A Modeling Approach to Studying the Influence of Grafting on the Anatomical Features and SAUR Gene Expression in Watermelons
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. The Applied Grafting Method and Field Work
2.3. Morpho-Anatomical Examinations
2.4. Quantitative RT-PCR Analysis
2.5. Statistical Analysis
3. Results
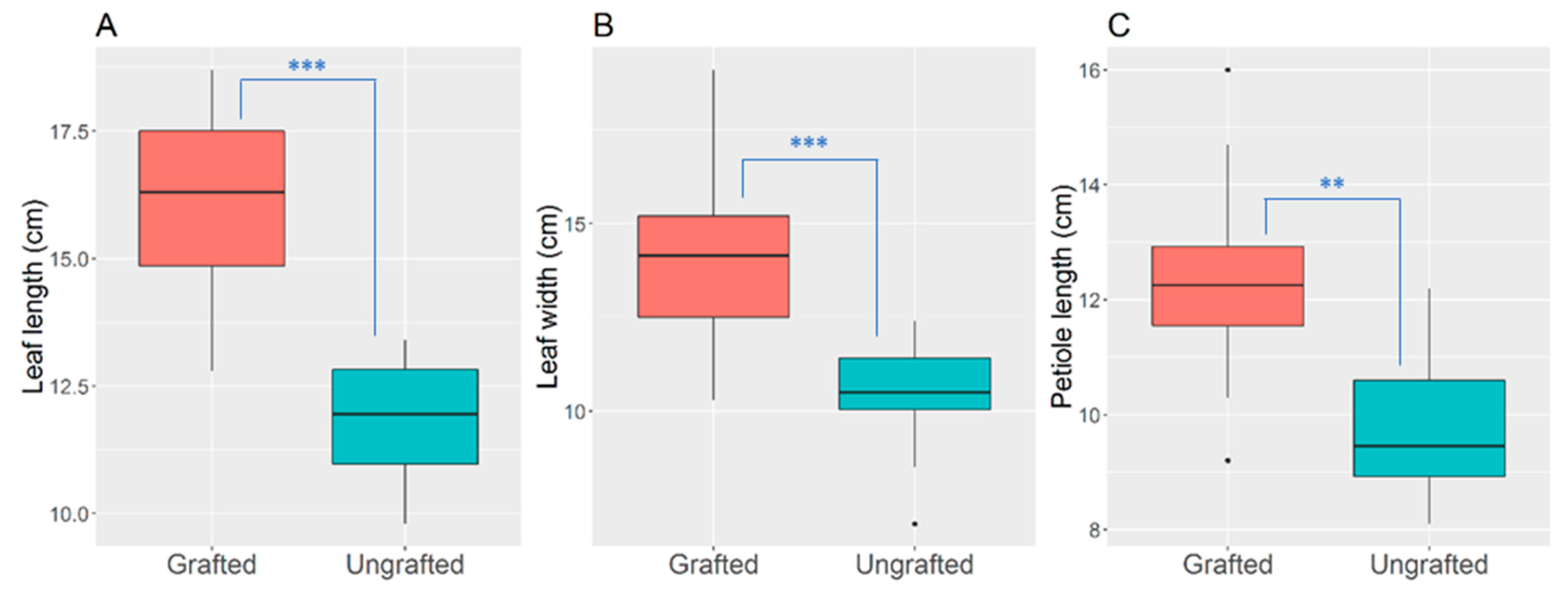
3.1. Leaf Morpho-Anatomical Changes
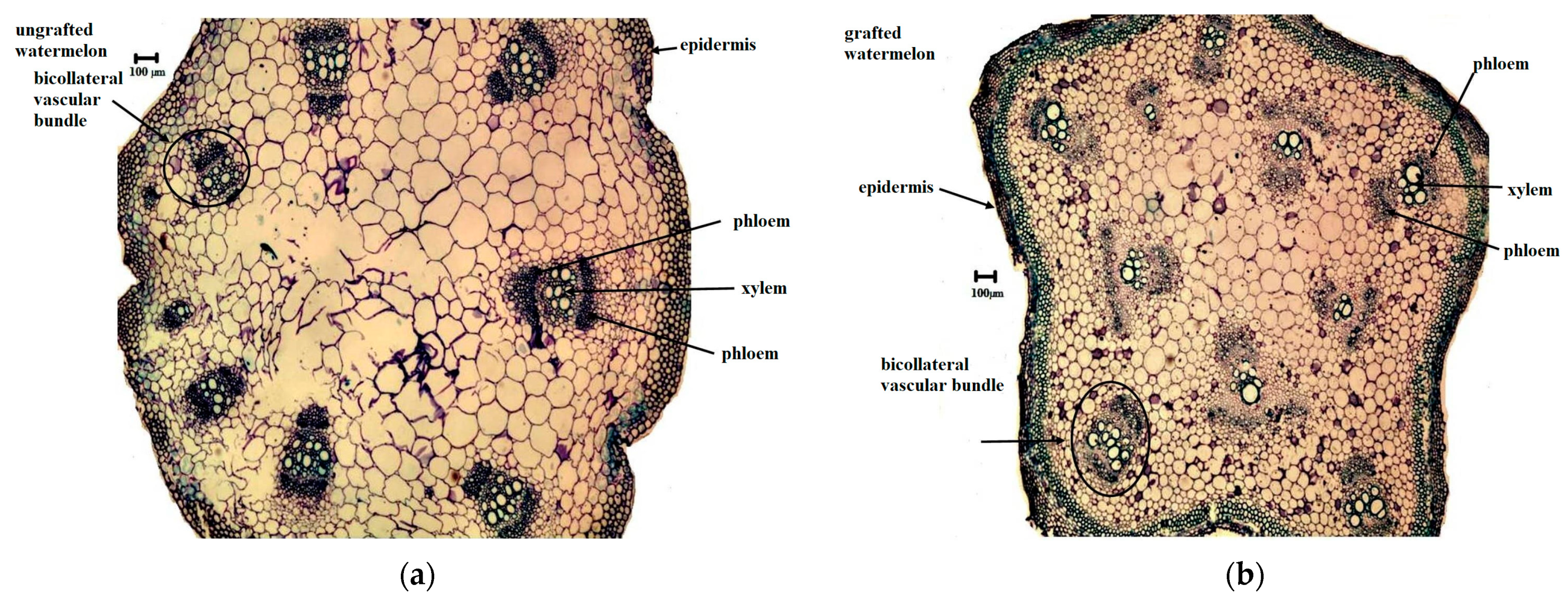
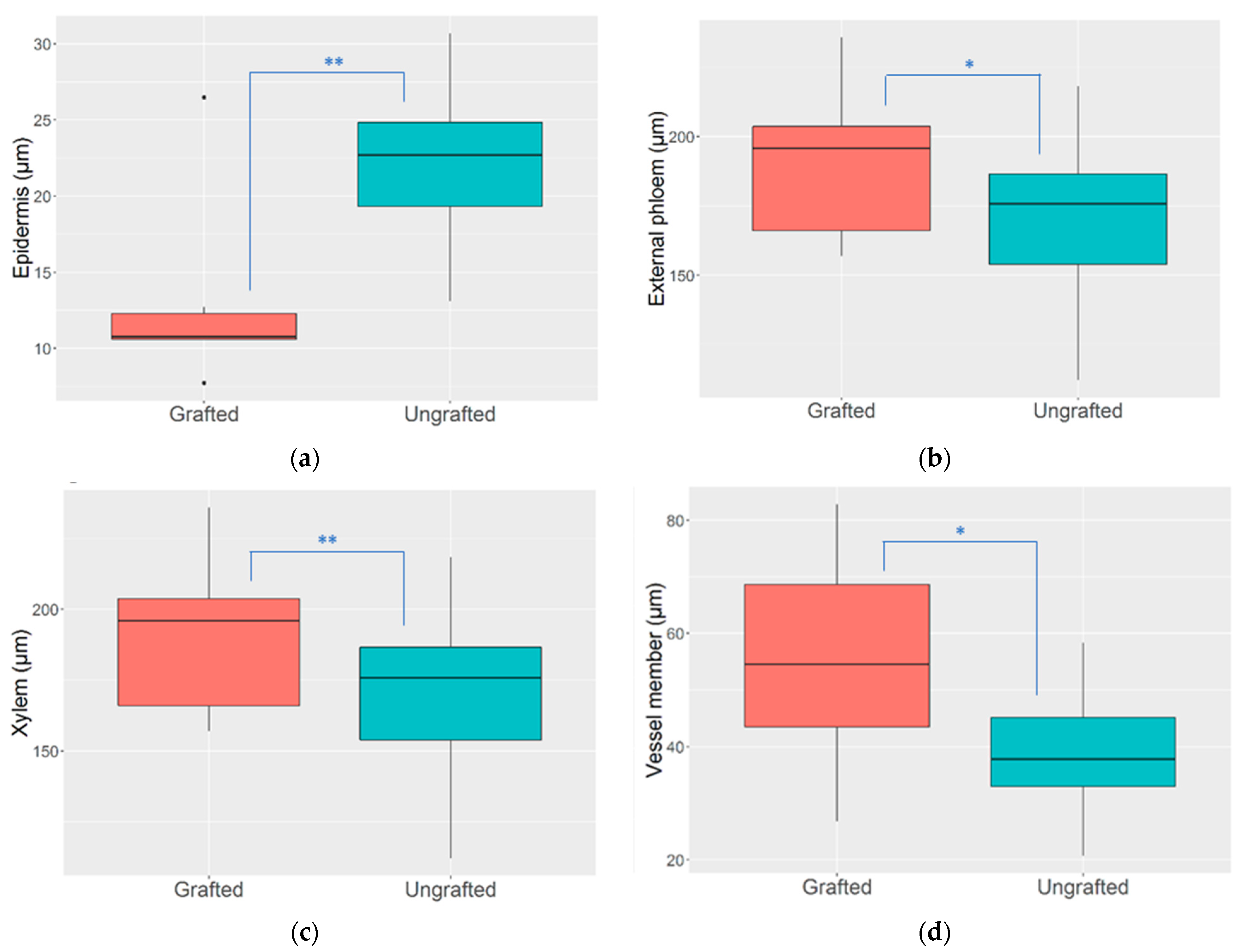
3.2. Stem Anatomical Changes
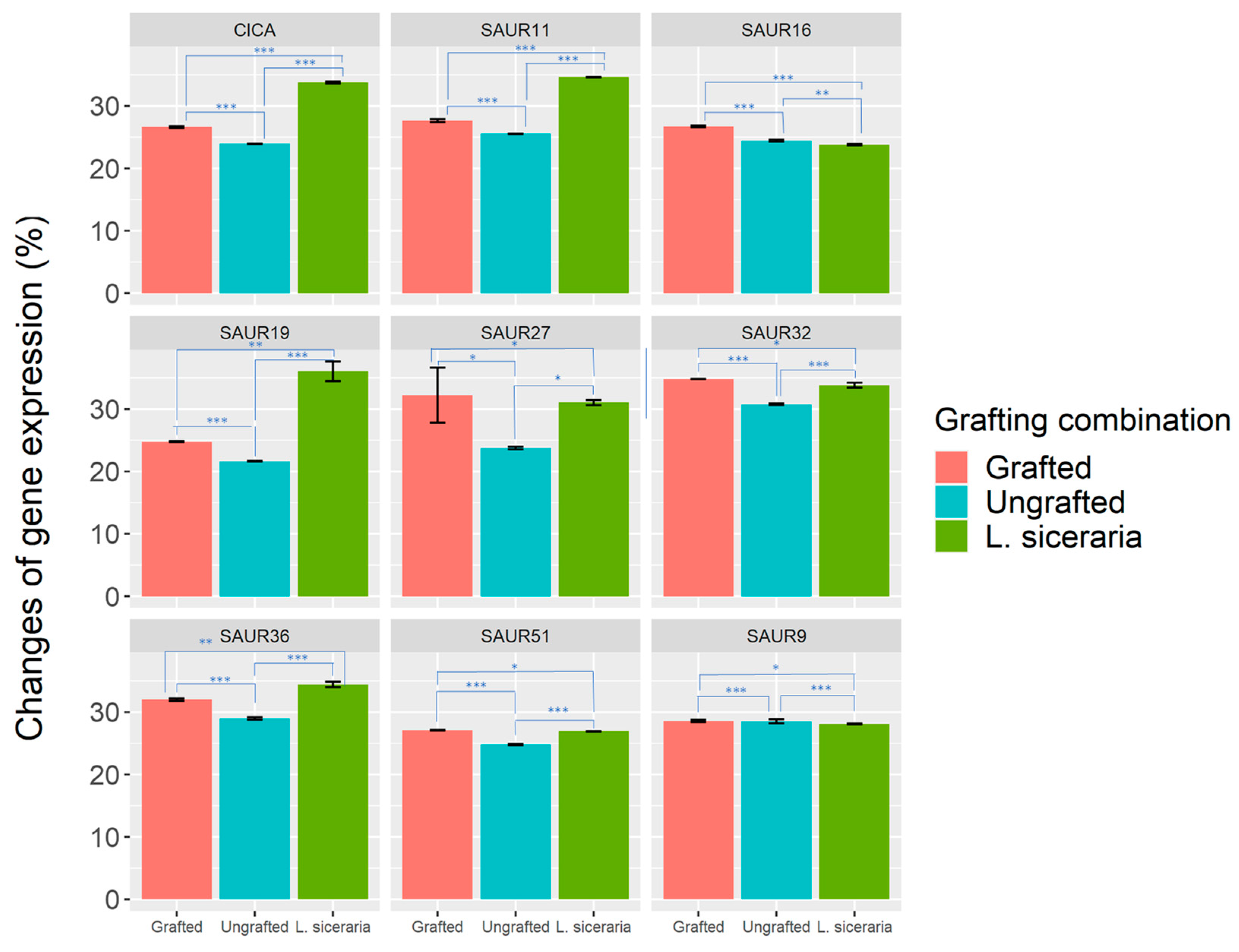
3.3. Expression Analysis of Grafted C. lanatus SAUR Genes
4. Discussion
4.1. Leaf Morpho-Anatomical Changes
4.2. Stem Anatomy Changes
4.3. Differentially Expressed ClaSAUR Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, J.M.; Oda, M. Grafting of herbaceous vegetable and ornamental crops. Hortic. Rev. 2003, 28, 61–124. [Google Scholar] [CrossRef]
- Goldschmidt, E. Plant grafting: New mechanisms, evolutionary implications. Front Plant Sci. 2014, 5, 727. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, S.R.; Shu, S.; Xu, Y.; Sun, J. Isolation and expression pattern analysis of CmRNF5 and CmNPH3L potentially involved in graft compatibility in cucumber/pumpkin graft combinations. Sci. Hortic. 2018, 227, 92–101. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, Q.; Wang, L.; Guo, S.; Shu, S.; Lu, N.; Sun, J. Involvement of metabolic, physiological and hormonal responses in the graft-compatible process of cucumber/pumpkin combinations was revealed through the integrative analysis of mRNA and miRNA expression. Plant Physiol. Biochem. 2018, 129, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Mullor, R.; Pascual-Seva, N.; Martínez-Gimeno, M.A.; López-Serrano, L.; Badal Marín, E.; Pérez-Pérez, J.G.; Bonet, L.; Padilla, Y.G.; Calatayud, Á.; Pascual, B.; et al. Grafting onto an Appropriate Rootstock Reduces the Impact on Yield and Quality of Controlled Deficit Irrigated Pepper Crops. Agronomy 2020, 10, 1529. [Google Scholar] [CrossRef]
- Otani, T.; Seike, N. Comparative effects of rootstock and scion on dieldrin and endrin uptake by grafted cucumber (Cucumis sativus). J. Pestic. Sci. 2006, 31, 316–321. [Google Scholar] [CrossRef]
