. If the second derivative of the Logistic equation is equal to zero, the inflection point t = lna/b of the curve can be obtained, and the value of t at this time is the semi-lethal temperature (LT50). The hydrogen peroxide (H2O2) detection kit provided by Suzhou Keming Biotechnology Co., Ltd. was used to determine the H2O2 content by spectrophotometry. Lipid peroxidation levels are an indicator of oxidative stress, and MDA levels are determined by the thiobarbiturate (TBA) reaction [25]. Anthrone colorimetry is used to determine the level of soluble sugars that indicate carbohydrate metabolism [26]. Soluble protein concentrations were assessed using the coomassie blue staining method [27]. Proline (Pro) concentrations, which signal osmoregulation, were evaluated using the sulfosalicylate-acid ninhydrin method [28].
Survival rate and Freezing damage index: Seedlings exhibiting uniform growth were selected for planting using “Prunus davidiana” as rootstock in 2019 spring. Each variety was planted with a total of 40 specimens per plot. The survival rate and freezing damage index statistics for three consecutive years from 2021 to 2023 are calculated as follows:
Survival rate = number of surviving plants/number of planted plants × 100%.
Freezing damage index = (1 × S1 + 2 × S2 + 3 × S3 + 4 × S4 + 5 × S5)/(Number of branches investigated × 5) × 100%.
Among them, S1, S2, S3, S4, and S5 are the number of new shoots representing levels 0–5. The lower the freezing damage index, the lighter the degree of damage.
Damage levels:
Level 0: No stripping, no impact on yield.
Level 1: Less than 1/3 one-branch stripping, no impact on yield.
Level 2: Less than 1/2 one-branch stripping, slight effects on yield.
Level 3: Less than 3/4 one-branch stripping, medium effects on yield.
Level 4: All one-branch stripping, serious effects on yield.
Level 5: All one-branch stripping, the trunk is damaged, no yield.
2.4. Statistical Analysis
Excel 2010 and SPSS Statistics 24.0 software were used for data collation and preliminary statistical analysis, and Origin 2018 64Bit was used for mapping. Three independent biological replicates were used for each experiment. Duncan’s multivariate range test was used to discriminate and statistically distinguish the mean values. A p-value less than 0.05 was considered statistically significant, and all data points are expressed as mean ± standard error.
3. Results
3.1. REC and LT50 Under Various Low-Temperature Induction Conditions
As illustrated in Figure 1, the REC of seven varieties exhibited a gradual increase with decreasing treatment temperatures above −20 °C. Most varieties experienced a slight decline at −25 °C, followed by a rapid increase in REC as the temperature continued to drop. The highest REC was recorded at −35 °C. Notably, the inflection point for ‘Ziyan Ruiyu’ occurred at −20 °C, while the remaining six varieties displayed their inflection points at −25 °C. Furthermore, five peach varieties did not exceed 50% relative conductivity when subjected to −25 °C. Quantitatively, the average REC at −35 °C was found to be 166.55% higher than that observed at −5 °C (see Figure 2).
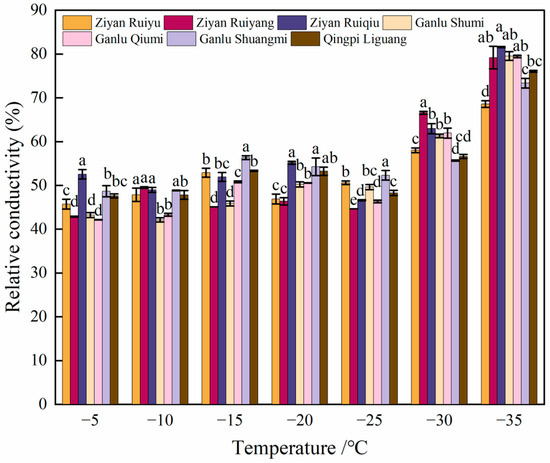
Figure 2.
Changes in relative electric conductivity of seven varieties of peach under different low temperatures. Data are mean ± SE (n = 3), and different letters indicated significant differences among different varieties (p < 0.05, Duncan test).
The REC measurements obtained from various low-temperature treatments, in conjunction with the LT50 derived from the Logistic equation, serve as effective indicators for assessing plant sensitivity and adaptability to cold stress. Fundamentally, a lower LT50 value signifies greater tolerance of a given plant to cold stress. A comprehensive analysis of LT50 values across seven peach source branches revealed a range from −32.24~−27.24 °C. The lowest correlation coefficient (R2) was 0.76, indicating a strong positive correlation between REC and LT50, which was statistically significant at p < 0.05 (Table 1). The observed differences in cold resistance among various resources highlight the genetic and physiological diversity within peach species, carrying important implications for agricultural practices across different climatic zones.

Table 1.
Logistics equation of the relative electric conductivity and the median lethal temperature (LT50) of seven peach varieties.
3.2. Changes in Lipid Membrane Peroxides (H2O2, MDA) Content
The analysis of H2O2 levels in annual shoots revealed a general trend characterized by an initial increase, followed by a decrease and then another rise. The first peak was observed at −15 °C (Figure 3A). At this temperature, the varieties ‘Ganlu Shumi’, ‘Ganlu Qiumi’, ‘Ganlu Shuangmi’, and ‘Qingpi Liguang’ exhibited their highest H2O2 content. Most varieties experienced a decline at −20 °C; however, the variety of ‘Ziyan Ruiyu’ demonstrated a rapid increase in H2O2 levels at this temperature, reaching a maximum of 44.34 μmol g−1 at −30 °C a value that is 2.60 times greater than that recorded at the control temperature. At −25 °C, H2O2 content continued to decrease with further reductions in temperature, while most varieties displayed their second peak at −30 °C; notably, ‘Ziyan Ruiyu’, ‘Ziyan Ruiyang’, and ‘Ziyan Ruiqiu’ achieved their highest concentrations. At −20 °C, the H2O2 levels for ‘Ganlu Shumi’, ‘Ziyan Ruiqiu’, and ‘Ziyan Ruiyang’ remained consistently low throughout the observation period.
