Metabolic Control of Sugarcane Internode Elongation and Sucrose Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Non-Destructive Measurements
Modeling Growth
2.3. Destructive Sampling
2.4. Biomass Composition
2.4.1. Extraction of Biomass Component
2.4.2. Cell Wall Constituents
2.4.3. Water Solubles
2.5. Extraction of Metabolites and Proteins
2.6. Metabolome
2.7. Proteome Data Collection and Processing
2.8. Statistical Analysis
3. Results
3.1. Crop Growth
3.2. Biomass Accumulation
3.3. Biomass Composition
3.4. Biomass Accumulation Rates
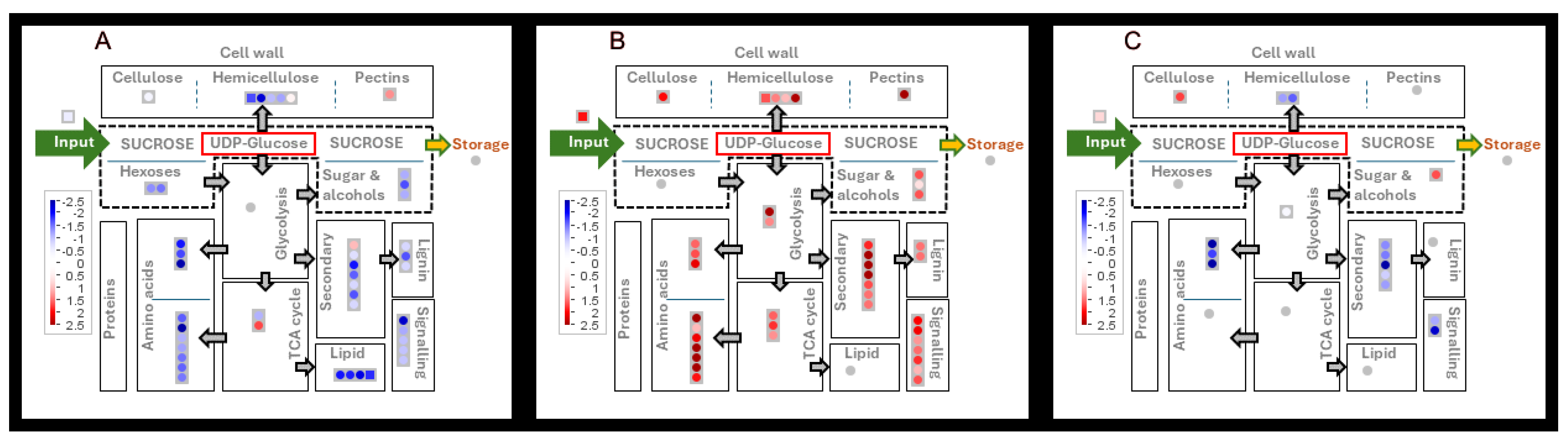
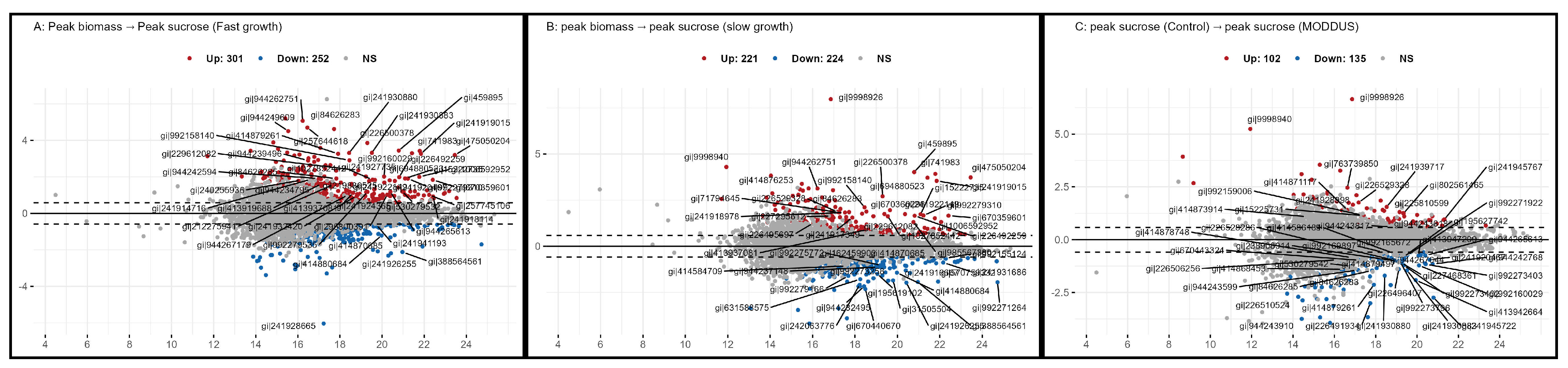
3.5. Metabolome
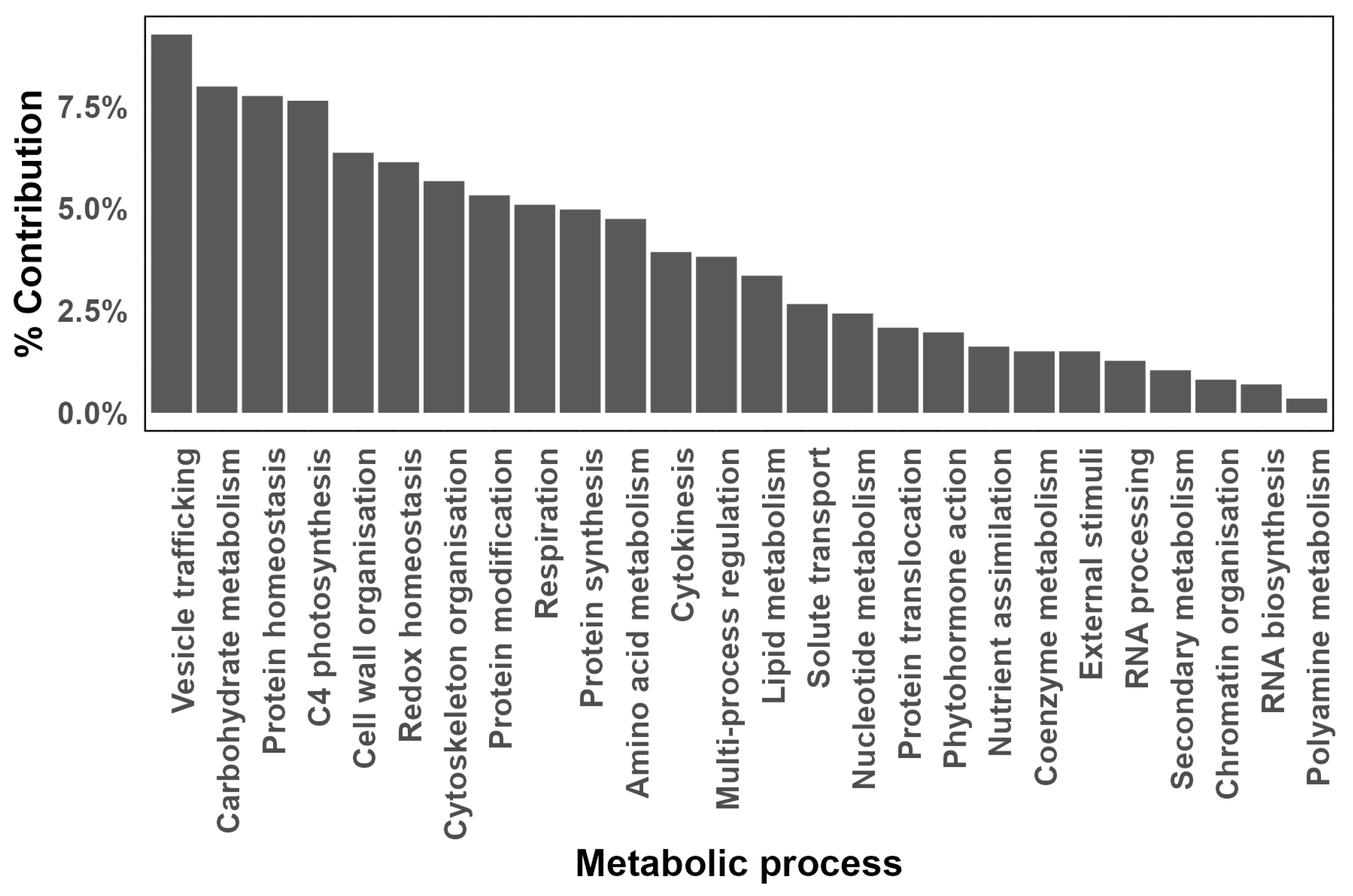
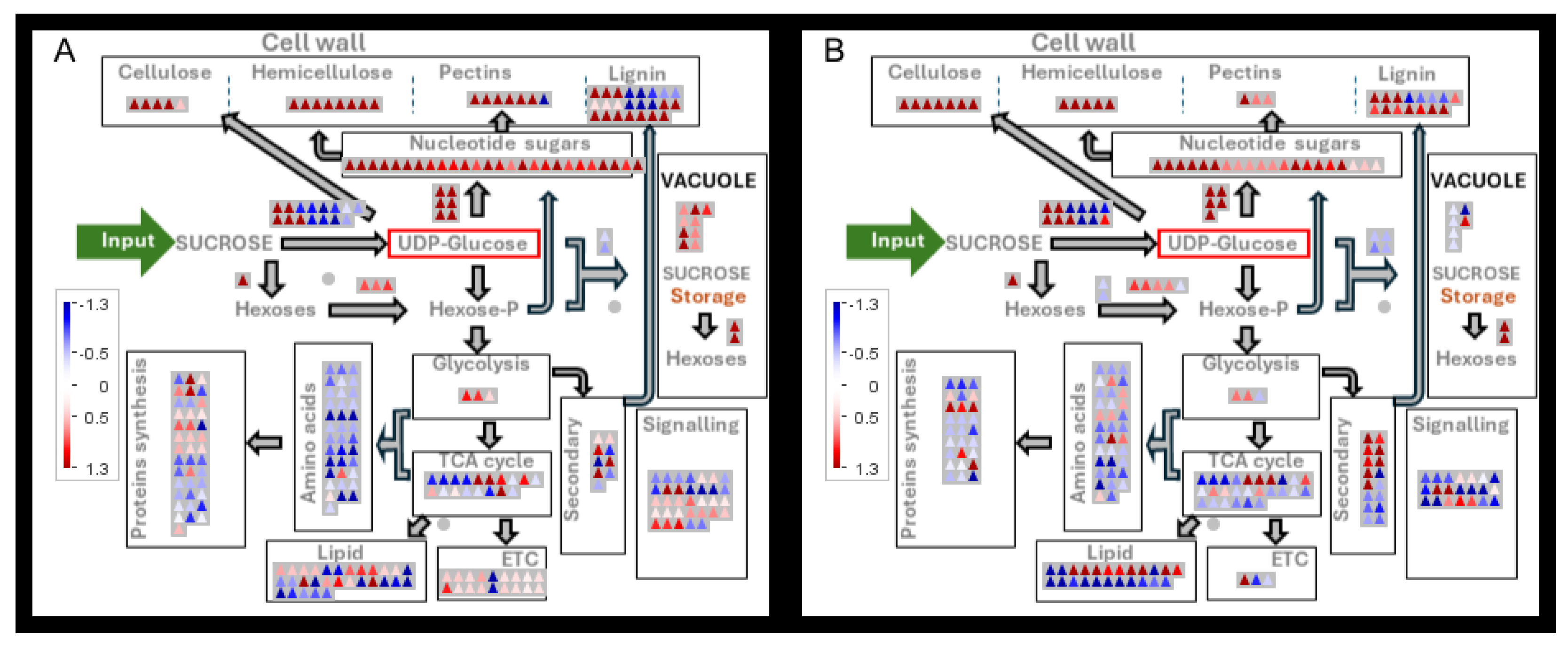
3.6. Protein Levels
3.6.1. Carbohydrate Metabolism and Glycolysis
3.6.2. Cell Wall Sugars
3.6.3. Lignin
3.6.4. Vesicle Trafficking
3.6.5. Vacuolar Metabolism
4. Discussion
4.1. Sink Strength
4.2. Carbon Availability and Regulation of Cell Wall Synthesis
4.3. Sucrose Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DEPs | Differentially expressed proteins |
References
- Martin, A.; Palmer, W.; Brown, C.; Abel, C.; Lunn, J.; Furbank, R.; Grof, C.P.L. A Developing Setaria Viridis Internode: An Experimental System for the Study of Biomass Generation in a C4 Model Species. Biotechnol. Biofuels 2016, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Kebrom, T.H.; McKinley, B.; Mullet, J.E. Dynamics of Gene Expression during Development and Expansion of Vegetative Stem Internodes of Bioenergy Sorghum. Biotechnol. Biofuels 2017, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Lingle, S.E. Seasonal Internode Development and Sugar Metabolism in Sugarcane. Crop. Sci. 1997, 37, 1222–1227. [Google Scholar] [CrossRef]
- Botha, F.C.; Scalia, G.; Marquardt, A.; Wathen-Dunn, K. Sink Strength During Sugarcane Culm Growth: Size Matters. Sugar Tech. 2023, 25, 1047–1060. [Google Scholar] [CrossRef]
- Martin, A.P.; Brown, C.W.; Nguyen, D.Q.; Palmer, W.M.; Furbank, R.T.; Byrt, C.S.; Lambrides, C.J.; Grof, C.P.L. Cell Wall Development in an Elongating Internode of Setaria. In Genetics and Genomics of Setaria; Doust, A., Diao, X., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 211–238. [Google Scholar]