- Dong, H.H.; Niu, Y.H.; Li, W.J.; Zhang, D.M. Effects of cotton rootstock on endogenous cytokinins and abscisic acid in xylem sap and leaves in relation to leaf senescence. J. Exp. Bot. 2008, 59, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, J.; Hua, B.; Liu, Z.; Fan, M.; Bie, Z. Grafting onto different rootstocks as a means to improve watermelon tolerance to low potassium stress. Sci. Hortic. 2013, 149, 80–85. [Google Scholar] [CrossRef]
- Pina, A.; Cookson, S.J.; Calatayud, A.; Errea, P. Physiological and molecular mechanisms underlying graft compatibility. In Vegetable Grafting: Principles and Practices, 2nd ed.; Colla, G., Pérez-Alfocea, F., Schwarz, D., Eds.; CABI: London, UK, 2017; pp. 132–154. [Google Scholar] [CrossRef]
- Lu, K.; Sun, J.; Li, Q.; Li, X.; Jin, S. Effect of Cold Stress on Growth, Physiological Characteristics, and Clavin-Cyle-Related Gene Expression of Grafted watermelon Seedlings of Different Gourd Rootstocks. Horticulturae 2021, 7, 391. [Google Scholar] [CrossRef]
- Yetisir, H.; Sari, N. Effect of different rootstock on plant growth, yield and quality of watermelon. Aust. J. Exp. Agric. 2003, 43, 1269–1274. [Google Scholar] [CrossRef]
- Yetisir, H.; Sari, N.; Yücel, S. Rootstock resistance to Fusarium wilt and effect on watermelon fruit yield and quality. Phytoparasitica 2003, 31, 163–169T. [Google Scholar] [CrossRef]
- Turhan, A.; Ozmen, N.; Kuscu, H.; Serbeci, M.S.; Seniz, V. Influence of rootstocks on yield and fruit characteristics and quality of watermelon. Hortic. Environ. Biotechnol. 2012, 53, 336–341. [Google Scholar] [CrossRef]
- Nisini, P.T.; Colla, G.; Grantani, E.; Temperini, O.; Crinó, P.; Saccado, F. Rootstock resistance to fusarium wilt and effect on fruit yield and quality of two muskmelon cultivars. Sci. Hortic. 2002, 93, 281–288. [Google Scholar] [CrossRef]
- Ombódi, A. Az oltás elméleti és gyakorlati szerepe a dinnyetermesztésben. Hajtásos Korai Termesztés 2005, 36, 9–12. [Google Scholar]
- Chouka, A.S.; Jaberi, H. Effect of grafting on watermelon vegetative and root development, production and fruit quality. Acta Hortic. 1999, 492, 85–94. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Salerno, A.; Rea, E. The effectiveness of grafting to improve alkalinity tolerance in watermelon. Environ. Exp. Bot. 2010, 68, 283–291. [Google Scholar] [CrossRef]
