Impact of Maize Nutrient Composition on the Developmental Defects of Spodoptera frugiperda
Abstract
1. Introduction
2. Materials and Methods
2.1. Planting of Maize Leaves
2.2. Rearing of S. frugiperda
2.3. Chemicals and Reagents
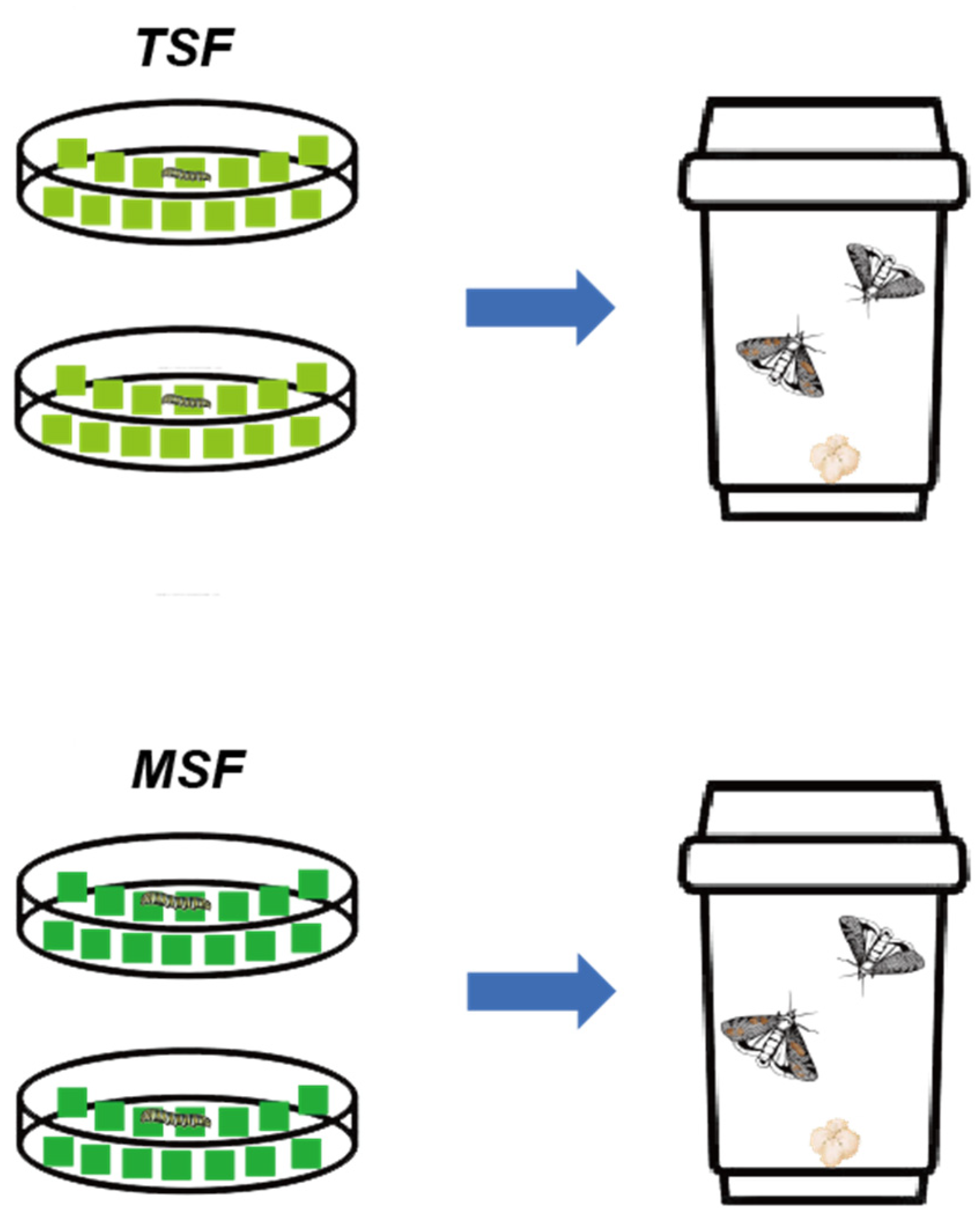
2.4. The Feeding Experiment of S. frugiperda Larvae
2.5. Determination of 20E, JH I, JH II, and JH III in S. frugiperda
2.6. Determination of 21 Amino Acids Content in Maize Leaves
2.7. Determination of 4 Soluble Sugars Content in Maize Leaves
2.8. Determination of 4 Sterols Content in Maize Leaves
2.9. Data Analysis
3. Results
3.1. Method Validation
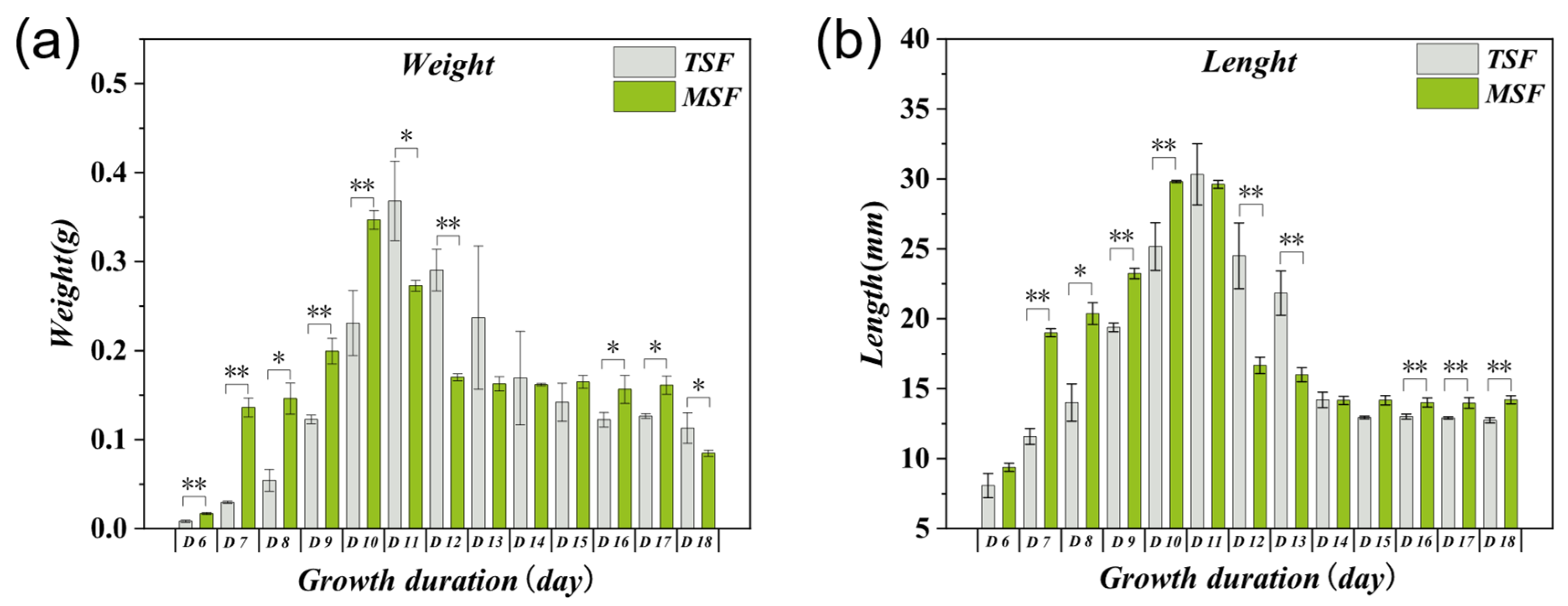
3.2. Effects of Feeding on Maize Leaves at Different Growth Stages on the Growth and Development of S. frugiperda
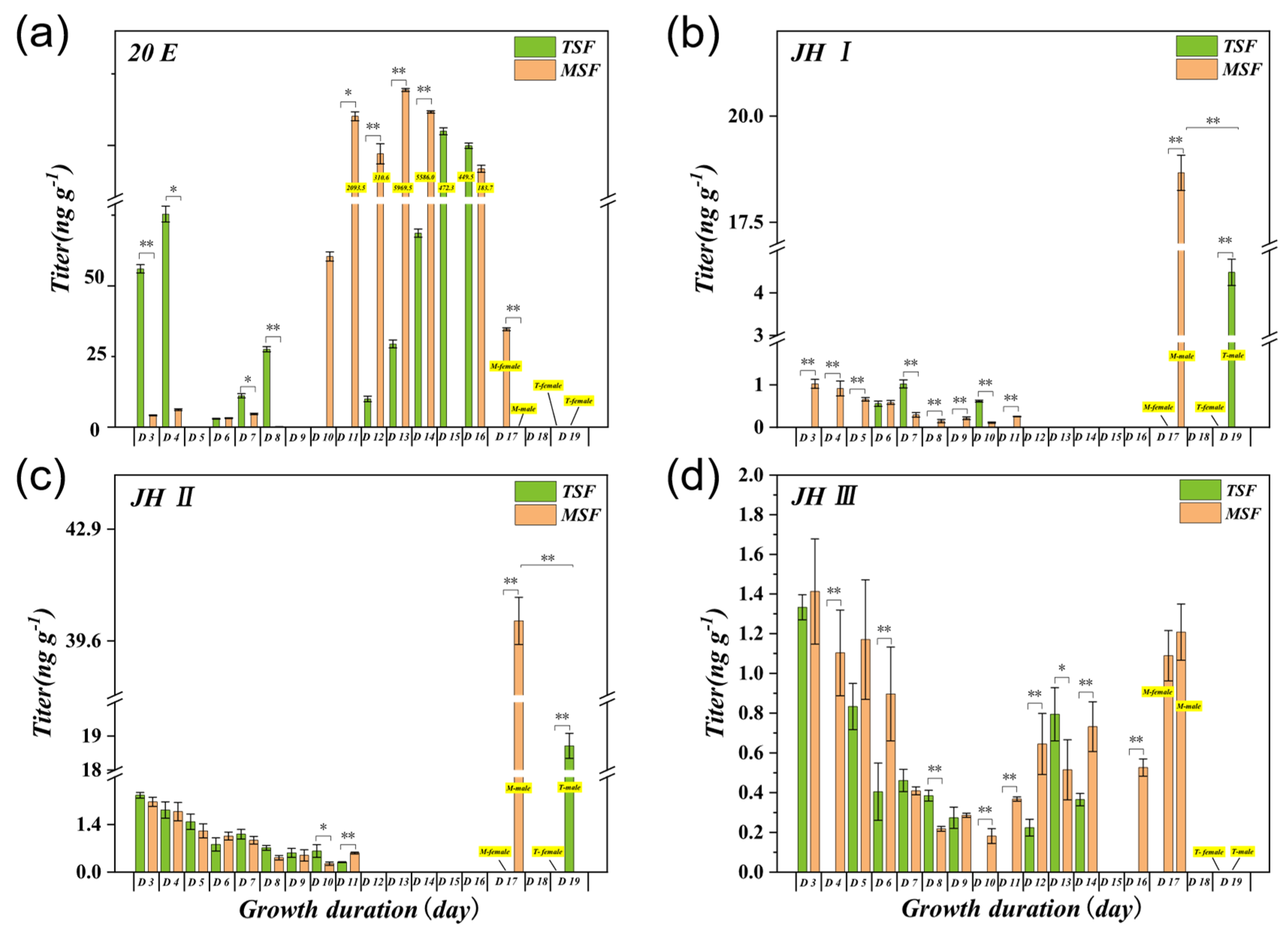
3.3. Effects of Feeding on Maize Leaves at Different Growth Stages on the Levels of 20E, JH I, JH II, and JH III in S. frugiperda
3.4. Differences in the Levels of 21 Amino Acids in Maize Leaves at Different Growth Stages
3.5. Differences in the Levels of Four Soluble Sugars in Maize Leaves at Different Growth Stages
3.6. Differences in the Levels of Four Sterols in Maize Leaves at Different Growth Stages
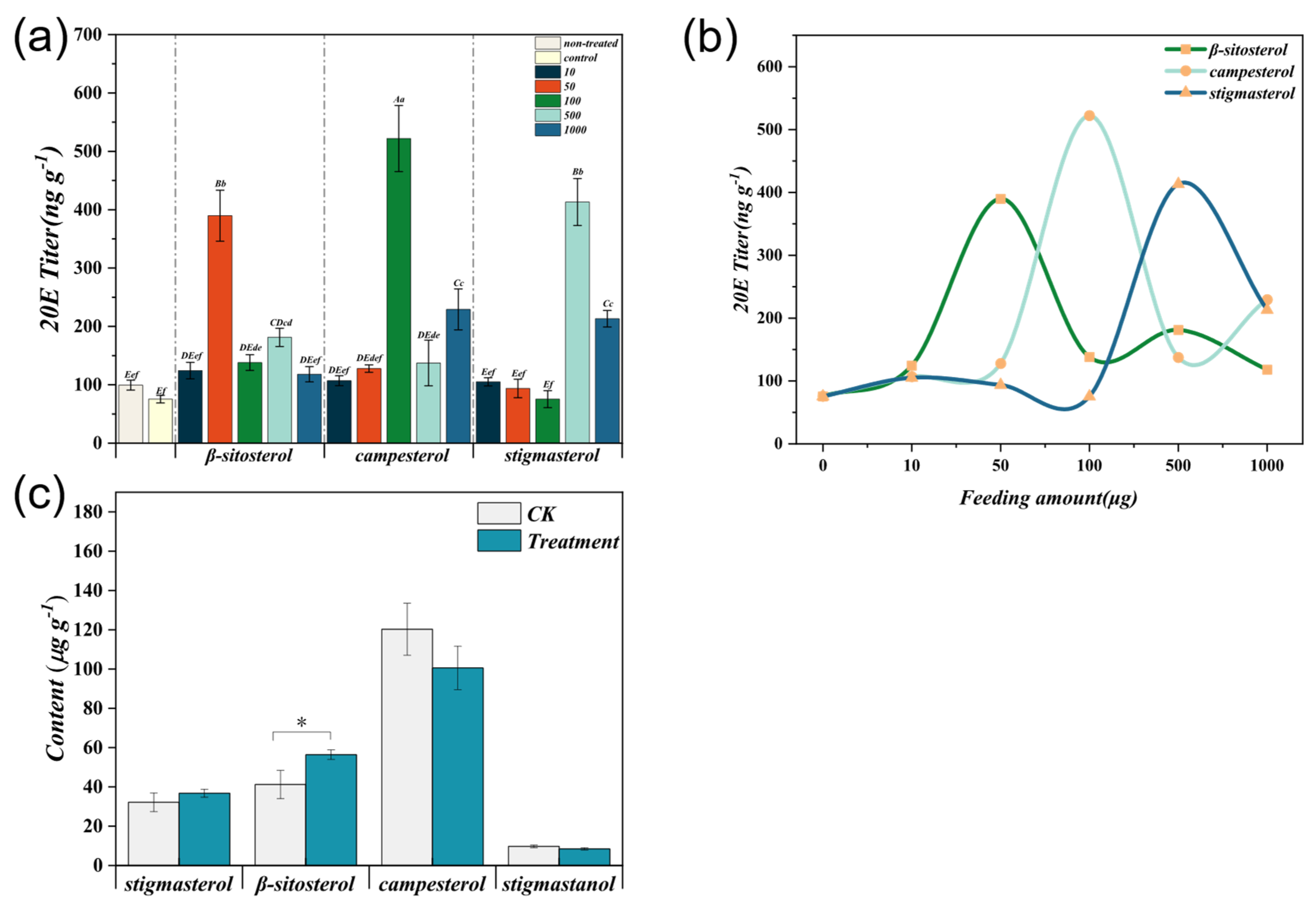
3.7. Effects of Different Sterol Levels on S. frugiperda
3.8. Defense Response of Maize Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Simpson, S.J.; Raubenheimer, D. The nature of nutrition: A unifying framework. Aust. J. Zool. 2011, 59, 350–368. [Google Scholar] [CrossRef]
- Moustafa, M.A.; Osman, E.A.; Mokbel, E.S.; Fouad, E.A. Biochemical and molecular characterization of chlorantraniliprole resistance in spodoptera littoralis (lepidoptera: Noctuidae). Crop Prot. 2024, 177, 106533. [Google Scholar] [CrossRef]
- Pashley, D.P. Host-associated Genetic Differentiation in Fall Armyworm (Lepidoptera: Noctuidae): A Sibling Species Complex? Ann. Entomol. Soc. Am. 1986, 79, 898–904. [Google Scholar] [CrossRef]
- Pashley, D.P.; Johnson, S.J.; Sparks, A.N. Genetic Population Structure of Migratory Moths: The Fall Armyworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1985, 78, 756–762. [Google Scholar] [CrossRef]
- Pashley, D.P.; Martin, J.A. Reproductive Incompatibility Between Host Strains of the Fall Armyworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1987, 80, 731–733. [Google Scholar] [CrossRef]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Lü, D.; Dong, Y.; Yan, Z.; Liu, X.; Zhang, Y.; Yang, D.; He, K.; Wang, Z.; Wang, P.; Yuan, X.; et al. Dynamics of gut microflora across the life cycle of Spodoptera frugiperda and its effects on the feeding and growth of larvae. Pest Manag. Sci. 2023, 79, 173–182. [Google Scholar] [CrossRef] [PubMed]
