Evaluation of Pepper (Capsicum spp.) Germplasm Collection for Bacterial Wilt (Ralstonia solanacearum) Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation
2.2. Pathogen and Inoculum Preparation
2.3. Bacterial Wilt Resistance Evaluation
2.4. Fruit-Related Traits and Carotenoid Content Analysis of Resistant Accessions
2.5. Statistical Analysis
3. Results
3.1. Disease Assessment
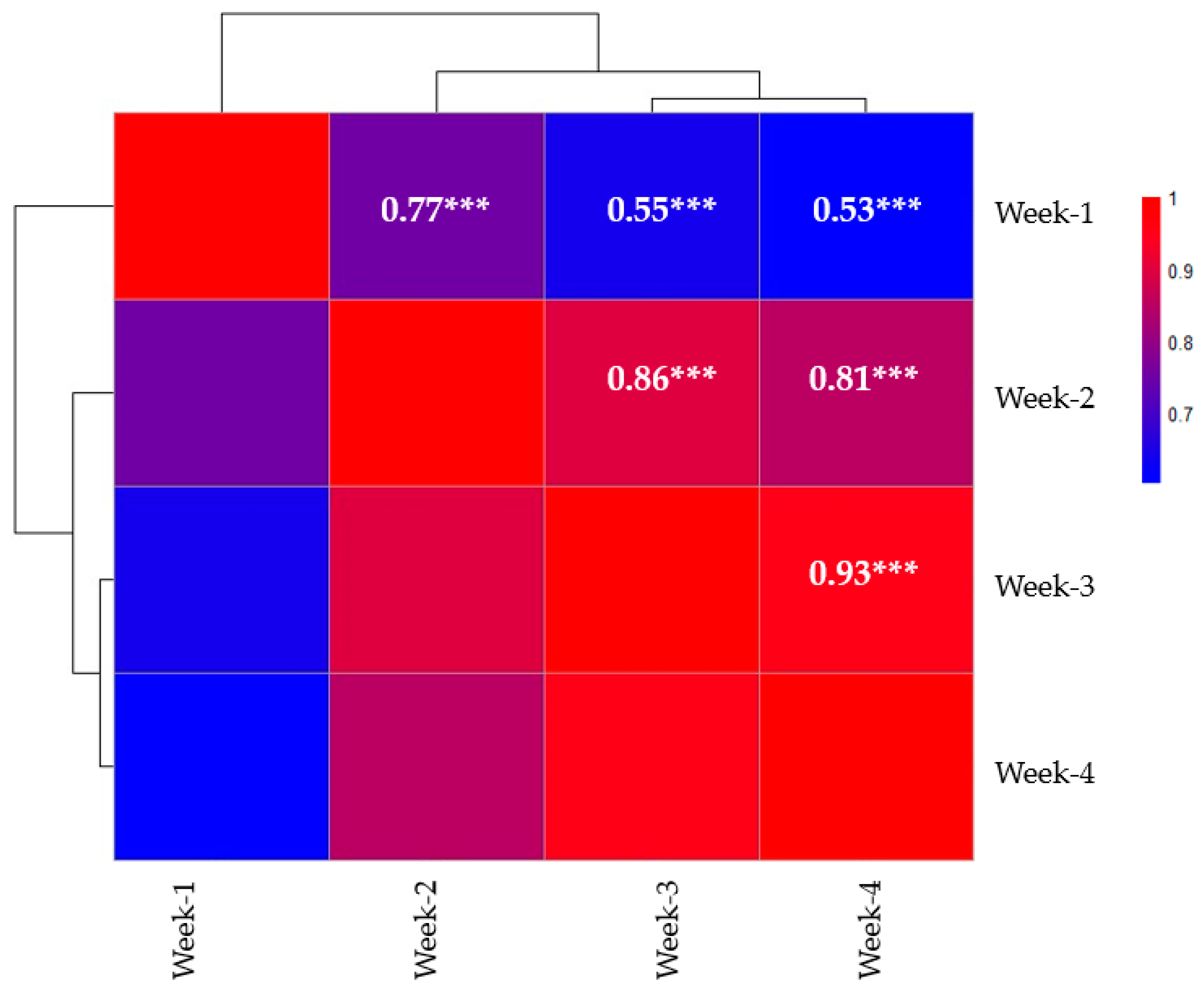
3.2. Correlation Analysis
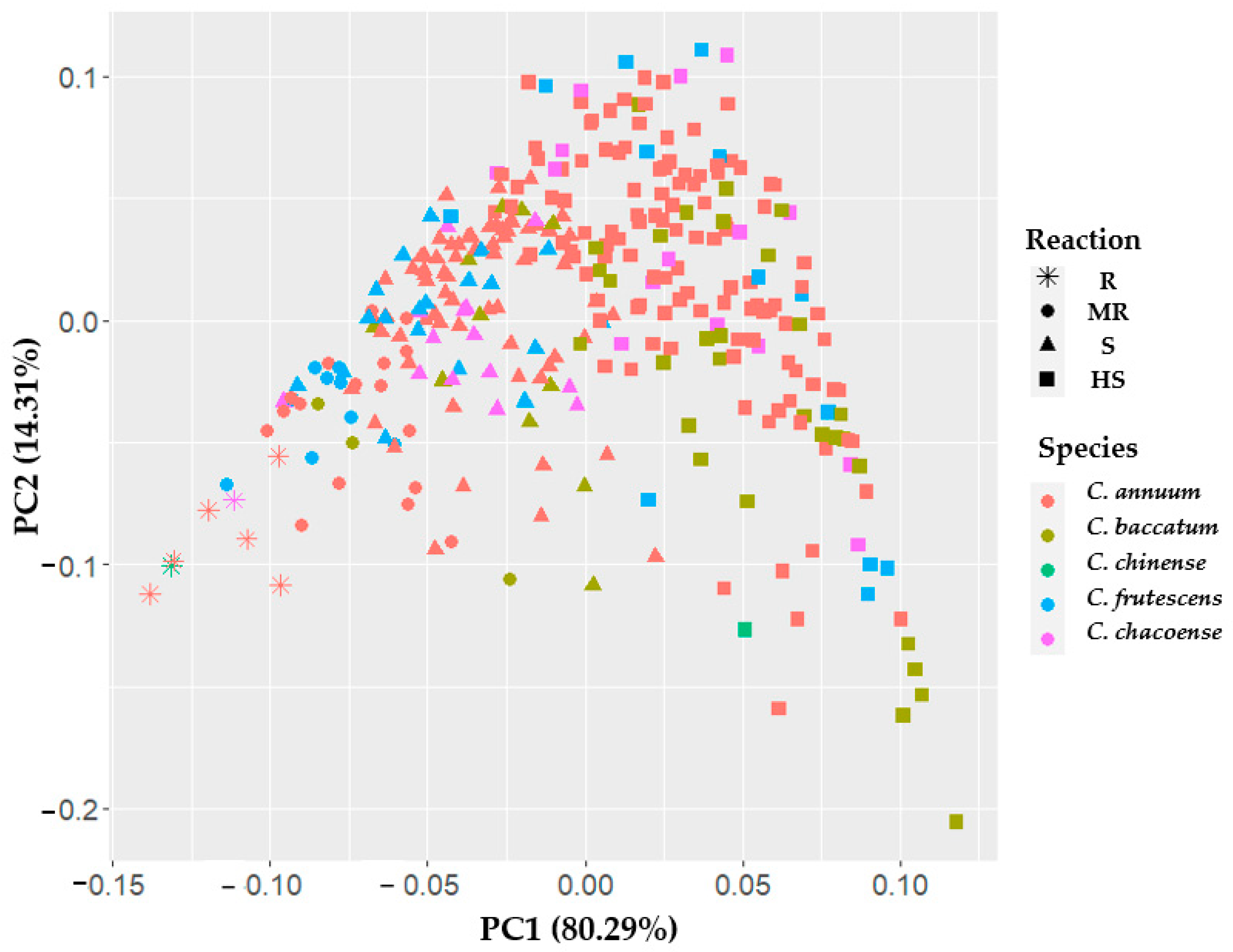
3.3. Principal Component Analysis (PCA)
3.4. Selection of Resistant Pepper Accessions against BW
3.5. Fruit-Related Traits and Bioactive Compounds in Selected Resistant Pepper Accessions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perry, L.; Dickau, R.; Zarrillo, S.; Holst, I.; Pearsall, D.M.; Piperno, D.R.; Berman, M.J.; Cooke, R.G.; Rademaker, K.; Ranere, A.J.; et al. Starch Fossils and the Domestication and Dispersal of Chili Peppers (Capsicum spp. L.) in the Americas. Science 2007, 315, 986–988. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 8 April 2024).
