Optimizing Fertilization Strategies to Promote Leaf-Use Ginkgo Productivity and Ecosystem Economic Benefits: An Integrated Evaluation of a Field Trial in Southern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experimental Materials
2.3. Experimental Design and Field Management
2.4. Methods for Sample Collection and Indicator Determination
2.5. Estimation of Environmental Risks Caused by Fertilization
2.6. Estimation of Profitability Derived from Fertilization
2.7. Comprehensive Evaluation with TOPSIS Model
2.8. Statistical Analysis
3. Results
3.1. Leaf-Use Ginkgo Leaves Yield
3.2. Main Characteristics of Leaf-Use Ginkgo Leaves
3.3. Effects of Fertilization on Flavonoid Content and Yield
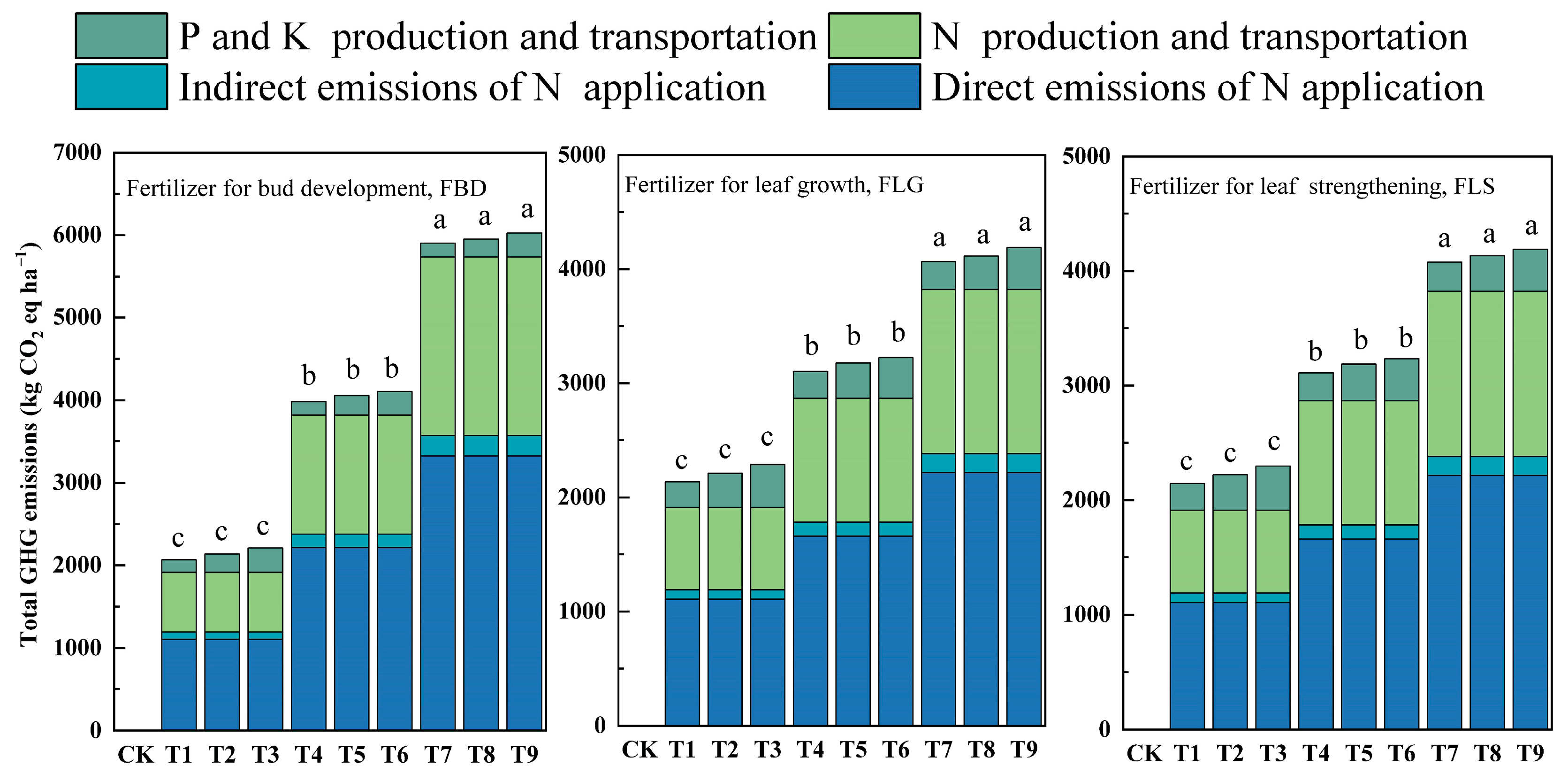
3.4. Analysis of Environmental Risks and Economic Benefits of Fertilization
3.5. Suitability Ranking of Fertilization Treatments Based on Game Theory Weighting and TOPSIS
4. Discussion
4.1. Effects of Different Fertilization Conditions on Leaf Growth of Leaf-Use Ginkgo
4.2. Concentration and Accumulation of Flavonoids in Ginkgo Leaves under Different Fertilization Schemes
4.3. Environmental Risks and Economic Benefits Derived from Different Fertilization Regimes
4.4. Suitable Fertilization-Management Strategies for Leaf-Use Ginkgo Plantations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Wang, M.; Gao, C.; Fu, F.; El-Kassaby, Y.A.; Wang, T.; Wang, G. Spatial prediction and delineation of Ginkgo biloba production areas under current and future climatic conditions. Ind. Crop. Prod. 2021, 166, 113444. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Wang, G.; Cao, F. Growth and flavonol accumulation of Ginkgo biloba leaves affected by red and blue light. Ind. Crop. Prod. 2022, 187, 115488. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhang, J.; Wang, S. Advances in the chemical constituents and chemical analysis of Ginkgo biloba leaf, extract, and phytopharmaceuticals. J. Pharmaceut. Biomed. 2021, 193, 113704. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, X.; Zhang, H.; Zhu, W.; Jiang, Z.; Shi, Y.; Li, L. In vitro anti-aging activities of Ginkgo biloba leaf extract and its chemical constituents. Food Sci. Technol. 2020, 40, 476–482. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Sang, Y.; Xing, S.; Wu, Q.; Liu, X. Identification and Characterization of MicroRNAs in Ginkgo biloba var. epiphylla Mak. PLoS ONE 2015, 10, 127184. [Google Scholar]
- Cao, F. Chinese Ginkgo; Jiangsu Science and Technology Press: Beijing, China, 2002. [Google Scholar]
- Zhou, Q.; Mu, K.; Ni, Z.; Liu, X.; Li, Y.; Xu, L. Analysis of genetic diversity of ancient Ginkgo populations using SSR markers. Ind. Crop. Prod. 2020, 145, 111942. [Google Scholar] [CrossRef]
- Lin, H.Y.; Li, W.H.; Lin, C.F.; Wu, H.R.; Zhao, Y.P. International Biological Flora: Ginkgo biloba. J. Ecol. 2022, 110, 951–982. [Google Scholar] [CrossRef]