- Botha, F. Advances in Understanding of Sugarcane Plant Growth and Physiology. In Achieving Sustainable Cultivation of Sugarcane; Rott, P., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; Volume 2. [Google Scholar]
- Mason, P.J.; Hoang, N.V.; Botha, F.C.; Furtado, A.; Marquardt, A.; Henry, R.J. Organ-Specific Expression of Genes Associated with the UDP-glucose Metabolism in Sugarcane (Saccharum Spp. Hybrids). BMC Genom. 2023, 24, 18. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.M.; Zhang, J.; Jones, T.C.; Nagai, C.; Ming, R. Cell Wall Metabolism and Hexose Allocation Contribute to Biomass Accumulation in High Yielding Extreme Segregants of a Saccharum Interspecific F2 Population. BMC Genom. 2017, 18, 773. [Google Scholar] [CrossRef] [PubMed]
- Casu, R.E.; Rae, A.L.; Nielsen, J.M.; Perroux, J.M.; Bonnett, G.D.; Manners, J.M. Tissue-Specific Transcriptome Analysis within the Maturing Sugarcane Stalk Reveals Spatial Regulation in the Expression of Cellulose Synthase and Sucrose Transporter Gene Families. Plant Mol. Biol. 2015, 89, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Casu, R.E.; Grof, C.P.; Rae, A.L.; McIntyre, C.L.; Dimmock, C.M.; Manners, J.M. Identification of a Novel Sugar Transporter Homologue Strongly Expressed in Maturing Stem Vascular Tissues of Sugarcane by Expressed Sequence Tag and Microarray Analysis. Plant Mol. Biol. 2003, 52, 371–386. [Google Scholar] [CrossRef]
- Dhungana, S.R.; Braun, D.M. Genomic Analyses of SUT and TST Sugar Transporter Families in Low and High Sugar Accumulating Sugarcane Species (Saccharum spontaneum and Saccharum officinarum). Trop. Plant Biol. 2022, 15, 181–196. [Google Scholar] [CrossRef]
