Abstract
Cereals are a staple food in many regions of the world and are essential for global food security. Lead is one of the most significant environmental stressors, impacting plants throughout their life cycle and causing substantial damage to plant growth and development. It disrupts intracellular processes, thereby reducing plant productivity. The aim of this study was to determine the effect of exogenously applied vitamin PP (100 µM) (nicotinamide) on the morphological, physiological, and biochemical parameters of spring barley var. Eunova under lead stress (1 mM Pb(NO3)2) and to determine the most effective method of applying this vitamin in a pot experiment. Vitamin PP was applied exogenously through three different methods: seed soaking, foliar application, and soil irrigation. The application of 1 mM Pb(NO3)2 resulted in decreased root (from 13.9% to 19.9%) and shoot length (from 16.2% to 24.8%) and increased catalase (CAT) activity from 45% to 106%, and peroxidase (POX) activity from 39% to 46% compared to the control. Lead stress led to an increase in proline (Pro) content from 30 to 63% and comparatively in malondialdehyde (MDA) content (rising from 61% to 79.4%), as well as elevated assimilatory pigment content (by 35%) in barley grown in the pot experiment. Exogenous vitamin PP significantly and positively influenced the improvement of the measured morphological, biochemical, and physiological parameters, reducing the toxicity of lead salts. It was shown that the most effective method of vitamin PP application was achieved through foliar spraying and irrigation.
1. Introduction
Global agriculture faces numerous challenges, notably the need to increase food production by more than 60% to meet the needs of a growing population [1,2]. To meet this growing demand, agriculture will need millions of additional hectares of arable land [1]. However, since a significant proportion of arable land is already contaminated, utilizing such land will play a significant role in modern agriculture. Advances and innovations in agriculture have the potential to prevent a global shortage of arable land [3].
Reduced agricultural productivity stems from various environmental factors, with heavy metals playing a significant role [4,5,6]. Heavy metal contamination poses a serious problem due to their toxicity and persistence in the environment [7,8], affecting both developed and developing countries [9].
Lead (Pb) ranks as the second most toxic heavy metal in the environment [10]. It can enter the environment through various sources, stemming from both natural processes and human activities, such as mining and metallurgy, industrial emissions, fossil fuel combustion, pesticide use, improper waste disposal in landfills, the presence of lead-based paints, and the use of lead in water pipes or sewerage systems [11,12]. These activities contribute to the distribution of lead across different environmental compartments, like air, soil, water, and sediments. Plants readily absorb this element from the soil, accumulating it in various organs [11,13]. Exposure to lead inhibits plant growth, biomass, and development, and has adverse effects on physiological and metabolic processes [14]. Subsequently, oxidative stress ensues in plants due to the generation of reactive oxygen species (ROS), such as hydroxyl radicals (OH−), hydrogen peroxide (H2O2), singlet oxygen (1O2), and superoxide anion radicals (O2−). ROS overproduction disrupts cellular redox homeostasis, leading to structural damage in plants [15,16]. All ROS can cause damage to assimilatory pigments, cell membranes, carbohydrates, DNA, proteins, disruption of oxidative phosphorylation, and damage to the mitochondrial respiratory chain [11,16].
Plants have evolved their own cellular defense mechanisms to avoid, tolerate, detoxify, or eliminate heavy metals [17,18]. These mechanisms involve changes at the molecular, biochemical, and physiological levels [18,19]. They encompass chelation by metallothionein or phytochelatin, compartmentalization in cell walls and intracellular vesicles, lignification, inhibition of direct uptake of heavy metals from the soil, and the neutralization mechanism for ROS [17]. This latter mechanism, incorporating both nonenzymatic and enzymatic elements, converts ROS into less toxic compounds [15,16,17].
Despite the array of defense mechanisms possessed by plants, their resilience is often overwhelmed by excessive levels of heavy metals. To support plant tolerance or alleviate heavy metal-induced stress at the physiological, biochemical, and molecular levels within the cell, various exogenous substances have been tested in numerous scientific studies [4,19,20,21]. According to Feng et al. (2023), exogenous substances are defined as various compounds necessary for plant growth and development. These substances are introduced from external sources and act to increase plant tolerance. The primary functions of exogenous substances are associated with regulatory dimensions, specifically, enhancing the effectiveness of the antioxidant system, inducing the production of osmoregulatory compounds, improving the efficiency of the photochemical pathway, redirecting the accumulation and movement of heavy metals, modulating endogenous hormone levels, and controlling gene expression [22].
In recent years, numerous scientific studies have explored the use of exogenous substances to mitigate the toxicity of various abiotic stresses, including those induced by heavy metals. Effective alleviation of lead stress has been achieved through the application of substances such as brassinosteroids [23,24,25,26], auxins, cyto-kinins [27], salicylic acid (SA) [28,29] jasmonic acid (JA) [30], various organic chelates [31,32], glutathione (GSH) [33], and vitamins [34,35,36]. Various methods of introducing exogenous substances into plants exposed to heavy metals are documented in the literature. For example, these substances can be applied in hydroponic systems [31,35,37], on Petri dishes [36], in vitro culture on MS media [35,38], or in soil [29,33]. They can also be applied as foliar sprays [29,37,39] or seed imbibition treatments [39].
Niacinamide, also known as niacin or vitamin PP, stands as one of the major nonenzymatic antioxidants. This compound acts as a precursor to the coenzymes NADH and NADPH, crucial in numerous enzymatic oxidation–reduction reactions in plant cells. These coenzymes are vital for energy metabolism, photosynthesis, and cellular respiration [40,41]. Niacinamide plays a role in the biosynthesis of secondary metabolites [40,42], contributing to plant defense, signaling, and adaptation to environmental challenges [43]. Therefore, vitamin PP is crucial for plant growth, development, and adaptation to changing environmental conditions, highlighting its importance.
Barley, scientifically known as Hordeum vulgare L., stands as one of the oldest cultivated crops. It is primarily grown for human consumption, as an ingredient in animal feed, and for the production of alcoholic beverages. Due to its rapid growth, low climatic requirements, adaptability to various environments, and clear response to stress factors, it serves as a valuable subject for abiotic stress research [44,45].
The aim of the research was to assess the impact of exogenously applied vitamin PP (100 µM) on the morphological, physiological, and biochemical parameters of spring barley (H. vulgare L.) var. Eunova under lead stress (1 mM Pb(NO3)2) and to establish the most effective method of applying this vitamin in the pot experiment.
2. Materials and Methods
2.1. Experiment Site and Conditions
The 2-year pot experiment took place during the spring in the growth chamber and laboratory of Microbiology and Environmental Biochemistry, West Pomeranian University of Technology in Szczecin (latitude 53°26′17″ N, longitude 14°32′32″ E).