- He, L.M.; Wu, Q.L.; Gao, X.W.; Wu, K.M. Population life tables for the invasive fall armyworm, Spodoptera frugiperda fed on major oil crops planted in China. J. Integr. Agric. 2021, 20, 745–754. [Google Scholar] [CrossRef]
- Guo, Z.; Jin, R.; Guo, Z.; Cai, T.; Zhang, Y.; Gao, J.; Huang, G.; Wan, H.; He, S.; Xie, Y.; et al. Insecticide susceptibility and mechanism of Spodoptera frugiperda on different host plants. J. Agric. Food Chem. 2022, 70, 11367–11376. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.L.; Zhang, H.W.; Wu, K.M. Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China. J. Integr. Agric. 2021, 20, 637–645. [Google Scholar] [CrossRef]
- Pantaleoni, R.A.; Pusceddu, M.; Tauber, C.A.; Theodorou, P.; Loru, L. How much does a drop of sugar solution benefit a hatchling of Chrysoperla pallida (Neuroptera Chrysopidae)? Biol. Control 2022, 172, 104963. [Google Scholar] [CrossRef]
- Gris, L.; Battershill, C.N.; Prinsep, M.R. Investigation of the dietary preferences of two Dorid nudibranchs by feeding-choice experiments and chemical analysis. J. Chem. Ecol. 2023, 49, 599–610. [Google Scholar] [CrossRef]
- Siva Prasad, S.; Madhavi, R. Impact of honey-enriched mulberry diet on the energy metabolism of the silkworm, Bombyx mori. J. Appl. Nat. Sci. 2020, 12, 133–145. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Schweikhard, E.S.; Ziegler, C.M. Amino acid secondary transporters: Toward a common transport mechanism. Curr. Top. Membr. 2012, 70, 1–28. [Google Scholar] [CrossRef]
- Su, S.; Wang, X.; Jian, C.; Ignatus, A.D.; Zhang, X.; Peng, X.; Chen, M. Life-history traits and flight capacity of grapholita molesta (lepidoptera: Tortricidae) using artificial diets with varying sugar Content. J. Econ. Entomol. 2021, 114, 112–121. [Google Scholar] [CrossRef]
- Urbaneja-Bernat, P.; González-Cabrera, J.; Hernández-Suárez, E.; Tena, A. Honeydew of HLB vector, trioza erytreae, increases longevity, egg load and parasitism of its main parasitoid tamarixia dryi. Biol. Control 2023, 179, 105169. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, D.; Xie, J.; Lei, Y.; Li, J.; Fang, G.; Tian, L.; Liu, J.; Cui, Y.; Zhang, M.; et al. Evolution of the cholesterol biosynthesis pathway in animals. Mol. Biol. Evol. 2019, 36, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Niwa, R.; Niwa, Y.S. The fruit fly Drosophila melanogaster as a model system to study cholesterol metabolism and homeostasis. Cholesterol 2011, 2011, 176802. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.B. The utilization of sterols by insects. J. Lipid Res. 1964, 5, 3–19. [Google Scholar] [CrossRef]