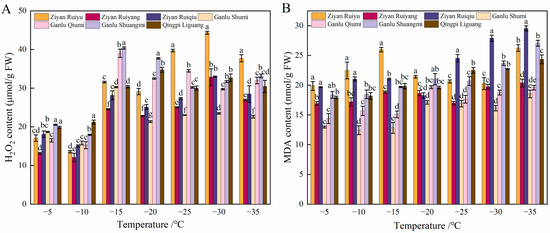
Figure 3.
Changes of H2O2 and MDA contents in six peach varieties at different low temperatures. (A,B) represent H2O2 and MDA content, respectively. Data are mean ± SE (n = 3), and different letters indicated significant differences among different varieties (p < 0.05, Duncan test).
The MDA content in annual shoots revealed a general trend characterized by an initial increase, followed by a decrease, and then another rise (Figure 3B). Seven varieties showed no significant difference in MDA content at −5 °C. When the temperature was higher than −20 °C, the MDA content of ‘Ziyan Ruiyu’ reached its peak, and it decreased under the low-temperature stress of −25 °C. As the temperature decreased, the MDA content increased sharply at −30 °C, and the MDA content of ‘Ziyan Ruiyang’, ‘Ganlu Shumi’ and ‘Ganlu Qiumi’ remained at a low level. The MDA content of ‘Ziyan Ruiyang’ and ‘Ganlu Shuangmi’ was relatively stable. Therefore, the overall performance was that the higher the LT50 of peach resources, the higher the MDA level after low-temperature stress, and more MDA was accumulated during the low-temperature treatment process.
3.3. Changes of SS, SP, and Contents of Proline(Pro) Under Different Low-Temperature Inductions
The results of SS under low-temperature induction are presented in Figure 4A. As the temperature decreased, the SS content in annual peach branches exhibited a trend of initially increasing and then decreasing. At −5 °C, there was no significant difference in SS content among all varieties (p > 0.05). Subsequently, the SS content increased with further decreases in temperature, experienced a slight decline at −20 °C, reached its peak at −25 °C, and then gradually decreased thereafter. Under the low-temperature stress of −25 °C, ‘Ganlu Qiumi’ exhibited the highest SS content among the six varieties at 48.07%, which is 2.07 times greater than that of the control group (−5 °C). Conversely, ‘Ziyan Ruiyu’ displayed the lowest SS content among these varieties at 35.93%, which is still 1.49 times higher than that observed in the control group (−5 °C).
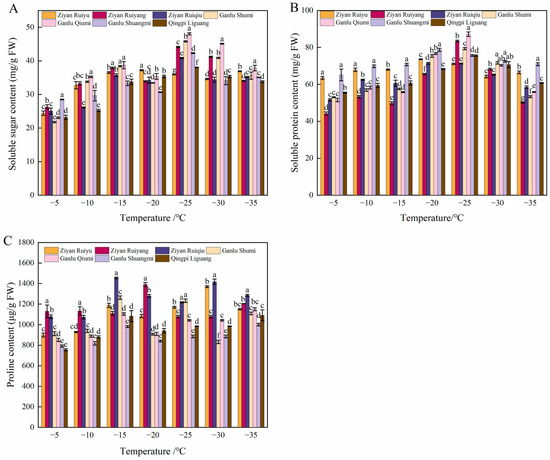
Figure 4.
Changes in soluble sugar content, soluble protein, and free proline in six varieties at different low temperatures. (A–C) respectively represent the content of soluble sugars, soluble proteins, and proline. Data are mean ± SE (n = 3), and different letters indicated significant differences among different varieties (p < 0.05, Duncan test).
The results of the SP content are presented in Figure 4B. During the low-temperature treatment, the soluble protein content of six peach varieties exhibited a trend of initially increasing and then decreasing as the temperature decreased. All varieties reached their peak SP content at temperatures ranging from −25 °C to −20 °C. Notably, ‘Ganlu Qiumi’ and ‘Ganlu Shuangmi’ maintained higher levels of soluble protein under low-temperature stress. The initial temperatures for ‘Ziyan Ruiyang’ and ‘Ganlu Qiumi’ were recorded as the lowest, measuring 44.21 mg g−1 and 51.53 mg g−1, respectively; however, these two varieties demonstrated the highest increase rates at −25 °C, with increases of 66% and 69%, respectively. In contrast, ‘Ziyan Ruiyu’ exhibited the smallest increase at only 12%.
In the process of low-temperature stress, the proline (Pro) content of six peach shoots showed an ascending–descending–ascending trend (Figure 4C). When the temperature was higher than −15 °C, the Pro content of all six varieties showed an increasing trend, and the content of ‘Ziyan Ruiyu’ increased faster. When low-temperature stress was −25~−20 °C, the Pro contents of ‘Ziyan Ruiyang’ and ‘Ziyan Ruiqiu’ decreased, and the other five varieties all increased. After −30 °C, the Pro content of all varieties generally increased with the decrease in temperature, and the changing trend of ‘Ganlu Shuangmi’ was the most significant. Pro content increased by 12.90% when −35 °C compared with −30 °C.