The soil used for the study was sourced from the arable–humus layer (Ap, 0–30 cm) in Ostoi, near Szczecin. This soil underwent sieving through a 2 mm mesh sieve and was divided into eight portions. Four portions were subjected to a solution of 1 mM Pb(NO3)2 (207.0 mg Pb+2), while the remaining portions were treated with water to attain 60% of the maximum water-holding capacity. The prepared soil was then placed into 3.50 kg pots.
The material for the study consisted of seeds of spring barley var. Eunova, acquired a class (C/1) certified seed from a specialized shop. Before sowing, the seeds underwent three rinses of 20 min each in sterile distilled water. Subsequently, they were immersed in a 7% sodium hypochlorite solution for 10 min, followed by rinsing in sterile distilled water for 15 min. After this initial sterilization process, some seeds were immersed in a solution of 100 µM Vit PP in the form of nicotinamide, while the remaining seeds were soaked in water for 24 h. An equal number of seeds (10 barley seeds per pot) were sown in each pot at a depth of 3.0 cm.
The pots received natural watering every 10 days (100 cm3 of distilled water) until the first leaves appeared. Thereafter, the barley var. Eunova plants were watered every 10 days (100 cm3 of distilled water or water supplemented with 100 µM Vit PP). Additionally, some plants were sprayed with a solution containing 100 µM Vit PP. Each plant in the pot was thoroughly sprayed with the solution until it dripped from the leaves into the pot, with 10 cm3 of solution per plant, amounting to a total of 100 cm3 per pot. All solutions used for spraying and watering included Tween 20 as a dispersing agent.
The experiment consisted of 8 combinations carried out in 3 replicates and detailed information on the compounds used in this study is given below: (1) control, (2) 100 µM Vit PP soaking seeds, (3) 100 µM Vit PP foliar application, (4) 100 µM Vit PP soil application, (5) 1 mM Pb(NO3)2, (6) 1 mM Pb(NO3)2 + 100 µM Vit PP soaking seeds, (7) 1 mM Pb(NO3)2 + 100 µM Vit PP foliar application, (8) 1 mM Pb(NO3)2 + 100 µM Vit PP soil application.
During the growing season, the growth and development of the plants were observed. biochemical parameters (catalase and peroxidase activity, malondialdehyde and proline content) and physiological parameters (total chlorophyll and carotenoid content) were measured at four stages related to the physiological development of the plants: tillering, stem elongation, heading and flowering. In addition, morphological measurements of the plants (root length, stem length, spike length, fresh root weight, fresh stem weight, fresh spike weight) were made after the flowering phase (Figure 1). The experiment was conducted in a complete randomization system. All measurements were performed in three replicates.
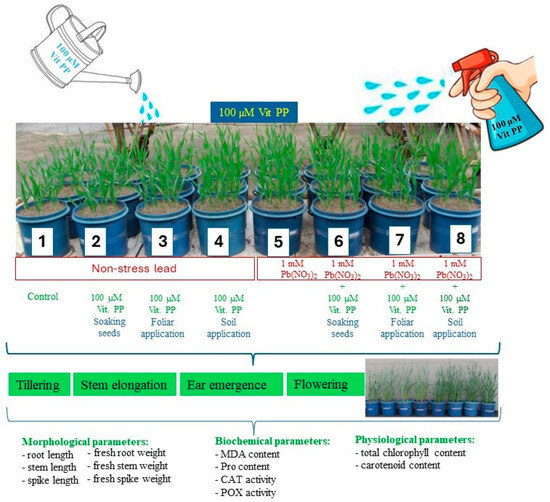
Figure 1.
Schematic of the pot experiment.
2.2. Determination of the Morphological Parameters
After the plants had flowered, they were harvested. The spike, stem, and roots of all plants in each replicate were measured in centimeters using a ruler, and the measurements were recorded. The spike, roots, and stem were separated to measure their mass, and the roots were thoroughly cleaned of soil before weighing them on a digital scale.
2.3. Determination of Biochemical and Physiological Parameters
To assess the activity of antioxidant enzymes and determine the levels of Pro and MDA, fresh plant tissue from fully developed apical leaves was collected and homogenized with an appropriate chilled extraction solution. The extraction solutions used were 0.0067 M phosphate buffer at pH 7.0 for CAT, 0.05 M acetate buffer at pH 5.6 for POX, 3% sulfosalicylic acid for Pro, and 0.1% trichloroacetic acid for MDA. The resulting homogenates were then centrifuged at 15,000× g at 40 °C for 15–25 min. The supernatants obtained were used for each assay.
Catalase activity (CAT [EC 1.11.1.6]) was determined using a spectrophotometer, (Merck Nova 400, Darmstadt, Germany) following Lück’s method [46]. The reaction mixture consisted of 1/15 M phosphate buffer (pH 7.0), 1.25 × 10−2 M H2O2, and the centrifuged enzyme extract. The assay involved measuring the decrease in ultraviolet light absorption over 60 s as H2O2 decomposed by CAT at a wavelength of λ = 240 nm. Enzyme activity was expressed as μM H2O2·g−1 FW of plant tissue·min−1.
Peroxidase activity (POX [EC 1.11.1.7]) activity was measured following the method of Chance and Maehly [47] using a spectrophotometer, (Merck Nova 400, Darmstadt, Germany). The method involved the colorimetric determination of purpurogallin formation during the oxidation of pyrogallol (0.02 M) in the presence of H2O2 (0.06 M) at a wavelength of λ = 430 nm over 4 min. Peroxidase activity was expressed in μM purpurogallol·g−1 FW of plant tissue·min−1.
The content of free proline was determined using the ninhydrin reaction per Bates method [48]. The reaction mixture consisted of extracted plant material in 3% sulfosalicylic acid, ice-cold acetic acid, and acidic ninhydrin. After boiling the mixture in a water bath at 100 °C for 60 min, the reaction was stopped by cooling the samples on ice. The samples were then extracted with toluene, and the absorbance of the colored chromophore against toluene was measured on a spectrophotometer, (Merck Nova 400, Darmstadt, Germany) at a wavelength of λ = 520 nm. The concentration of proline was read from the standard curve prepared for L-proline and expressed in μmol·g−1 FW of plant tissue.