- Wang, G.; Cao, F.; Chang, L.; Guo, X.; Wang, J. Temperature has more effects than soil moisture on biosynthesis of flavonoids in Ginkgo (Ginkgo biloba L.) leaves. New For. 2014, 45, 797–812. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, J.; Shen, X.; Wang, G.; Wang, T. Predicting the Bioclimatic Habitat Suitability of Ginkgo biloba L. in China with Field-Test Validations. Forests 2019, 10, 705. [Google Scholar] [CrossRef]
- Chu, S.; Ouyang, J.; Liao, D.; Zhou, Y.; Liu, S.; Shen, D.; Wei, X.; Zeng, S. Effects of enriched planting of native tree species on surface water flow, sediment, and nutrient losses in a Eucalyptus plantation forest in southern China. Sci. Total Environ. 2019, 675, 224–234. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, C.; Wang, M.; Fu, F.; El-Kassaby, Y.A.; Wang, T.; Wang, G. Metabolome and transcriptome analyses reveal flavonoids biosynthesis differences in Ginkgo biloba associated with environmental conditions. Ind. Crop. Prod. 2020, 158, 112963. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Ferreira, I.Q.; Rodrigues, M.A. Leaf nutrient concentration standards for lemon verbena (Aloysia citrodora Paláu) obtained from field and pot fertilization experiments. J. Appl. Res. Med. Aroma. Plants 2018, 8, 33–40. [Google Scholar] [CrossRef]
- Abou-Sreea, A.I.B.; Rady, M.M.; Roby, M.H.H.; Ahmed, S.M.A.; Majrashi, A.; Ali, E.F. Cattle manure and bio-nourishing royal jelly as alternatives to chemical fertilizers: Potential for sustainable production of organic Hibiscus sabdariffa L. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100334. [Google Scholar] [CrossRef]
- Pouyanfar, M.; Alipour, N.M.; Nourafcan, H.; Faramarzi, A. Role of foliar nutrition on the growth, yield, and fatty acid compositions of linseed (Linum usitatissimum L.). J. Appl. Res. Med. Aromat. Plants 2022, 31, 100414. [Google Scholar] [CrossRef]
- Guo, J.; Wu, Y.; Wang, B.; Lu, Y.; Cao, F.; Wang, G. The Effects of Fertilization on the Growth and Physiological Characteristics of Ginkgo biloba L. Forests 2016, 7, 293. [Google Scholar] [CrossRef]
- Wang, L.; Shi, H.; Wu, J.; Cao, F. Alternative partial root-zone irrigation enhances leaf flavonoid accumulation and water use efficiency of Ginkgo biloba. New For. 2016, 47, 377–391. [Google Scholar] [CrossRef]
- Wu, D.; Feng, J.; Lai, M.; Ouyang, J.; Liao, D.; Yu, W.; Wang, G.; Cao, F.; Jacobs, D.F.; Zeng, S. Combined application of bud and leaf growth fertilizer improves leaf flavonoids yield of Ginkgo biloba. Ind. Crop. Prod. 2020, 150, 112379. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, N.; Ma, Q.; Zhou, J.; Sun, T.; Zhang, X.; Wu, L. Applying Nutrient Expert system for rational fertilisation to tea (Camellia sinensis) reduces environmental risks and increases economic benefits. J. Clean. Prod. 2021, 305, 127197. [Google Scholar] [CrossRef]
- Ding, Z.; Jia, S.; Wang, Y.; Xiao, J.; Zhang, Y. Phosphate stresses affect ionome and metabolome in tea plants. Plant Physiol. Biochem. 2017, 120, 30–39. [Google Scholar] [CrossRef]