- Sarath Babu, B.; Pandravada, S.R.; Prasada Rao, R.D.V.J.; Anitha, K.; Chakrabarty, S.K.; Varaprasad, K.S. Global Sources of Pepper Genetic Resources against Arthropods, Nematodes and Pathogens. Crop Protect. 2011, 30, 389–400. [Google Scholar] [CrossRef]
- Parisi, M.; Alioto, D.; Tripodi, P. Overview of Biotic Stresses in Pepper (Capsicum spp.): Sources of Genetic Resistance, Molecular Breeding and Genomics. Int. J. Mol. Sci. 2020, 21, 2587. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Faridi, N.; Raj, S.M.P.; Agarwal, A.; Punetha, M. Recent Advances in Immuno-Based Methods for the Detection of Ralstonia solanacearum. J. Microbiol. Methods 2024, 217–218, 106889. [Google Scholar] [CrossRef] [PubMed]
- Planas-Marquès, M.; Kressin, J.P.; Kashyap, A.; Panthee, D.R.; Louws, F.J.; Coll, N.S.; Valls, M. Four Bottlenecks Restrict Colonization and Invasion by the Pathogen Ralstonia solanacearum in Resistant Tomato. J. Exp. Bot. 2020, 71, 2157–2171. [Google Scholar] [CrossRef] [PubMed]
- Genin, S. Molecular Traits Controlling Host Range and Adaptation to Plants in Ralstonia solanacearum. New Phytol. 2010, 187, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Peeters, N.; Guidot, A.; Vailleau, F.; Valls, M. Ralstonia solanacearum, a Widespread Bacterial Plant Pathogen in the Post-Genomic Era. Mol. Plant Pathol. 2013, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kim, J.; Kang, Y.; Lee, S.; Hwang, I. Genetic Diversity and Distribution of Korean Isolates of Ralstonia solanacearum. Plant Dis. 2007, 91, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Kang, H.W. Physiological, Biochemical and Genetic Characteristics of Ralstonia solanacearum Strains Isolated from Pepper Plants in Korea. Res. Plant Dis. 2013, 19, 265–272. [Google Scholar] [CrossRef]
- Jiang, G.; Peyraud, R.; Remigi, P.; Guidot, A.; Berthomé, R.; Ding, W.; Jousset, A.; Genin, S.; Peeters, N. The Population Dynamics of a Bacterial Pathogen after Host Re-Infection Affects the Founding Population Size. bioRxiv 2016, 061408. [Google Scholar] [CrossRef]
- Yuliar; Nion, Y.A.; Toyota, K. Recent Trends in Control Methods for Bacterial Wilt Diseases Caused by Ralstonia solanacearum. Microbes Environ. 2015, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Elphinstone, J.G. The current bacterial wilt situation: A global overview. In Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex; Phytopathology: St. Paul, MN, USA, 2005; ISBN 978-0-89054-329-0. [Google Scholar]
- Huet, G. Breeding for Resistances to Ralstonia solanacearum. Front. Plant Sci. 2014, 5, 715. [Google Scholar] [CrossRef] [PubMed]
- French, E.R. Bacterial Wilt: The Disease and Its Causative Agent, Pseudomonas solanacearum; Proceedings of the International Symposium on Bacterial Wilt Held in Taiwan in October 1992; CAB International: Oxon, UK, 1994; ISBN 978-0-85198-875-7. [Google Scholar]
- Lopez, M.M.; Biosca, E.G. Potato bacterial wilt management: New prospects for an old problem. In Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex; Phytopathology: St. Paul, MN, USA, 2005; ISBN 978-0-89054-329-0. [Google Scholar]