Malondialdehyde levels were determined through reaction with thio-barbituric acid following Sudhakar et al. [49] method (2001). Homogenates containing 0.5% TBA dissolved in 20% TCA were boiled at 100 °C for 10 min and rapidly cooled on ice. After centrifugation (10,000× g, 10 min), the absorbance of the supernatant was measured using a Merck Nova 400 spectrophotometer at wavelengths of λ = 532 nm and λ = 600 nm. The concentration of MDA was calculated using the millimolar absorption coefficient ε = 155 mM−1 cm−1. The result was adjusted by subtracting the absorbance of the sample at 600 nm, which accounts for nonspecific reaction products with TBA. Malondialdehyde content was expressed as nmol MDA·g−1 FW of plant tissue.
Assimilatory pigments (chlorophylls and carotenoids) were extracted from plant material using chilled 80% acetone. Chlorophyll content was determined by the method of Arnon et al. [50] as modified by Lichtenthaler and Wellburn [51], while carotenoid content was determined by the method of Hager and Meyer-Berthenrath [52]. To extract assimilatory pigments from leaves, green samples weighing approximately 0.05 g were ground in a mortar with 10 cm3 of 80% acetone. The homogenates were then centrifuged at 1500 rpm for 10 min.
2.4. Statistical Analysis of Results
Statistical analyses were performed using Statistica 13 (TIBCO Software Inc., Krakow, Poland). The results were analyzed using descriptive statistics, including mean and standard deviation. Means were compared using Tukey’s HSD test at a significance level of p < 0.05.
3. Results
3.1. Plant Growth and Biomass
The statistical analysis of barley’s morphological parameters, conducted after the flowering phase, showed that in both the first and second seasons, as well as in the combined data from both seasons, there were generally no significant differences in the measured parameters among the tested combinations (Figure 2 and Figure 3). However, significant differences were observed in root length and stem length between the tested combinations. Specifically, the addition of lead resulted in a 13.9% decrease in root length in the first season and a 19.9% decrease in the second season, with stem length decreasing by 24.8% and 16.2%, respectively, compared to the control. Moreover, the application of exogenous vitamin PP had a significant and positive impact on root and stem length, mitigating the toxicity of lead salts. The most effective application of vitamin PP was through foliar spraying, demonstrating its beneficial effects on plant growth and lead stress reduction.
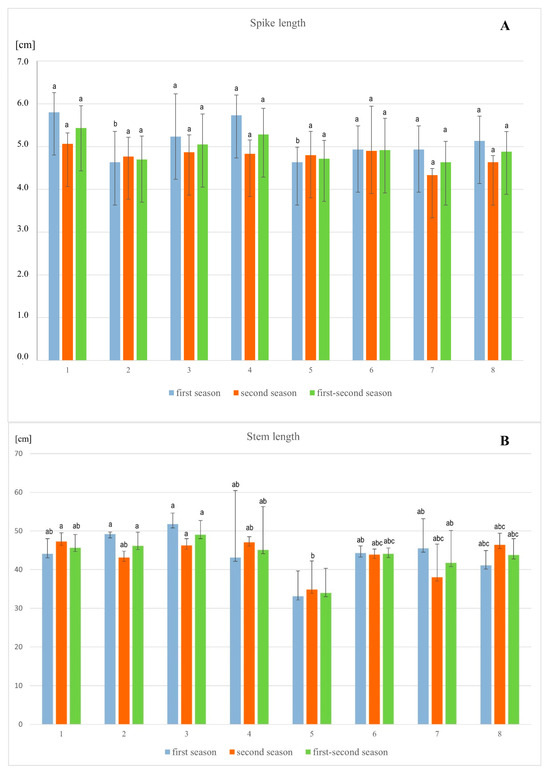
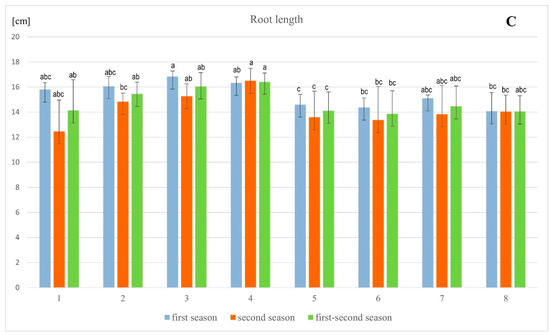
Figure 2.
Influence of different methods of vitamin PP application on the length (A–C) of spring barley var. Eunova growing in soil with 1 mM Pb(NO3)2; (A)–spike, (B)–stem, (C)–root; (1) control, (2) 100 µM Vit PP soaking seeds, (3) 100 µM Vit PP foliar application, (4) 100 µM Vit PP soil application, (5) 1 mM Pb(NO3)2, (6) 1 mM Pb(NO3)2 + 100 µM Vit PP soaking seeds, (7) 1 mM Pb(NO3)2 + 100 µM Vit PP foliar application, (8) 1 mM Pb(NO3)2 + 100 µM Vit PP soil application; a–c—homogeneous groups.
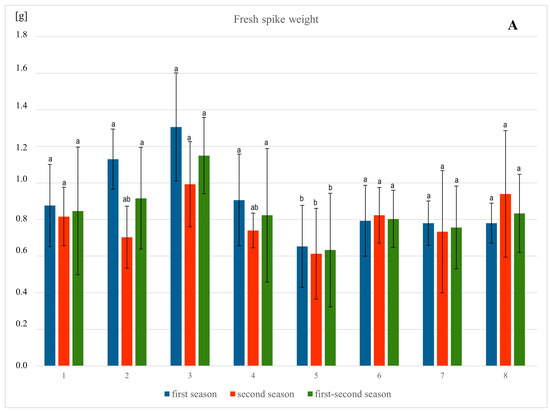
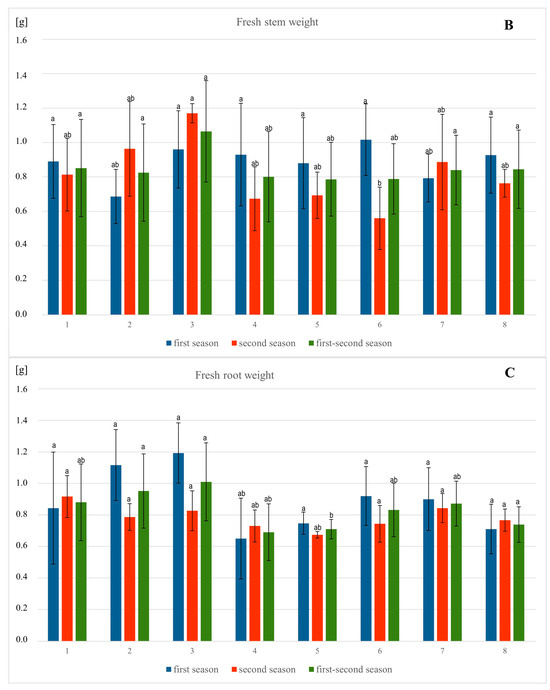
Figure 3.