- Mohamed, F.H.; El-Hamed, K.E.A.; Elwan, M.W.M.; Hussien, M.N.E. Impact of grafting on watermelon growth, fruit yield and quality. Horticulturae 2012, 76, 99–118. [Google Scholar] [CrossRef]
- Farhadi, A.; Aroeii, H.; Nemati, H.; Salehi, R.; Giuffrida, F. The effectiveness of different rootstocks for improving yield and growth of cucumber cultivated hydroponically in a greenhouse. Horticulturae 2016, 2, 1. [Google Scholar] [CrossRef]
- Yetisir, H.; Sari, N. Effect of Hypocotyl Morphology on Survival Rate and Growth of Watermelon Seedlings Grafted on Rootstocks with Different Emergence Performance at Various Temperatures. Turk. J. Agric. For. 2004, 28, 231–236. [Google Scholar]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Rea, E. Effect of salinity on yield, fruit quality, leaf gas exchange, and mineral composition of grafted watermelon plants. Hort. Sci. 2006, 41, 622–627. [Google Scholar] [CrossRef]
- Proietti, S.; Rouphael, Y.; Colla, G.; Cardarelli, M.; De Agazio, M.; Zacchini, M.; Rea, E.; Moscatello, S.; Battistelli, A. Fruit quality of mini-watermelon as affected by grafting and irrigation regimes. J. Sci. Food Agric. 2008, 88, 1107–1114. [Google Scholar] [CrossRef]
- Melnyk, C.W. Plant grafting: Insights into tissue regeneration. Regeneration 2016, 4, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Lucas, W.J.; Groover, A.; Lichtenberger, R.; Furuta, K.; Yadav, S.R.; Helariutta, Y.; He, X.Q.; Fukuda, H.; Kang, J.; Brady, S.M.; et al. The plant vascular system: Evolution, development and functions. J. Integr. Plant Biol. 2013, 55, 294–388. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zheng, B. Molecular Responses during Plant Grafting and Its Regulation by Auxins, Cytokinins, and Gibberellins. Biomolecules 2019, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Köse, C.; Güleryüz, M. Effects of auxins and cytokinins on graft union of grapevine (Vitis vinifera). N. Z. J. Crop Hortic. Sci. 2006, 34, 145–150. [Google Scholar] [CrossRef]