- Bentz, B.J.; Six, D.L. Ergosterol content of fungi associated with dendroctonus ponderosae and dendroctonus rufipennis (coleoptera: Curculionidae, scolytinae). Ann. Entomol. Soc. Am. 2006, 99, 189–194. [Google Scholar] [CrossRef]
- Spindler, K.-D.; Hönl, C.; Tremmel, C.; Braun, S.; Ruff, H.; Spindler-Barth, M. Ecdysteroid hormone action. Cell. Mol. Life Sci. 2009, 66, 3837–3850. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Chang, W.; Sui, X.; Liu, Y.; Song, G.; Song, F.; Feng, F. Changes in rhizobacterial community mediating atrazine dissipation by arbuscular mycorrhiza. Chemosphere 2020, 256, 127046. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, K.; Gao, Y.; Liu, X.; Chen, W.; Ge, W.; Feng, Q.; Palli, S.R.; Li, S. Antagonistic actions of juvenile hormone and 20-hydroxyecdysone within the ring gland determine developmental transitions in Drosophila. Proc. Natl. Acad. Sci. USA 2018, 115, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, S.; Jia, Q.; Wu, L.; Yuan, D.; Li, E.Y.; Feng, Q.; Wang, G.; Palli, S.R.; Wang, J.; et al. Juvenile hormone membrane signaling phosphorylates USP and thus potentiates 20-hydroxyecdysone action in Drosophila. Sci. Bull. 2022, 67, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Liu, S. Juvenile hormone studies in Drosophila melanogaster. Front. Physiol. 2021, 12, 785320. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, I.T.; Grötzner, M.; Meyering-Vos, M.; Hoffmann, K.H. Neuropeptides affecting the transfer of juvenile hormones from males to females during mating in Spodoptera frugiperda. J. Insect Physiol. 2014, 66, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Horodyski, F.M. Effects of starvation and mating on corpora allata activity and allatotropin (manse-AT) gene expression in Manduca sexta. Peptides 2006, 27, 567–574. [Google Scholar] [CrossRef]
- Mirth, C.K.; Shingleton, A.W. The roles of juvenile hormone, insulin/target of rapamycin, and ecydsone signaling in regulating body size in Drosophila. Commun. Integr. Biol. 2014, 7, e971568. [Google Scholar] [CrossRef]
- Bernays, E.A. Chapter 201—Phytophagous insects. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 798–800. [Google Scholar]
- Pan, L.-L.; Miao, H.; Wang, Q.; Walling, L.L.; Liu, S.-S. Virus-induced phytohormone dynamics and their effects on plant-insect interactions. New Phytol. 2021, 230, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.-L.; Guo, G.-X.; Ji, X.-L. Volatile emission in wheat and parasitism by Aphidius avenae after exogenous application of salivary enzymes of Sitobion avenae. Entomol. Exp. Appl. 2009, 130, 215–221. [Google Scholar] [CrossRef]