Influence of different methods of vitamin PP application on the fresh weight (A–C) of spring barley var. Eunova growing in soil with 1 mM Pb(NO3)2; A–spike, B–stem, C–root; (1) control, (2) 100 µM Vit PP soaking seeds, (3) 100 µM Vit PP foliar application, (4) 100 µM Vit PP soil application, (5) 1 mM Pb(NO3)2, (6) 1 mM Pb(NO3)2 + 100 µM Vit PP soaking seeds, (7) 1 mM Pb(NO3)2 + 100 µM Vit PP foliar application, (8) 1 mM Pb(NO3)2 + 100 µM Vit PP soil application; a–b—homogeneous groups.
3.2. Antioxidant Enzyme Activities, Malondialdehyde, and Proline Contents
It was observed that the activity of enzymes involved in antioxidant defense significantly increased (p > 0.05) in the presence of lead salts. CAT activity increased from 45% to 106% compared to the control, while POX activity showed a similar level of increase (from 39% to 46%) during the developmental stages of barley studied (across both years). The application of vitamin PP in various forms slightly increased the enzyme activity compared to the control, but these differences were described as insignificant. However, applying this vitamin to plants on lead-contaminated soil led to decreased enzyme levels compared to plants exposed to lead alone. The most significant decrease in enzyme activity was observed with foliar spraying and irrigation using vitamin PP, in both the first and second years of the study (Table 1).
Lead salts contributed to an increase in Pro content in both years of the experiment. However, significant differences were observed only at the heading and flowering stages of barley, with no notable differences in Pro content found at earlier stages of plant development. The application of vitamin PP resulted in a reduction of Pro in barley var. Eunova plants are compared to plants growing solely on lead. The most significant effect was observed during spraying and irrigation (Table 2).
Lead stress contributed significantly (p > 0.05) to an increase in MDA content compared to the control (rising from 61% to 79.4%) during the developmental stages studied in barley var. Eunova (across both years). The exogenous application of vitamin PP, particularly through watering and in most cases, spraying, significantly (p > 0.05) reduced the MDA content compared to plants grown with lead alone (Table 2).
3.3. Total Chlorophyll and Carotenoid Contents
The lead salts used in the experiment significantly (p > 0.05) reduced the content of assimilatory pigments, including total chlorophyll and carotenoids (Table 3). As the plants grew, there was a gradual decrease in total chlorophyll content (ranging from 20.3% to 35.3% lower than the control), while carotenoids decreased by 22.4% to 28.7% (across both years). Vitamin PP, applied in all forms, increased the content of both total chlorophyll and carotenoids compared to plants growing with lead alone, but the increase was significant only when applied through irrigation.

Table 1.
Influence of different methods of application of vitamin PP on the enzyme activity of barley growing in soil with lead.
Table 1.
Influence of different methods of application of vitamin PP on the enzyme activity of barley growing in soil with lead.
| Catalase [μmol∙H2O2∙g−1 FW∙min−1] (% Control) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination | First Season | Second Season | Synthesis First—Second Season | |||||||||
| Tillering | Stem Elongation | Ear Emergence | Flowering | Tillering | Stem Elongation | Ear Emergence | Flowering | Tillering | Stem Elongation | Ear Emergence | Flowering | |
| 1 | 104.8 ± 5.49 c (100) | 100.4 ± 5.30 d (100) | 84.7 ± 2.74 c (100) | 60.7 ± 0.12 d (100) | 115.4 ± 10.26 b (100) | 110.6 ± 8.83 d (100) | 63.36 ± 5.28 c (100) | 48.9 ± 11.45 c (100) | 110.1 ± 8.71 c (100) | 105.5 ± 8.59 d (100) | 74.1 ± 12.30 c (100) | 54.8 ± 9.69 c (100) |
| 2 | 119.3 ± 5.14 bc (113.9) | 107.9 ± 7.53 cd (107.5) | 92.6 ± 4.54 c (109.3) | 67.7 ± 1.87 c (111.5) | 121.5 ± 3.12 b (105.30) | 114.4 ± 1.77 cd (103.34) | 68.9 ± 1.69 c (108.9) | 52.5 ± 4.17 c (107.3) | 120.4 ± 3.98 c (109.3) | 111.2 ± 6.02 d (105.4) | 80.8 ± 13.27 c (109.0) | 60.1 ± 8.80 c (109.7) |
| 3 | 108.1 ± 9.93 c (103.2) | 102.4 ± 1.84 d (102.02) | 85.0 ± 3.93 c (100.35) | 60.7 ± 1.97 d (100.1) | 113.2 ± 4.87 b (98.1) | 110.2 ± 5.67 d (99.6) | 61.9 ± 2.76 c (97.7) | 51.7 ± 5.39 c (105.56) | 110.6 ± 7.53 c (100.5) | 106.3 ± 5.70 d (100.7) | 73.5 ± 13.04 c (99.2) | 56.1 ± 6.15 c (102.4) |