- Mitchell, J.; Van Staden, J. Cytokinins and the wounding response in potato tissue. Z. Pflanzenphysiol. 1983, 109, 1–5. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, L.; Huang, M.; He, X.; Yang, Y.; Liu, X.; Li, Y.; Hou, X. Gibberellins play an essential role in late embryogenesis of Arabidopsis. Nat. Plants. 2018, 4, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ragni, L.; Nieminen, K.; Pacheco-Villalobos, D.; Sibout, R.; Schwechheimer, C.; Hardtke, C.S. Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion. Plant Cell 2011, 23, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Regnault, T.; Daviere, J.M.; Wild, M.; Sakvarelidze-Achard, L.; Heintz, D.; Carrera Bergua, E.; Lopez Diaz, I.; Gong, F.; Hedden, P.; Achard, P. The gibberellin precursor GA12 acts as a long-distance growth signal in Arabidopsis. Nat. Plants. 2015, 1, 15073. [Google Scholar] [CrossRef]
- Růžička, K.; Ursache, R.; Hejátko, J.; Helariutta, Y. Xylem development-from the cradle to the grave. New Phytol. 2015, 207, 519–535. [Google Scholar] [CrossRef]
- Aloni, R. The role of cytokinin in organised differentiation of vascular tissues. Funct. Plant Biol. 1993, 20, 601–608. [Google Scholar] [CrossRef]
- Yin, H.; Yan, B.; Sun, J.; Jia, P.; Zhang, Z.; Yan, X.; Chai, J.; Ren, Z.; Zheng, G.; Liu, H. Graft-union development: A delicate process that involves cell-cell communication between scion and stock for local auxin accumulation. J. Exp. Bot. 2012, 11, 4219–4232. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Schuster, C.; Leyser, O.; Meyerowitz, E.M. A development framework for graft formation and vascular reconnection in Arabidopsis thaliana. Curr. Biol. 2015, 25, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Aloni, B.; Karni, L.; Deventurero, G.; Levin, Z.; Cohen, R.; Katzir, N.; Lotan-Pompan, M.; Edelstein, M.; Aktas, H.; Turhan, E.; et al. Physiological and biochemical changes at the rootstock-scion interface in graft combinations between Cucurbita root stocks and a melon scion. J. Hortic. Sci. Biotechnol. 2008, 83, 777–783. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Cano-Delgado, A.; Lee, J.Y.; Demura, T. Regulatory Mechanisms for specification and patterning of plant vascular tissues. Annu. Rev. Cell Dev. Biol. 2010, 26, 605–637. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Gray, W.M. SAUR proteinsas effectors of hormonal and environmental signals in plant growth. Mol. Plant. 2015, 8, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Huang, X.; Bao, Y.; Wang, B.; Zeng, H.; Cheng, W.; Tang, M.; Li, Y.; Ren, J.; Sun, Y. Genome-wide identification of SAUR genes in watermelon (Citrullus lanatus). Physiol. Mol. Biol. Plants 2017, 23, 619–628. [Google Scholar] [CrossRef]