- Kim, G.W.; Gwon, H.S.; Jeong, S.T.; Hwang, H.Y.; Kim, P.J. Different responses of nitrogen fertilization on methane emission in rice plant included and excluded soils during cropping season. Agric. Ecosyst. Environ. 2016, 230, 162–168. [Google Scholar] [CrossRef]
- Zhou, P.; Bai, X.; Wang, H.; Yang, M.; Bao, L.; Deng, X.; Chen, Z.; Zhou, J. Optimizing nitrogen and water management for sustainable greenhouse vegetable production with less greenhouse gas emissions. Agric. Ecosyst. Environ. 2023, 352, 108529. [Google Scholar] [CrossRef]
- Wu, J.; Ying, Y.; Cao, F. Advances in Research on Fertilization for Ginkgo biloba. Acta Agric. Univ. Jiangxiensis 2003, 25, 402–406. [Google Scholar]
- Gomaa, M.A.; Kandil, E.E.; El-Dein, A.A.M.Z.; Abou-Donia, M.E.M.; Ali, H.M.; Abdelsalam, N.R. Increase maize productivity and water use efficiency through application of potassium silicate under water stress. Sci. Rep. 2021, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Chattha, M.U.; Hassan, M.U.U.; Khan, I.; Nawaz, M.; Shah, A.N.; Sattar, A.; Hashem, M.; Alamri, S.; Aslam, M.T.; Alhaithloul, H.A.S.; et al. Hydrogen peroxide priming alleviates salinity induced toxic effect in maize by improving antioxidant defense system, ionic homeostasis, photosynthetic efficiency and hormonal crosstalk. Mol. Biol. Rep. 2022, 49, 5611–5624. [Google Scholar] [CrossRef]
- Paolo, B.; Lara, R.; Martina, C.; Emma, T.; Giacomo, T.; Beatrice, F.; Adolfo, R. Nitrogen fertilization levels and timing affect the plasticity of yield components in bread wheat (Triticum aestivum L.). Field. Crops Res. 2022, 289, 108734. [Google Scholar]
- Chitwood, D.H.; Sinha, N.R. Evolutionary and Environmental Forces Sculpting Leaf Development. Curr. Biol. 2016, 26, R297–R306. [Google Scholar] [CrossRef]
- Bu, W.; Wang, F.; Zhang, C.; Bruelheide, H.; Fang, X.; Wang, H.; Chen, F. The contrasting effects of nitrogen and phosphorus fertilizations on the growth of Cunninghamia lanceolata depend on the season in subtropical China. For. Ecol. Manag. 2021, 482, 118874. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2015; pp. 1491–1494. [Google Scholar]
- Zhang, W.; Dou, Z.; He, P.; Ju, X.; Powlson, D.; Chadwick, D.; Norse, D.; Lu, Y.; Zhang, Y.; Wu, L.; et al. New technologies reduce greenhouse gas emissions from nitrogenous fertilizer in China. Proc. Natl. Acad. Sci. USA 2013, 110, 8375–8380. [Google Scholar] [CrossRef]
- Cui, Z.; Yue, S.; Wang, G.; Zhang, F.; Chen, X. In-Season Root-Zone N Management for Mitigating Greenhouse Gas Emission and Reactive N Losses in Intensive Wheat Production. Environ. Sci. Technol. 2013, 47, 6015–6022. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, Z.; Hu, Y.; Schmidhalter, U.; Zhang, W.; Ruan, S.; Chen, X. Integrated assessment of agronomic, environmental and ecosystem economic benefits of blending use of controlled-release and common urea in wheat production. J. Clean. Prod. 2021, 287, 125572. [Google Scholar] [CrossRef]
