Abstract
Agricultural practices profoundly influence soil microbial populations and physicochemical properties, vital for crop growth and quality. This study aims to explore the impact of diverse agrochemical applications on soil microbial dynamics, physicochemical properties, and maize yield and proximate properties. Topsoil samples, collected at depths of 1 to 15 cm, were transported to Jimma University for maize cultivation. Over 120 days, soil and maize samples were collected at specified intervals for analysis, including soil pH, microbial populations, and nutrient content. Statistical analysis using one-way ANOVA (p < 0.05) was conducted. Soil bacterial and fungal populations were measured on days 5, 10, 20, 40, 80, and 120. The highest total mesophilic bacterial count (TMBC) was in compost-treated pots (G) and the lowest in those receiving macronutrient fertilizers and glyphosates (B). The highest total mesophilic fungal count (TMFC) was in pots with glyphosates and compost (F), and the lowest was in pots treated with macronutrient fertilizers and glyphosates (B). Pots treated with macronutrient fertilizers and glyphosates (B), macronutrient fertilizers (A), and micronutrient fertilizers (C) showed the lowest Fe and Zn levels. Maize in pots treated with macronutrient fertilizer combined with glyphosate (B) exhibited the lowest protein, fats, and carbohydrates. Notably, compost-treated soils showed the highest bacterial and fungal counts, Fe, and Zn concentrations, while micro-mineral fertilizer combined with glyphosate (B) depleted the soil. Agrochemical treatments negatively affected maize yield quality, indicating complex treatment-related changes in soil parameters.
1. Introduction
Agriculture stands at the intersection of human survival and economic prosperity, with maize (Zea mays L.) being one of the most vital crops globally [1]. As a staple food, a key livestock feed, and a raw material for industrial products, maize significantly influences local economies and global markets. Enhancements in agricultural practices, particularly through agrochemicals, have led to remarkable increases in crop productivity and have helped meet the growing food demand [2].
However, agrochemicals such as fertilizers and pesticides raise concerns about their long-term impact on soil health and sustainability. Their extensive application can lead to soil degradation, reduced soil fertility, and environmental pollution, thus posing a threat to agricultural sustainability [3]. One primary concern is the effect of agrochemicals on soil microbiological activity. Soil microorganisms are essential for maintaining soil health as they contribute to nutrient cycling, organic matter decomposition, and suppression of soil-borne diseases [4]. Agrochemicals can disrupt these microbial communities, leading to reduced soil fertility and negatively impacting plant growth [5].
Currently, micronutrient deficiencies have emerged as a significant challenge to crop productivity in Ethiopia, with widespread deficiencies of zinc (Zn), iron (Fe), manganese (Mn), and copper (Cu) noted across various regions [6,7]. Practices such as increased cropping intensity, use of high-yielding varieties, and extensive fertilizer and pesticide application are expected to exacerbate these problems [6,8]. Inadequate food crop concentration and, thus, human intake of these elements can result in nutritional deficiency, anemia, compromised immune function, skin diseases, delayed child development, and intelligence-related issues [9,10,11]. This issue poses a growing threat to food and nutrition security in developing countries [12,13].
Moreover, soil salinity, exacerbated by improper irrigation practices and excessive agrochemical use, poses a significant threat to agricultural productivity. High salinity levels can inhibit plant growth, reduce crop yields, and lead to long-term soil degradation [14]. Furthermore, decreased pH [15] and the use of pesticides can influence the availability of soil micronutrients (Cu, Fe, Mn, and Zn) and microbial populations [16,17]. In particular, glyphosate, a commonly used herbicide, strongly binds to soil components, potentially affecting soil microbial communities and, thus, the general agroecosystem sustainability [18]. Despite certain prokaryotic and fungal species metabolizing glyphosate to protect susceptible species, the effectiveness of this mechanism in mitigating the herbicide’s impact is still under scrutiny [19].
Several studies reveal that glyphosate concentrations exceed approved rates, possibly concealing the buffering effects of resistant members [18]. An overuse of glyphosate can affect soil microbial respiration and biomass [20]. Furthermore, long-term glyphosate use can lead to substantial changes in vegetation composition and growth by interfering with soil ecosystems and micronutrient availability [21].
The use of mineral fertilizers and pesticides, aiming to increase soil fertility and crop productivity, may also negatively impact the soil health and environment [22] by altering soil physicochemical properties, disrupting soil ecological balances, and disturbing the soil nutrient balance, decomposition rates, and nutrient bioavailability [23]. Furthermore, overuse of these chemicals can reduce the populations of beneficial soil microorganisms and enzyme activities that are essential for soil health and plant productivity [23]. Although mineral fertilizers alone may increase crop production, concerns exist regarding their environmental and long-term soil sustainability implications [24]. Mineral fertilizers can increase crop disease incidence and reduce soil micronutrient availability [25].
In addition, prolonged and uninterrupted cropping practices over several decades often result in modifications to soil properties, e.g., leading to a reduction in the levels of organic matter and essential micronutrients such as iron (Fe), manganese (Mn), zinc (Zn), and copper (Cu) both as plant available species and bound to soil organic matter and other surfaces of the soil particles [26].
Ethiopia currently confronts numerous soil fertility challenges, including organic matter and micronutrient depletion due to factors such as topsoil erosion, acidity, and salinity [27]. Additionally, prolonged use of inorganic fertilizers and excessive pesticide application exacerbate these challenges [28]. The micronutrient deficiency in the soil system directly impacts plant uptake and, indirectly, human health [6,10]. Prolonged continuous cropping modifies soil physicochemical and biological characteristics and diminishes micronutrient availability, exacerbating soil fertility challenges in Ethiopia [27]. While widespread micronutrient deficiencies are evident in the western part of Ethiopia, data regarding the effects of agrochemicals on soil micronutrient availability and plant uptake are lacking [29]. This study aims to fill this gap by investigating the impacts of different agrochemical treatments on soil micronutrient availability, microbial dynamics, and maize proximate properties in western Ethiopia.
2. Materials and Methods
2.1. The Intervention Approach of the Study
Initially, soil samples were gathered from three districts within the Kellem Wellega zone: Sayo, Hawa Gelan, and Dale Wabara, all three from small-scale farmers’ cultivated fields. Due to the environmental homogeneity at these three sites, the soil samples were combined. The pot experiment was conducted, implementing the intervention approaches of the study in the following manner.
This study involves a split-plot experimental design to assess the impact of different soil treatments on various soil properties and microbial activities. Initially, soil samples were collected and subjected to different treatment allocations. These treatments included macronutrient fertilizers (A), macronutrient fertilizers and glyphosates (B), micronutrient fertilizers (C), glyphosates (D), a control group with no additives (E), compost combined with glyphosates (F), and compost (G). Following the application of these treatments, the soil samples were monitored for changes in mesophilic bacteria and fungus populations, along with measurements of ash, pH, electrical conductivity (EC), moisture content (MC), and organic matter (OM). Additionally, the levels of copper (Cu), zinc (Zn), and iron (Fe) were evaluated.