- Dafoe, N.J.; Huffaker, A.; Vaughan, M.M.; Duehl, A.J.; Teal, P.E.; Schmelz, E.A. Rapidly Induced Chemical Defenses in Maize Stems and Their Effects on Short-term Growth of Ostrinia nubilalis. J. Chem. Ecol. 2011, 37, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Kaplan, F.; Huffaker, A.; Dafoe, N.J.; Vaughan, M.M.; Ni, X.; Rocca, J.R.; Alborn, H.T.; Teal, P.E. Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc. Natl. Acad. Sci. USA 2011, 108, 5455–5460. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Ba, R.; Luo, J.; Zou, L.; Huang, M.; Li, Y.; Li, H.; Li, X. Simultaneous detection and distribution of five juvenile hormones in 58 insect species and the absolute configuration in 32 insect species. J. Agric. Food Chem. 2023, 71, 7878–7890. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Szołtysik, R.; Tang, J.; Bajkacz, S. Efficient extraction and sensitive HPLC-MS/MS quantification of selected ecdysteroids in plants. J. Food Compos. Anal. 2022, 110, 104580. [Google Scholar] [CrossRef]
- GBT30987-2020; Determination of Free Amino Acids in Plant. State Administration for Market Regulation; Standardization Administration of China: Beijing, China, 2020. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=4BF0E4CD3BD859FBC7DA3805D7A2F8A0 (accessed on 29 July 2024).
- Gailing, M.F.; Guibert, A.; Combes, D. Sensitive and reproducible method for neutral monosaccharides and uronic acid determination in sugar beet pulp enzymatic treatment media and press juices by high performance anion exchange chromatography with pulsed amperometric detection. Biotechnol. Tech. 1998, 12, 165–169. [Google Scholar] [CrossRef]
- Asl, P.J.; Niazmand, R.; Molaveisi, M.; Mousavi Kalajahi, S.E. Determination of vitamin E and β-sitosterol in vegetable oil wastes by graphene-based magnetic solid-phase extraction method coupled with GC-MS. J. Food Meas. Charact. 2021, 15, 5630–5636. [Google Scholar] [CrossRef]
- Kaspi, R.; Mossinson, S.; Drezner, T.; Kamensky, B.; Yuval, B. Effects of larval diet on development rates and reproductive maturation of male and female Mediterranean fruit flies. Physiol. Entomol. 2002, 27, 29–38. [Google Scholar] [CrossRef]
- Balzan, M.V.; Wäckers, F.L. Flowers to selectively enhance the fitness of a host-feeding parasitoid: Adult feeding by Tuta absoluta and its parasitoid Necremnus artynes. Biol. Control 2013, 67, 21–31. [Google Scholar] [CrossRef]
- Teulier, L.; Weber, J.-M.; Crevier, J.; Darveau, C.-A. Proline as a fuel for insect flight: Enhancing carbohydrate oxidation in hymenopterans. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160333. [Google Scholar] [CrossRef] [PubMed]
- Friend, W.G.; Dadd, R.H. Insect nutrition: A comparative perspective. Adv. Nutr. Res. 1982, 4, 205–247. [Google Scholar] [CrossRef] [PubMed]
- Lipke, H.; Fraenkel, G. Insect nutrition. Annu. Rev. Entomol. 1956, 1, 17–44. [Google Scholar] [CrossRef]