- Garner, R.J. The Grafter’s Handbook, 6th ed.; Octopus Publishing Group: London, UK, 2013; p. 320. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Crawley, M.J. Statistics: An Introduction Using R, 2nd ed.; John Wiley and Sons: Bognor Regis, UK, 2014; p. 360. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; p. 497. [Google Scholar]
- Huang, X.; Chen, J.; Bao, Y.; Liu, L.; Jiang, H.; An, X.; Dai, L.; Wang, B.; Peng, D. Transcript profiling reveals auxin and cytokinin signaling pathways and transcription regulation during in vitro organogenesis of ramie (Boehmeria nivea L. Gaud). PLoS ONE 2014, 9, e113768. [Google Scholar] [CrossRef]
- Kong, Q.; Yuan, J.; Gao, L.; Zhao, S.; Jiang, W.; Huang, Y.; Bie, Z. Identification of suitable reference genes for gene expression normalization in qRT-PCR analysis in watermelon. PLoS ONE 2014, 9, e90612. [Google Scholar] [CrossRef]
- Yetisir, H.; Caliskan, M.E.; Soylu, S.; Sakar, M. Some physiological and growth responses of watermelon (Citrullus lanatus) Matsum and Nakai) grafted onto Lagenaria siceraria to flooding. Environ. Exp. Bot. 2006, 58, 1–8. [Google Scholar] [CrossRef]
- Sáenz-Pérez, C.A.; Osorio-Hernández, E.; Estrada-Drouaillet, B.; Castro-Nava, S.; Delgado-Martínez, R.; López-Badillo, C.M.; Rodríguez-Herrera, R. Rootstock Influence on Growth and Mineral Content of Citrus limon and Citrus sinensis cv. Valencia Inoculated with Candidatus Liberibacter Asiaticus. Agronomy 2020, 10, 1564. [Google Scholar] [CrossRef]
- Rahmatian, A.; Delshad, M.; Salehi, R. Effect of grafting on growth, yield and fruit quality of single and double stemmed tomato plants grown hydroponically. Prot. Hortic. 2014, 55, 115–119. [Google Scholar] [CrossRef]
- Navas, M.L.; Garnier, E. Plasticity of whole plant and leaf traits in Rubia peregrine in response to light, nutrient and water availability. Acta Oecol. 2002, 23, 375–383. [Google Scholar] [CrossRef]
- Lawson, T.; Morison, J. Visualising patterns of CO2 diffusion in leaves. New Phyt. 2006, 169, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Read, J.; Stokes, A. Plant biomechanics in an ecological context. Am. J. Bot. 2006, 93, 1546–1565. [Google Scholar] [CrossRef] [PubMed]