After this follow-up period, a comprehensive analysis was conducted to further assess ash content, pH, EC, moisture, and organic matter. The analysis also included an evaluation of total protein, fat, fibers, carbohydrates, and gross energy content, along with the levels of zinc (Zn), iron (Fe), manganese (Mn), and copper (Cu). This thorough approach ensures a detailed understanding of how different soil treatments influence both the chemical and biological properties of the soil.
2.2. Study Setting
The pot experiment was carried out at Jimma University. However, the soil was collected from the Kellem Wellega zone, in the western part of Ethiopia’s Oromia region, during June 2023. This zone lies approximately 672 km from Addis Ababa. Comprising 10 districts with a total population of 965,000 individuals, nearly half of whom are female [30]. Within this zone, there are approximately 175,000 households, each with an average size of 4.5 members [31].
For our study, we deliberately selected three specific districts: Sayo, Hawa Gelan, and Dale Wabara. These districts were chosen due to their adoption of high-yielding crop varieties, intensive cultivation methods, prolonged fertilizer use, soil acidification, and the absence of organic manure, as well as management practices contributing to the observed micronutrient deficiencies.
The selected areas were positioned at an elevation ranging from 1701 to 1830 m above sea level. The climate pattern is characterized by abundant summer rains, a brief wet season, and a dry winter. Annual precipitation varies from 800 to 1200 mm, while daily temperatures range between 15 and 25 degrees Celsius [32]. Renowned for its prolific agricultural output, the region produces a variety of crops, including coffee, maize, teff, wheat, barley, bean seed, and sorghum. The study area map is depicted in Figure 1.
Figure 1.
Geographical location of the study area for Sayo, Hawa Gelan, and Dale Wabara districts in Kellem Wellega zone, Western Ethiopia.
2.3. Sample Collection from Farming Sites
Using a soil auger, topsoil samples were collected from the three selected districts of Kellem Wellega, i.e., Sayo, Hawa Gelan, and Dale Wabara, extracting topsoil from depths of 1 to 15 cm. Before the collection process, surface debris and plant materials were carefully cleared by hand and stored in polyethylene bags for later disposal. The gathered samples were subsequently transported to Jimma University of Agricultural College, where they were mixed and utilized to cultivate maize within an open greenhouse environment.
2.4. Experimental Design and Treatment
The experiment employed a completely split-plot experimental design, each treatment consisting of three replications (n = 3) and labeled treatments A to G. Pots were arranged in an open greenhouse to ensure exposure to sunlight. The soils were collected from the farmers’ fields during the period from September to November 2022. A survey has identified glyphosate as a prevalent herbicide in the local community [7]. Commonly used concentrations of active ingredients were combined in the plastic pots containing soil (10 cm deep) and homogenized. Plastic pots in seven treatment combinations, including a control group, all in three replications, were arranged in a split-plot experimental design. Maize, identified as a commonly consumed plant, was planted and allowed to germinate and grow until maturity, in which the temperature requirements can vary depending on the type of environment, and watered twice daily, in the morning and the evening.
The soil was sampled during the experiment. Parameters including total aerobic mesophilic bacteria count (TMBC), total mesophilic fungi count (TMFC), organic matter, moisture content, pH, electrical conductivity (EC), ash content, as well as iron, zinc, manganese, and copper levels were measured at intervals of 5, 10, 20, 40, 80, and 120 days.
Glyphosate quantities added were calculated based on soil density and volume. For glyphosate, 0.75 pounds or 0.34 kg of glyphosate is used per hectare, which translates to 2.67 × 10−6 kg or 2.61 mg of glyphosate per 16 kg of soil for each pot. The treatments included a control group that contains only the local soil or free of any treatments, as well as the application of an herbicide, i.e., glyphosate, at a rate of 0.75 pounds per acre of soil, which is equivalent to 2.67 mg of glyphosate per 16 kg of soil. As per our experimental design, the soil was divided into seven equal-weight portions of 16 kg each, which were transferred into plastic pots given in Table 1.

Table 1.
Designation of treatments and types of fertilizers utilized in the soil pot experiment.
This study investigates the effects of macro and micronutrient fertilizers on maize growth and soil health, applying precise rates per 16 kg soil pots. Key nutrients include nitrogen, phosphorus, and potassium, alongside zinc, iron, copper, and manganese. Furthermore, the added amounts for all kinds of fertilizers are shown in Table 2.

Table 2.
Types, sources, and rate of fertilizers required for the soil pot experiment.
2.5. Preparation of Compost Samples
Compost was prepared according to the methods outlined in [28], and it was mixed in a ratio of one part compost to four parts soil for maize cultivation, as described in [29].
2.6. Moisture Contents (MC), Organic Matter (OM), and Ash Contents (AC)
The moisture content (MC), organic matter (OM), and ash contents (AC) of soil and crop yield samples were determined by methods [30].
2.7. Determination of pH and Electrical Conductivity from Soil Samples
Soil pH was measured using a pH meter [31], and electrical conductivity was determined with a digital multi-parameter device (Bante 900-Bante Instruments, Shanghai, China).
2.8. Determination of Micronutrients from Soil Samples
Soil samples were collected on days 5, 10, 20, 40, 80, and 120 after pesticide application. Samples were dried at 105 °C for 24 h, ground to a fine powder, and then dry-ashed at 550 °C for 6 h. After cooling, the ash was digested with HNO3 and HCl, filtered, and analyzed for Zn, Fe, Mn, and Cu using an atomic absorption spectrophotometer [32].
2.9. Determination of Total Mesophilic Bacteria and Fungi Counts in Soil Samples
Polyethylene bags were sterilized for 12 h, glassware at 160 °C for 2 h, and growth media autoclaved at 121 °C for 15 min. A gram of fresh-weight soil samples was collected on days 5, 10, 20, 40, 80, and 120 and analyzed using serial dilution and pour plate techniques [33], asparagus mannitol agar [34], and potato dextrose agar [35]. Bacterial colonies were incubated at 28 ± 2 °C for 18–24 h, and fungi on PDA with chloramphenicol at room temperature for 48–72 h.
2.10. Quantification and Quality Analysis of Harvested Maize
2.10.1. Sample Preparation for Proximate Composition
Sample preparation converted the samples into homogeneous material for various quantity and quality analyses. Maize plant samples were dried and ground. Specific sample preparation was then conducted according to the sample type and analyses requested. Protein, fat, crude fiber, moisture, and ash were determined by the methods of [33].
2.10.2. Total Carbohydrates and Energy Gross
Total carbohydrates were determined using proteins, fats, ash, fibers, and moisture proportions, which were added and subtracted from 100 . The gross energy value (expressed in kilocalories) was calculated using Atwater’s conversion factors of 4 kcal/g for protein, 9 kcal/g for fat, 4 kcal/g for carbohydrates, and 2 kcal/g for fiber [34].
2.11. Statistical Analysis
Initially, the data were entered into EPI-INFO version 7 and subsequently imported into the Statistical Package for the Social Sciences version 26 (SPSS). Following this, descriptive statistics, encompassing mean, range, and standard deviations, were computed. Furthermore, the nutritional composition of agrochemical-treated maize was analyzed. Finally, a non-parametric test, specifically the Kruskal–Wallis’s test of one-way ANOVA, was used to compare the primary effects of the treatments.