3.4. Correlation Analysis Between Physiological and Biochemical Indices and LT50
The electrical conductivity of annual branches subjected to various temperature stresses was measured, and the low-temperature LT50 for each variety was determined using the Logistic equation. This analysis facilitated the identification of cold resistance among different peach varieties. We used SPSS to conduct a correlation analysis between the eight physiological and biochemical indicators of cold resistance (REC, H2O2, MDA, SS, SP, Pro) measured in this study and the LT50.
From the correlation matrix (Table 2) of the derived antifreeze index correlation coefficients, it can be seen that LT50 is significantly positively correlated with REC, MDA content, and H2O2 content (p < 0.01), with correlation coefficients of 0.824, 0.752, and 0.833, respectively. It is significantly negatively correlated with SS, SP and Pro content (p < 0.01), with correlation coefficients of −0.874, −0.733, and −0.878, respectively.

Table 2.
The correlation analysis of physiological and biochemical indexes for cold resistance of peaches.
3.5. Evaluation and Analysis of Cold Resistance in Peach Resources (Varieties) Using the Mean Membership Function Method
Calculate the membership functions of the six physiological and biochemical indicators that are significantly correlated with cold resistance at −25 °C for seven peach cultivars (Table 3). The average membership function method was employed for a comprehensive evaluation of these indices. The results indicated that the average membership function values of the seven identified peach resources (varieties) ranged from 0.21 to 0.91, demonstrating considerable variation in cold resistance among them. Notably, the cold resistance of ‘Ganlu Qiumi’, ‘Ziyan Ruiyang’, ‘Ganlu Shumi’, and ‘Ziyan Ruiqiu’ surpassed that of the control variety of ‘Qingpi Liguang’.

Table 3.
Identification results of cold resistances of peach resources.
3.6. Observation of Adaptability of Different Peach Varieties
The freezing damage indexes among different varieties under low-temperature stress vary greatly (Figure 5). The freezing damage indexes of ‘Ganlu Shumi’ and ‘Ziyan Ruiyang’ were below 20%, with ‘Ziyan Ruiyang’ exhibiting the lowest freezing damage index at 17.74%, which was 3.13% lower than that of the control variety, ‘Qingpi Liguang’, a difference that is statistically significant. Following this, ‘Ganlu Shumi’ had a freezing damage index of 18.52%. Both varieties of ‘Ziyan Ruiyang’ and ‘Ganlu Shumi’ were significantly lower in their freezing damage indexes compared to other varieties, and in terms of survival rate, both varieties demonstrated resilience with a survival rate of 100%, indicating strong cold resistance. Varieties such as ‘Ganlu Qiumi’ and ‘Ziyan Ruiqiu’ followed closely behind, with their freezing damage indexes remaining below 30% and survival rates recorded at 90% and 100%, respectively, thus showcasing robust cold resistance performance (Table 4).
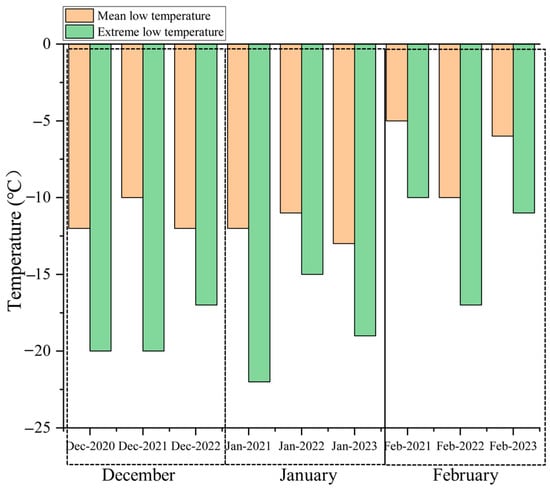
Figure 5.
Winter temperature of Jiayuguan.

Table 4.
Cold resistance test of seven peach varieties (resources) in the Jiayuguan area.
3.7. Correlation Analysis of Freezing Damage Index, LT50, and Membership Function Across Various Peach Varieties
The analysis of field adaptability among peach varieties revealed that a smaller freezing damage index of peaches correlates with a higher survival rate of the trees (Figure 6). The Pearson product–moment correlation coefficient method was employed to conduct a correlation analysis involving the peach’s freezing damage index, LT50 (the semi-lethal temperature), and the average degree of membership function.
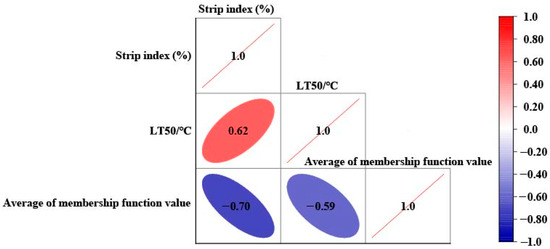
Figure 6.
Correlation analysis of stripe index, LT50, and membership function of different peach varieties.
The findings indicated a positive correlation between the freezing damage index and semi-lethal temperature LT50, with a correlation value reaching 0.62. Specifically, as the freezing damage index decreased, the semi-lethal temperature also decreased, suggesting enhanced cold resistance in these trees. Conversely, there was a negative correlation between the freezing damage index and the average degree of membership function, with a phase relation value of −0.7. This indicates that as the freezing damage index decreases, the average degree of membership function increases. Furthermore, it was observed that LT50 also exhibits a negative correlation with the average degree of membership function; as LT50 decreases, there is an increase in this degree of membership function, further signifying improved cold resistance among various peach cultivars.