- Qu, F.; Zhang, Q.; Jiang, Z.; Zhang, C.; Zhang, Z.; Hu, X. Optimizing irrigation and fertilization frequency for greenhouse cucumber grown at different air temperatures using a comprehensive evaluation model. Agric. Water. Manag. 2022, 273, 107876. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Tang, Y.; Shao, J.; Xu, T.; Ma, R.; Jiang, Y.; Cheng, D. Optimizing Fertilizer Management Based on Controlled-Release Fertilizer to Improve Yield, Quality, and Reduce Fertilizer Application on Apples. J. Plant Nutr. Soil Sci. 2022, 22, 393–405. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Ren, F.; Jiang, Q.; Zhou, X.; Zhao, J.; Liu, X. Integrated Physiological, Transcriptomic, and Metabolomic Analyses of the Response of Peach to Nitrogen Levels during Different Growth Stages. Int. J. Mol. Sci. 2022, 23, 10876. [Google Scholar] [CrossRef]
- Xue, J.; Kimberley, M.O.; McKinley, R.B. Impact of nitrogen input from biosolids application on carbon sequestration in a Pinus radiata forest. For. Ecosyst. 2022, 9, 100020. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; Zhang, J.; Ma, K. An optimum combination of irrigation amount, irrigation water salinity and nitrogen application rate can improve cotton (for fiber) nitrogen uptake and final yield. Ind. Crops Prod. 2022, 187, 115386. [Google Scholar] [CrossRef]
- Radicetti, E.; Campiglia, E.; Marucci, A.; Mancinelli, R. How winter cover crops and tillage intensities affect nitrogen availability in eggplant. Nutr. Cycl. Agroecosys. 2017, 108, 177–194. [Google Scholar] [CrossRef]
- Pal, P.K.; Prasad, R.; Pathania, V. Effect of decapitation and nutrient applications on shoot branching, yield, and accumulation of secondary metabolites in leaves of Stevia rebaudiana Bertoni. J. Plant Physiol. 2013, 170, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Vanderzande, S.; Hias, N.; Edge-Garza, D.; Costes, E.; Davey, M.W.; Keulemans, J. Sylleptic branching in winter-headed apple (Malus × domestica) trees: Accession-dependent responses and their relationships with other tree architectural characteristics. Tree Genet. Genomes 2016, 12, 87. [Google Scholar] [CrossRef]
- Van Do, T.; Thang, N.T.; Lam, V.T.; Van Thuyet, D.; Trung, P.D.; Quy, T.H.; Phuong, N.T.T.; Huyen, L.T.T.; Thinh, N.H.; Van Tuan, N.; et al. Monitoring fine root growth to identify optimal fertilization timing in a forest plantation: A case study in Northeast Vietnam. PLoS ONE 2019, 14, 225567. [Google Scholar] [CrossRef]
- Pandey, V.; Patel, A.; Patra, D.D. Amelioration of mineral nutrition, productivity, antioxidant activity and aroma profile in marigold (Tagetes minuta L.) with organic and chemical fertilization. Ind. Crop. Prod. 2015, 76, 378–385. [Google Scholar] [CrossRef]
- Deng, K.; Li, L.; Li, L.; Xu, F.; Yuan, H.; Zha, S.; Xiao, X.; Yu, J.; Cheng, S.; Cheng, H. Molecular Mechanism of Selenium Affecting the Synthesis of Flavonoids in G. biloba Leaves. Plant Mol. Biol. Report. 2022, 40, 232–246. [Google Scholar] [CrossRef]