3. Results
3.1. Soil Physicochemical Properties
3.1.1. Soil Moisture (MC), Ash (AC), and Organic Matter (OM) Content
When comparing the results across treatments labeled A to G (Table 1), notable variations emerge in the parameters measured. For soil moisture content, treatment of macronutrient fertilizers (A) consistently exhibits lower levels compared to other treatments, while treatments of micronutrient fertilizers(C), glyphosates (D), and control (E) generally display intermediate moisture levels. Conversely, the treatment of compost and glyphosates (F) consistently resulted in the highest moisture content, particularly at longer time intervals (Table 3).

Table 3.
Moisture, ash, and organic matter of soil samples from pot experiments were recorded at 5, 10, 20, 80, and 120th days of intervals.
Regarding ash contents, the treatment of compost (G) consistently presents lower levels compared to other treatments. Treatment F tends to show relatively higher ash content, especially in extended durations (Table 3).
In terms of organic matter, the treatment of compost (G) consistently displays higher levels compared to other treatments, especially over longer periods, while the treatment of compost and glyphosates (F) shows relatively lower organic matter content, particularly in extended durations (Table 3). The non-parametric, one-way ANOVA analysis revealed notable variations in ash (p = 0.01) and organic matter (p = 0.01) contents.
3.1.2. Electrical Conductivity (EC) and pH
Comparing the results across different sampling days for treatments labeled A to G reveals significant variations. For pH levels, compost (G) treatment consistently gave higher pH values than other treatments across various sampling days (Table 4). In contrast, treatments of macronutrient fertilizers (A), micronutrient fertilizers, and glyphosates (B) tend to exhibit lower pH levels, particularly at longer trial durations. Notably, treatments of micronutrient fertilizers (C) and glyphosates (D) display fluctuating pH levels, showing both higher and lower values throughout the trial period (p = 0.00).

Table 4.
Electrical conductivity (EC, µs/cm) and pH physicochemical properties of soil samples from pot experiments that were recorded at 5, 10, 20, 80, and 120th days of intervals.
Regarding electrical conductivity (EC), the treatment of compost (G) consistently displays the highest values across most trial days, indicating greater conductivity compared to other treatments (Table 4). Treatments of micronutrient fertilizers (C) and glyphosates (D) also show relatively high EC values, especially at certain time points, while treatments of macronutrient fertilizers (A), micronutrient fertilizers, and glyphosates (B) generally exhibit lower conductivity levels. The treatment of compost and glyphosates (F) stands out with significantly elevated EC values, particularly at later trial days, suggesting unique effects compared to other treatments (p = 0.03). (Table 4).
3.1.3. Total Mesophilic Bacterial Count (TMBC) and Fungus (TMFC)
Comparing the results across sampling days and treatments labeled A to G reveals intricate patterns in TMBC (Table 5). At day 5, treatment compost and glyphosates (F) demonstrate the highest counts for both TMBC and TMFC, with counts of 2.4 × 106 and 7 × 103 colony-forming units (cfu), respectively. Treatment of compost (G) also shows notably high TMBC counts (8 × 105 cfu). However, control treatment E displays the lowest TMBC count (2 × 105), while treatment macronutrient fertilizers (A) record the lowest TMFC (9.8 × 104 cfu).

Table 5.
Microbial count of soil samples from pot experiments that were recorded at 5, 10, 20, 80, and 120th days of intervals.
By day 10, TMBC counts peak in the treatment of compost and glyphosates (F) at 2.5 × 106, whereas the treatment of macronutrient fertilizers and glyphosates (B) exhibits the lowest count at 3 × 105. For TMFC, treatment compost and glyphosates (F) again lead with a count of 2.9 × 104, while treatment of micronutrient fertilizers (C) shows the lowest count at 9 × 103 cfu.
By day 20, treatment of compost G emerges with the highest TMBC count at 4 × 106, contrasting with treatment macronutrient fertilizers A’s count of 1.4 × 106 cfu. Similarly, treatment of compost G leads to TMFC counts at 5.7 × 105, while treatment of macronutrient fertilizers and glyphosates B presents the lowest count at 4.2 × 104 cfu.
By day 40, treatment of compost G maintains dominance with the highest TMBC count at 9 × 106, contrasting sharply with treatment A’s count of 5 × 106 colony-forming units (cfu). For TMFC, all treatments exhibit relatively low counts compared to previous days.
By day 80, TMBC counts decreased across all treatments, with treatment of compost G retaining the highest count at 1.5 × 106, and treatment macronutrient fertilizers and glyphosates B showing the lowest count at 7 × 105 cfu. TMFC counts also decline, with treatment of compost G leading at 6 × 103 and treatment of micronutrient fertilizers C recording the lowest count at 3 × 103 cfu.
At day 120, TMBC counts decrease further, with Treatment of compost G maintaining the highest count at 5 × 104 and treatment macronutrient fertilizers A recording the lowest count at 7 × 104 cfu (p = 0.00).
Similarly, TMFC counts diminish, with the treatment of compost G leading at 5 × 103 and treatments of macronutrient fertilizers A, macronutrient fertilizers and glyphosates B, and control E displaying the lowest count at 2 × 103 cfu (p = 0.03) (Table 5).
In general, the graphical representation of total mesophilic bacteria count (TMBC), and total mesophilic fungus count (TMFC) (Figure 2 show that mesophilic bacterial found in pots initially increase up to 40 days after the startup of a growing season, followed by a significant decline until the harvesting period while mesophilic fungal population count tended to increase up to day 20 and then gradually decline (Figure 2).
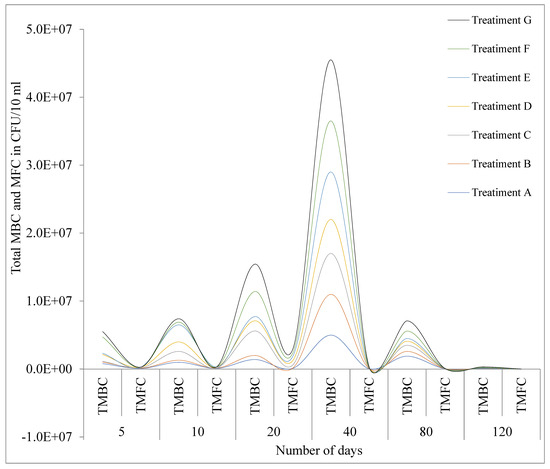
Figure 2.
A graphical representation illustrating the colony formation of total mesophilic bacteria count (TMBC) and mesophilic fungi (TMFC) in soil samples from pot experiments, recorded at intervals of 5, 10, 20, 80, and 120 days. Keys: exponent(E), fertilizers (A), fertilizers and glyphosates (B), micronutrient fertilizers (C), pesticides (D), control (E), compost and pesticides (F), compost (G), total mesophilic bacteria count (TMBC), total mesophilic fungus count (TMFC), and E(Exponent).