- da Silva, N.R.; Florindo, J.B.; Gómez, M.C.; Rossatto, D.R.; Kolb, R.M.; Bruno, O.M. Plant Identification Based on leaf Midrib Cross-Section Images Using Fractal Descriptors. PLoS ONE 2015, 10, e0130014. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.A.M.; Chitwood, D.H.; Azevedo, A.A.; Araújo, W.L.; Ribeiro, D.M.; Peres, L.E.; Martins, S.C.V.; Zsögön, A. Bundle sheath extensions affect leaf structural and physiological plasticity in response to irradiance. Plant Cell Environ. 2019, 42, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N.; Sack, L.; Gilbert, M.E. The role of bundle sheath extensions and life form in stomatal responses to leaf water status. Plant Physiol. 2011, 156, 962–973. [Google Scholar] [CrossRef]
- Day, T.A.; Martin, G.; Vogelmann, T.C. Penetration of UV-B radiation in foliage: Evidence that the epidermis behaves as a non-uniform filter. Plant Cell Environ. 1993, 16, 735–741. [Google Scholar] [CrossRef]
- Savaldi-Goldstein, S.; Peto, C.; Chory, J. The epidermis both drives and restricts plant shoot growth. Nature 2007, 446, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Omara, R.I.; Abdelaal, K.A.A. Biochemical, histopathological and genetic analysis associated with leaf rust infection in wheat plants (Triticum aestivum L.). Physiol. Mol. Plant Pathol. 2018, 104, 48–57. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.; Qi, Z. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef]
- Oda, M.; Nagaoka, M.; Mori, T.; Sei, M. Effect of hypocotyl morphology on survival rate and growth of cucumber seedling grafted on Cucurbita spp. Jpn. Agric. Res. Q. 1993, 26, 259–263. [Google Scholar]
- Makesh Kumar, B.; Veni, P.; Vijayalakshmi, M.; Selvaraj, K.; Stephan, J. Stem Anatomy of Four Genera of Family Cucurbitaceae. Int. J. Adv. Res. Bot. 2020, 6, 15–18. [Google Scholar] [CrossRef]
- Sachs, T. Pattern Formation in Plant Tissues, 25th ed.; Developmental and cell Biology Series; Cambridge University Press: Cambridge, UK, 2005; 248p. [Google Scholar]