- Akov, S. A qualitative and quantitative study of the nutritional requirements of Aedes aegypti L. larvae. J. Insect Physiol. 1962, 8, 319–335. [Google Scholar] [CrossRef]
- Singh, K.R.; Brown, A.W. Nutritional requirements of Aedes aegypti L. J. Insect Physiol. 1957, 1, 199–220. [Google Scholar] [CrossRef]
- Doi, M.; Yamaoka, I.; Nakayama, M.; Mochizuki, S.; Sugahara, K.; Yoshizawa, F. Isoleucine, a blood glucose-lowering amino acid, increases glucose uptake in rat skeletal muscle in the absence of increases in AMP-activated protein kinase activity. J. Nutr. 2005, 135, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Li, S.H.; Tang, L.; Kuang, S.Y.; Feng, L.; Zhou, X.Q. Flesh quality loss in response to dietary isoleucine deficiency and excess in fish: A link to impaired Nrf2-dependent antioxidant defense in muscle. PLoS ONE 2014, 9, e115129. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Sun, S.P.; Li, Y.X.; Wang, L.; Dong, D.J.; Wang, J.X.; Zhao, X.F. 20-hydroxyecdysone reprograms amino acid metabolism to support the metamorphic development of Helicoverpa armigera. Cell Rep. 2023, 42, 112644. [Google Scholar] [CrossRef] [PubMed]
- Parra, J.R.; Mihsfeldt, L. Comparison of artificial diets for rearing the sugarcane borer. In Advances in Insect Rearing for Research and Pest Management; CRC Press: Boca Raton, FL, USA, 2021; pp. 195–209. [Google Scholar]
- Parra, J.R.; Milano, P.; Consoli, F.L.; Zerio, N.G.; Haddad, M.L. Efeito da nutrição de adultos e da umidade na fecundidade de diatraea saccharalis (fabr.) (lepidoptera: Crambidae). An. Soc. Entomológica Bras. 1999, 28, 49–57. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Jerud, A.; Reinherz, A.T. The hungry caterpillar: An analysis of how carbohydrates stimulate feeding in Manduca sexta. J. Exp. Biol. 2007, 210, 3054–3067. [Google Scholar] [CrossRef][Green Version]
- Vera, M.T.; Oviedo, A.; Abraham, S.; Ruiz, M.J.; Mendoza, M.; Chang, C.L.; Willink, E. Development of a larval diet for the South American fruit fly Anastrepha fraterculus (Diptera: Tephritidae). Int. J. Trop. Insect Sci. 2014, 34, S73–S81. [Google Scholar] [CrossRef]
- Cho, I.K.; Chang, C.L.; Li, Q.X. Diet-induced over-expression of flightless-I protein and its relation to flightlessness in mediterranean fruit fly, ceratitis capitata. PLoS ONE 2013, 8, e81099. [Google Scholar] [CrossRef]
- He, X.; Sigsgaard, L. A floral diet increases the longevity of the coccinellid adalia bipunctata but does not allow molting or reproduction. Front. Ecol. Evol. 2019, 7, 6. [Google Scholar] [CrossRef]
- Williams, L., 3rd; Deschodt, P.; Pointurier, O.; Wyckhuys, K.A. Sugar concentration and timing of feeding affect feeding characteristics and survival of a parasitic wasp. J. Insect Physiol. 2015, 79, 10–18. [Google Scholar] [CrossRef]