3.1.4. Soil Micronutrient Level Analyzed from Pot Experiment
Comparing the results across trial days and treatments labeled A to G revealed variations in soil micronutrient concentration (Table 6). On day 5, the treatment of compost and glyphosates (F) stands out with notably higher values in Fe, Mn, and Zn, suggesting its efficacy in promoting the accumulation of these elements. Conversely, treatment of macronutrient fertilizers and glyphosates (B) show to exhibit lower values, particularly noticeable in the Fe and Mn content.

Table 6.
Fe, Mn, Cu, and Zn micronutrients of soil samples were analyzed from pot experiments that were recorded at 5, 10, 20, 80, and 120th days of intervals.
By day 10, treatment of compost (G) displays elevated levels of Fe and Mn, while treatment of glyphosates (D) shows a significant increase in Zn content. In contrast, treatment of micronutrient fertilizers (C) demonstrates relatively lower values across most parameters.
Moving to day 20, the treatment of compost (G) maintains its dominance with consistently high micronutrient values, especially evident for Fe and Mn. However, the control (E) presents lower values for Fe and Mn compared to the other treatments.
By day 40, treatment compost and glyphosates (F) continue to exhibit the highest values for Fe and Mn, while treatment of compost G surpasses others in Zn content. Notably, treatment of micronutrient fertilizers C consistently displays relatively lower values across all parameters.
As the trial progresses to day 80, the treatment of compost (G) maintains its lead with consistently high values for Fe, Mn, and Zn. Treatments of macronutrient fertilizers and glyphosates (B) and glyphosates (D) show varying patterns with fluctuations with time.
Finally, by day 120, the treatment of compost (G) remains prominent, with notably high values of Fe, Mn, and Zn. Treatment micronutrient fertilizers (C), on the other hand, consistently demonstrate lower values.
Overall, compost treatment (G) consistently demonstrates higher values of Fe (p = 0.00 *) and Zn (p = 0.01), indicating its potential efficacy in promoting the accumulation of these essential elements in the soil. Conversely, Treatment A, B, and D consistently display lower values across all parameters. The highest concentrations of Mn were found in the treatments of micronutrient fertilizers (C), while the lowest was observed in the treatments of macronutrient fertilizers (A), macronutrient fertilizers, and glyphosates (B), and glyphosates (D) (p = 0.00) (Table 6).
3.2. Maize Yield Quality
Analyzing the results across different sampling days for treatments A to G reveals significant variations (Table 7). The lowest plant moisture contents (MC) and ash content (AC) were found in maize grown in the compost (G) treatment, with values of 12.63 ± 0.13 and 1.69 ± 0.1 in g/100 g of samples, respectively. The highest significance of plant moisture contents (p = 0.00) was found in the macronutrient fertilizer treatment (A), and the highest plant ash content was observed for micronutrient fertilizer treatment (C) (p = 0.00) in g/100 g of maize.

Table 7.
Physicochemical characteristics and proximate composition of maize plants grown under fertilizer and chemical soil treatments in pot experiments.
Regarding crude protein content (p = 0.01), in g/100 g, the highest significant concentration was found in maize from the treatment of compost (G) (11.42 ± 0.8). For total crude fats (TCF), the highest level was seen in the glyphosate treatment (D) (3.98 ± 0.01). For total carbohydrates (p = 0.00) and crude fibers (p = 0.00), the highest contents were found in the micronutrient fertilizer treatment (C), with values of 66.23 ± 0.1 and 8.21 ± 0.02 in g/100 g of maize samples, respectively. Lastly, the highest total gross energy (kcal/100 g) level was found in compost treatment (G) (348.465 ± 0.08) (p = 0.00).
The lowest concentration of crude protein (PC) was observed in treatment C (8.57 ± 0.25). For total crude fats (TCF), the lowest level was found in macronutrient fertilizer and glyphosate treatment B (2.21 ± 0.2), while the total carbohydrates (CHO) exhibited the lowest concentration in glyphosate treatment D (57.34 ± 0.1) in g/100 g of maize samples. The lowest total gross energy (kcal/100 g) and crude fibers (CF) in g/100 g were recorded for maize from macronutrient treatment A, with values of (308.375 ± 0.1) and (6.265 ± 0.1), respectively.
In terms of plant micronutrient concentrations, Fe (p = 0.00), Cu (p = 0.03), and Zn (p = 0.00) concentrations were the highest for compost treatment (G) and the lowest in the glyphosate treatment (D) and the treatments with macronutrient fertilizers and glyphosates (B). Finally, plant Mn (p = 0.00) concentration in ppm was the highest in micronutrient treatment (C) and lowest in glyphosate treatment (D) (Table 7).
4. Discussion
This study highlights the significant impact of commonly used effort factors used in intensive agricultural management. The use of chemical fertilizer and glyphosate showed clear effects on soil biological and physicochemical properties, soil micronutrient concentration, as well as maize yield and quality. This experiment illustrates how excessive use of inorganic fertilizers diminishes the population of beneficial soil microorganisms by altering the functional diversity of the soil microbial community.
The total mesophilic bacterial count (TMBC) and total mesophilic fungal count (TMFC) exhibited significant differences among treatment groups. The compost-treated soil exhibited the highest TMBC and TMFC, suggesting a positive influence on soil bacterial populations (Table 5). Compost is known to enrich soil microbial diversity and activity, providing organic substrates and favorable conditions for bacterial growth [35]. Additionally, the presence of glyphosates in compost-treated soil did not seem to hinder bacterial populations, actually indicating potential synergistic effects or tolerance to glyphosates [36]. Likewise, the introduction of inorganic fertilizers alters the structure of the soil microbial community, thereby impacting the composition and diversity of mesophilic bacteria, leading to periodic decreases in their population [37,38].
Furthermore, we observed a significant drop in mesophilic bacterial and fungal count in the pots treated with both macro and micronutrient fertilizers, as well as glyphosate, compared to untreated pots. This decrease may be attributed to the addition of glyphosate, a non-selective organophosphate herbicide that has been found to reduce phosphate enzyme activity [39]. The reduction in phosphate enzyme activity causes the death of sensitive microorganisms [40]. Glyphosate, a common herbicide in agrochemicals, disrupts soil microbial communities by inhibiting specific microbial pathways and reducing microbial biomass [41]. Conversely, compost amendments positively influenced bacterial populations, while chemical inputs had detrimental effects.
Furthermore, our study shows that glyphosate and inorganic fertilizer treatment initially may increase mesophilic bacterial up to 40 days after the startup of a growing season, followed by a significant decline until the harvesting period, while mesophilic fungal population count tended to increase up to day 20 and then gradually decline (Figure 2). The microbial counts may temporarily rise due to their ability to mineralize glyphosate and fertilizers for energy, but later on, these chemicals can selectively promote the growth of certain microbes while inhibiting others, leading to shifts in microbial diversity and overall abundance [42]. Additionally, inorganic fertilizers and glyphosate can be toxic to certain microorganisms and contribute to the decrease in microbial count in farming soil [43]. Moreover, the synergistic interactions between glyphosates, macro, and micronutrient fertilizers can further inhibit microbial soil community functions, ultimately leading to a decrease in microbial populations [44,45,46].