4. Discussion
The impact of low temperatures on trees and their internal changes is a complex phenomenon that encompasses physiological, structural, and biochemical levels [29]. By measuring the physiological and biochemical indicators of peach trees under low-temperature induction, we can identify feasible identification indicators that are of great significance for judging the occurrence and severity of fruit tree frost damage, selecting breeding parents for hybrid breeding, and cultivating new cold-resistant varieties. Plant responses to low temperatures are primarily manifested at the cellular membrane level, which serves as a critical site for both cold injury and penetration responses. The cell membrane is composed of a phospholipid bilayer interspersed with various proteins. Its structural integrity and fluidity are essential for normal cellular function; however, exposure to low temperatures diminishes membrane fluidity. This reduction leads to the formation of intracellular ice crystals and a decrease in the rate of biochemical reactions, ultimately affecting substance transport and signal transduction [30,31]. The conductivity method is an approach used to assess the freeze tolerance of isolated tissues. The permeability of membranes, along with ion channel functionality, can be reflected through REC measurements [32]. Notably, REC exhibits an S-shaped increase as temperature decreases; this trend can be accurately modeled using the Logistic equation. The inflection point on this fitted S-shaped curve corresponds to the critical temperature approximately aligned with LT50 thresholds below which physiological metabolism undergoes significant alterations, placing plants in a semi-lethal state characterized by irreversible damage [33]. This relationship quantitatively illustrates how temperature influences water dynamics alongside cold resistance mechanisms [34]. In this study, the REC demonstrates a gradual increase as the temperature decreases above −25 °C. At approximately −25 °C, there is a slight decrease followed by a rapid increase, resulting in an “S” shaped curve throughout the process. This trend aligns with the findings of Reynolds [35] et al., which indicate that varieties exhibiting strong cold resistance tend to have lower relative conductivity. The LT50 serves as a physiological index for assessing plant cold resistance; specifically, a lower LT50 signifies greater cold tolerance in plants. When temperatures fall below LT50, cellular tissues are susceptible to rapid freezing, and subsequent freeze damage occurs. The application of this method across various plant species can accurately and intuitively reflect their levels of cold resistance [36]. Liu [37] et al. evaluated the cold resistance of 82 peach varieties using the conductivity method and concluded that the critical temperature for cold resistance in dormant annual peach branches ranged from −27.0 °C to −19.0 °C. The tested varieties in this study comprised local resources from Gansu or their hybrid varieties, all demonstrating LT50 values below −27 °C, indicates that the local resources of Gansu have strong resistance to cold, which is a characteristic formed by their long-term adaptation to low temperatures and has the potential for inheritance, making them suitable as parental materials for breeding cold-resistant varieties.
H2O2 plays a crucial role as a signaling molecule involved in regulating various physiological processes within plants. Typically, H2O2 is eliminated through diverse enzymatic systems present in plants; however, under stress conditions, alterations occur in enzyme activity leading to H2O2 accumulation. Concurrently, H2O2 can react with other cellular molecules, resulting in membrane lipid peroxidation products such as MDA [38]. MDA serves as a marker of oxidative stress, and both MDA and H2O2 play critical roles in the antioxidative stress response and cellular protection mechanisms in plants. An increase in MDA content indicates that cells are undergoing oxidative stress [39]. Furthermore, MDA is a byproduct of polyunsaturated fatty acid accumulation; excessive levels can compromise membrane integrity and impair cellular function. In this study, the H2O2 content across all peach varieties decreased at temperatures ranging from −30 to −25 °C with decreasing stress temperature, followed by a reduction in MDA content at −35 to −30 °C. This observation is consistent with Wang’s findings [40], which reported an overall increase in MDA content at −25 °C. These results suggest that as the LT50 threshold is approached, the functionality of the plant enzymatic system becomes constrained, leading to a progressive accumulation of H2O2 that surpasses the regulatory capacity of plants. Consequently, this culminates in elevated levels of MDA and heightened oxidative stress.
Under low-temperature stress, plants actively accumulate a variety of organic and inorganic substances to enhance cellular fluid concentration, reduce osmotic potential, and mitigate excessive water loss from cells. SS, SP, and Pro are classified as osmotic regulatory substances; the accumulation of them can effectively prevent damage caused by low temperatures [41]. In this study, the levels of SS and SP exhibited an initial increase followed by a subsequent decrease, peaking at −25 °C. This observation suggests that the elevation in SS and SP content plays a crucial regulatory role in enhancing the cold resistance of peach trees [42]. However, when the temperature is further lowered (below −25 °C) and causes cell damage (with significant increases in MDA and REC), the soluble sugar content decreases; below −30 °C, the SS content of most varieties decreases, indicating that the accumulation of SS and SP at the semi-lethal temperature of low-temperature stress is the highest, thus playing a protective role. These findings are consistent with those reported by Xing [43] et al. regarding oxidative damage reduction in Cabernet Sauvignon grapes. Among osmoregulatory substances, Pro accumulation is recognized as a physiological adaptation mechanism for plants under low-temperature stress. This process activates signal transduction pathways such as proline synthetase synthesis under cold stimulation and promotes the conversion of glutamic acid or glutamine to proline [44]. The accumulation of Pro functions as an osmoregulatory agent that protects cells stabilizes protoplasmic colloids and metabolic processes within tissues, lowers the freezing point, and prevents cell dehydration. Additionally, it can alleviate oxidative stress induced by low temperatures through the synthesis of antifreeze proteins [45]. In this study, fluctuations in proline content were observed under cold stress, reflecting trends of Pro accumulation noted in rapes [46] and almonds [47] under similar low-temperature conditions. These findings suggest that various osmoregulatory substances play distinct roles in peach plants’ responses to environmental stress. SS accumulated during the early stages of low-temperature stress may form protective compounds via metabolic pathways associated with glucose metabolism, thereby enhancing cold resistance [48]. Furthermore, SP increased water content in branches while simultaneously reducing freezing-induced cracking and mortality of protoplasts due to low temperatures [49]. By analyzing the correlation between physiological and biochemical indices and LT50, it was determined that LT50 exhibited a positive correlation with REC, MDA content, and H2O2 levels (p < 0.01) while showing a negative correlation with SS content, SP levels, and free Pro concentrations. Consequently, increases in REC, as well as MDA and H2O2 concentrations, are likely primary contributors to cellular oxidative damage and membrane disruption under low-temperature stress. The elevation of SS, SP, and Pro indicates enhanced plant adaptability to cold stress conditions, which subsequently improves survival rates.