- Rae, A.L.; Grof, C.P.; Casu, R.E.; Bonnett, G.D. Sucrose Accumulation in the Sugarcane Stem: Pathways and Control Points for Transport and Compartmentation. Field Crop. Res. 2005, 92, 159–168. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Schroeder, B.L.; Hurney, A.P.; Wood, A.W.; Moody, P.W.; Allsopp, P.G. Concepts and Value of the Nitrogen Guidelines Contained in the Australian Sugar Industry’s “Six Easy Steps” Nutrient Management Program. In Proceedings of the International Society of Sugar Cane Technologists: Proceedings of the XXVIIth Congress, Veracruz, Mexico, 7–11 March 2010; Volume 27, pp. 1–13. [Google Scholar]
- Berding, N.; Marston, D.H. Operational Validation of the Efficacy of Spectracanetm, a High-Speed Analytical System for Sugarcane Quality Components. In Proceedings of the 32nd Annual Conference of the Australian Society of Sugar Cane Technologists 2010, ASSCT 2010, Bundaberg, Australia, 11–14 May 2010; pp. 445–459. [Google Scholar]
- Pisanó, I.; Gottumukkala, L.; Hayes, D.J.; Leahy, J.J. Characterisation of Italian and Dutch Forestry and Agricultural Residues for the Applicability in the Bio-Based Sector. Ind. Crop. Prod. 2021, 171, 113857. [Google Scholar] [CrossRef]
- Sluiter, A.; Sluiter, J. Determination of Starch in Solid Biomass Samples by HPLC: Laboratory Analytical Procedure (LAP): Issue Date, 07/17/2005; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Hayes, D.J. Development of near Infrared Spectroscopy Models for the Quantitative Prediction of the Lignocellulosic Components of Wet Miscanthus Samples. Bioresour. Technol. 2012, 119, 393–405. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Bhagia, S.; Nunez, A.; Wyman, C.E.; Kumar, R. Robustness of Two-Step Acid Hydrolysis Procedure for Composition Analysis of Poplar. Bioresour. Technol. 2016, 216, 1077–1082. [Google Scholar] [CrossRef]
- Dillewijn, C.V. Botany of Sugarcane; Chronica Botanica Co.: Leyden, The Netherlands, 1960. [Google Scholar]
- Whittaker, A.; Botha, F. Carbon Partitioning during Sucrose Accumulation in Sugarcane Internodal Tissue. Plant Physiol. 1997, 115, 1651–1659. [Google Scholar] [CrossRef]
- Bindon, K.A.; Botha, F.C. Carbon Allocation to the Insoluble Fraction, Respiration and Triose-phosphate Cycling in the Sugarcane Culm. Physiol. Plant. 2002, 116, 12–19. [Google Scholar] [CrossRef]