Compost-treated soil (G) exhibited the highest pH values, indicating alkaline conditions favorable for nutrient availability and microbial activity. The alkalinity of compost-amended soil may be attributed to the buffering capacity of organic matter, which helps maintain pH stability [47]. Additionally, the decomposition of organic materials releases alkaline substances, further contributing to elevated pH levels [48]. In contrast, treatments of macronutrient fertilizers (A), micronutrient fertilizers, and glyphosates (B) tend to exhibit lower pH levels, particularly at longer trial durations (Table 4). The acidifying effect of chemical fertilizers may stem from the presence of ammonium-based nitrogen sources, which undergo nitrification and release protons, lowering soil pH [49]. Moreover, acidic conditions can inhibit the growth and activity of beneficial soil microbes, disrupting soil biological processes [50]. Compost application promotes alkaline conditions conducive to nutrient availability and microbial activity, while chemical fertilizers may contribute to soil acidification and nutrient imbalances.
Electrical conductivity (EC) values exhibited significant variation among treatments, which reflects differences in soil salinity and nutrient concentrations. Compost-treated soil recorded the highest conductivity levels, indicating higher nutrient concentrations and organic matter content. The elevated EC in compost-amended soil may result from the decomposition of organic materials, which releases soluble ions and increases the electrical conductivity of soil solutions [51]. Additionally, the presence of organic matter enhances cation exchange capacity, facilitating the retention and release of nutrients, further contributing to elevated EC levels [52].
Conversely, treatments involving macronutrient fertilizers (A) and macronutrient fertilizers combined with glyphosate application (B) generally exhibited lower EC levels (Table 4). This observation is consistent with the notion that chemical fertilizers may not contribute significantly to soil ion concentration compared to organic inputs like compost [53]. Additionally, glyphosate, a common herbicide used in conjunction with fertilizers, might further influence soil conductivity due to its impact on microbial activity and nutrient cycling [54]. This finding aligns with previous research suggesting that organic matter decomposition, as facilitated by compost application, can increase soil ion concentration and, consequently, electrical conductivity [53].
Similarly, soil organic matter content showed significant variation among treatments. Treatment of compost (G) consistently displays higher levels compared to other treatments, especially over longer periods, while treatment of micronutrient fertilizers (C) and glyphosates (D) shows relatively lower organic matter content, particularly in extended durations, indicating limited organic inputs and microbial activity [55]. Compost amendments promote organic matter accumulation, stimulating microbial activity and enhancing soil productivity [44]. Conversely, treatment of micronutrient fertilizers and glyphosates shows relatively lower organic matter content, particularly in extended durations due to the chemical nature, which may not contribute as significantly to organic matter accumulation as compost does [56,57] (Table 3).
Moreover, micronutrient levels (Fe, Zn, and Mn) displayed significant variation by the treatments. The application of compost enriches the soil with organic matter, which serves as a reservoir for micronutrients and promotes their release for plant uptake [58]. Additionally, compost enhances soil microbial activity, facilitating the mineralization of organic matter and the mobilization of micronutrients bound to soil particles [59]. Moreover, chemical fertilizers and glyphosate applications may also contribute to micronutrient deficiencies by disrupting soil microbial communities involved in nutrient-cycling processes [60].
Furthermore, glyphosate, a broad-spectrum herbicide, can form complexes with micronutrients in the soil through chemical interactions, reducing their bioavailability for plant uptake. Glyphosate can also chelate with micronutrients like Fe and Mn, leading to deficiencies in plants [61]. Moreover, glyphosate usage alters microbial populations, impacting micronutrient uptake by plants and inhibits soil microorganisms crucial for organic matter decomposition, thereby reducing mineralization essential for micronutrient availability [62].
Concerning the maize plant’s moisture and ash content, the results support suggestions of maintaining a moisture level between 12 and 14%, which is recommended for maize, as it ensures optimal conditions for storage [63]. The moisture content observed in this study was lower compared to some previous research [64] (9.76–10.6%), [65] (12.01%), and [66] (30–34%), but higher than that reported for Pakistan’s maize [67] (6.09–11.57%). This may be due to the compost treatment likely contributing to lower moisture content in maize due to improved soil structure and water retention capabilities [68]. Conversely, the macronutrient fertilizer treatment may have resulted in higher moisture content in maize due to the specific nutrient composition and application method of the fertilizer. Furthermore, macronutrient fertilizers typically contain nitrogen, phosphorus, and potassium, which can influence plant metabolism and water uptake, potentially leading to higher moisture levels in harvested grains [69]. This variability in moisture and ash content can be attributed to differences in soil water retention capacity and the water-holding capacity of organic amendments like compost [68], while the variation in ash content likely reflects differences in mineral content and soil fertility levels across treatments [70].
The ash content (lowest in the compost treatment) generally fell outside the range reported by several other studies, including [71] (1.1–2.95%), [72] (3.76–4.39%), and [67] (91.09–5.46%). The yield of ash content is often correlated with the presence of essential macro and micronutrients in food samples, as highlighted by [73], and, thus, has significant implications for human health and well-being [10,74]. Furthermore, the inorganic fertilizer treatment may have led to higher ash content in maize due to the specific micronutrients provided, such as iron, zinc, copper, and manganese that are essential for various physiological processes in plants, including enzyme activation and metabolism, which can affect the mineral composition of maize grains. Additionally, the compost may have facilitated nutrient uptake and utilization, leading to increased mineral content and, thus, higher ash content in maize grains.
Maize yield quality parameters such as crude protein, crude fat contents, crude fiber, total carbohydrate, and gross energy indicated significant differences among the treatments. In terms of crude protein content, the highest value was observed in maize flour from pots containing compost. These findings are in line with previous studies by [72] (9.24–11.58%), [65] (4.58–7.24%), and [64] (7.71–14.60). This suggests that compost amendments may enhance nitrogen availability and promote higher protein synthesis in maize plants [75] and the genetic variability of maize seeds [76]. Furthermore, environmental factors, including soil type, climate, and agricultural practices, can influence their nutritional composition [77].
Also crude fat content was the highest in maize in the compost treatment and then in line with results from other studies [64] (2.17–4.43%), [65] (3.84–4.61%) and [67] (2.87–12.54%). The observed differences in nutrient levels may be attributed to various factors, including genetic variability of maize and environmental factors such as soil moisture and temperature, which can influence the fiber content of maize grains [78].
Furthermore, the highest amount of crude fiber was found in maize from pots receiving inorganic micronutrient fertilizers and glyphosate, also in line with findings by [72] (64.02–67.68), [65] (76.85–80.31%), and greater than [64] (69.66–74.55) and [79](82.40%). The variations in total fiber content (TF) may be associated with variances in soil organic matter content and microbial activity [80]. Carbohydrate and gross energy content varied significantly among treatment groups. Such variability could stem from differences in nutrient availability and soil microbial activity, ultimately affecting the carbohydrate content and energy content of maize grains [81].