- Ou, Q.; King-Jones, K. What goes up must come down: Transcription factors have their say in making ecdysone pulses. Curr. Top. Dev. Biol. 2013, 103, 35–71. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Feng, Q. Fat body biology in the last decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Z.; Yang, Y.; Wang, P.; Zhou, X.; Zhao, T.; Guo, M.; Meng, M.; Zhang, T.; Qian, W.; et al. Comparative transcriptome analysis provides novel insight into morphologic and metabolic changes in the fat body during silkworm metamorphosis. Int. J. Mol. Sci. 2018, 19, 3525. [Google Scholar] [CrossRef]
- Svoboda, J.A.; Robbins, W.E. Conversion of beta sitosterol to cholesterol blocked in an insect by hypocholesterolemic agents. Science 1967, 156, 1637–1638. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, J.A.; Weirich, G.F. Sterol metabolism in the tobacco hornworm, Manduca sexta—A review. Lipids 1995, 30, 263–267. [Google Scholar] [CrossRef]
- Su, Q.; Preisser, E.L.; Zhou, X.M.; Xie, W.; Liu, B.M.; Wang, S.L.; Wu, Q.J.; Zhang, Y.J. Manipulation of host quality and defense by a plant virus improves performance of whitefly vectors. J. Econ. Entomol. 2015, 108, 11–19. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Dutta, B.; Coolong, T.; Srinivasan, R. A non-persistent aphid-transmitted Potyvirus differentially alters the vector and non-vector biology through host plant quality manipulation. Sci. Rep. 2019, 9, 2503. [Google Scholar] [CrossRef] [PubMed]




| Analytes | ESI Mode | Rt (min) | TM (m/z) | CV (V) | Quantifying Ion | CE (eV) | Qualitative Ion | CE (eV) |
|---|---|---|---|---|---|---|---|---|
| JH I | positive | 5.72 | 295.3 | 45.0 | 263.3 | 10 | 295.3 | 17 |
| JH II | positive | 5.51 | 281.3 | 40.0 | 249.2 | 9 | 57.1 | 40 |
| JH III | positive | 5.27 | 267.3 | 55.0 | 235.2 | 8 | 147.3 | 16 |
| 20E | positive | 2.67 | 481.2 | 81.5 | 445.2 | 19 | 371.2 | 24 |
| Matrix | Analytes | Standard Curve | Calibration Curve | Linearity Range (ng mL−1) | LOD (ng mL−1) | LOQ (ng mL−1) | Matrix Effect | ||
|---|---|---|---|---|---|---|---|---|---|
| Equation | r | Equation | r | ||||||
| Spodopterafrugiperda | JH I | y = 7079.5 x − 49,273 | 0.9992 | y = 5101.2 x − 11,454.4 | 0.9995 | 1–200 | 0.05 | 0.15 | 0.72 |
| JH II | y = 2938.1 x − 11,233.5 | 0.9989 | y = 2069.9 x − 9409.4 | 0.9991 | 1–200 | 0.04 | 0.12 | 0.70 | |
| JH III | y = 2997.7 x − 51,902.1 | 0.9992 | y = 1916.6 x – 29,367.1 | 0.9987 | 1–200 | 0.07 | 0.21 | 0.64 | |
| 20E | y = 2943.2 x − 3244.6 | 0.9995 | y = 1644.8 x − 3547.4 | 0.9991 | 1–4000 | 0.30 | 0.90 | 0.56 | |