The fuzzy membership function method was employed to calculate the weighted average values of measurement indices, thereby providing a comprehensive and systematic reflection of the evaluation criteria. Luo [50] et al. used the membership function method to evaluate the cold resistance of fresh grape varieties and concluded that this method is superior to the single index evaluation method and can fully reflect the actual cold resistance of grapes. Building on this methodology, Wang [40] utilized six physiological indices as evaluation criteria for branch cold resistance in grapes, conducting a thorough analysis and classification of their cold resilience. In this study, we applied the membership function method to comprehensively evaluate the cold resistance of seven peach varieties. The varieties identified as exhibiting significant cold resistance included ‘Ganlu Qiumi’, ‘Ziyan Ruiyang’, ‘Ganlu Shumi’, and ‘Ziyan Ruiqiu’, all demonstrating an average membership function degree exceeding 0.6. Furthermore, these cold-resistant varieties were validated through comparisons with LT50 assessments. While all selected varieties exhibited notable cold resilience, variations were observed in their ranking order based on this criterion. In our previous study on cold resistance of local peach varieties in Gansu province, LT50 and average membership function [51] were used from physiological, biochemical, and anatomical perspectives using REC, MDA, Pro, SP, SS, cork layer ratio, and xylem thickness/cortical thickness (X/C) as indicators. Peach resources with strong cold resistance under cold conditions were comprehensively evaluated, and the overall results were consistent with field performance. However, there were still differences in some resources, indicating that although the comprehensive identification method effectively improved the screening efficiency and reduced the bias related to a single index, the cold resistance of peaches could not be accurately judged by laboratory indicators alone. It is still a meaningful research direction to find a convenient, fast, and accurate cold resistance identification method in future studies.
Cold resistance is a complex physiological process influenced by numerous factors, making it challenging to comprehensively and objectively assess the cold resistance of plants through any single physiological index. Although our early test indicators are laboratory results [51], the cold resistance results will eventually be applied to actual production, and it is the final significance of the evaluation of cold resistance resources. Therefore, in addition to the laboratory physiological index, a field investigation of cold resistance of varieties was added to mutually confirm the accuracy of the two methods. To provide a more convenient and accurate reference for the identification of peach cold resistance in the future. In the cultivation conditions of the Hexi Corridor, the absolute minimum temperature can reach −35 °C [52]. Common cultivars often struggle to survive winter in the Jiayuguan area, where local resources are frequently compromised by low temperatures and freezing events. Consequently, this region serves as an ideal site for field identification of cold resistance, with freezing damage indexes as indicators of fruit tree resilience. In this study, various cultivars were evaluated in the Jiayuguan area, revealing differing degrees of freeze damage among their branches. A comprehensive analysis of field adaptability and LT50 demonstrated a significant positive correlation between the freezing damage index and LT50; specifically, a lower freezing damage index corresponded to a smaller LT50. These findings suggest that fruit trees exhibiting high freezing damage indexes are more susceptible to winter sap flow loss, which can lead to increased water loss from their branches [53]. The highest survival rate was observed at the lowest freezing damage index for ‘Ziyan Ruiyang’ and ‘Ganlu Shumi’. When compared to the control variety ‘Qingpi Liguang’, ‘Ganlu Shumi’ exhibited a higher LT50 value; however, it also displayed a lower freezing damage index along with reduced survival rates in freezing conditions. The results were inconsistent with Amuti et al. analysis of the difference between the extraction rate and cold resistance [54]. We suspect that this may be because the physiological adaptability of Amomum villosum (soil conditions, environmental factors, management) is relatively weak; therefore, its survival rate is lower under variable climate conditions, thus forming characteristics related to environmental adaptation. The correlation results of LT50 and membership function showed a negative correlation, but the correlation was not significant; hence, the comprehensive evaluation results were not completely consistent. The LT50 of ‘Ganlu Shumi’ was the lowest at −32.24 °C, showing the strongest cold resistance, which was different from the membership function results. Wang et al. and Ma et al. also showed a similar situation in peach and wine grape studies [55,56]. Both the field adaptability index (freezing damage index) and the laboratory membership function index can help to understand the growth performance of fruit trees under different environmental conditions, and the significant negative correlation between the two indexes indicates that membership function can be an important evaluation index reflecting the field adaptability of peach. Therefore, both LT50 assessments and membership function methodologies can be employed to evaluate cold resistance in ‘Ziyan Ruiyang’ and ‘Ganlu Shumi’, with ‘Ziyan Ruiyu’ demonstrating inferior cold resistance among them. The results indicated that LT50, membership function evaluation, and field adaptability observation could quickly determine the cold resistance of peach varieties and further improve the screening criteria on the basis of previous screening. However, due to limitations related to experimental materials and control conditions, discrepancies may arise when compared to actual cold resistance observed in peach varieties. Additionally, given the complex environmental factors present at introduction sites, relying solely on these methods may not fully capture outcomes related to field adaptability. In future research work, we will further verify the cold resistance of the two varieties. The cold resistance of the branches is an important factor determining the overall viability of plants. The cold resistance of the flower buds of peach trees is usually lower than that of the branches, and the common problem in cold areas is that the branches freeze and the flower buds fall off. Therefore, the study of branch cold tolerance under low-temperature stress can provide basic data and theoretical support for the subsequent study of flower bud cold tolerance. We hope to understand the physiological changes of branches under low-temperature conditions through in-depth study and lay a foundation for further flower bud research. By studying the molecular response of peach varieties to low-temperature stress, we hope to develop more effective identification techniques.