| 4 | 109.8 ± 4.93 c (104.8) | 104.9 ± 4.27 d (104.6) | 88.8 ± 6.66 c (104.8) | 66.4 ± 1.24 cd (109.4) | 119.2 ± 7.76 b (103.3) | 121.2 ± 4.43 cd (109.5) | 67.7 ± 5.69 c (106.9) | 51.1 ± 1.88 c (104.4) | 114.5 ± 7.77 c (103.9) | 113.1 ± 9.69 d (107.2) | 78.3 ± 12.30 c (105.7) | 58.7 ± 8.52 c (107.1) |
| 5 | 150.2 ± 20.80 a (143.3) | 185.6 ± 2.34 a (184.8) | 178.4 ± 8.21 a (210.5) | 122.5 ± 2.38 a (201.8) | 170.63 ± 3.28 a (147.91 | 167.2 ± 2.04 a (151.1) | 121.6 ± 3.51 a (191.9) | 102.3 ± 2.43 a (209.1) | 160.4 ± 17.39 a (145.7) | 176.4 ± 10.14 a (167.2) | 150.0 ± 31.59 a (202.4) | 112.4 ± 11.25 a (205.1) |
| 6 | 143.6 ± 1.79 ab (137.0) | 132.9 ± 4.16 b (132.4) | 143.9 ± 6.39 b (169.9) | 90.6 ± 3.68 b (149.3) | 159.0 ± 4.95 ab (137.8) | 143.7 ± 3.73 b (129.9) | 91.4 ± 8.72 b (144.2) | 77.3 ± 7.44 b (157.9) | 151.3 ± 9.08 ab (137.4) | 138.3 ± 6.87 b (131.1) | 117.7 ± 29.61 b (158.8) | 83.9 ± 8.98 b (153.1) |
| 7 | 126.9 ± 2.17 abc (121.13) | 121.0 ± 4.48 bc (120.5) | 139.1 ± 5.75 b (164.1) | 91.2 ± 1.81 b (150.2) | 155.5 ± 6.56 ab (134.8) | 124.4 ± 8.78 c (112.4) | 83.3 ± 3.13 b (131.5) | 76.1 ± 5.3 b(155.6) | 141.2 ± 16.26 b (128.2) | 122.7 ± 3.41 c (116.3) | 111.2 ± 30.82 b (150.1) | 83.6 ± 8.96 b (152.5) |
| 8 | 122.5 ± 5.11 bc (116.9) | 126.0 ± 6.23 b (125.5) | 135.4 ± 5.52 b (159.7) | 87.9 ± 4.01 b (144.8) | 157.7 ± 4.23 ab (136.7) | 138.4 ± 11.02 b (125.1) | 94.5 ± 4.79 b (149.1) | 74.1 ± 2.09 b (151.4) | 140.1 ± 19.71 b (127.2) | 132.2 ± 7.86 b (125.3) | 114.9 ± 22.86 b (155.1) | 80.9 ± 8.07 b- (147.6) |
| Peroxidase [μM Purpurogallin∙g−1 FW∙min−1] (% Control) | ||||||||||||
| Combination | First Season | Second Season | Synthesis First—Second Season | |||||||||
| Tillering | Stem Elongation | Ear Emergence | Flowering | Tillering | Stem Elongation | Ear Emergence | Flowering | Tillering | Stem Elongation | Ear Emergence | Flowering | |
| 1 | 3.7 ± 0.84 b (100) | 6.0 ± 0.51 c (100) | 8.0 ± 0.32 b (100) | 7.9 ± 1.08 c (100) | 3.6 ± 0.22 c (100) | 6.4 ± 0.31 b (100) | 7.4 ± 0.12 c (100) | 8.1 ± 1.32 b (100) | 3.7 ± 0.53 d (100) | 6.2 ± 0.43 b (100) | 7.7 ± 0.39 d (100) | 8.0 ± 0.84 d (100) |
| 2 | 4.1 ± 0.46 ab (110.4) | 6.2 ± 1.36 bc (103.7) | 8.4 ± 0.45 b (105.6) | 8.3 ± 0.61 bc (105.1) | 3.9 ± 0.22 bc (107.9) | 6.6 ± 1.24 b (102.8) | 7.6 ± 0.22 bc (103.5) | 8.3 ± 0.69 b (102.6) | 4.0 ± 0.32 cd (108.1) | 6.4 ± 0.84 b (103.2) | 8.0 ± 0.53 cd (103.9) | 8.3 ± 0.58 cd (103.7) |
| 3 | 4.1 ± 0.15 ab (109.6) | 6.2 ± 0.12 bc (102.6) | 7.9 ± 0.71 b (99.4) | 8.1 ± 0.68 bc (101.4) | 3.91 ± 0.19 bc (107.7) | 6.3 ± 0.74 b (98.2) | 7.5 ± 0.72 bc (102.1) | 8.1 ± 0.23 b (100.3) | 4.0 ± 0.15 cd (108.1) | 6.2 ± 0.48 b (100.1) | 7.7 ± 0.67 d (100.1) | 8.1 ± 0.45 d (101.2) |
| 4 | 4.1 ± 0.45 ab (108.9) | 6.1 ± 0.53 bc (102.3) | 7.9 ± 0.45 b (99.4) | 8.0 ± 0.38 c (101.6) | 3.8 ± 0.13 bc (105.2) | 6.5 ± 0.21 b (101.2) | 7.5 ± 0.71 bc (101.1) | 8.2 ± 0.44 b (100.8) | 3.9 ± 0.33 cd (105.4) | 6.3 ± 0.40 b (101.6) | 7.7 ± 0.59 d (100.1) | 8.1 ± 0.37 d (101.2) |
| 5 | 5.4 ± 0.49 a (144.8) | 8.4 ± 0.54 a (140.4) | 11.1 ± 1.03 a (138.7) | 10.9 ± 0.96 a (137.9) | 5.3 ± 0.19 a (146.38) | 8.8 ± 0.85 a (136.9) | 10.3 ± 0.82 a (139.6) | 12.1 ± 1.50 a (149.7) | 5.4 ± 0.34 a (145.9) | 8.6 ± 0.66 a (138.7) | 10.7 ± 0.95 a (138.9) | 11.5 ± 1.16 a (143.7) |
| 6 | 5.1 ± 0.49 ab (135.3) | 7.8 ± 0.29 a (130.8) | 9.6 ± 0.33 ab (120.1) | 9.9 ± 0.89 ab (125.8) | 4.6 ± 0.52 ab (126.1) | 8.1 ± 0.39 ab (125.8) | 9.1 ± 0.32 ab (123.1) | 9.9 ± 0.72 ab (122.0) | 4.8 ± 0.52 ab (129.7) | 7.9 ± 0.32 a (127.4) | 9.3 ± 0.39 b (120.8) | 9.9 ± 0.68 b (123.7) |
| 7 | 4.5 ± 0.39 ab (120.4) | 7.3 ± 0.99 abc (121.8) | 9.5 ± 1.24 ab (119.54) | 9.7 ± 0.82 abc (122.1) | 4.5 ± 0.43 b (123.2) | 7.9 ± 0.74 ab (123.5) | 8.8 ± 0.88 abc (118.8) | 9.7 ± 0.65 b (119.7) | 4.5 ± 0.28 bc (121.6) | 7.6 ± 0.85 a (122.5) | 9.2 ± 1.05 bc (119.5) | 9.7 ± 0.66 bc (121.2) |
| 8 | 4.7 ± 0.58 ab (126.6) | 7.58 ± 0.36 ab (126.3) | 9.1 ± 0.54 ab (114.0) | 9.57 ± 1.15 abc (120.5) | 4.3 ± 0.39 bc (119.9) | 7.8 ± 0.21 ab (122.7) | 8.7 ± 0.25 bc (117.5) | 9.4 ± 0.42 b (115.3) | 4.5 ± 0.49 bc (121.6) | 7.7 ± 0.29 a (124..2) | 8.9 ± 0.44 bcd (115.6) | 9.5 ± 0.78 bcd (118.7) |
(1) Control, (2) 100 µM Vit PP soaking seeds, (3) 100 µM Vit PP foliar application, (4) 100 µM Vit PP soil application, (5) 1 mM Pb(NO3)2, (6) 1 mM Pb(NO3)2 + 100 µM Vit PP soaking seeds, (7) 1 mM Pb(NO3)2 + 100 µM Vit PP foliar application, (8) 1 mM Pb(NO3)2 + 100 µM Vit PP soil application; a–d—homogeneous groups.