- Lin, Y.; Lou, K.; Wu, G.; Wu, X.; Zhou, X.; Feng, Y.; Zhang, H.; Yu, P. Bioactive metabolites in of Ginkgo biloba leaves: Variations by seasonal, meteorological and soil. Braz. J. Biol. 2020, 80, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W.; Wang, Z.; Zhang, W.; Yan, X.; Yang, J.; et al. Producing more grain with lower environmental costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Z.; He, X.; Wang, X.; Shi, X.; Zou, C.; Chen, X. The effects of controlled release urea on maize productivity and reactive nitrogen losses: A meta-analysis. Environ. Pollut. 2019, 246, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; He, P.; Pampolino, M.F.; Li, Y.; Liu, S.; Xie, J.; Hou, Y.; Zhou, W. Narrowing yield gaps and increasing nutrient use efficiencies using the Nutrient Expert system for maize in Northeast China. Field Crop. Res. 2016, 194, 75–82. [Google Scholar] [CrossRef]
- Zeng, S.; Jacobs, D.F.; Sloan, J.L.; Xue, L.; Li, Y.; Chu, S. Split fertilizer application affects growth, biomass allocation, and fertilizer uptake efficiency of hybrid Eucalyptus. New For. 2013, 44, 703–718. [Google Scholar] [CrossRef]
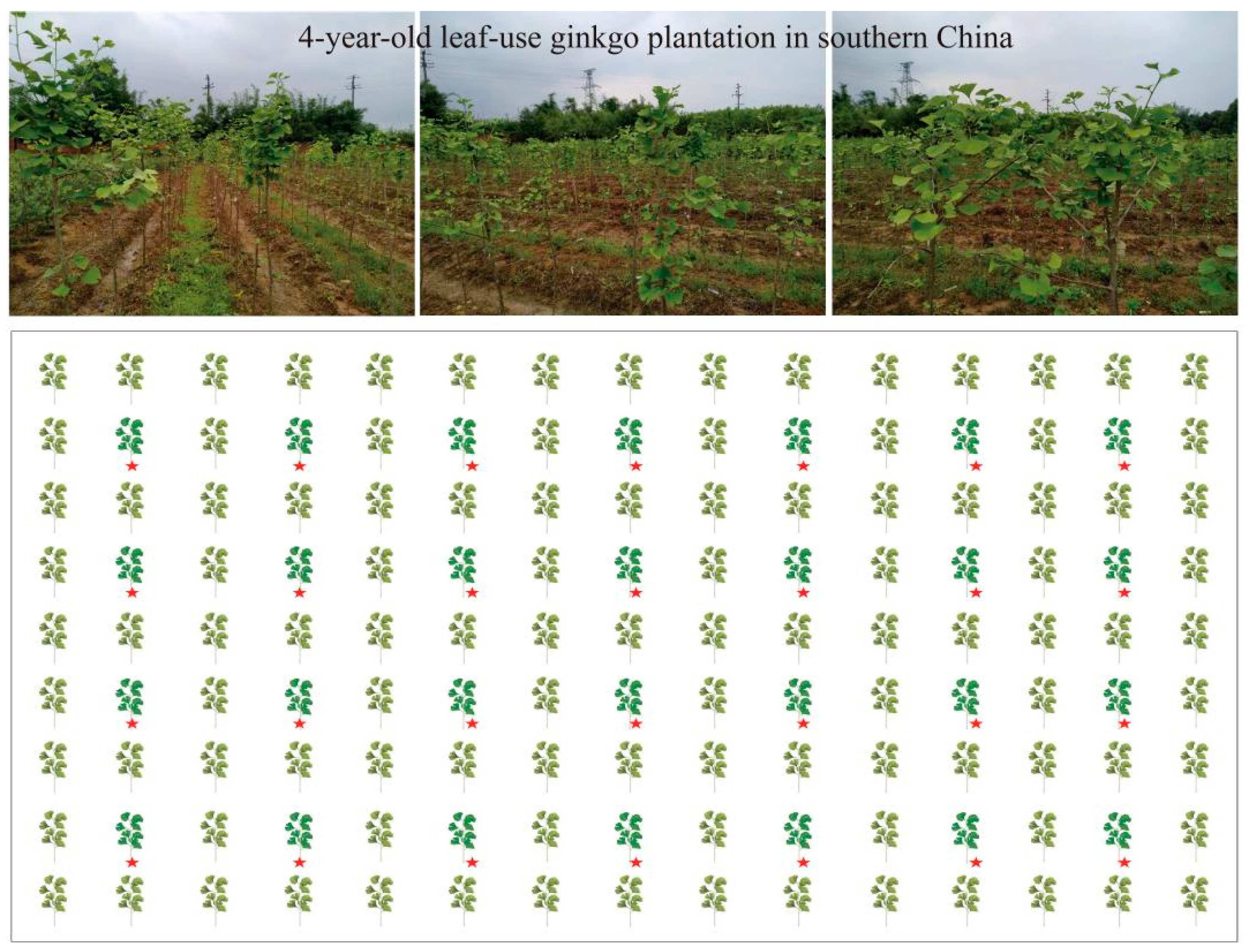
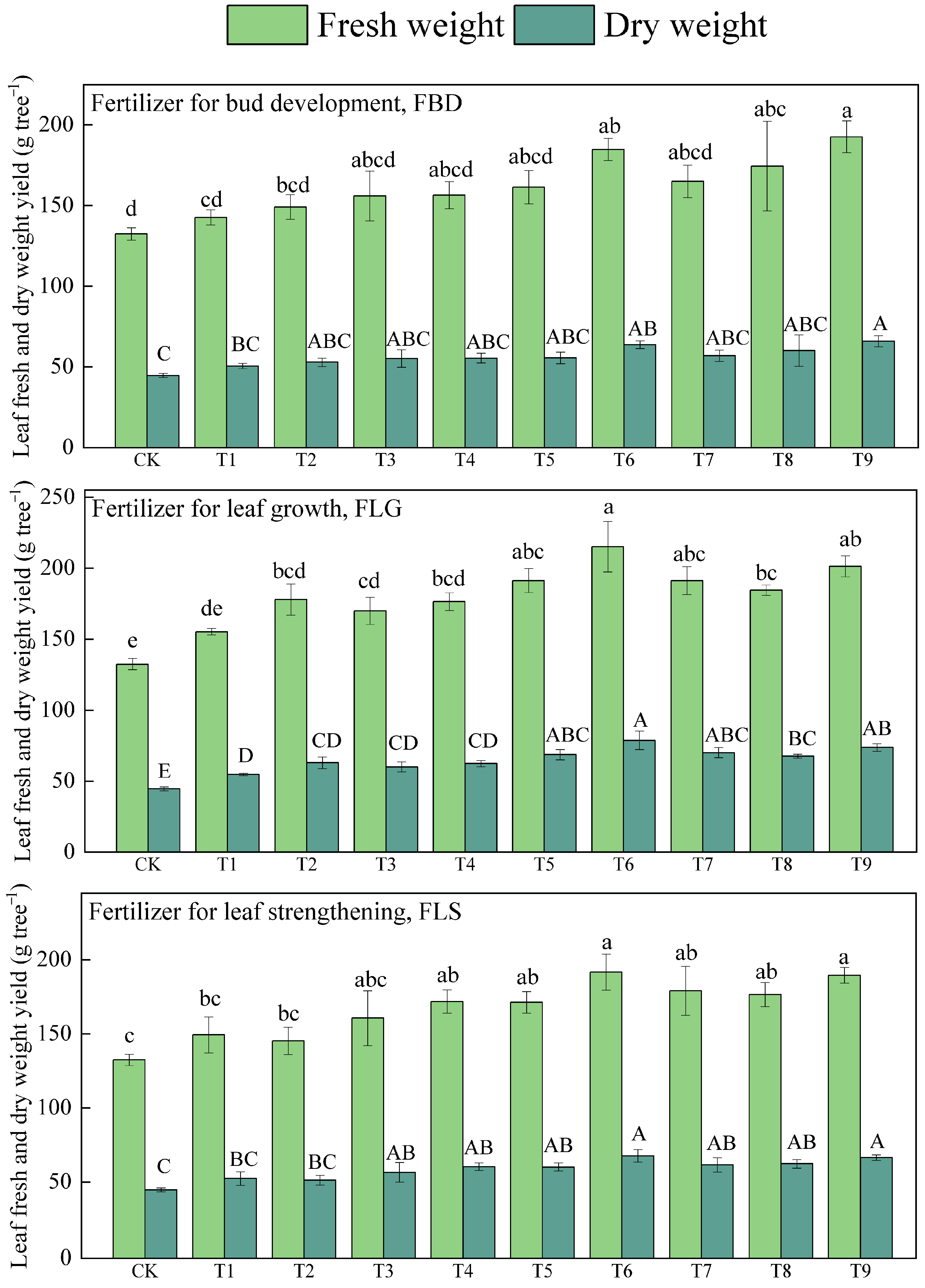
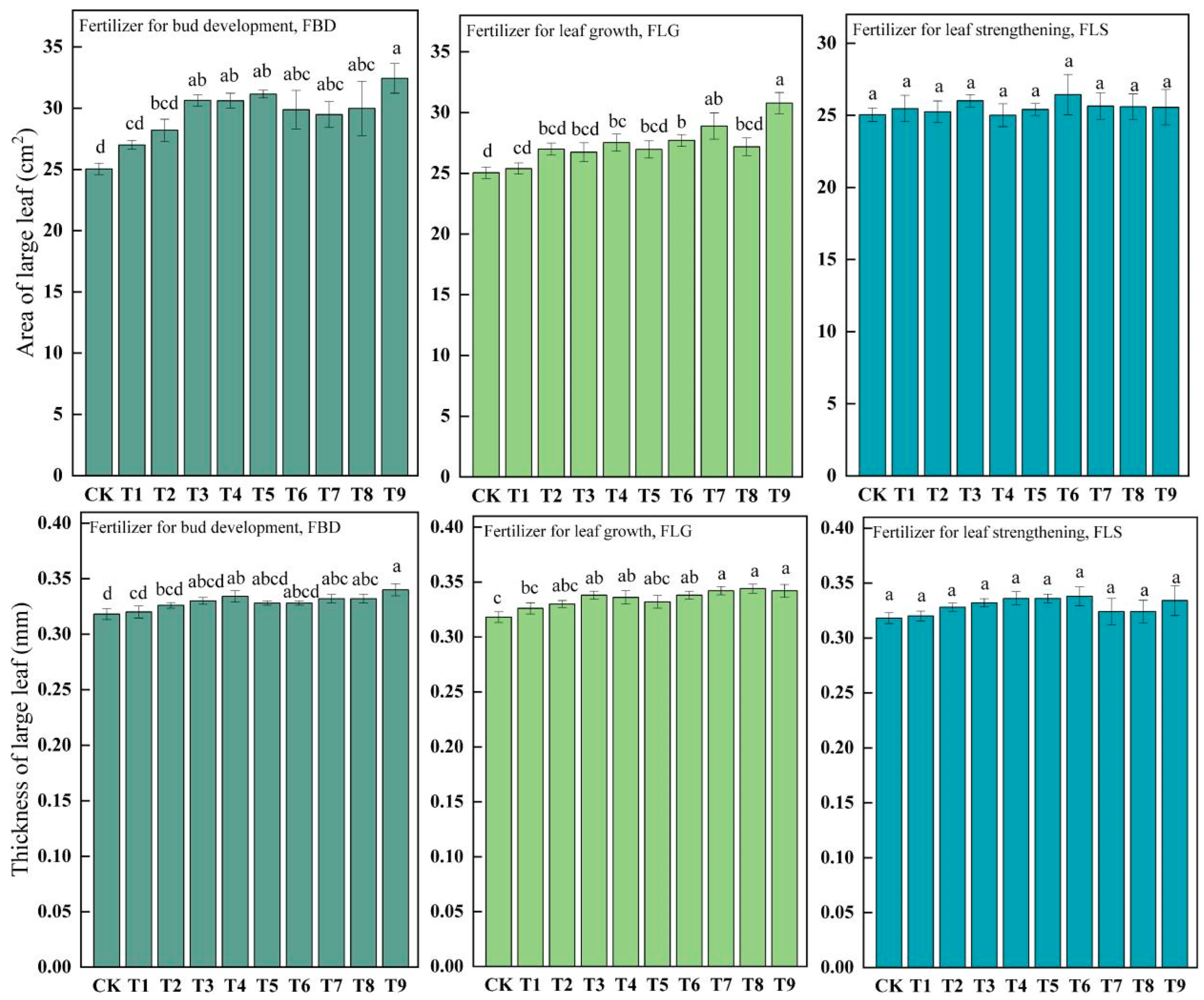
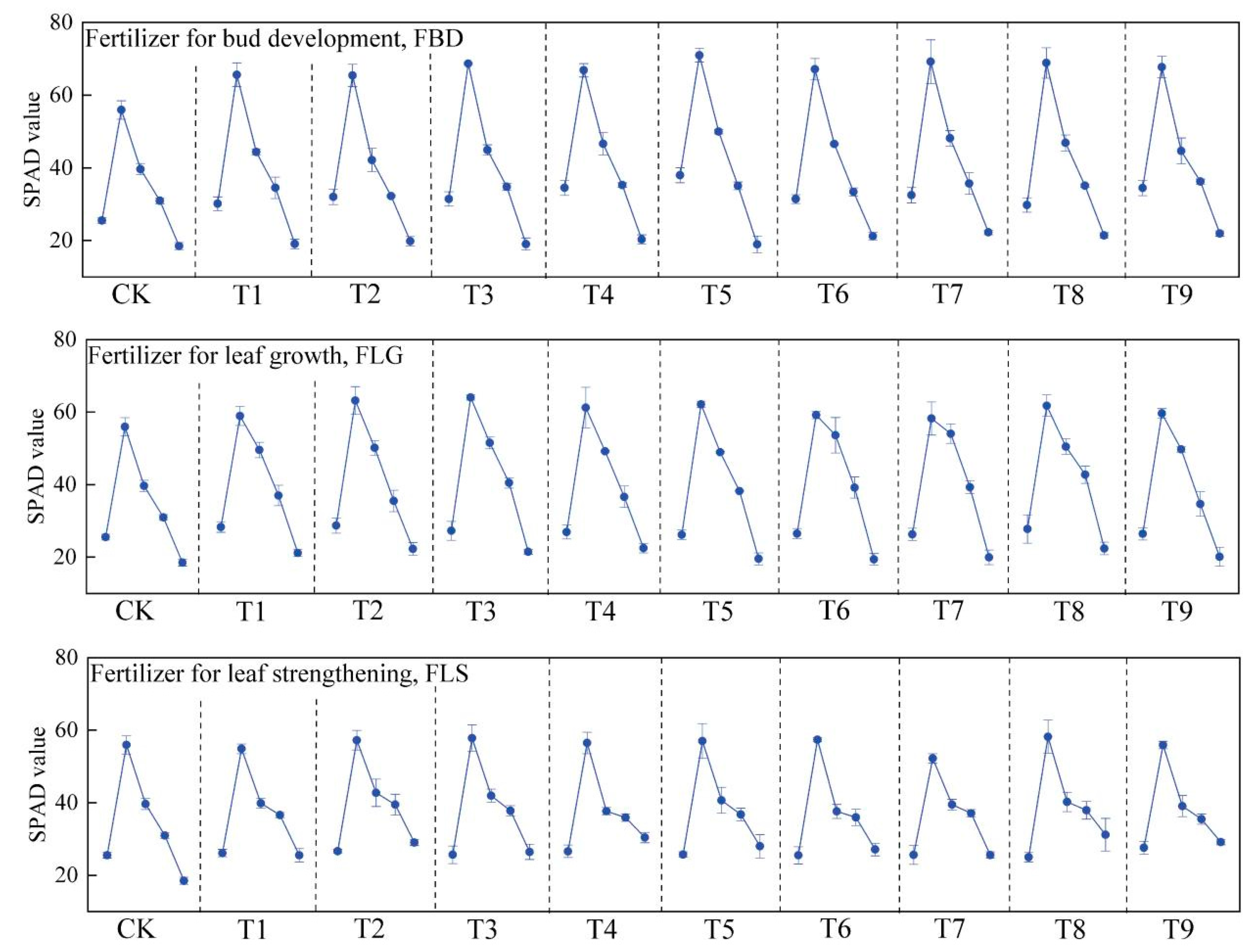
| Treatments | Fertilizer for Bud Development FBD (Applied in March) | Fertilizer for Leaf Growth FLG (Applied in May) | Fertilizer for Leaf Strengthening FLS (Applied in July) |
|---|---|---|---|
| CK | 0 | 0 | 0 |
| T1 | 2.0 g N + 1.0 g P2O5 + 1.0 g K2O | 2.0 g N + 1.5 g P2O5 + 1.5 g K2O | 2.0 g N + 1.5 g P2O5 + 2.0 g K2O |
| T2 | 2.0 g N + 1.5 g P2O5 + 1.5 g K2O | 2.0 g N + 2.0 g P2O5 + 2.0 g K2O | 2.0 g N + 2.0 g P2O5 + 2.5 g K2O |
| T3 | 2.0 g N + 2.0 g P2O5 + 2.0 g K2O | 2.0 g N + 2.5 g P2O5 + 2.5 g K2O | 2.0 g N + 2.5 g P2O5 + 3.0 g K2O |
| T4 | 4.0 g N + 1.0 g P2O5 + 1.5 g K2O | 3.0 g N + 1.5 g P2O5 + 2.0 g K2O | 3.0 g N + 1.5 g P2O5 + 2.5 g K2O |
| T5 | 4.0 g N + 1.5 g P2O5 + 2.0 g K2O | 3.0 g N + 2.0 g P2O5 + 2.5 g K2O | 3.0 g N + 2.0 g P2O5 + 3.0 g K2O |
| T6 | 4.0 g N + 2.0 g P2O5 + 1.0 g K2O | 3.0 g N + 2.5 g P2O5 + 1.5 g K2O | 3.0 g N + 2.5 g P2O5 + 2.0 g K2O |
| T7 | 6.0 g N + 1.0 g P2O5 + 2.0 g K2O | 4.0 g N + 1.5 g P2O5 + 2.5 g K2O | 4.0 g N + 1.5 g P2O5 + 3.0 g K2O |
| T8 | 6.0 g N + 1.5 g P2O5 + 1.0 g K2O | 4.0 g N + 2.0 g P2O5 + 1.5 g K2O | 4.0 g N + 2.0 g P2O5 + 2.0 g K2O |
| T9 | 6.0 g N + 2.0 g P2O5 + 1.5 g K2O | 4.0 g N + 2.5 g P2O5 + 2.0 g K2O | 4.0 g N + 2.5 g P2O5 + 2.5 g K2O |
| Treatment | Quercetin (mg g−1) | Kaempferol (mg g−1) | Isorhamnetin (mg g−1) | Total Flavonoid (mg g−1) | Flavonoid Yield (mg tree−1) | |
|---|---|---|---|---|---|---|
| Fertilizer for bud development, FBD | CK | 0.83 ± 0.11 ab | 1.22 ± 0.12 b | 0.06 ± 0.01 a | 5.28 ± 0.59 b | 238.09 ± 30.34 b |
| T1 | 1.08 ± 0.07 a | 1.37 ± 0.05 b | 0.06 ± 0.01 a | 6.30 ± 0.14 ab | 318.25 ± 6.04 ab | |