| Maize leaves | Asp | y = 456,614 x + 361,750 | 0.9977 | — | — | 50–10,000 | 10.03 | 30.09 | — |
| Glu | y = 324,736 x + 184,075 | 0.9998 | — | — | 50–10,000 | 7.54 | 22.62 | — | |
| Asn | y = 456,201 x + 385,934 | 0.9971 | — | — | 50–10,000 | 4.65 | 13.95 | — | |
| Ser | y = 986,938 x + 56,129 | 0.9999 | — | — | 50–10,000 | 5.21 | 15.63 | — | |
| Gln | y = 189,5451 x − 25,323 | 1.0000 | — | — | 50–10,000 | 2.87 | 8.61 | — | |
| His | y = 1,991,836 x + 75,798 | 1.0000 | — | — | 50–10,000 | 4.65 | 13.95 | — | |
| Gly | y = 1,666,200 x + 151,059 | 1.0000 | — | — | 50–10,000 | 2.14 | 6.42 | — | |
| Arg | y = 1,779,518 x + 155,562 | 1.0000 | — | — | 50–10,000 | 7.03 | 21.09 | — | |
| Thr | y = 1,802,003 x + 517,783 | 0.9999 | — | — | 50–10,000 | 2.68 | 8.04 | — | |
| Ala | y = 1,433,572 x + 620,812 | 0.9999 | — | — | 50–10,000 | 1.04 | 3.12 | — | |
| GABA | y = 1,077,132 x + 749,942 | 0.9988 | — | — | 50–10,000 | 1.68 | 5.04 | — | |
| Pro | y = 660,450 x + 132,951 | 1.0000 | — | — | 50–10,000 | 4.78 | 14.34 | — | |
| The | y = 1,259,897 x + 221,518 | 0.9999 | — | — | 50–10,000 | 3.47 | 10.41 | — | |
| Cys | y = 1,549,093 x + 150,932 | 0.9998 | — | — | 50–10,000 | 15.12 | 45.36 | — | |
| Tyr | y = 343,025 x + 33,085 | 0.9998 | — | — | 50–10,000 | 3.41 | 10.23 | — | |
| Val | y = 2,484,236 x + 420,372 | 1.0000 | — | — | 50–10,000 | 1.58 | 4.74 | — | |
| Met | y = 1,631,826 x − 31,975 | 0.9994 | — | — | 50–10,000 | 3.43 | 10.29 | — | |
| Lys | y = 2,943,732 x + 504,176 | 0.9999 | — | — | 50–10,000 | 2.33 | 6.99 | — | |
| Ile | y = 950,400 x + 878,610 | 0.9987 | — | — | 50–10,000 | 0.78 | 2.34 | — | |
| Leu | y = 3,098,555 x + 1,750,375 | 1.0000 | — | — | 50–10,000 | 0.59 | 1.77 | — | |
| Phe | y = 4,524,886 x + 682,101 | 0.9996 | — | — | 50–10,000 | 0.45 | 1.35 | — | |
| Glucose | y = 2.9232 x + 0.2718 | 0.9998 | — | — | 100–5000 | 12.36 | 37.08 | — | |
| Fructose | y = 1.4463 x + 0.2255 | 0.9995 | — | — | 100–5000 | 33.25 | 99.75 | — | |
| Sucrose | y = 0.9074 x + 0.3696 | 0.9957 | — | — | 100–5000 | 26.32 | 78.96 | — | |
| Maltose | y = 1.1931 x + 0.2524 | 0.9993 | — | — | 100–5000 | 30.74 | 92.22 | — | |
| Stigmasterol | y = 56,689 x − 7666 | 0.9911 | — | — | 50–5000 | 10.39 | 31.17 | — | |
| β-sitosterol | y = 805,511 x − 105,048 | 0.9943 | — | — | 50–5000 | 15.47 | 46.41 | — | |
| Campesterol | y = 47,850 x − 4799 | 0.9941 | — | — | 50–5000 | 13.56 | 40.68 | — | |
| Stigmastanol | y = 58,737 x − 8348 | 0.9926 | — | — | 50–5000 | 9.69 | 29.07 | — | |
| Matrix | Analytes | Spiked (ng g−1) | ||
|---|---|---|---|---|
| 1 | 10 | 100 | ||
| S. frugiperda | JH I | 85 (8) a | 87 (11) | 95 (10) |
| JH II | 81 (9) | 88 (7) | 91 (12) | |
| JH III | 88 (11) | 85 (9) | 83 (14) | |
| 20E | 75 (10) | 80 (13) | 89 (12) | |