- Saddler, G.S. Management of bacterial wilt disease. In Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex; Phytopathology: St. Paul, MN, USA, 2005; ISBN 978-0-89054-329-0. [Google Scholar]
- Denny, T. Plant Pathogenic Ralstonia Species. In Plant-Associated Bacteria; Gnanamanickam, S.S., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 573–644. ISBN 978-1-4020-4536-3. [Google Scholar]
- Hayward, A.C. Biology and Epidemiology of Bacterial Wilt Caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 1991, 29, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, H.; Sato, T.; Monma, S. In Inheritance of Bacterial Wilt Resistance in the Sweet Pepper Cv. Mie-Midori. In Proceedings of the 10th Eucarpia Meeting on Genetics and Breeding of Capsicum and Eggplant, Avignon, France, 7–11 September 1998; p. 172. [Google Scholar]
- Kim, B.-S.; Cheung, J.-D.; Cha, Y.-S.; Hwang, H.-S. Resistance to Bacterial Wilt of Introduced Peppers. Korean J. Plant Pathol. 1998, 14, 217–219. [Google Scholar]
- Lopes, C.A.; Boiteux, L.S. Biovar-Specific and Broad-Spectrum Sources of Resistance to Bacterial Wilt (Ralstonia solanacearum) in Capsicum. Crop Breed. Appl. Biotechnol. 2004, 4, 350–355. [Google Scholar] [CrossRef][Green Version]
- Mimura, Y.; Yoshikawa, M.; Hirai, M. Pepper Accession LS2341 Is Highly Resistant to Ralstonia solanacearum Strains from Japan. HortScience 2009, 44, 2038–2040. [Google Scholar] [CrossRef]
- Wang, J.-F.; Olivier, J.; Thoquet, P.; Mangin, B.; Sauviac, L.; Grimsley, N.H. Resistance of Tomato Line Hawaii7996 to Ralstonia solanacearum Pss4 in Taiwan Is Controlled Mainly by a Major Strain-Specific Locus. Mol. Plant-Microbe Interact. 2000, 13, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-F.; Ho, F.-I.; Truong, H.T.H.; Huang, S.-M.; Balatero, C.H.; Dittapongpitch, V.; Hidayati, N. Identification of Major QTLs Associated with Stable Resistance of Tomato Cultivar ‘Hawaii 7996’ to Ralstonia solanacearum. Euphytica 2013, 190, 241–252. [Google Scholar] [CrossRef]
- Carmeille, A.; Caranta, C.; Dintinger, J.; Prior, P.; Luisetti, J.; Besse, P. Identification of QTLs for Ralstonia solanacearum Race 3-Phylotype II Resistance in Tomato. Theor. Appl. Genet. 2006, 113, 110–121. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, X.; Wang, D.; Zhang, L.; Zu, C.; Gao, Z.; Zhang, H.; Wang, Z.; Sun, X.; Yao, D. The Detection of QTLs Controlling Bacterial Wilt Resistance in Tobacco (N. Tabacum L.). Euphytica 2013, 192, 259–266. [Google Scholar] [CrossRef]
- Lebeau, A.; Gouy, M.; Daunay, M.C.; Wicker, E.; Chiroleu, F.; Prior, P.; Frary, A.; Dintinger, J. Genetic Mapping of a Major Dominant Gene for Resistance to Ralstonia solanacearum in Eggplant. Theor. Appl. Genet. 2013, 126, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Lafortune, D.; Béramis, M.; Daubèze, A.-M.; Boissot, N.; Palloix, A. Partial Resistance of Pepper to Bacterial Wilt Is Oligogenic and Stable Under Tropical Conditions. Plant Dis. 2005, 89, 501–506. [Google Scholar] [CrossRef]
- Mimura, Y.; Kageyama, T.; Minamiyama, Y.; Hirai, M. QTL Analysis for Resistance to Ralstonia solanacearum in Capsicum Accession ‘LS2341’. J. Jpn. Soc. Hortic. Sci. 2009, 78, 307–313. [Google Scholar] [CrossRef]