- Lingle, S.E.; Thomson, J.L. Sugarcane Internode Composition During Crop Development. Bioenergy Res. 2012, 5, 168–178. [Google Scholar] [CrossRef]
- Marquardt, A.; Scalia, G.; Wathen-Dunn, K.; Botha, F. Yellow Canopy Syndrome (YCS) in Sugarcane Is Associated with Altered Carbon Partitioning in the Leaf. Sugar Tech. 2017, 19, 647–655. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. MetaboAnalystR: An R Package for Flexible and Reproducible Analysis of Metabolomics Data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed]
- Usadel, B.; Poree, F.; Nagel, A.; Lohse, M.; Czedik-Eysenberg, A.N.; Stitt, M. A Guide to Using MapMan to Visualize and Compare Omics Data in Plants: A Case Study in the Crop Species, Maize. Plant Cell Environ. 2009, 32, 1211–1229. [Google Scholar] [CrossRef]
- Marquardt, A.; Henry, R.J.; Botha, F.C. Effect of Sugar Feedback Regulation on Major Genes and Proteins of Photosynthesis in Sugarcane Leaves. Plant Physiol. Biochem. 2021, 158, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Schwacke, R.; Ponce-Soto, G.Y.; Krause, K.; Bolger, A.M.; Arsova, B.; Hallab, A.; Gruden, K.; Stitt, M.; Bolger, M.E.; Usadel, B. MapMan4: A Refined Protein Classification and Annotation Framework Applicable to Multi-Omics Data Analysis. Mol. Plant 2019, 12, 879–892. [Google Scholar] [CrossRef]
- De Mendiburu, F.; Reinhard, S. Agricolae-Ten Years of an Open Source Statistical Tool for Experiments in Breeding, Agriculture and Biology. PeerJ PrePrints 2015, 3, e1404v1. [Google Scholar]
- Steel, R.; Torrie, J.; Dickey, D. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1981. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-seq Data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Bindon, K.A.; Botha, F.C. Tissue Discs as an Experimental System for Metabolic Flux Analysis in the sugarcane culm. South Afr. J. Bot. 2001, 67, 244–249. [Google Scholar] [CrossRef][Green Version]
- Benjamini, Y.; Hochberg, Y. On the Adaptive Control of the False Discovery Rate in Multiple Testing with Independent Statistics. J. Educ. Behav. Stat. 2000, 25, 60–83. [Google Scholar] [CrossRef]