Moreover, iron, manganese, copper, and zinc content differed significantly among treatment groups. This variation may be attributed to differences in soil pH and organic matter content [82,83]. Compost amendments positively impacted nutrient content and composition, emphasizing their importance in enhancing soil fertility and crop quality. Conversely, glyphosate treatment and micronutrient fertilizer treatment showed contrasting effects on nutrient availability and composition.
5. Conclusions
The study emphasizes the substantial impact of inorganic mineral fertilizers and agrochemicals on soil properties, maize yield, and quality, including soil and plant micronutrient concentrations. Compost-treated soils exhibited the highest mesophilic bacterial and fungal count with higher Fe and Zn micronutrient concentrations. The glyphosate-treated soil showed the lowest micronutrient levels. Additionally, the presence of inorganic fertilizers and glyphosate resulted in a decrease in key soil parameters such as pH, electrical conductivity, and organic matter. Maize from the agrochemical-treated pots generally displayed low crude protein, total fat, total carbohydrates, gross energy, and total fiber levels.
These findings suggest that soil fertility in the western Ethiopia region, where small-scale farmers farm, may be compromised due to the extensive use of inorganic fertilizers and agrochemicals. To address this issue, it is recommended to implement sustainable agricultural practices that reduce reliance on agrochemical inputs, promote soil conservation measures, and adopt soil fertility enhancement techniques such as organic farming methods. Additionally, educating farmers on proper agrochemical usage through farmer education programs can help mitigate the adverse effects on soil health and crop productivity in the long term.
Author Contributions
Methodology, T.A.S. and M.K.P.; Data curation, T.A.S.; Writing—original draft, T.N.A.; Writing—review & editing, T.N.A.; Visualization, S.M., T.A.S., M.K.P., E.J. and G.T.T.; Supervision, M.K.P., E.J. and G.T.T.; Project administration, G.T.T.; Funding acquisition, G.T.T. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for writing the study results was provided by the Norwegian Directorate for Higher Education and Skills, NORPART/WASH4ONEHEALTH/Grant no.10070/2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Edmeades, G.O.; Trevisan, W.L.; Prasanna, B.M.; Campos, H. Tropical maize (Zea mays L.). In Genetic Improvement of Tropical Crops; Springer: Berlin/Heidelberg, Germany, 2017; pp. 57–109. [Google Scholar]
- Nadarajah, K.; Abdul Rahman, N.S.N. The Microbial Connection to Sustainable Agriculture. Plants 2023, 12, 2307. [Google Scholar] [CrossRef] [PubMed]
- Tefera, M.L.; Carletti, A.; Altea, L.; Rizzu, M.; Migheli, Q.; Seddaiu, G. Land degradation and the upper hand of sustainable agricultural intensification in sub-Saharan Africa–A systematic review. J. Agric. Rural. Dev. Trop. Subtrop. 2024, 125, 63–68. [Google Scholar]
- Idowu, J.; Ghimire, R.; Flynn, R.; Ganguli, A. Soil Health: Importance, Assessment, and Management; College of Agricultural, Consumer and Environmental Sciences: Urbana, IL, USA, 2019. [Google Scholar]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of agrochemicals on soil microbiota and management: A review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Abera, Y.; Kassa, S. Status of soil micronutrients in Ethiopian soils: A review. J. Environ. Earth Sci. 2017, 7, 85–90. [Google Scholar]
- Afata, T.N.; Mekonen, S.; Shekelifa, M.; Tucho, G.T. Prevalence of pesticide use and occupational exposure among small-scale farmers in Western Ethiopia. Environ. Health Insights 2022, 16, 11786302211072950. [Google Scholar] [CrossRef] [PubMed]
- Pogrzeba, M.; Rusinowski, S.; Sitko, K.; Krzyżak, J.; Skalska, A.; Małkowski, E.; Ciszek, D.; Werle, S.; McCalmont, J.P.; Mos, M.; et al. Relationships between soil parameters and physiological status of Miscanthus x giganteus cultivated on soil contaminated with trace elements under NPK fertilisation vs. microbial inoculation. Environ. Pollut. 2017, 225, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.P.; Binod, K.B.; Prajna, P.G.; Sonali, S.; Prajna, C.; Sanjeeta, R.; Kasturi, P.; Sourav, B. (Eds.) Micronutrient Deficiency; Newredmars Education Pvt Ltd.: Bhubaneswar, India, 2023. [Google Scholar]
- Afata, T.N.; Mekonen, S.; Tucho, G.T. Serum concentration of zinc, copper, iron, and its associated factors among pregnant women of small-scale farming in western Ethiopia. Sci. Rep. 2023, 13, 4197. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Akash, S.; Jony, M.H.; Alam, M.N.; Nowrin, F.T.; Rahman, M.M.; Rauf, A.; Thiruvengadam, M. Exploring the potential function of trace elements in human health: A therapeutic perspective. Mol. Cell. Biochem. 2023, 478, 2141–2171. [Google Scholar] [CrossRef] [PubMed]
- Jatav, H.S.; Sharma, L.D.; Sadhukhan, R.; Singh, S.K.; Singh, S.; Rajput, V.D.; Parihar, M.; Jatav, S.S.; Jinger, D.; Kumar, S.; et al. An overview of micronutrients: Prospects and implication in crop production. In Plant Micronutrients: Deficiency and Toxicity Management; Springer: Cham, Switzerland, 2020; pp. 1–30. [Google Scholar]
- Kumar, D.; Patel, K.P.; Ramani, V.P.; Shukla, A.K.; Meena, R.S. Management of micronutrients in soil for the nutritional security. In Nutrient Dynamics for Sustainable Crop Production; Springer: Singapore, 2020; pp. 103–134. [Google Scholar]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Ul Hussan, M.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Losak, T.; Hlusek, J.; Martinec, J.; Jandak, J.; Szostkova, M.; Filipcik, R.; Manasek, J.; Prokes, K.; Peterka, J.; Varga, L.; et al. Nitrogen fertilization does not affect micronutrient uptake in grain maize (Zea mays L.). Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 543–550. [Google Scholar]
- Devi, K.D.; Beena, S.; Abraham, C. Effect of 2, 4-D residues on soil microflora. J. Trop. Agric. 2008, 46, 76–78. [Google Scholar]
- Paul, N.; Sur, P.; Das, D.K.; Mukherjee, D. Effect of pesticides on available cationic micronutrients along with viable bacteria and fungi in soil. Afr. J. Microbiol. Res. 2013, 7, 2764–2769. [Google Scholar] [CrossRef][Green Version]