- Du, H.; Wen, C.; Zhang, X.; Xu, X.; Yang, J.; Chen, B.; Geng, S. Identification of a Major QTL (qRRs-10.1) That Confers Resistance to Ralstonia solanacearum in Pepper (Capsicum annuum) Using SLAF-BSA and QTL Mapping. Int. J. Mol. Sci. 2019, 20, 5887. [Google Scholar] [CrossRef] [PubMed]
- Kelman, A. The Relationship of Pathogenicity of Pseudomonas solanacearum to Colony Appearance in a Tetrazolium Medium. Phytopathology 1954, 44, 693–695. [Google Scholar]
- Kim, S.G.; Hur, O.-S.; Ro, N.-Y.; Ko, H.-C.; Rhee, J.-H.; Sung, J.S.; Ryu, K.-Y.; Lee, S.-Y.; Baek, H.J. Evaluation of Resistance to Ralstonia solanacearum in Tomato Genetic Resources at Seedling Stage. Plant Pathol. J. 2016, 32, 58–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, C.-H.; Tsai, K.-C.; Prior, P.; Wang, J.-F. Phylogenetic Relationships and Population Structure of Ralstonia Solanacearum Isolated from Diverse Origins in Taiwan. Plant Pathol. 2014, 63, 1395–1403. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.; Oh, D.-G. Resistance of Pepper Cultivars to Ralstonia solanacearum Isolates from Major Cultivated Areasof Chili Peppers in Korea. Hortic. Sci. Technol. 2018, 36, 569–576. [Google Scholar] [CrossRef]
- Roberts, D.P.; Denny, T.P.; Schell, M.A. Cloning of the Egl Gene of Pseudomonas solanacearum and Analysis of Its Role in Phytopathogenicity. J. Bacteriol. 1988, 170, 1445–1451. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, S.Y.; Chu, S.M.; Lim, S.H.; Suh, S.-C.; Lee, Y.-T.; Cho, H.S.; Ha, S.-H. Variation and Correlation Analysis of Flavonoids and Carotenoids in Korean Pigmented Rice (Oryza Sativa L.) Cultivars. J. Agric. Food Chem. 2010, 58, 12804–12809. [Google Scholar] [CrossRef]
- Tran, N.H.; Kim, B.-S. Sources of Resistance to Bacterial Wilt Found in Vietnam Collections of Pepper (Capsicum annuum) and Their Nuclear Fertility Restorer Genotypes for Cytoplasmic Male Sterility. Plant Pathol. J. 2012, 28, 418–422. [Google Scholar] [CrossRef][Green Version]
- Jiang, G.; Wei, Z.; Xu, J.; Chen, H.; Zhang, Y.; She, X.; Macho, A.P.; Ding, W.; Liao, B. Bacterial Wilt in China: History, Current Status, and Future Perspectives. Front. Plant Sci. 2017, 8, 1549. [Google Scholar] [CrossRef] [PubMed]
- Phiri, T.M.; Bhattarai, G.; Chiwina, K.E.; Fan, Q.; Xiong, H.; Alatawi, I.; Dickson, R.; Joshi, N.K.; Rojas, A.; Ling, K.-S.; et al. An Evaluation of Bacterial Wilt (Ralstonia solanacearum) Resistance in a Set of Tomato Germplasm from the United States Department of Agriculture. Agronomy 2024, 14, 350. [Google Scholar] [CrossRef]
- Thakur, H.; Sharma, A.; Sharma, P.; Rana, R.S. An Insight into the Problem of Bacterial Wilt in Capsicum Spp. with Special Reference to India. Crop Prot. 2021, 140, 105420. [Google Scholar] [CrossRef]
- Lebeau, A.; Daunay, M.-C.; Frary, A.; Palloix, A.; Wang, J.-F.; Dintinger, J.; Chiroleu, F.; Wicker, E.; Prior, P. Bacterial Wilt Resistance in Tomato, Pepper, and Eggplant: Genetic Resources Respond to Diverse Strains in the Ralstonia solanacearum Species Complex. Phytopathology 2011, 101, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.W.; Wang, J.F.; Hanson, P.M. Breeding Tomatoes for Resistance to Bacterial Wilt, A Global View. Acta Hortic. 2005, 695, 161–172. [Google Scholar] [CrossRef]