- Lingle, S. Sugar Metabolism during Growth and Development in Sugarcane Internodes. Crop. Sci. 1999, 39, 480–486. [Google Scholar] [CrossRef]
- Bonnett, G.D. Developmental Stages (Phenology). In Sugarcane: Physiology, Biochemistry, and Functional Biology; Moore, P.H., Botha, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013; pp. 35–53. [Google Scholar]
- Shameer, S.; Vallarino, J.; Fernie, A.; Ratcliffe, R.G.; Sweetlove, L. Flux Balance Analysis of Metabolism during Growth by Osmotic Cell Expansion and Its Application to Tomato Fruits. Plant J. 2020, 103, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar]
- Perlo, V.; Botha, F.C.; Furtado, A.; Hodgson-Kratky, K.; Henry, R.J. Metabolic Changes in the Developing Sugarcane Culm Associated with High Yield and Early High Sugar Content. Plant Direct 2020, 4, e00276. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Dong, F.; Pang, Z.; Fallah, N.; Zhou, Y.; Li, Z.; Hu, C. Integrated Metabolomics and Transcriptome Analyses Unveil Pathways Involved in Sugar Content and Rind Color of Two Sugarcane Varieties. Front. Plant Sci. 2020, 13, 921536. [Google Scholar] [CrossRef] [PubMed]
- Glassop, D.; Roessner, U.; Bacic, A.; Bonnett, G.D. Changes in the Sugarcane Metabolome with Stem Development. Are They Related to Sucrose Accumulation? Plant Cell Physiol. 2007, 48, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Perlo, V.; Furtado, A.; Botha, F.C.; Margarido, G.R.; Hodgson-Kratky, K.; Choudhary, H.; Gladden, J.; Simmons, B.; Henry, R.J. Transcriptome and Metabolome Integration in Sugarcane through Culm Development. Food Energy Secur. 2022, 11, e421. [Google Scholar] [CrossRef]
- Casu, R.E.; Jarmey, J.M.; Bonnett, G.D.; Manners, J.M. Identification of Transcripts Associated with Cell Wall Metabolism and Development in the Stem of Sugarcane by Affymetrix GeneChip Sugarcane Genome Array Expression Profiling. Funct. Integr. Genom. 2007, 7, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Haigler, C.H.; Ivanova-Datcheva, M.; Hogan, P.S.; Salnikov, V.V.; Hwang, S.; Martin, K.; Delmer, D.P. Carbon Partitioning to Cellulose Synthesis. Plant Cell Walls 2001, 47, 29–51. [Google Scholar]