5. Conclusions
In this study, we systematically evaluated the physiological responses of cold-resistant peach varieties under low-temperature stress. By integrating LT50 assessments, membership function methods, and observations of peach variety adaptability, we conducted a comprehensive evaluation of various indices to quantify the cold resistance capabilities of different cultivars. The cold-resistant peach varieties ‘Ziyan Ruiyang’ and ‘Ganlu Shumi’ demonstrated significant physiological adaptability under low-temperature conditions and were able to mitigate damage caused by low temperatures through the regulation of endogenous substance synthesis. This research provides a theoretical foundation for breeding cold-resistant peach trees and underscores the importance of physiological indices in assessing plant stress resistance.
Author Contributions
Formal analysis, R.N.; Funding acquisition, R.N. and C.W.; Investigation, R.N., Y.Z. and C.W.; Resources, F.W.; Software, J.H.; Supervision, C.W.; Validation, R.N.; Visualization, R.N. and J.H.; Writing—original draft, R.N. and J.H.; Writing—review and editing, R.N., J.H. and C.W. All authors have read and agreed to the published version of the manuscript.
Funding
Subproject under the National Key R&D Plan (2022YFD1602108); National Natural Science Foundation of China (Project 32060651); Modern Agricultural Industrial Technology System (CARS-30-17); Special Project of the Gansu Science and Technology Mission (22CX8NA025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article. The data presented in this study are available in the insert article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Toumi, I.; Zarrouk, O.; Ghrab, M.; Nagaz, K. Improving peach fruit quality traits using deficit irrigation strategies in southern Tunisia arid area. Plants 2022, 11, 1656. [Google Scholar] [CrossRef]
- Jia, W. Temporal and spatial variations in extreme temperatures in the Qilian Mountains-Hexi Corridor over the period 1960–2013. J. Mt. Sci. 2016, 13, 2224–2236. [Google Scholar] [CrossRef]
- Wang, L.R. Historical review and prospect of peach variety improvement in China. J. Fruit Sci. 2021, 38, 2178–2195. [Google Scholar]
- Niu, R.; Cheng, Y.; Wang, F.; Zhang, Y.; Wang, C. Transcriptome Analysis Provides Insights into the Safe Overwintering of Local Peach Flower Buds. Curr. Issues Mol. Biol. 2024, 46, 13903–13921. [Google Scholar] [CrossRef]
- Li, C.; Junttila, O.; Palva, E.T. Environmental regulation and physiological basis of freezing tolerance in woody plants. Acta Physiol. Plant. 2004, 26, 213–222. [Google Scholar] [CrossRef]
- Yang, Y.J.; Hu, H.; Huang, W. The light dependence of mesophyll conductance and relative limitations on photosynthesis in evergreen Sclerophyllous Rhododendron species. Plants 2020, 9, 1536. [Google Scholar] [CrossRef]
- Yan, L.; Liu, S.; Li, R.; Li, Z.; Piao, J.; Zhou, R. Calcium enhanced the resistance against Phoma arachidicola by improving cell membrane stability and regulating reactive oxygen species metabolism in peanut. BMC Plant Biol. 2024, 24, 501. [Google Scholar] [CrossRef]
- Li, R.X.; Jin, X.L.; Hu, X.J.; Chai, Y.X.; Cai, M.Y.; Luo, F.; Zhang, F.J. Analysis and comprehensive evaluation on cold resistance of six varieties of Michelia. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2017, 28, 1464–1472. [Google Scholar]
- Recalde, L.; Vázquez, A.; Groppa, M.D.; Benavides, M.P. Reactive oxygen species and nitric oxide are involved in polyamine-induced growth inhibition in wheat plants. Protoplasma 2018, 255, 1295–1307. [Google Scholar] [CrossRef]
- Maruta, T.; Sawa, Y.; Shigeoka, S.; Ishikawa, T. Diversity and evolution of ascorbate peroxidase functions in chloroplasts: More than just a classical antioxidant enzyme? Plant Cell Physiol. 2016, 57, 1377–1386. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T. Formation and biological targets of quinones: Cytotoxic versus cytoprotective effects. Chem. Res. Toxicol. 2017, 30, 13–37. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, H.; Wang, L.; Zhao, Q.; Wang, D.; Zhang, T. Cold acclimation alleviates cold stress-induced PSII inhibition and oxidative damage in tobacco leaves. Plant Signal. Behav. 2022, 17, 2013638. [Google Scholar] [CrossRef]
- Kour, J.; Bhardwaj, T.; Chouhan, R.; Singh, A.D.; Gandhi, S.G.; Bhardwaj, R.; Alsahli, A.A.; Ahmad, P. Phytomelatonin maintained chromium toxicity induced oxidative burst in Brassica juncea L. through improving antioxidant system and gene expression. Environ. Pollut. 2024, 356, 124256. [Google Scholar] [CrossRef]