- Kepler, R.M.; Schmidt, D.J.E.; Yarwood, S.A.; Cavigelli, M.A.; Reddy, K.N.; Duke, S.; Bradley, C.; Williams, M.M.; Buyer, J.S.; Maul, J.E. Soil Microbial Communities in Diverse Agroecosystems Exposed to the Herbicide Glyphosate. Appl. Environ. Microbiol. 2020, 86, e01744-19. [Google Scholar] [CrossRef]
- Dill, G.M.; Sammons, R.D.; Feng, P.C.C.; Kohn, F.; Kretzmer, K.; Mehrsheikh, A.; Bleeke, M.; Honegger, J.L.; Farmer, D.; Wright, D.; et al. Glyphosate: Discovery, development, applications, and properties. Glyphosate Resist. Crops Weeds Hist. Dev. Manag. 2010, 1, 344. [Google Scholar]
- Imfeld, G.; Vuilleumier, S. Measuring the effects of pesticides on bacterial communities in soil: A critical review. Eur. J. Soil Biol. 2011, 49, 22–30. [Google Scholar] [CrossRef]
- Davet, P. Microbial Ecology of Soil and Plant Growth; CRC Press: London, UK, 2004. [Google Scholar]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Chapter 2—Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 25–54. [Google Scholar]
- Prashar, P.; Shah, S. Impact of fertilizers and pesticides on soil microflora in agriculture. Sustain. Agric. Rev. 2016, 19, 331–361. [Google Scholar]
- Lane, M.; Lorenz, N.; Saxena, J.; Ramsier, C.; Dick, R.P. Microbial activity, community structure and potassium dynamics in rhizosphere soil of soybean plants treated with glyphosate. Pedobiologia 2012, 55, 153–159. [Google Scholar] [CrossRef]
- Luo, P.; Han, X.; Wang, Y.; Han, M.; Shi, H.; Liu, N.; Bai, H. Influence of long-term fertilization on soil microbial biomass, dehydrogenase activity, and bacterial and fungal community structure in a brown soil of northeast China. Ann. Microbiol. 2014, 65, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Zhou, Q.; Zhang, T. Effects of continuous cropping Jiashi muskmelon on rhizosphere microbial community. Front. Microbiol. 2023, 13, 1086334. [Google Scholar] [CrossRef]
- Girma, K. Minerals and trace elements in the soil-plant-animal continuum in Ethiopia: A review. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 11219–11235. [Google Scholar]
- Laekemariam, F.; Tsehai, K.K.; Mamo, T.; Gebrekidan, H. Soil–plant nutrient status and their relations in maize-growing fields of Wolaita Zone, southern Ethiopia. Commun. Soil Sci. Plant Anal. 2016, 47, 1343–1356. [Google Scholar] [CrossRef]
- Laekemariam, F.; Kibret, K. Explaining Soil Fertility Heterogeneity in Smallholder Farms of Southern Ethiopia. Appl. Environ. Soil Sci. 2020, 2020, 6161059. [Google Scholar] [CrossRef]
- PSA. Summary and Statistical Report of the 2007 Population and Housing Census. Population Size by Age and Sex. 2008. Available online: https://www.ethiopianreview.com/pdf/001/Cen2007 (accessed on 17 May 2024).
- EDHS. Central Statistical Agency (CSA) [Ethiopia] and ICF. In Ethiopia Demographic and Health Survey 2016; CSA: Addis Ababa, Ethiopia; ICF: Rockville, MD, USA, 2016; pp. 1–551. [Google Scholar]
- Gonfa, R.; Gadisa, T.; Habitamu, T. The diversity, abundance and habitat association of medium and large-sized mammals of Dati Wolel National Park, Western Ethiopia. Int. J. Biodivers. Conserv. 2015, 7, 112–118. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- FAO. Food energy-methods of analysis and conversion factors. In Report of a Technical Workshop; FAO: Rome, Italy, 2003. [Google Scholar]
- Aulakh, C.S.; Sharma, S.; Thakur, M.; Kaur, P. A review of the influences of organic farming on soil quality, crop productivity and produce quality. J. Plant Nutr. 2022, 45, 1884–1905. [Google Scholar] [CrossRef]
- Somenahally, A.C.; Hollister, E.B.; Loeppert, R.H.; Yan, W.; Gentry, T.J. Microbial communities in rice rhizosphere altered by intermittent and continuous flooding in fields with long-term arsenic application. Soil Biol. Biochem. 2011, 43, 1220–1228. [Google Scholar] [CrossRef]
- Onet, A.; Dincă, L.C.; Grenni, P.; Laslo, V.; Teusdea, A.C.; Vasile, D.L.; Enescu, R.E.; Crisan, V.E. Biological indicators for evaluating soil quality improvement in a soil degraded by erosion processes. J. Soils Sediments 2019, 19, 2393–2404. [Google Scholar] [CrossRef]
- Ashworth, A.; DeBruyn, J.; Allen, F.; Radosevich, M.; Owens, P. Microbial community structure is affected by cropping sequences and poultry litter under long-term no-tillage. Soil Biol. Biochem. 2017, 114, 210–219. [Google Scholar] [CrossRef]
- Sannino, F.; Gianfreda, L. Pesticide influence on soil enzymatic activities. Chemosphere 2001, 45, 417–425. [Google Scholar] [CrossRef]
- Govedarica, M.M.; Jarak, M.N.; Milošević, N.A.; Đurić, S.; Đorđević, S.; Najdenovska, O.; Milošev, D.S. Herbicides and microbiological activity in soil under the corn. Letop. Naučnih Rad. Poljopr. Fak. 2002, 26, 24–31. [Google Scholar]
- Wolmarans, K. The Effect of Glyphosate and Glyphosate-Resistant Maize and Soyabeans on Soil Micro-Organisms and the Incedence of Disease. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2013. [Google Scholar]
- Srinivasulu, M.; Ortiz, D.R. Effect of Pesticides on Bacterial and Fungal Populations in Ecuadorian Tomato Cultivated Soils. Environ. Process. 2017, 4, 93–105. [Google Scholar] [CrossRef]
- Baboo, M.; Pasayat, M.; Samal, A.; Kujur, M.; Maharana, J.K.; Patel, A.K. Effect of four herbicides on soil organic carbon, microbial biomass-c, enzyme activity and microbial populations in agricultural soil. Int. J. Res. Environ. Sci. 2013, 3, 100–112. [Google Scholar]
- Chen, F.; Dixon, R.A. Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 2007, 25, 759–761. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef]
- Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A. Chapter 5 Impact of Pesticides on Soil Microbial Diversity, Enzymes, and Biochemical Reactions. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2009; pp. 159–200. [Google Scholar]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Nardi, S.; Concheri, G.; Pizzeghello, D.; Sturaro, A.; Rella, R.; Parvoli, G. Soil organic matter mobilization by root exudates. Chemosphere 2000, 41, 653–658. [Google Scholar] [CrossRef]
- Hassink, J. The capacity of soils to preserve organic C and N by their association with clay and silt particles. Plant Soil 1997, 191, 77–87. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Brevik, E.C.; Abowaly, M.; Ali, R.; Moghanm, F.S.; Gharib, M.S.; Mansour, H.; Fawzy, Z.F.; Prokisch, J. Soil Degradation under a Changing Climate: Management from Traditional to Nano-Approaches. Egypt. J. Soil Sci. 2024, 64, 287–298. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 961–1010. [Google Scholar]