Table 2.
Influence of different methods of application of vitamin PP on the Pro and MDA content of barley growing in soil with lead.
Table 2.
Influence of different methods of application of vitamin PP on the Pro and MDA content of barley growing in soil with lead.
| Proline [μmol∙g−1∙FW] (% Control) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination | First Season | Second Season | Synthesis First—Second Season | |||||||||
| Tillering | Stem Elongation | Ear emergence | Flowering | Tillering | Stem Elongation | Ear Emergence | Flowering | Tillering | Stem Elongation | Ear Emergence | Flowering | |
| 1 | 2.5 ± 0.20 a (100) | 2.6 ± 0.79 a (100) | 3.4 ± 1.44 d (100) | 3.4 ± 0.42 b (100) | 2.1 ± 0.68 a (100) | 2.3 ± 0.27 a (100) | 2.9 ± 0.13 d (100) | 2.6 ± 0.21 bc (100) | 2.3 ± 0.49 b (100) | 2.5 ± 0.57 cd (100) | 3.2 ± 0.95 c (100) | 3.0 ± 0.51 c (100) |
| 2 | 2.5 ± 0.11 a (98.7) | 2.7 ± 0.49 a (102.7) | 3.7 ± 0.18 cd (107.7) | 3.6 ± 0.38 b (106.3) | 2.1 ± 0.21 a (98.7) | 2.5 ± 0.54 a (107.2) | 3.1 ± 0.37 bcd (105.0) | 2.8 ± 0.16 b (105.5) | 2.3 ± 0.25 b (99.1) | 2.6 ± 0.49 bcd (104.8) | 3.4 ± 0.39 bc (106.3) | 3.2 ± 0.52 c (105.6) |
| 3 | 2.4 ± 0.64 a (96.93) | 2.6 ± 0.11 a (98.0) | 3.6 ± 1.13 cd (104.5) | 3.5 ± 0.36 b (102.9) | 2.0 ± 0.11 a (94.4) | 2.3 ± 0.47 a (101.4) | 3.0 ± 0.12 cd (102.4) | 2.7 ± 0.43 b (102.9) | 2.2 ± 0.47 b (96.1) | 2.5 ± 0.34 d (99.6) | 3.3 ± 0.78 bc (103.4) | 3.1 ± 0.55 c (102.6) |
| 4 | 2.5 ± 0.24 a (99.6) | 2.6 ± 0.16 a (99.9) | 3.7 ± 0.32 cd (107.0) | 3.4 ± 0.25 b (100.6) | 2.1 ± 0.17 a (96.9) | 2.3 ± 0.18 a (101.5) | 2.9 ± 0.15 d (99.6) | 2.7 ± 0.22 b (102.0) | 2.3 ± 0.30 b (98.7) | 2.5 ± 0.23 cd (100.8) | 3.3 ± 0.45 bc (103.4) | 3.0 ± 0.45 c (100.7) |
| 5 | 3.2 ± 0.89 a (130.3) | 3.9 ± 0.37 a (147.9) | 5.1 ± 0.33 a (149.5) | 5.5 ± 0.59 a (163.2) | 2.8 ± 0.15 a (135.5) | 3.3 ± 0.35 a (143.4) | 4.2 ± 0.38 a (142.6) | 3.8 ± 0.38 a (145.5) | 3.1 ± 0.60 a (133.0) | 3.6 ± 0.48 a (146.1) | 4.6 ± 0.60 a (146.2) | 4.7 ± 0.96 a (154.8) |
| 6 | 2.9 ± 0.52 a (119.9) | 3.7 ± 0.88 a (140.4) | 4.7 ± 0.14 b (138.0) | 4.9 ± 0.76 ab (145.4) | 2.8 ± 0.42 a (130.6) | 3.1 ± 0.31 a (133.9) | 3.8 ± 0.29 ab (130.2) | 3.5 ± 0.29 ab (130.2) | 2.9 ± 0.44 ab (125.2) | 3.4 ± 0.69 ab (137.6) | 4.3 ± 0.50 ab (134.3) | 4.2 ± 0.96 ab (139.2) |
| 7 | 2.7 ± 0.15 a (107.1) | 3.8 ± 0.36 a (142.2) | 4.4 ± 0.27 b (127.7) | 4.8 ± 0.33 ab (141.1) | 2.7 ± 0.21 a (129.2) | 2.9 ± 0.38 a (129.5) | 3.7 ± 0.21 ab (126.0) | 3.2 ± 0.46 ab (120.1) | 2.7 ± 0.17 ab (117.8) | 3.4 ± 0.55 ab (136.4) | 4.1 ± 0.43 abc (126.7) | 3.9 ± 0.96 b (131.5) |
| 8 | 2.8 ± 0.14 a (113.6) | 3.7 ± 0.25 a (138.5) | 4.6 ± 0.79 b (133.5) | 4.7 ± 0.18 ab (140.3) | 2.7 ± 0.22 a (127.1) | 2.9 ± 0.52 a (128.4) | 3.6 ± 0.32 abc (124.0) | 3.0 ± 0.34 ab (130.8) | 2.8 ± 0.18 ab (120.4) | 3.3 ± 0.54 abc (134.0) | 4.1 ± 0.74 abc (128.9) | 3.9 ± 0.96 b (129.2) |
| MDA [μmol∙g−1 FW] (% Control) | ||||||||||||
| Combination | First Season | Second Season | Synthesis First—Second Season | |||||||||