- Floyd, S.K.; Bowman, J.L. Gene regulation: Ancient microRNA target sequences in plants. Nature 2004, 428, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Lewsey, M.G.; Hardcastle, T.J.; Melnyk, C.W.; Molnár, A.; Valli, A.; Ulrich, M.A.; Nery, J.R.; Baulcombe, D.C.; Ecker, J.R. Mobile small RNAs regulate genome-wide DNA methylation. Proc. Natl. Acad. Sci. USA 2016, 113, E801–E810. [Google Scholar] [CrossRef]
- Michurin, I.V. Selected Works; Foreign Languages Publishing House: Soviet Socialist Republics, Moscow, 1949; p. 527. [Google Scholar]
- Rosell, J.A.; Olson, M.E.; Anfodillo, T. Scaling of xylem vessel diameter with plant size: Causes, predictions, and outstanding questions. Curr. For. Rep. 2017, 3, 46–59. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, X.; Ayre, B.G.; Turgeon, R. The origin and composition of cucurbit “phloem” exudate. Plant Physiol. 2012, 158, 1873–1882. [Google Scholar] [CrossRef]
- Gaupels, F.; Ghirardo, A. The extrafascicular phloem is made for fighting. Front. Plant Sci. 2013, 4, 187. [Google Scholar] [CrossRef]
- De Rybel, B.; Mahönen, A.P.; Helariutta, Y.; Weijers, D. Plant vascular development: From early specification to differentiation. Nat. Rev. Mol. Cell Biol. 2016, 17, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Nakaune, M.; Ma, J.F.; Kojima, M.; Takebayashi, Y.; Sakakibara, H.; Otagaki, S.; Matsumoto, S.; Shiratake, K. Plant Hormone and Inorganic Ion Concentrations in the Xylem Exudate of Grafted Plants Depend on the Scion–Rootstock Combination. Plants 2022, 11, 2594. [Google Scholar] [CrossRef] [PubMed]
- Shireen, F.; Nawaz, M.A.; Xiong, M.; Ahmad, A.; Sohail, H.; Chen, Z.; Abouseif, Y.; Huang, Y.; Bie, Z. Pumpkin rootstock improves the growth and development of watermelon by enhancing uptake and transport of boron and regulating the gene expression. Plant Physiol. Biochem. 2020, 154, 204–218. [Google Scholar] [CrossRef] [PubMed]
- van Mourik, H.; Dijk, A.D.J.; Stortenbeker, N.; Angenent, G.C.; Bemer, M. Divergent regulation of Arabidopsis SAUR genes: A focus on the SAUR10-clade. BMC Plant Biol. 2017, 17, 245. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Qi, M.; Ding, X.; Zheng, Y.; Zhou, T.; Chen, Y.; Ning, H.; Zhu, M.; Bian, H.; Wang, J. The SAUR41 subfamily of SMALL AUXIN UP RNA genes is abscisic acid inducible to modulate cell expansion and salt tolerance in Arabidopsis thaliana seedlings. Ann. Bot. 2020, 125, 805–819. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, Y.; Li, M.; Lamin-Samu, A.T.; Yang, D.; Yu, X.; Izhar, M.; Jan, I.; Ali, M.; Lu, G. The Arabidopsis SMALL AUXIN UP RNA32 protein regulates ABA-mediated responses to drought stress. Front. Plant Sci. 2021, 12, 625493. [Google Scholar] [CrossRef]