- Cohen, R.; Horev, C.; Burger, Y.; Shriber, S.; Hershenhorn, J.; Katan, J.; Edelstein, M. Horticultural and Pathological Aspects of Fusarium Wilt Management Using Grafted Melons. HortScience 2002, 37, 1069–1073. [Google Scholar] [CrossRef]
- Lee, J.-M. Cultivation of Grafted Vegetables I. Current Status, Grafting Methods, and Benefits. HortScience 1994, 29, 235–239. [Google Scholar] [CrossRef]
- Duan, X.; Liu, F.; Bi, H.; Ai, X. Grafting Enhances Bacterial Wilt Resistance in Peppers. Agriculture 2022, 12, 583. [Google Scholar] [CrossRef]
- King, S.R.; Davis, A.R.; Liu, W.; Levi, A. Grafting for Disease Resistance. HortScience 2008, 43, 1673–1676. [Google Scholar] [CrossRef]
- Duan, X.; Bi, H.G.; Li, T.; Wu, G.X.; Li, Q.M.; Ai, X.Z. Root Characteristics of Grafted Peppers and Their Resistance to Fusarium Solani. Biol. Plant. 2017, 61, 579–586. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Effect of Nickel and Grafting Combination on Yield, Fruit Quality, Antioxidative Enzyme Activities, Lipid Peroxidation, and Mineral Composition of Tomato. J. Plant Nutr. Soil Sci. 2015, 178, 848–860. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable Grafting as a Tool to Improve Drought Resistance and Water Use Efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.A.T.S.; Hongal, S.; Harshavardhan, M.; Chandan, K.; Kumar, A.J.S.; Ashok; Kyriacou, M.C.; Rouphael, Y.; Kumar, P. Productive Characteristics and Fruit Quality Traits of Cherry Tomato Hybrids as Modulated by Grafting on Different Solanum spp. Rootstocks under Ralstonia solanacearum Infested Greenhouse Soil. Agronomy 2021, 11, 1311. [Google Scholar] [CrossRef]
- Sanwal, S.K.; Mann, A.; Kumar, A.; Kesh, H.; Kaur, G.; Rai, A.K.; Kumar, R.; Sharma, P.C.; Kumar, A.; Bahadur, A.; et al. Salt Tolerant Eggplant Rootstocks Modulate Sodium Partitioning in Tomato Scion and Improve Performance under Saline Conditions. Agriculture 2022, 12, 183. [Google Scholar] [CrossRef]
- Carlos Álvarez-Hernández, J. Grafting in Horticultural Crop Species: Effective Pest and Disease Management Technique with Potential in Michoacan, Mexico. In Horticultural Crops; Kossi Baimey, H., Hamamouch, N., Adjiguita Kolombia, Y., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-83880-421-3. [Google Scholar]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of Hydrogen Peroxide in Plant Extracts Using Titanium (IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.-Y.; Lee, K.; Do, J.-W.; Hong, S.-C.; Lee, K.-H.; Cho, M.-C.; Yang, E.-Y.; Yoon, J.-B. QTL Mapping of Resistance to Bacterial Wilt in Pepper Plants (Capsicum annuum) Using Genotyping-by-Sequencing (GBS). Horticulturae 2022, 8, 115. [Google Scholar] [CrossRef]
- Lee, S.; Chakma, N.; Joung, S.; Lee, J.M.; Lee, J. QTL Mapping for Resistance to Bacterial Wilt Caused by Two Isolates of Ralstonia solanacearum in Chili Pepper (Capsicum annuum L.). Plants 2022, 11, 1551. [Google Scholar] [CrossRef]
- Lee, J.-H.; Siddique, M.I.; Jang, S.; Kim, G.-W.; Choi, G.J.; Kwon, J.-K.; Kang, B.-C. Identification of QTLs Associated with Resistance to Bacterial Wilt in Pepper (Capsicum annuum L.) through Bi-Parental QTL Mapping and Genome-Wide Association Analysis. Sci. Hortic. 2024, 329, 112987. [Google Scholar] [CrossRef]