| Tillering | Stem Elongation | Ear Emergence | Flowering | Tillering | Stem Elongation | Ear Emergence | Flowering | Tillering | Stem Elongation | Ear Emergence | Flowering | |
| 1 | 18.3 ± 1.11 b (100) | 25.3 ± 1.54 c (100) | 28.2 ± 3.68 b (100) | 30.1 ± 2.52 c (100) | 11.1 ± 1.29 c (100.0) | 24.9 ± 1.79 c (100) | 33.3 ± 1.07 b (100) | 36.5 ± 2.11 c (100) | 14.7 ± 4.08 d (100) | 25.1 ± 1.51 c (100) | 30.8 ± 3.71 b (100) | 33.3 ± 4.07 d (100) |
| 2 | 19.3 ± 1.15 b (105.2) | 26.2 ± 0.25 c (103.4) | 29.2 ± 0.69 b (103.6) | 32.1 ± 2.51 c (106.5) | 11.9 ± 1.58 bc (106.6) | 25.1 ± 2.23 c (96.5) | 36.8 ± 3.79 b (110.5) | 38.5 ± 4.09 c (105.4) | 15.5 ± 4.12 cd (105.7) | 25.6 ± 1.55 c (101.9) | 33.0 ± 4.82 b (107.3) | 35.3 ± 4.64 d (105.9) |
| 3 | 18.3 ± 0.56 b (100.1) | 25.2 ± 1.16 c (99.7) | 28.9 ± 1.69 b (102.4) | 31.1 ± 0.75 c (103.2) | 11.8 ± 1.48 bc (105.8) | 25.1 ± 2.62 c (96.5) | 34.8 ± 1.89 b (104.4) | 36.9 ± 4.23 c (101.2) | 15.1 ± 3.73 d (102.3) | 25.1 ± 1.82 c (100.1) | 31.8 ± 3.61 b (103.5) | 33.9 ± 4.21 d (102.1) |
| 4 | 18.5 ± 1.25 b (100.9) | 25.7 ± 0.92 c (101.6) | 28.5 ± 3.14 b (101.2) | 31.2 ± 1.55 c (103.8) | 11.1 ± 1.44 c (99.4) | 25.6 ± 0.63 c (98.5) | 35.2 ± 6.48 b (105.6) | 36.8 ± 3.37 c (100.8) | 14.7 ± 4.24 d (100.3) | 25.6 ± 0.71 c (101.9) | 31.9 ± 5.83 b (103.6) | 34.0 ± 3.83 d (102.2) |
| 5 | 28.9 ± 2.9 a (158.3) | 41.4 ± 1.21 a (163.4) | 45.4 ± 5.4 a (160.1) | 51.7 ± 5.12 a (171.6) | 18.6 ± 3.01 a (167.1) | 46.9 ± 4.07 a (180.8) | 62.9 ± 5.39 a (188.9) | 67.8 ± 2.19 a (185.8) | 23.8 ± 6.27 a (161.9) | 44.2 ± 4.05 a (175.6) | 54.2 ± 10.78 a (176.0) | 59.7 ± 9.52 a (179.4) |
| 6 | 26.9 ± 3.11 a (146.8) | 37.3 ± 2.32 ab (147.2) | 42.6 ± 2.62 a (150.9) | 46.6 ± 2.89 ab (154.7) | 16.9 ± 1.85 ab (151.5) | 41.6 ± 4.26 ab (160.4) | 57.5 ± 2.21 a (172.6) | 61.4 ± 5.88 ab (168.4) | 21.9 ± 5.95 ab (148.6) | 39.4 ± 3.88 b (156.9) | 50.0 ± 8.47 a (162.6) | 54.0 ± 9.13 ab (162.2) |
| 7 | 26.1 ± 3.71 a (142.6) | 33.9 ± 4.20 b (134.1) | 40.3 ± 4.51 ab (142.9) | 45.1 ± 2.52 ab (149.8) | 16.4 ± 2.62 ab (147.3) | 38.6 ± 3.34 b (148.8) | 56.2 ± 3.95 ab (168.8) | 53.7 ± 1.01 b (147.1) | 21.3 ± 6.05 ab (144.4) | 36.3 ± 4.25 b (144.3) | 48.3 ± 9.52 ab (156.9) | 49.4 ± 5.01 bc (148.3) |
| 8 | 23.2 ± 2.19 ab (126.76) | 32.7 ± 0.92 b (129.3) | 40.6 ± 5.82 ab (143.9) | 42.4 ± 4.85 b (141.0) | 15.2 ± 1.23 abc (137.1) | 38.3 ± 2.07 b (147.7) | 56.5 ± 5.12 ab (169.6) | 52.5 ± 2.21 b (143.9) | 19.2 ± 4.64 bc (130.7) | 35.5 ± 3.34 b (141.3) | 48.6 ± 10.01 ab (157.9) | 47.5 ± 6.47 c (142.6) |
(1) Control, (2) 100 µM Vit PP soaking seeds, (3) 100 µM Vit PP foliar application, (4) 100 µM Vit PP soil application, (5) 1 mM Pb(NO3)2, (6) 1 mM Pb(NO3)2 + 100 µM Vit PP soaking seeds, (7) 1 mM Pb(NO3)2 + 100 µM Vit PP foliar application, (8) 1 mM Pb(NO3)2 + 100 µM Vit PP soil application; a–d—homogeneous groups.

Table 3.
Influence of different methods of application of vitamin PP on the assimilation pigments content of barley growing in soil with lead.
Table 3.
Influence of different methods of application of vitamin PP on the assimilation pigments content of barley growing in soil with lead.