- Markakis, M.N.; Boron, A.K.; Van Loock, B.; Saini, K.; Cirera, S.; Verbelen, J.P.; Vissenberg, K. Characterization of a small auxin-up RNA (SAUR)-like gene involved in Arabidopsis thaliana development. PLoS ONE 2013, 8, e82596. [Google Scholar] [CrossRef]


| Gene | Ungrafted C. lanatus | Grafted C. lanatus | L. siceraria |
|---|---|---|---|
| ClCAC | 1 | 1 | 1 |
| ClaSAUR9 | 1 | 1 | 1 |
| ClaSAUR10 | 1 | 0 | 0 |
| ClaSAUR11 | 1 | 1 | 1 |
| ClaSAUR16 | 1 | 1 | 1 |
| CLaSAUR19 | 1 | 1 | 1 |
| ClaSAUR27 | 1 | 1 | 1 |
| ClaSAUR32 | 1 | 1 | 1 |
| ClaSAUR36 | 1 | 1 | 1 |
| ClaSAUR41 | 1 | 0 | 0 |
| ClaSAUR51 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márkus, R.; Kocsis, M.; Farkas, Á.; Nagy, D.U.; Helfrich, P.; Kutyáncsánin, D.; Nyitray, G.; Czigle, S.; Stranczinger, S. A Modeling Approach to Studying the Influence of Grafting on the Anatomical Features and SAUR Gene Expression in Watermelons. Agronomy 2024, 14, 1472. https://doi.org/10.3390/agronomy14071472
Márkus R, Kocsis M, Farkas Á, Nagy DU, Helfrich P, Kutyáncsánin D, Nyitray G, Czigle S, Stranczinger S. A Modeling Approach to Studying the Influence of Grafting on the Anatomical Features and SAUR Gene Expression in Watermelons. Agronomy. 2024; 14(7):1472. https://doi.org/10.3390/agronomy14071472
Chicago/Turabian StyleMárkus, Rita, Marianna Kocsis, Ágnes Farkas, Dávid U. Nagy, Paul Helfrich, Damir Kutyáncsánin, Gergely Nyitray, Szilvia Czigle, and Szilvia Stranczinger. 2024. "A Modeling Approach to Studying the Influence of Grafting on the Anatomical Features and SAUR Gene Expression in Watermelons" Agronomy 14, no. 7: 1472. https://doi.org/10.3390/agronomy14071472
APA StyleMárkus, R., Kocsis, M., Farkas, Á., Nagy, D. U., Helfrich, P., Kutyáncsánin, D., Nyitray, G., Czigle, S., & Stranczinger, S. (2024). A Modeling Approach to Studying the Influence of Grafting on the Anatomical Features and SAUR Gene Expression in Watermelons. Agronomy, 14(7), 1472. https://doi.org/10.3390/agronomy14071472