| T2 | 0.94 ± 0.07 ab | 1.53 ± 0.07 ab | 0.06 ± 0.02 a | 6.36 ± 0.26 ab | 335.02 ± 14.94 a | |
| T3 | 0.98 ± 0.07 ab | 1.81 ± 0.22 ab | 0.08 ± 0.01 a | 7.20 ± 0.45 a | 393.10 ± 31.94 a | |
| T4 | 1.06 ± 0.12 ab | 1.39 ± 0.10 b | 0.08 ± 0.01 a | 6.36 ± 0.38 ab | 355.80 ± 35.67 a | |
| T5 | 0.84 ± 0.04 ab | 1.33 ± 0.15 b | 0.09 ± 0.02 a | 5.69 ± 0.39 b | 320.28 ± 36.82 ab | |
| T6 | 0.80 ± 0.06 b | 1.37 ± 0.08 b | 0.07 ± 0.01 a | 5.62 ± 0.11 b | 358.83 ± 16.40 a | |
| T7 | 0.89 ± 0.09 ab | 1.6 ± 0.17 ab | 0.07 ± 0.01 a | 6.42 ± 0.3 ab | 365.27 ± 27.95 a | |
| T8 | 0.81 ± 0.05 ab | 1.37 ± 0.14 b | 0.07 ± 0.01 a | 5.66 ± 0.40 b | 331.17 ± 39.07 ab | |
| T9 | 0.86 ± 0.1 ab | 1.47 ± 0.13 ab | 0.08 ± 0.01 a | 6.04 ± 0.45 ab | 399.33 ± 40.98 a | |
| Fertilizer for leaf growth, FLG | CK | 0.83 ± 0.11 c | 1.22 ± 0.12 d | 0.06 ± 0.01 b | 5.28 ± 0.59 b | 238.09 ± 30.34 b |
| T1 | 1.49 ± 0.24 a | 1.49 ± 0.08 bcd | 0.07 ± 0.01 ab | 7.65 ± 0.77 a | 419.10 ± 39.13 a | |
| T2 | 1.46 ± 0.12 ab | 1.67 ± 0.1 abc | 0.08 ± 0.01 ab | 8.07 ± 0.46 a | 512.72 ± 53.17 a | |
| T3 | 1.12 ± 0.07 bc | 1.88 ± 0.13 a | 0.07 ± 0.01 ab | 7.73 ± 0.27 a | 465.23 ± 33.01 a | |
| T4 | 1.04 ± 0.04 c | 1.68 ± 0.14 abc | 0.07 ± 0.01 ab | 7.01 ± 0.31 a | 437.60 ± 22.51 a | |
| T5 | 1.08 ± 0.12 c | 1.85 ± 0.14 ab | 0.09 ± 0.01 ab | 7.58 ± 0.65 a | 514.22 ± 29.88 a | |
| T6 | 1.00 ± 0.10 c | 1.64 ± 0.15 abc | 0.07 ± 0.01 ab | 6.79 ± 0.62 ab | 524.08 ± 42.02 a | |
| T7 | 1.02 ± 0.05 c | 1.65 ± 0.11 abc | 0.21 ± 0.13 a | 7.22 ± 0.49 a | 507.00 ± 45.55 a | |
| T8 | 0.98 ± 0.14 c | 1.52 ± 0.07 abcd | 0.08 ± 0.01 ab | 6.49 ± 0.43 ab | 438.96 ± 27.89 a | |
| T9 | 1.01 ± 0.11 c | 1.43 ± 0.1 cd | 0.07 ± 0.01 ab | 6.31 ± 0.51 ab | 466.13 ± 41.62 a | |
| Fertilizer for leaf strengthening, FLS | CK | 0.83 ± 0.11 c | 1.22 ± 0.12 a | 0.06 ± 0.01 a | 5.28 ± 0.59 c | 238.09 ± 30.34 c |
| T1 | 1.15 ± 0.05 abc | 1.67 ± 0.08 a | 0.08 ± 0.01 a | 7.27 ± 0.29 ab | 382.09 ± 41.97 ab | |
| T2 | 1.13 ± 0.06 abc | 1.58 ± 0.10 a | 0.08 ± 0.01 a | 6.98 ± 0.28 abc | 355.93 ± 17.46 b | |
| T3 | 1.39 ± 0.15 a | 1.39 ± 0.18 a | 0.09 ± 0.04 a | 7.20 ± 0.73 bc | 390.03 ± 21.1 ab | |
| T4 | 1.33 ± 0.14 abc | 1.55 ± 0.19 a | 0.21 ± 0.14 a | 7.73 ± 0.68 a | 460.30 ± 24.15 a | |
| T5 | 1.04 ± 0.06 bc | 1.52 ± 0.13 a | 0.07 ± 0.01 a | 6.59 ± 0.27 abc | 394.79 ± 16.7 ab | |
| T6 | 0.90 ± 0.11 c | 1.41 ± 0.20 a | 0.07 ± 0.01 a | 5.98 ± 0.75 bc | 398.38 ± 40.78 ab | |
| T7 | 0.93 ± 0.09 c | 1.59 ± 0.15 a | 0.05 ± 0.01 a | 6.46 ± 0.51 abc | 390.68 ± 24.24 ab | |
| T8 | 0.90 ± 0.10 c | 1.35 ± 0.06 a | 0.22 ± 0.14 a | 6.19 ± 0.33 abc | 388.96 ± 35.11 ab | |
| T9 | 0.87 ± 0.03 c | 1.48 ± 0.10 a | 0.22 ± 0.14 a | 6.45 ± 0.48 abc | 427.37 ± 27.77 ab |
| Treatment | Total Income A | Fertilizer Cost B | Labor Costs B | Ecological Costs B | Health Costs B | Net Income | Ecosystem Economic Benefits C | |
|---|---|---|---|---|---|---|---|---|