- Barratt, D.P.; Barber, L.; Kruger, N.J.; Smith, A.M.; Wang, T.L.; Martin, C. Multiple, Distinct Isoforms of Sucrose Synthase in Pea. Plant Physiol. 2001, 127, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, D.; Kossmann, J.; Botha, F.; Groenewald, J.H. Reduced Neutral Invertase Activity in the Culm Tissues of Transgenic Sugarcane Plants Results in a Decrease in Respiration and Sucrose Cycling and an Increase in the Sucrose to Hexose Ratio. Funct. Plant Biol. 2010, 37, 22–31. [Google Scholar] [CrossRef]
- Verbančič, J.; Lunn, J.E.; Stitt, M.; Persson, S. Carbon Supply and the Regulation of Cell Wall Synthesis. Mol. Plant 2018, 11, 75–94. [Google Scholar]
- Ruprecht, C.; Mendrinna, A.; Tohge, T.; Sampathkumar, A.; Klie, S.; Fernie, A.R.; Nikoloski, Z.; Persson, S.; Mutwil, M. FamNet: A Framework to Identify Multiplied Modules Driving Pathway Expansion in Plants. Plant Physiol. 2016, 170, 1878–1894. [Google Scholar]
- Roach, M.; Arrivault, S.; Mahboubi, A.; Krohn, N.; Sulpice, R.; Stitt, M.; Niittylä, T. Spatially Resolved Metabolic Analysis Reveals a Central Role for Transcriptional Control in Carbon Allocation to Wood. J. Exp. Bot. 2017, 68, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- McKinley, B.; Rooney, W.; Wilkerson, C.; Mullet, J. Dynamics of Biomass Partitioning, Stem Gene Expression, Cell Wall Biosynthesis, and Sucrose Accumulation during Development of Sorghum Bicolor. Plant J. 2016, 88, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Piques, M.; Schulze, W.X.; Höhne, M.; Usadel, B.; Gibon, Y.; Rohwer, J.; Stitt, M. Ribosome and Transcript Copy Numbers, Polysome Occupancy and Enzyme Dynamics in Arabidopsis. Mol. Syst. Biol. 2009, 5, 314. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Gibon, Y. Why Measure Enzyme Activities in the Era of Systems Biology? Trends Plant Sci. 2014, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Nelson, C.J.; Trösch, J.; Castleden, I.; Huang, S.; Millar, A.H. Protein Degradation Rate in Arabidopsis Thaliana Leaf Growth and Development. Plant Cell 2017, 29, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nayak, S.; Koch, K.; Ming, R. Carbon Partitioning in Sugarcane (Saccharum Species). Front. Plant Sci. 2013, 4, 201. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.M. Phloem Loading and Unloading of Sucrose: What a Long, Strange Trip from Source to Sink. Annu. Rev. Plant Biol. 2022, 73, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, W.E.; Rohwer, J.M.; Botha, F.C. Protein-level Expression and Localization of Sucrose Synthase in the Sugarcane Culm. Physiol. Plant. 2004, 121, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambandam, P.P.; Mason, P.J.; Hoang, N.V.; Furtado, A.; Botha, F.C.; Henry, R.J. Analysis of the Diversity and Tissue Specificity of Sucrose Synthase Genes in the Long Read Transcriptome of Sugarcane. BMC Plant Biol. 2019, 19, 160. [Google Scholar] [CrossRef]
- Hoepfner, S.W.; Botha, F.C. Expression of Fructokinase Isoforms in the Sugarcane Culm. Plant Physiol. Biochem. 2003, 41, 741–747. [Google Scholar] [CrossRef]
- Bieniawska, Z.; Paul Barratt, D.H.; Garlick, A.P.; Thole, V.; Kruger, N.J.; Martin, C.; Zrenner, R.; Smith, A.M. Analysis of the Sucrose Synthase Gene Family in Arabidopsis. Plant J. 2007, 49, 810–828. [Google Scholar] [CrossRef] [PubMed]
- Lingle, S.E.; Smith, R.C. Sucrose Metabolism Related to Growth and Ripening in Sugarcane Internodes. Crop Sci. 1991, 31, 172–177. [Google Scholar] [CrossRef]
- Vorster, D.J.; Botha, F.C. Partial Purification and Characterisation of Sugarcane Neutral Invertase. Phytochemistry 1998, 49, 651–655. [Google Scholar] [CrossRef] [PubMed]
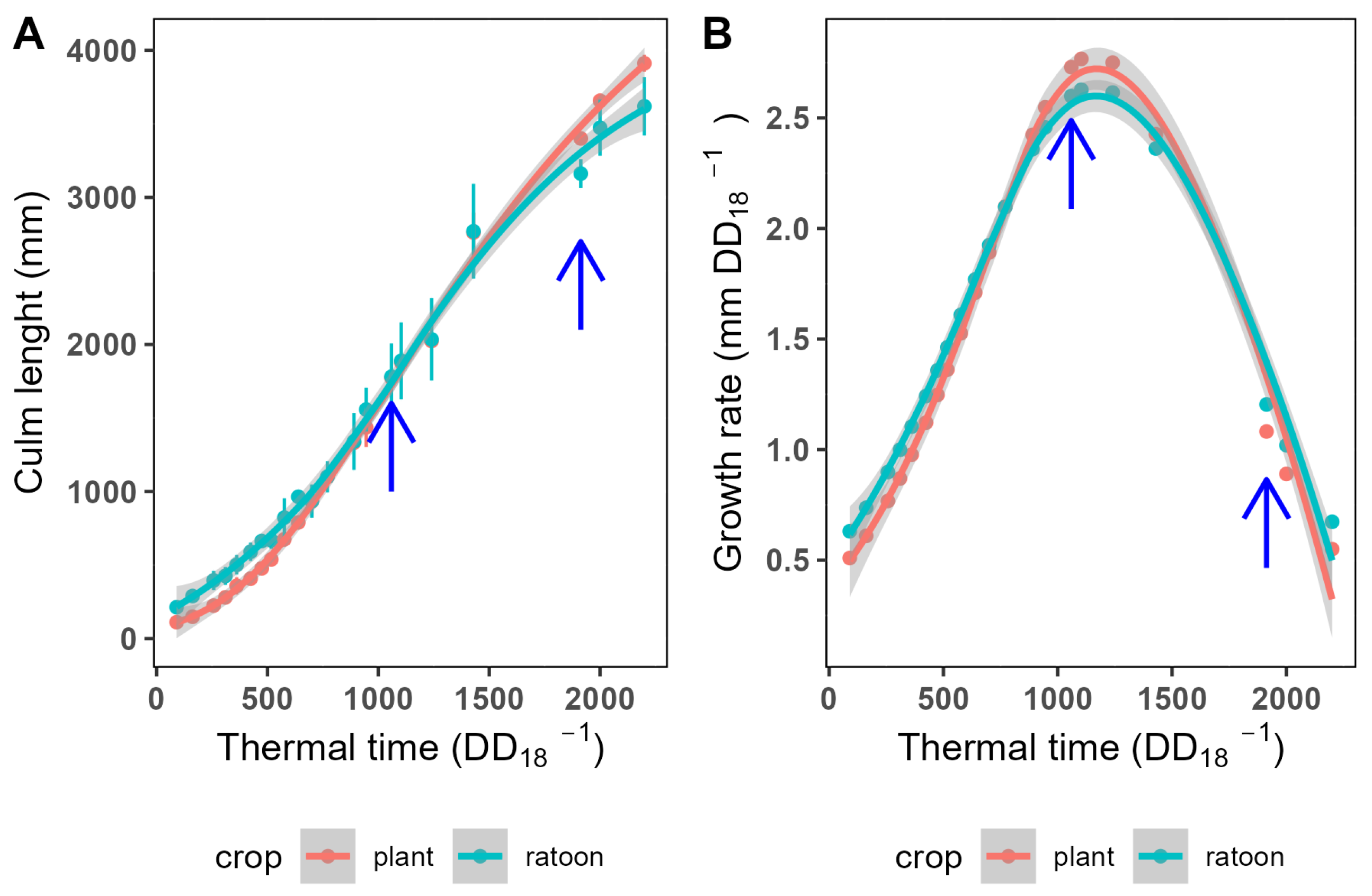
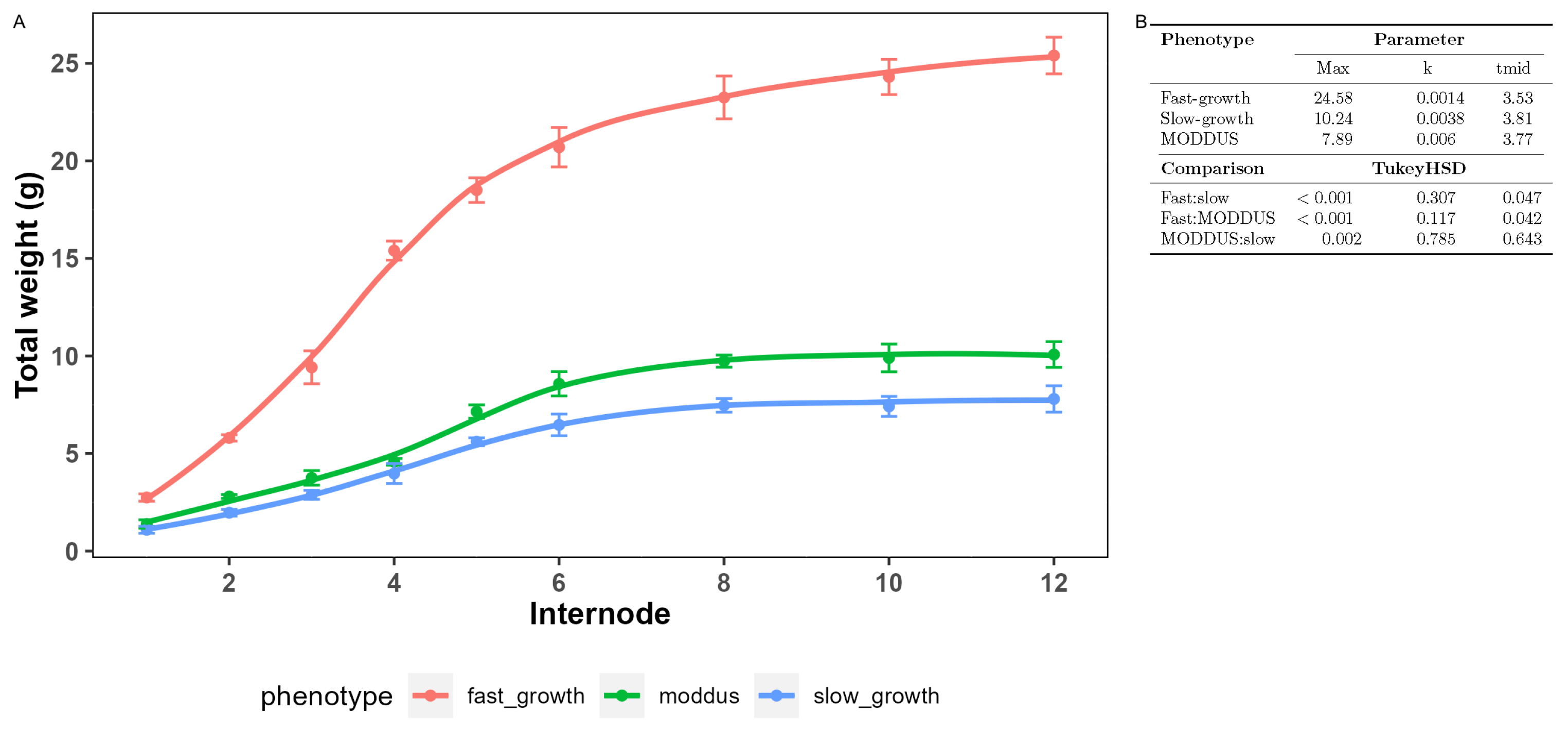
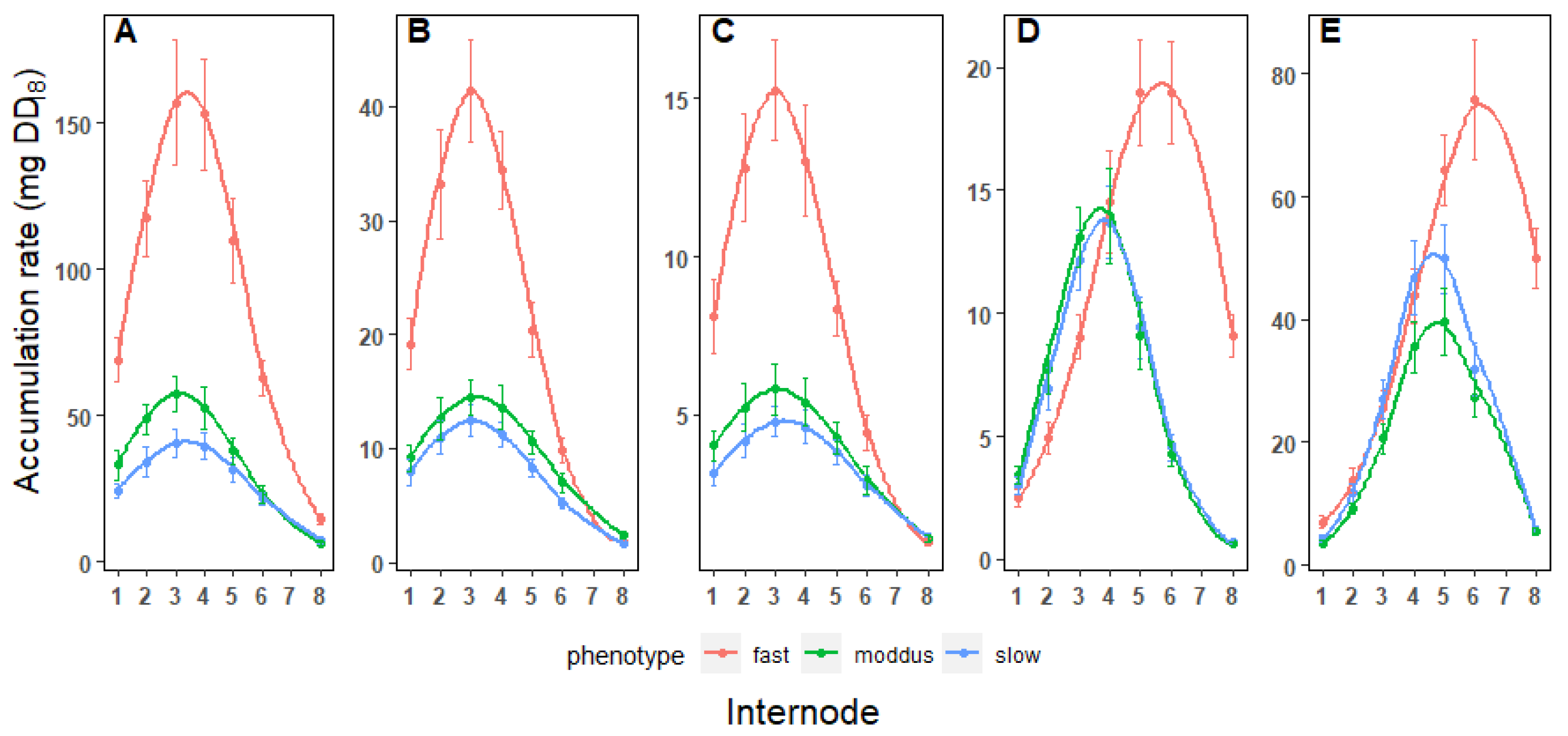
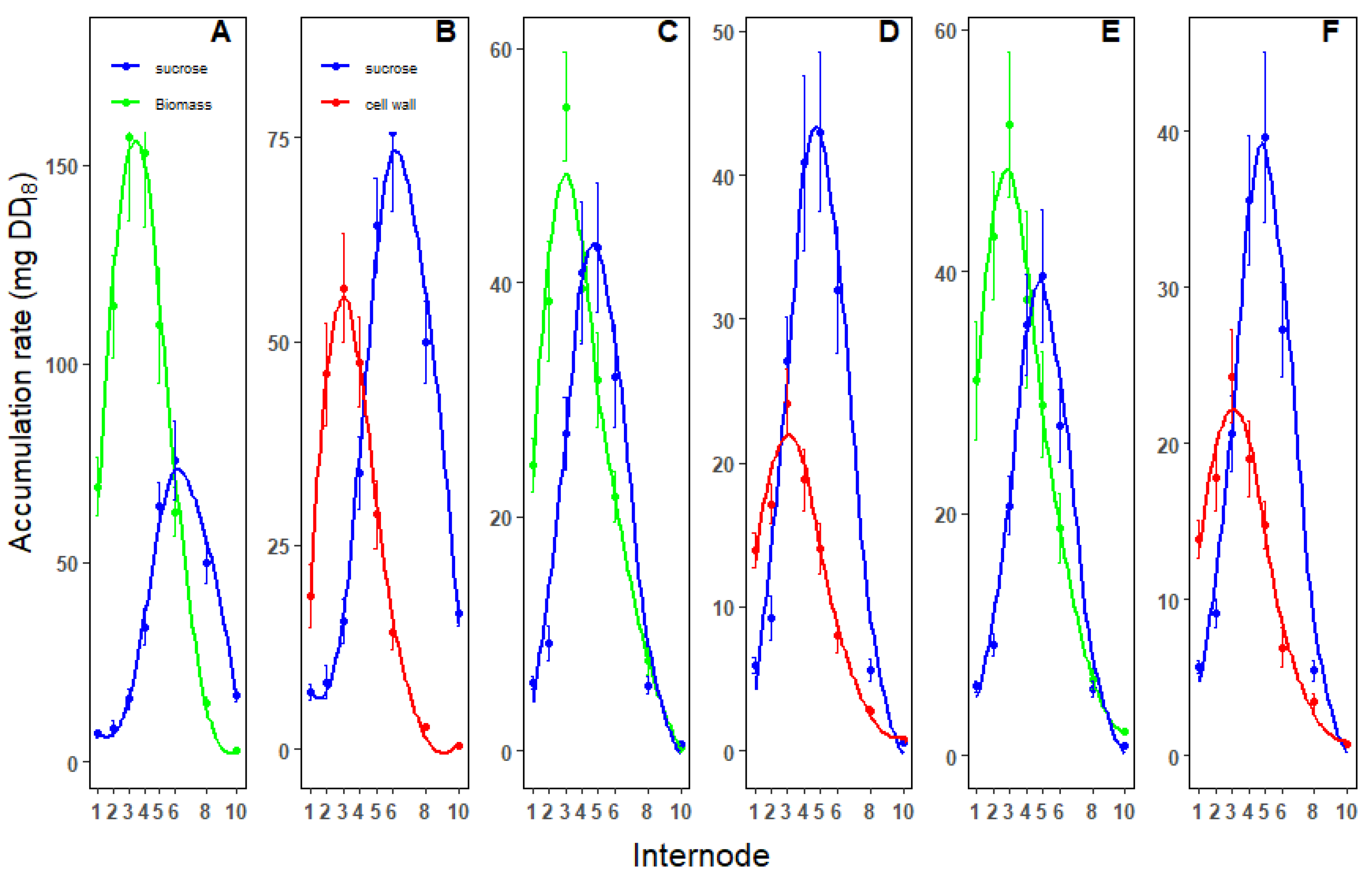
| Component | Phenotype | Parameter 1 | ||
|---|---|---|---|---|
| Max | k | tmid 2 | ||
| dry weight | fast_growth | 24.58 a | 0.03 a | 3.42 a |
| dry weight | moddus | 10.25 b | 0.02 b | 3.14 a |
| dry weight | slow_growth | 7.89 c | 0.02 b | 3.39 a |