- El Bey, N.; Maazoun, A.M.; Nahdi, O.; Krima, N.B.; Aounallah, M.K.; Mahdy, H.A.; Abdelmawgoud, A.M.; Fawzy, Z.F.; Ibrahim, H.A.; Kumar, M.; et al. Department of Horticulture & Postharvest Technology, Institute of Agriculture, Visva-Bharati, Sriniketan-731236, West Bengal. Corresponding e-mail: Debprld@ yahoo. com. J. Appl. Hortic. 2024, 26, 1. [Google Scholar]
- Lane, M.; Lorenz, N.; Saxena, J.; Ramsier, C.; Dick, R.P. The effect of glyphosate on soil microbial activity, microbial community structure, and soil potassium. Pedobiologia 2012, 55, 335–342. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Benjamin, J.G. Impacts of soil organic carbon on soil physical behavior. Quantifying Model. Soil Struct. Dyn. 2013, 3, 11–40. [Google Scholar]
- Baweja, P.; Kumar, S.; Kumar, G. Fertilizers and pesticides: Their impact on soil health and environment. Soil Health 2020, 59, 265–285. [Google Scholar]
- Sebiomo, A.; Ogundero, V.; Bankole, S. Effect of four herbicides on microbial population, soil organic matter and dehydrogenase activity. Afr. J. Biotechnol. 2011, 10, 770–778. [Google Scholar]
- Shu, X.; He, J.; Zhou, Z.; Xia, L.; Hu, Y.; Zhang, Y.; Zhang, Y.; Luo, Y.; Chu, H.; Liu, W.; et al. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef] [PubMed]
- Laetitia, C.; van Beek, C.; Elias, E.; Selassie, Y.G.; Gebresamuel, G.; Tsegaye, A.; Hundessa, F.; Tolla, M.; Munaye, M.; Yemane, G.; et al. Soil organic matter depletion as a major threat to agricultural intensification in the highlands of Ethiopia. Ethiop. J. Sci. Technol. 2018, 11, 271–285. [Google Scholar]
- Bàrberi, P. Weed management in organic agriculture: Are we addressing the right issues? Weed Res. 2002, 42, 177–193. [Google Scholar] [CrossRef]
- Franz, J.E.; Mao, M.K.; Sikorski, J.A. Glyphosate: A Unique Global Herbicide; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Khan, Z.I.; Hussain, A.; Ashraf, M.; McDowell, L.R. Mineral status of soils and forages in Southwestern Punjab-Pakistan: Micro-minerals. Asian-Australas. J. Anim. Sci. 2006, 19, 1139–1147. [Google Scholar] [CrossRef]
- Bala, B.K. Drying and Storage of Cereal Grains; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Ullah, I.; Ali, M.; Farooqi, A. Chemical and nutritional properties of some maize (Zea mays L.) varieties grown in NWFP, Pakistan. Pak. J. Nutr. 2010, 9, 1113–1117. [Google Scholar] [CrossRef]
- Ogunyemi, A.M.; Otegbayo, B.O.; Fagbenro, J.A. Effects of NPK and biochar fertilized soil on the proximate composition and mineral evaluation of maize flour. Food Sci. Nutr. 2018, 6, 2308–2313. [Google Scholar] [CrossRef]
- Tizhe, T.D.; Alonge, S.O.; Iortsuun, D.N.; Adekpe, D.I.; Batta, K. Evaluation of the effect of nicosulfuron at different times of application on the chemical component of maize (Zea mays). Nusant. Biosci. 2022, 14, 122–127. [Google Scholar] [CrossRef]
- Sagbo, F.S.; Aïssi, M.V.; Hounkpatin, W.A.; Houedo, C.; Dansi, A.; Soumanou, M.M. Physicochemical and pasting properties of some local and improved maize varieties cultivated in Benin. Int. J. Biol. Chem. Sci. 2017, 11, 1753–1765. [Google Scholar] [CrossRef][Green Version]
- Adugna, G. A review on impact of compost on soil properties, water use and crop productivity. Acad. Res. J. Agric. Sci. Res. 2016, 4, 93–104. [Google Scholar]
- Ray, K.; Banerjee, H.; Dutta, S.; Sarkar, S.; Murrell, T.S.; Singh, V.K.; Majumdar, K. Macronutrient management effects on nutrient accumulation, partitioning, remobilization, and yield of hybrid maize cultivars. Front. Plant Sci. 2020, 11, 535999. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Enyisi, I.S.; Umoh, V.J.; Whong, C.M.Z.; Abdullahi, I.O.; Alabi, O. Chemical and nutritional values of maize and maize products obtained from selected markets in Kaduna. J. Pharm. Allied Sci. 2014, 11, 2106–2113. [Google Scholar]
- Adegbite, J.A.; Lajide, L.; Aladesanwa, R.D.; Aiyesanmi, A.F.; Abiodun, O.A.; Adepeju, A.B.; Oladapo, S.A. Effect of herbicide application on residue content and nutritional composition of maize from a pilot maize farm. Am. J. Agric. Sci. 2016, 3, 35–39. [Google Scholar]
- Ndukwe, O.K.; Edeoga, H.; Omosun, G. Varietal differences in some nutritional composition of ten maize (Zea mays L.) varieties grown in Nigeria. Int. J. Acad. Res. Reflect. 2015, 3, 1–11. [Google Scholar]
- Mohajan, H.K. Food Insecurity and Malnutrition of Africa: A Combined Attempt Can Reduce Them. J. Econ. Dev. Environ. People 2022, 11, 24–34. [Google Scholar] [CrossRef]
- Mäder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil fertility and biodiversity in organic farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef]
- Fahrurrozi, F.; Muktamar, Z.; Dwatmadji, D.; Setyowati, N.; Sudjatmiko, S.; Chozin, M. Growth and yield responses of three sweet corn (Zea mays L. var. Saccharata) varieties to local-based liquid organic fertilizer. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 6, 319–323. [Google Scholar]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 2011, 32 (Suppl. 1), S31–S40. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, L.; Sun, D.; Luo, L.; Banson, K.E. The Impact of Climate Change on Yield Potential of Maize across China. Int. J. Plant Prod. 2017, 11, 47–64. [Google Scholar]
- Kabir, S.; Das, A.; Rahman, M.; Singh, M.; Morshed, M.; Marma, A. Effect of genotype on proximate composition and biological yield of maize (Zea mays L.). Arch. Agric. Environ. Sci. 2019, 4, 185–189. [Google Scholar] [CrossRef]
- Cumming, G.S.; Buerkert, A.; Hoffmann, E.M.; Schlecht, E.; Von Cramon-Taubadel, S.; Tscharntke, T. Implications of agricultural transitions and urbanization for ecosystem services. Nature 2014, 515, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, K.; Dey, P.; Tewatia, R. Current nutrient management approaches. Indian J. Fertil. 2014, 10, 14–27. [Google Scholar]
- Abadía, J.; Vázquez, S.; Rellán-Álvarez, R.; El-Jendoubi, H.; Abadía, A.; Álvarez-Fernández, A.; López-Millán, A.F. Towards a knowledge-based correction of iron chlorosis. Plant Physiol. Biochem. 2011, 49, 471–482. [Google Scholar] [CrossRef]
- Mukherjee, A.B.; Kabata Pendias, A. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).