| Fertilizer for bud development, FBD | CK | 3327.32 ± 98.82 c | 0 | 0 | 0 | 0 | 3327.32 ± 98.82 a | - |
| T1 | 3766.87 ± 120.84 bc | 125.45 | 86.64 | 72.22 | 39.48 | 3443.08 ± 120.84 a | 115.77 ± 45.62 b | |
| T2 | 3936.5 ± 196.66 abc | 170.78 | 86.64 | 73.75 | 39.48 | 3565.85 ± 196.66 a | 238.54 ± 98.11 b | |
| T3 | 4113.72 ± 400.63 abc | 216.12 | 86.64 | 75.28 | 39.48 | 3696.21 ± 400.63 a | 368.89 ± 304.27 ab | |
| T4 | 4127.56 ± 219.44 abc | 166.4 | 86.64 | 141.56 | 78.97 | 3654.00 ± 219.44 a | 326.68 ± 135.32 ab | |
| T5 | 4140.06 ± 269.48 abc | 211.73 | 86.64 | 143.09 | 78.97 | 3619.63 ± 269.48 a | 292.32 ± 184.47 ab | |
| T6 | 4748.36 ± 179.34 ab | 238.57 | 86.64 | 144.06 | 78.97 | 4200.13 ± 179.34 a | 872.81 ± 95.07 a | |
| T7 | 4235.89 ± 261.92 abc | 207.35 | 86.64 | 210.9 | 118.45 | 3612.55 ± 261.92 a | 285.23 ± 168.49 ab | |
| T8 | 4479.62 ± 721.49 ab | 234.18 | 86.64 | 211.87 | 118.45 | 3828.48 ± 721.49 a | 501.16 ± 642.40 a | |
| T9 | 4907.87 ± 260.97 a | 279.51 | 86.64 | 213.4 | 118.45 | 4209.86 ± 260.97 a | 882.55 ± 194.88 a | |
| Fertilizer for leaf growth, FLG | CK | 3327.32 ± 98.82 c | 0 | 0 | 0 | 0 | 3327.32 ± 98.82 e | - |
| T1 | 4084.26 ± 61.96 d | 170.78 | 86.64 | 73.75 | 39.48 | 3713.61 ± 61.96 de | 386.29 ± 40.09 e | |
| T2 | 4696.57 ± 297.19 cd | 216.12 | 86.64 | 75.28 | 39.48 | 4279.06 ± 297.19 bcd | 951.74 ± 226.68 bcde | |
| T3 | 4477.99 ± 259.45 cd | 261.45 | 86.64 | 76.81 | 39.48 | 4013.61 ± 259.45 cde | 686.29 ± 194.49 de | |
| T4 | 4654.91 ± 168.15 cd | 194.34 | 86.64 | 108.51 | 59.23 | 4206.19 ± 168.15 bcd | 878.88 ± 78.22 cde | |
| T5 | 5122.14 ± 265.4 abc | 239.67 | 86.64 | 110.04 | 59.23 | 4626.56 ± 265.40 abc | 1299.25 ± 176.10 bcd | |
| T6 | 5865.25 ± 480.34 a | 266.51 | 86.64 | 111.01 | 59.23 | 5341.87 ± 480.34 a | 2014.55 ± 402.34 a | |
| T7 | 5221.24 ± 264.83 abc | 217.90 | 86.64 | 143.27 | 78.97 | 4694.46 ± 264.83 abc | 1367.14 ± 181.72 bc | |
| T8 | 5040.15 ± 99.94 bc | 244.73 | 86.64 | 144.24 | 78.97 | 4485.57 ± 99.94 bcd | 1158.25 ± 43.42 bcd | |
| T9 | 5492.51 ± 198.12 ab | 290.07 | 86.64 | 145.77 | 78.97 | 4891.06 ± 198.12 ab | 1563.74 ± 106.34 ab | |
| Fertilizer for leaf strengthening, FLS | CK | 3327.32 ± 98.82 c | 0 | 0 | 0 | 0 | 3327.32 ± 98.82 c | - |
| T1 | 3896.63 ± 338.61 bc | 176.95 | 86.64 | 73.93 | 39.48 | 3519.62 ± 338.61 bc | 192.3 ± 258.11 c | |
| T2 | 3817.02 ± 241.53 bc | 222.28 | 86.64 | 75.46 | 39.48 | 3393.15 ± 241.53 c | 65.83 ± 170.19 c | |
| T3 | 4208.81 ± 493.03 ab | 267.62 | 86.64 | 76.99 | 39.48 | 3738.08 ± 493.03 abc | 410.76 ± 418.94 bc | |
| T4 | 4488.4 ± 192.38 ab | 200.51 | 86.64 | 108.7 | 59.23 | 4033.33 ± 192.38 abc | 706.02 ± 93.61 abc | |
| T5 | 4474.42 ± 205.18 ab | 245.84 | 86.64 | 110.23 | 59.23 | 3972.48 ± 205.18 abc | 645.17 ± 109.94 abc | |
| T6 | 5036.14 ± 315.34 a | 272.67 | 86.64 | 111.2 | 59.23 | 4506.40 ± 315.34 a | 1179.08 ± 216.82 a | |
| T7 | 4585.87 ± 361.16 ab | 224.07 | 86.64 | 143.46 | 78.97 | 4052.73 ± 361.16 abc | 725.42 ± 279.27 abc | |