| Descriptive Statistics | Week 1 | Week 2 | Week 3 | Week 4 |
|---|---|---|---|---|
| Mean | 0.50 | 2.02 | 2.78 | 3.06 |
| Standard Error | 0.03 | 0.06 | 0.06 | 0.05 |
| Median | 0.33 | 2.00 | 2.95 | 3.20 |
| Standard Deviation | 0.58 | 1.17 | 1.03 | 0.84 |
| Sample Variance | 0.34 | 1.38 | 1.07 | 0.71 |
| Kurtosis | 1.94 | −1.07 | −0.08 | 0.76 |
| Skewness | 1.46 | 0.02 | −0.80 | −0.94 |
| Minimum | 0 | 0 | 0 | 0 |
| Maximum | 3 | 4 | 4 | 4 |
| Count | 338 | 338 | 338 | 338 |
| Weeks | Species | Disease Severity Index | Total | |||
|---|---|---|---|---|---|---|
| 0–1 | 1–2 | 2–3 | 3–4 | |||
| Week-1 | C. annuum | 182 | 32 | 3 | 0 | 217 |
| C. baccatum | 25 | 16 | 5 | 1 | 47 | |
| C. chinense | 39 | 3 | 3 | 0 | 45 | |
| C. frutescens | 24 | 3 | 0 | 0 | 27 | |
| C. chacoense | 1 | 1 | 0 | 0 | 2 | |
| Total | 271 | 55 | 11 | 1 | 338 | |
| Week-2 | C. annuum | 39 | 63 | 61 | 54 | 217 |
| C. baccatum | 3 | 9 | 15 | 20 | 47 | |
| C. chinense | 20 | 11 | 6 | 8 | 45 | |
| C. frutescens | 8 | 8 | 6 | 5 | 27 | |
| C. chacoense | 1 | 0 | 1 | 0 | 2 | |
| Total | 71 | 91 | 89 | 87 | 338 | |
| Week-3 | C. annuum | 11 | 26 | 65 | 115 | 217 |
| C. baccatum | 1 | 2 | 13 | 31 | 47 | |
| C. chinense | 8 | 14 | 9 | 14 | 45 | |
| C. frutescens | 2 | 3 | 14 | 8 | 27 | |
| C. chacoense | 1 | 0 | 0 | 1 | 2 | |
| Total | 23 | 45 | 101 | 169 | 338 | |
| Week-4 | C. annuum | 6 | 18 | 66 | 127 | 217 |
| C. baccatum | 0 | 3 | 11 | 33 | 47 | |
| C. chinense | 2 | 9 | 19 | 15 | 45 | |
| C. frutescens | 1 | 0 | 15 | 11 | 27 | |
| C. chacoense | 1 | 0 | 0 | 1 | 2 | |
| Total | 10 | 30 | 111 | 187 | 338 | |
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| Week 1 | 42.34 | −84.45 | 32.22 | −5.94 |
| Week 2 | 52.78 | −6.17 | −80.95 | 24.92 |
| Week 3 | 52.66 | 34.86 | 7.93 | −77.12 |
| Week 4 | 51.45 | 40.16 | 48.41 | 58.26 |
| Eigenvalue | 3.21 | 0.57 | 0.15 | 0.07 |
| Variance % | 80.29 | 14.31 | 3.77 | 1.63 |
| Cumulative variance % | 80.29 | 94.59 | 98.37 | 100.00 |
| No. | IT No. | Species Name | Accession Name | Week 1 | Week 2 | Week 3 | Week 4 | Reaction |
|---|---|---|---|---|---|---|---|---|
| 1 | 236738 | C. chinense | C04417 | 0.0 | 0.0 | 0.0 | 0.0 | R |
| 2 | 283498 | C. chinense | C04433 | 0.0 | 0.0 | 0.0 | 0.0 | R |
| 3 | 240012 | C. annuum | Chili bangi #3 | 0.0 | 0.0 | 0.2 | 0.2 | R |
| 4 | 158713 | C. chacoense | PI 260429 | 0.0 | 0.0 | 0.0 | 0.4 | R |
| 5 | 221919 | C. frutescens | P 82005 | 0.1 | 0.6 | 0.6 | 0.6 | R |
| 6 | 240642 | C. annuum | NPL-GYS-2004-39 | 0.0 | 0.3 | 0.5 | 0.8 | R |
| 7 | 236398 | C. annuum | PX23595 (No.8) | 0.4 | 0.4 | 0.8 | 0.8 | R |
| 8 | 247232 | C. annuum | P 088-39 | 0.0 | 0.0 | 0.0 | 1.0 | R |
| 9 | 236340 | C. annuum | New Mexico | 0.0 | 0.0 | 0.0 | 1.0 | R |
| 10 | 228634 | C. annuum | 9852-193 AVRDC 211 | 0.0 | 0.5 | 1.0 | 1.0 | R |
| 11 | Control-1 | C. annuum | Muhanjilju | 0.0 | 1.11 | 3.56 | 3.56 | S |
| 12 | Control-2 | C. annuum | Meotjinsanai | 0.10 | 1.10 | 3.30 | 3.40 | S |
| 13 | Control-3 | C. annuum | Daekwonseoneon | 0.50 | 2.80 | 3.30 | 3.50 | S |