- Sofy, M.; Mohamed, H.; Dawood, M.; Abu-Elsaoud, A.; Soliman, M. Integrated usage of Trichoderma harzianum and biochar to ameliorate salt stress on spinach plants. Arch. Agron. Soil Sci. 2022, 68, 2005–2026. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, L.; Fu, X.; Mei, X.; Cheng, S.; Liao, Y.; Deng, R.; Xu, X.; Jiang, Y.; Duan, X.; et al. The sphingolipid biosynthetic enzyme Sphingolipid delta8 desaturase is important for chilling resistance of tomato. Sci. Rep. 2016, 6, 38742. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, J.; Ibrahim, M.; Jiao, X.; Song, X.; Bai, P.; Li, J. Effects of the interaction between vapor-pressure deficit and potassium on the photosynthesis system of tomato seedlings under low temperature. Sci. Hortic. 2021, 283, 110089. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, J.; Li, J.; Lu, G.Y.; Li, C.S.; Fu, G.P.; Zhang, X.K.; Ma, H.Q.; Liu, Q.Y.; Zou, X.L.; et al. Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. J. Integr. Agric. 2018, 17, 328–335. [Google Scholar]
- Li, Q.; Guan, C.; Zhao, Y.; Duan, X.; Yang, Z.; Zhu, J. Salicylic acid alleviates Zn-induced inhibition of growth via enhancing antioxidant system and glutathione metabolism in alfalfa. Ecotoxicol. Environ. Saf. 2023, 265, 115500. [Google Scholar] [CrossRef]
- Zhang, W.; Kumar, M.; Zhou, Y.; Yang, J.; Mao, Y. Analytically derived fuzzy membership functions. Clust. Comput. 2019, 22 (Suppl. S5), 11849–11876. [Google Scholar] [CrossRef]
- Dunne, J.C.; Tuong, T.D.; Livingston, D.P.; Reynolds, W.C.; Milla-Lewis, S.R. Field and laboratory evaluation of bermudagrass germplasm for cold hardiness and freezing tolerance. Crop Sci. 2019, 59, 392–399. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.S.; Tan, R.; Zhang, Y.B.; Chen, L. Optimization and evaluation of identification method for branch cold resistance of apricot germplasm under natural overwintering conditions. Agric. Technol. 2023, 43, 72–75. [Google Scholar]
- Gao, D.T.; Bai, R.; Lu, X.Y.; Wei, Z.F.; Guo, J.N. Study on the cold resistance of five grapevine rootstocks introduced to Shihezi. J. Fruit Sci. 2015, 32, 232–237. [Google Scholar]
- Yang, H.F. Cultivation technology of yellow bud green onion in Jiayuguan City. Gansu Agric. Sci. Technol. 2014, 01, 61–62. [Google Scholar]
- Xu, H.; Wang, X.D.; Zhou, Y.N.; Du, Z.J.; Zhuo, H. Study on the cold resistance of grape rootstocks and wine grape cultivars. Sino-Overseas Grapevine Wine 2003, 6, 20–23. [Google Scholar]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- John, A.; Barnett, G.; Miller, T.B. The determination of soluble carbohydrate in dried samples of grass silage by the anthrone method. J. Sci. Food Agric. 1950, 1, 336–339. [Google Scholar] [CrossRef]
- Zhang, Z.L. Experimental Guidance of Plant Physiology; Higher Education Press: Beijing, China, 2003. [Google Scholar]
- Schweet, R.S. The quantitative determination of proline and pipecolic acid with ninhydrin. J. Biol. Chem. 1954, 208, 603–613. [Google Scholar] [CrossRef]
- Mehrotra, S.; Verma, S.; Kumar, S.; Kumari, S.; Mishra, B.N. Transcriptional regulation and signalling of cold stress response in plants: An overview of current understanding. Environ. Exp. Bot. 2020, 180, 104243. [Google Scholar] [CrossRef]
- Dhaliwal, L.K.; Angeles-Shim, R.B. Cell membrane features as potential breeding targets to improve cold germination ability of seeds. Plants 2022, 11, 3400. [Google Scholar] [CrossRef]
- Passot, S.; Bouix, M.; Gautier, J.; Lieben, P.; Cenard, S.; Ghorbal, S.; Fonseca, F. 50. Relevance of cell biophysical behaviour and membrane fluidity for explaining freezing resistance of lactic acid bacteria. Cryobiology 2012, 65, 355. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Hill, R.S. Increases in water potential gradient reduce xylem conductivity in whole plants. Evidence from a low-pressure conductivity method. Plant Physiol. 2000, 123, 1021–1028. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Warmund, M.R. Ice Distribution inEarliglow’Strawberry Crowns and Tissue Recovery following Extracellular Freezing. J. Am. Soc. Hortic. Sci. 1993, 118, 644–648. [Google Scholar] [CrossRef]
- Lindén, L.; Palonen, P.; Lindén, M. Relating freeze-induced electrolyte leakage measurements to lethal temperature in red raspberry. J. Am. Soc. Hortic. Sci. 2000, 125, 429–435. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Niu, L.; Savigny, D.C. Use of electrical conductivity to assess irrigation impacts on grapevine winter hardiness. Int. J. Fruit Sci. 2014, 14, 267–283. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Kang, Y.; Wang, Y.; Liu, R.; Dong, S. Physiological Response to Low-Temperature Stress and Cold Resistance Evaluation of Ziziphus jujuba var. spinosa Clones from Different Provenances. Forests 2024, 15, 1130. [Google Scholar] [CrossRef]