| T8 | 4638.54 ± 211.95 ab | 257.07 | 86.64 | 144.62 | 78.97 | 4071.25 ± 211.95 abc | 743.93 ± 150.29 abc | |
| T9 | 4946.26 ± 130.96 a | 290.07 | 86.64 | 145.77 | 78.97 | 4344.81 ± 130.96 ab | 1017.5 ± 38.23 ab |
| Treatment | Ci | Ranking Number | |||
|---|---|---|---|---|---|
| Fertilizer for bud development, FBD | CK | 356.63 | 0 | 0 | 28 |
| T1 | 331.83 | 36.53 | 0.1 | 27 | |
| T2 | 309.57 | 58.2 | 0.16 | 24 | |
| T3 | 286.25 | 82.11 | 0.22 | 19 | |
| T4 | 294.19 | 70.54 | 0.19 | 23 | |
| T5 | 299.26 | 72.73 | 0.2 | 22 | |
| T6 | 198.47 | 162.15 | 0.45 | 10 | |
| T7 | 300.46 | 73.13 | 0.2 | 21 | |
| T8 | 262.72 | 105.42 | 0.29 | 17 | |
| T9 | 196.28 | 168.14 | 0.46 | 9 | |
| Fertilizer for leaf growth, FLG | T1 | 284.1 | 78.51 | 0.22 | 20 |
| T2 | 185.7 | 172.86 | 0.48 | 8 | |
| T3 | 230.87 | 134.2 | 0.37 | 14 | |
| T4 | 198.51 | 159.52 | 0.45 | 11 | |
| T5 | 125.09 | 232.51 | 0.65 | 4 | |
| T6 | 12.48 | 355.07 | 0.97 | 1 | |
| T7 | 113.78 | 243.01 | 0.68 | 3 | |
| T8 | 149.04 | 209.49 | 0.58 | 6 | |
| T9 | 78.51 | 280.02 | 0.78 | 2 | |
| Fertilizer for leaf strengthening, FLS | T1 | 317.42 | 53.93 | 0.15 | 25 |
| T2 | 338.58 | 54.14 | 0.14 | 26 | |
| T3 | 278.51 | 95.42 | 0.26 | 18 | |
| T4 | 228.13 | 131.71 | 0.37 | 15 | |
| T5 | 237.92 | 126.49 | 0.35 | 16 | |
| T6 | 145.34 | 214.59 | 0.6 | 5 | |
| T7 | 224.18 | 137.25 | 0.38 | 13 | |
| T8 | 220.56 | 143.31 | 0.39 | 12 | |
| T9 | 173.03 | 189.8 | 0.52 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.; Chu, S.; Zheng, F.; Xian, L.; Lu, J.; Liao, D.; Ouyang, J.; Long, M.; Jacobs, D.F.; Hu, D.; et al. Optimizing Fertilization Strategies to Promote Leaf-Use Ginkgo Productivity and Ecosystem Economic Benefits: An Integrated Evaluation of a Field Trial in Southern China. Agronomy 2024, 14, 1956. https://doi.org/10.3390/agronomy14091956
Xiao M, Chu S, Zheng F, Xian L, Lu J, Liao D, Ouyang J, Long M, Jacobs DF, Hu D, et al. Optimizing Fertilization Strategies to Promote Leaf-Use Ginkgo Productivity and Ecosystem Economic Benefits: An Integrated Evaluation of a Field Trial in Southern China. Agronomy. 2024; 14(9):1956. https://doi.org/10.3390/agronomy14091956
Chicago/Turabian StyleXiao, Mengrui, Shuangshuang Chu, Fenglin Zheng, Lihua Xian, Jie Lu, Dandan Liao, Jianhui Ouyang, Mandi Long, Douglass F. Jacobs, Dongnan Hu, and et al. 2024. "Optimizing Fertilization Strategies to Promote Leaf-Use Ginkgo Productivity and Ecosystem Economic Benefits: An Integrated Evaluation of a Field Trial in Southern China" Agronomy 14, no. 9: 1956. https://doi.org/10.3390/agronomy14091956
APA StyleXiao, M., Chu, S., Zheng, F., Xian, L., Lu, J., Liao, D., Ouyang, J., Long, M., Jacobs, D. F., Hu, D., & Zeng, S. (2024). Optimizing Fertilization Strategies to Promote Leaf-Use Ginkgo Productivity and Ecosystem Economic Benefits: An Integrated Evaluation of a Field Trial in Southern China. Agronomy, 14(9), 1956. https://doi.org/10.3390/agronomy14091956