| 14 | Control-4 | C. annuum | Manitta | 0.60 | 3.30 | 3.70 | 3.90 | S |
| No. | IT Number | Species Name | Fruit Length (cm) | Fruit Width (mm) | Fruit Wall Thickness (mm) | Fruit Weight (g) | °Brix |
|---|---|---|---|---|---|---|---|
| 1 | IT 236738 | C. chinense | 2.43 ± 0.28 | 23.90 ± 0.89 | 3.07 ± 0.76 | 5.20 ± 0.36 | 7.2 ± 2.42 |
| 2 | IT 283498 | C. chinense | 5.67 ± 0.38 | 21.53 ± 1.76 | 3.07 ± 0.31 | 10.30 ± 1.65 | 10.8 ± 1.63 |
| 3 | IT 240012 | C. annuum | 9.88 ± 0.52 | 17.77 ± 3.00 | 1.70 ± 0.36 | 12.23 ± 2.90 | 9.7 ± 1.13 |
| 4 | IT 221919 | C. frutescens | 4.05 ± 0.14 | 9.93 ± 0.12 | 1.13 ± 0.25 | 1.67 ± 0.12 | 7.8 ± 1.59 |
| 5 | IT 240642 | C. annuum | 6.65 ± 0.15 | 7.30 ± 1.25 | 0.60 ± 0.20 | 1.93 ± 0.23 | 7.4 ± 0.64 |
| 6 | IT 236398 | C. annuum | 9.57 ± 0.39 | 8.57 ± 0.70 | 0.87 ± 0.06 | 3.50 ± 0.44 | 14.8 ± 4.78 |
| 7 | IT 247232 | C. annuum | 14.00 ± 0.46 | 23.53 ± 0.86 | 2.37 ± 0.31 | 27.73 ± 2.57 | 9.6 ± 0.46 |
| 8 | IT 228634 | C. annuum | 5.20 ± 0.57 | 10.73 ± 0.32 | 0.70 ± 0.17 | 2.80 ± 0.26 | 6.2 ± 0.70 |
| IT Number | Species Name | α-Carotene | Antheraxanthin | β-Carotene | β-Cryptoxanthin | Capsanthin | Capsorubin | Lutein | Violaxanthin | Zeaxanthin | Total Carotenoids |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IT 236738 | C. chinense | 1.94 | 0.00 | 10.24 | 13.44 | 107.80 | 30.86 | 0.00 | 0.00 | 23.30 | 187.57 |
| IT 283498 | C. chinense | 2.86 | 0.00 | 17.99 | 194.43 | 364.58 | 41.66 | 0.00 | 0.00 | 49.38 | 670.90 |
| IT 240012 | C. annuum | 4.02 | 0.00 | 57.30 | 94.19 | 233.64 | 10.52 | 0.00 | 0.00 | 94.22 | 493.88 |
| IT 221919 | C. frutescens | 0.52 | 0.00 | 9.45 | 8.52 | 28.43 | 2.75 | 0.00 | 0.00 | 7.02 | 56.69 |
| IT 240642 | C. annuum | 6.68 | 0.00 | 125.36 | 212.31 | 140.73 | 15.11 | 0.00 | 0.00 | 164.82 | 665.01 |
| IT 236398 | C. annuum | 13.82 | 11.35 | 230.72 | 130.86 | 322.65 | 30.07 | 0.00 | 3.14 | 165.96 | 908.57 |
| IT 247232 | C. annuum | 4.94 | 0.00 | 31.57 | 49.38 | 275.54 | 27.80 | 0.00 | 0.00 | 51.34 | 440.58 |
| IT 228634 | C. annuum | 2.50 | 0.00 | 60.47 | 80.47 | 285.63 | 34.22 | 0.00 | 0.63 | 53.59 | 517.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ro, N.; Haile, M.; Oh, H.; Ko, H.-C.; Yi, J.; Na, Y.-W.; Hur, O. Evaluation of Pepper (Capsicum spp.) Germplasm Collection for Bacterial Wilt (Ralstonia solanacearum) Resistance. Agronomy 2024, 14, 1753. https://doi.org/10.3390/agronomy14081753
Ro N, Haile M, Oh H, Ko H-C, Yi J, Na Y-W, Hur O. Evaluation of Pepper (Capsicum spp.) Germplasm Collection for Bacterial Wilt (Ralstonia solanacearum) Resistance. Agronomy. 2024; 14(8):1753. https://doi.org/10.3390/agronomy14081753
Chicago/Turabian StyleRo, Nayoung, Mesfin Haile, Hyeonseok Oh, Ho-Cheol Ko, Jungyoon Yi, Young-Wang Na, and Onsook Hur. 2024. "Evaluation of Pepper (Capsicum spp.) Germplasm Collection for Bacterial Wilt (Ralstonia solanacearum) Resistance" Agronomy 14, no. 8: 1753. https://doi.org/10.3390/agronomy14081753
APA StyleRo, N., Haile, M., Oh, H., Ko, H.-C., Yi, J., Na, Y.-W., & Hur, O. (2024). Evaluation of Pepper (Capsicum spp.) Germplasm Collection for Bacterial Wilt (Ralstonia solanacearum) Resistance. Agronomy, 14(8), 1753. https://doi.org/10.3390/agronomy14081753