| cellulose | fast_growth | 5.44 a | 0.03 a | 3.05 a |
| cellulose | moddus | 2.71 b | 0.02 b | 3.21 b |
| cellulose | slow_growth | 2.22 c | 0.02 c | 3.06 b |
| hemicellulose | fast_growth | 2.36 a | 0.03 a | 3.03 a |
| hemicellulose | moddus | 1.21 b | 0.02 b | 3.13 a |
| hemicellulose | slow_growth | 0.91 c | 0.02 b | 3.32 a |
| lignin | fast_growth | 3.12 a | 0.02 a | 5.51 a |
| lignin | moddus | 1.62 b | 0.01 b | 3.63 b |
| lignin | slow_growth | 1.51 b | 0.02 b | 3.73 b |
| lignocellulose | fast_growth | 10.80 a | 0.02 a | 3.67 a |
| lignocellulose | moddus | 5.98 b | 0.03 a | 5.42 a |
| lignocellulose | slow_growth | 5.06 b | 0.03 a | 5.65 a |
| sucrose | fast_growth | 12.63 a | 0.02 a | 6.14 a |
| sucrose | moddus | 4.82 b | 0.03 b | 4.71 b |
| sucrose | slow_growth | 5.84 c | 0.04 b | 4.62 b |
| Phenotype 1 | Change 1 | |||
|---|---|---|---|---|
| Stage | Comparison | Up | Down | Insignificant |
| Mid season | peak biomass:peak sucrose | 5 | 41 | 28 |
| Mid season:late season | peak biomass:peak biomass | 41 | 2 | 31 |
| Mid season:late season | peak sucrose:peak sucrose | 38 | 2 | 34 |
| Late season | peak sucrose:peak sucrose | 1 | 32 | 41 |
| Peak season MODDUS | peak biomass:peak biomass | 8 | 19 | 24 |
| Peak season MODDUS | peak sucrose:peak sucrose | 3 | 17 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botha, F.C.; Marquardt, A. Metabolic Control of Sugarcane Internode Elongation and Sucrose Accumulation. Agronomy 2024, 14, 1487. https://doi.org/10.3390/agronomy14071487
Botha FC, Marquardt A. Metabolic Control of Sugarcane Internode Elongation and Sucrose Accumulation. Agronomy. 2024; 14(7):1487. https://doi.org/10.3390/agronomy14071487
Chicago/Turabian StyleBotha, Frederik C., and Annelie Marquardt. 2024. "Metabolic Control of Sugarcane Internode Elongation and Sucrose Accumulation" Agronomy 14, no. 7: 1487. https://doi.org/10.3390/agronomy14071487
APA StyleBotha, F. C., & Marquardt, A. (2024). Metabolic Control of Sugarcane Internode Elongation and Sucrose Accumulation. Agronomy, 14(7), 1487. https://doi.org/10.3390/agronomy14071487