- Liu, T.M.; Zhang, Z.W.; Li, H.; Ren, Z.B.; Zhou, C.T. Study on cold tolerance of peach varieties. Fruit Sci. 1998, 15, 107–111. [Google Scholar]
- Valizadeh-Kamran, R.; Toorchi, M.; Mogadam, M.; Mohammadi, H.; Pessarakli, M. Effects of freeze and cold stress on certain physiological and biochemical traits in sensitive and tolerant barley (Hordeum vulgare) genotypes. J. Plant Nutr. 2018, 41, 102–111. [Google Scholar] [CrossRef]
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, S.W.; Pan, Y.; Li, Y.L.; Li, S.D. Identification and comprehensive evaluation of cold resistance of wine grape germplasms in Northern Tianshan Region, Xinjiang. J. Fruit Sci. 2019, 41, 1933–1946. [Google Scholar]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef]
- Kwon, J.H.; Nam, E.Y.; Yun, S.K.; Kim, S.J.; Yu, D.J.; Lee, H.J. Comparative carbohydrate metabolism in the shoots of a cold-hardy and a cold-sensitive peach (Prunus persica) cultivar during cold acclimation and deacclimation. Hortic. Environ. Biotechnol. 2022, 63, 39–53. [Google Scholar] [CrossRef]
- Han, X.; Yao, F.; Xue, T.; Wang, Z.L.; Wang, Y.; Cao, X.; Hui, M.; Wu, D.; Li, Y.; Wang, H.; et al. Sprayed biodegradable liquid film improved the freezing tolerance of cv. Cabernet Sauvignon by up-regulating soluble protein and carbohydrate levels and alleviating oxidative damage. Front. Plant Sci. 2022, 13, 1021483. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Zuo, Y.; Gu, W.; Wei, S.; Li, J. Exogenous Proline Optimizes Osmotic Adjustment Substances and Active Oxygen Metabolism of Maize Embryo under Low-Temperature Stress and Metabolomic Analysis. Processes 2022, 10, 1388. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F.S.; Sharif, R.; Anand, A.; Corpas, F.; Jin, W.; Varshney, R.K. Assessment of proline function in higher plants under extreme temperatures. Plant Biol. 2023, 25, 379–395. [Google Scholar] [CrossRef]
- Jankovska-Bortkevič, E.; Gavelienė, V.; Koryznienė, D.; Jankauskienė, J.; Mockevičiūtė, R.; Sigita, J. Response of winter oilseed rape to imitated temperature fluctuations in autumn-winter period. Environ. Exp. Bot. 2019, 166, 103801. [Google Scholar] [CrossRef]
- Yu, Z.F. Study on Response Mechanism of ‘Late Abundance’ Almond Under Low Temperature Stress Based on Combined Transcriptional Metabolism. Ph.D. thesis, Xinjiang Agricultural University, Urumqi, China, 2023. [Google Scholar]
- Farooq, M.; Hussain, M.; Nawaz, A.; Lee, D.; Alghamdi, S.S.; Siddique, K.H. Seed priming improves chilling tolerance in chickpea by modulating germination metabolism, trehalose accumulation and carbon assimilation. Plant Physiol. Biochem. 2017, 111, 274–283. [Google Scholar] [CrossRef]
- Ma, X.Y.; Hu, H.Y.; Li, J.Y.; Hou, C.Y.; Li, D.M. Comparative study on cold resistance and drought tolerance of different grape rootstocks. J. Shanxi Agric. Univ. (Nat. Sci. Ed.) 2024, 44, 41–51. [Google Scholar]
- Luo, Y.X.; Guo, R.R.; Li, X.X.; Liu, R.C.; Wang, Y.J. Evaluation of cold resistance of 7 table grape varieties based on membership function method. J. Guizhou Agric. Sci. 2018, 46, 38–44. [Google Scholar]
- Niu, R.; Zhao, X.; Wang, C.; Wang, F. Physiochemical responses and ecological adaptations of peach to low-temperature stress: Assessing the cold resistance of local peach varieties from Gansu, China. Plants 2023, 12, 4183. [Google Scholar] [CrossRef]
- Ma, Y.; Niu, Z.; Sun, D.; Wang, X. Spatiotemporal evolution of dry and wet and quantitative analysis of the influence of meteorological factors based on MI and the FAO P–M model. Sci. Rep. 2024, 14, 21343. [Google Scholar] [CrossRef]
- Sellin, A. The dependence of water potential in shoots of Picea abies on air and soil water status. In Annales Geophysicae; Springer: Göttingen, Germany, 1998; Volume 16, pp. 470–476. [Google Scholar]
- Amuti, A. Study on Dry and Cold Resistance of Fruiting Branches of Early Fruiting Walnut in Wen 185 and Xin Xin2. Master’s Thesis, Tarim University, Alar, China, 2017. [Google Scholar]
- Wang, Z.Y.; Zhang, L.S.; Chang, R.F.; Liu, G.J.; Han, J.C.; Chen, H. Study on the relationship between tissue structure and cold resistance of peach branches. J. Hebei Agric. Sci. 2014, 18, 29–33. [Google Scholar]
- Ma, X.H.; Tang, X.P.; Dong, Z.G.; Zhao, Q.F.; Li, X.M.; Wang, M.; Ren, R. Comparison of cold resistance for 6 grapewine cultivars. J. Shanxi Agric. Univ. Nat. Sci. Ed. 2013, 33, 1–5. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).