| Stage | Developmental Duration (d) | |
|---|---|---|
| TSF | MSF | |
| 1st instar | 2.11 ± 0.19 a | 1.33 ± 0.33 b |
| 2nd instar | 2.11 ± 0.19 a | 2 ± 0.33 a |
| 3rd instar | 2.33 ± 0.33 a | 1.78 ± 0.19 a |
| 4th instar | 2.11 ± 0.19 a | 1.33 ± 0.33 b |
| 5th instar | 2 ± 0.33 a | 1.89 ± 0.19 a |
| 6th instar | 1.89 ± 0.19 a | 1.89 ± 0.19 a |
| Larval stage (1st–6th instars) | 12.56 ± 0.19 A | 10.22 ± 0.19 B |
| Prepupal stage | 1.33 ± 0.00 a | 1.11 ± 0.19 a |
| Pupal stage | 6.44 ± 0.38 a | 6 ± 0.33 a |
| Adult stage | 6.11 ± 0.19 A | 7.33 ± 0.33 B |
| Total instars (larva adult) | 26.44 ± 0.19 a | 24.67 ± 0.67 b |
| Rate | TSF (%) | MSF (%) |
|---|---|---|
| Pupation rate | 55.74 ± 1.70 A | 88.33 ± 0.56 B |
| Eclosion rate | 32.05 ± 0.77 A | 49.64 ± 1.11 B |
| Teratological rate of eclosion | 33.40 ± 1.07 A | 0.00 ± 0.00 B |
| Rate | Sterol | Quality (μg/Head) | |||||
|---|---|---|---|---|---|---|---|
| 0 (Control) | 10 | 50 | 100 | 500 | 1000 | ||
| Pupation rate (%) | β-sitosterol | 52.78 ± 2.78 Bc | 55.56 ± 2.78 ABbc | 66.67 ± 4.81 ABab | 69.44 ± 2.78 Aa | 66.67 ± 4.81 ABab | 66.67 ± 4.81 ABab |
| campesterol | 58.33 ± 4.81 ABabc | 66.67 ± 4.81 ABab | 69.44 ± 2.78 Aa | 66.67 ± 4.81 ABab | 63.89 ± 2.78 ABabc | ||
| stigmasterol | 58.33 ± 4.81 ABabc | 61.11 ± 2.78 ABabc | 63.89 ± 7.35 ABabc | 61.11 ± 2.78 ABabc | 66.67 ± 4.81 ABab | ||
| Eclosion rate (%) | β-sitosterol | 33.33 ± 4.81 Aa | 30.56 ± 2.78 Aa | 36.11 ± 2.78 Aa | 30.56 ± 2.78 Aa | 36.11 ± 2.78 Aa | 36.11 ± 2.78 Aa |
| campesterol | 30.56 ± 2.78 Aa | 36.11 ± 2.78 Aa | 36.11 ± 2.78 Aa | 36.11 ± 2.78 Aa | 33.33 ± 4.81 Aa | ||
| stigmasterol | 30.56 ± 2.78 Aa | 33.33 ± 9.62 Aa | 33.33 ± 4.81 Aa | 33.33 ± 4.81 Aa | 36.11 ± 2.78 Aa | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, H.; Zhang, C.; Hou, J.; Guo, X.; Dong, D.; Li, X. Impact of Maize Nutrient Composition on the Developmental Defects of Spodoptera frugiperda. Agronomy 2024, 14, 1690. https://doi.org/10.3390/agronomy14081690
Zhang W, Li H, Zhang C, Hou J, Guo X, Dong D, Li X. Impact of Maize Nutrient Composition on the Developmental Defects of Spodoptera frugiperda. Agronomy. 2024; 14(8):1690. https://doi.org/10.3390/agronomy14081690
Chicago/Turabian StyleZhang, Wenjie, Haolin Li, Cuifang Zhang, Jiangan Hou, Xiaxia Guo, Dengfeng Dong, and Xuesheng Li. 2024. "Impact of Maize Nutrient Composition on the Developmental Defects of Spodoptera frugiperda" Agronomy 14, no. 8: 1690. https://doi.org/10.3390/agronomy14081690
APA StyleZhang, W., Li, H., Zhang, C., Hou, J., Guo, X., Dong, D., & Li, X. (2024). Impact of Maize Nutrient Composition on the Developmental Defects of Spodoptera frugiperda. Agronomy, 14(8), 1690. https://doi.org/10.3390/agronomy14081690