| Total Chlorophyll [μg·g−1 FW] (% Control) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination | First Season | Second Season | Synthesis First—Second Season | |||||||||
| Tillering | Stem Elongation | Ear Emergence | Flowering | Tillering | Stem Elongation | Ear Emergence | Flowering | Tillering | Stem Elongation | Ear Emergence | Flowering | |
| 1 | 122.7 ± 5.42 ab (100) | 119.5 ± 12.69 ab (100) | 130.4 ± 15.65 ab (100) | 107.2 ± 3.36 a (100) | 120.4 ± 2.46 ab (100) | 108.8 ± 13.95 abc (100) | 130.2 ± 6.64 a (100) | 104.9 ± 10.84 ab (100) | 121.6 ± 3.95 ab (100) | 114.2 ± 10.73 ab (100) | 130.3 ± 10.75 a (100) | 106.1 ± 7.28 a (100) |
| 2 | 131.6 ± 4.67 a (107.3) | 125.4 ± 2.73 ab (104.9) | 132.8 ± 3.54 ab (101.9) | 111.8 ± 2.93 a (104.3) | 123.2 ±14.96 ab (102.3) | 112.5 ± 7.38 ab (103.4) | 133.5 ± 4.06 a (102.5) | 106.7 ± 1.66 ab (101.6) | 127.4 ± 10.93 a (104.10 | 118.9 ± 8.64 a (104.1) | 133.1 ± 3.43 a (102.1) | 109.2 ± 3.52 a (102.9) |
| 3 | 133.8 ± 3.45 a (109.1) | 125.9 ± 23.28 ab (105.3) | 135.8 ± 5.84 ab (104.2) | 108.6 ± 9.8 a (101.3) | 128.8 ± 1.69 a (106.9) | 120.7 ± 9.31 a (110.9) | 146.9 ± 17.28 a (112.9) | 112.1 ± 16.02 ab (106.8) | 131.3 ± 8.17 a (107.9) | 123.3 ± 16.11 a (107.9 | 141.4 ± 13.04 a (108.5) | 110.3 ± 12.05 a (103.9) |
| 4 | 135.3 ± 3.78 a (110.3) | 129.7 ± 5.26 a (108.5) | 140.8 ± 5.31 a (108.0) | 115.8 ± 2.55 a (107.9 | 130.0 ± 6.83 a (107.9) | 120.6 ± 1.44 a (110.9) | 143.1 ± 7.46 a (109.9) | 115.8 ± 5.72 a (110.3) | 132.7 ± 5.72 a (109.1) | 125.2 ± 6.03 a (109.6) | 141.9 ± 5.92 a (108.9) | 115.8 ± 3.96 a (109.1) |
| 5 | 99.2 ± 4.18 c (80.8) | 86.7 ± 0.97 c (72.59) | 92.9 ± 4.92 c (71.2) | 71.2 ± 6.16 c (66.4) | 94.5 ± 4.56 c (78.4) | 76.9 ± 3.68 d (70.73) | 88.0 ± 10.42 b (67.6) | 66.2 ± 8.99 c (63.1) | 96.8 ± 4.67 c (79.7) | 81.8 ± 5.87 d (71.6) | 90.4 ± 7.76 b (69.4) | 68.7 ± 7.43 c (64.7) |
| 6 | 104.9 ± 2.77 c (85.5) | 97.6 ± 10.76 bc (81.7) | 104.6 ± 13.34 bc (80.27) | 72.1 ± 1.24 c (67.2) | 102.1 ± 5.51 bc (84.7) | 85.5 ± 1.78 d (78.6) | 91.4 ± 6.83 b (70.2) | 82.9 ± 5.63 bc (78.9) | 103.5 ± 4.19 c (85.1) | 91.6 ± 9.56 cd (80.2) | 98.0 ± 11.92 b (75.2) | 77.5 ± 6.96 bc (73.0) |
| 7 | 107.4 ± 8.88 c (87.5) | 98.2 ± 3.49 bc (82.1) | 114.9 ± 11.60 abc (88.2) | 76.6 ± 3.37 bc (71.4) | 105.0 ± 13.67 abc (87.2) | 91.4 ± 6.45 cd (83.9) | 96.6 ± 14.43 b (74.2) | 84.7 ± 12.76 bc (80.7) | 106.2 ± 10.39 c (87.3) | 94.8 ± 5.94 cd (83.0) | 105.7 ± 15.42 b (81.1) | 80.7 ± 9.45 bc (76.1) |
| 8 | 111.1 ± 3.37 bc (90.6) | 101.6 ± 11.23 abc (85.0) | 116.1 ± 20.93 abc (89.1) | 81.1 ± 1.48 b (75.6) | 108.4 ± 3.02 abc (89.9) | 95.4 ±3.36 bcd (87.7) | 97.1 ± 5.21 b (74.6) | 90.0 ± 16.27 abc (85.8) | 109.7 ± 3.23 bc (89.6) | 98.5 ± 8.16 bc (86.2 | 106.6 ± 17.13 b (81.8) | 85.5 ± 11.44 b (80.6) |
| Carotenoids [μg·g−1FW] (% Control) | ||||||||||||
| Combination | First Season | Second Season | Synthesis First—Second Season | |||||||||
| Tillering | Stem Elongation | Ear Emergence | Flowering | Tillering | Stem Elongation | Ear Emergence | Flowering | Tillering | Stem Elongation | Ear Emergence | Flowering | |
| 1 | 44.3 ± 3.93 ab (100) | 42.8 ± 3.56 a (100) | 47.7 ± 4.37 ab (100) | 37.4 ± 2.24 ab (100) | 40.6 ± 2.48 ab (100) | 39.8 ± 8.61 a (100) | 36.8 ± 3.94 a (100) | 30.9 ± 6.47 ab (100) | 42.5 ± 3.57 abc (100) | 41.3 ± 6.13 a (100) | 42.2 ± 7.03 ab (100) | 34.2 ± 5.45 ab (100) |
| 2 | 46.3 ± 1.90 ab (104.6) | 45.5 ± 1.65 a (106.3) | 49.9 ± 1.62 a (104.6) | 39.6 ± 5.57 a (105.6) | 42.3 ± 6.49 ab (104.2) | 40.1 ± 2.49 a (100.7) | 38.0 ± 2.32 a (103.3) | 32.4 ± 5.01 ab (104.8) | 44.3 ±4.81 abc (104.2) | 42.8 ± 3.53 a (103.6) | 43.9 ± 6.75 a (104.0) | 35.9 ± 5.62 a (104.9) |
| 3 | 47.9 ± 1.83 a (108.0) | 44.1 ± 1.96 a (102.9) | 37.9 ± 4.01 ab (101.3) | 37.4 ± 9.11 ab (101.6) | 43.6 ± 3.23 ab (107.4) | 40.6 ± 8.91 a (101.9) | 38.3 ± 2.53 a (104.1) | 31.6 ± 3.91 ab (102.4) | 45.7 ± 3.30 ab (107.5) | 42.3 ± 6.07 a (102.4) | 43.4 ± 8.15 ab (102.8) | 34.8 ± 6.18 ab (101.7) |
| 4 | 47.6 ± 1.12 a (107.4) | 44.4 ± 3.35 a (103.6) | 48.5 ± 2.32 ab (101.7) | 38.4 ± 3.39 a (102.7) | 44.8 ± 4.26 a (110.3) | 41.8 ± 5.24 a (105.1) | 38.0 ± 2.20 a (103.4) | 34.2 ± 2.63 a (110.7) | 46.2 ± 3.18 a (108.7) | 43.1 ± 4.17 a (104.3) | 43.3 ± 6.07 ab (102.6) | 36.3 ± 4.94 a (106.1) |
| 5 | 34.9 ± 2.48 c (78.9) | 29.5 ± 3.03 bc (68.9) | 33.8 ± 7.94 b (70.9) | 25.8 ± 5.74 c (68.8) | 31.1 ± 4.09 b (76.5) | 29.1 ± 2.51 b (73.2) | 26.3 ± 3.06 b (71.5) | 22.9 ± 1.57 b (74.1) | 33.0 ± 3.70 e (77.6) | 29.3 ± 2.49 b (70.2) | 30.1 ± 6.07 c (71.3) | 24.4 ± 3.57 c (71.3) |
| 6 | 38.8 ± 3.02 bc (87.6) | 30.1 ± 5.21 bc (70.3) | 37.7 ± 2.98 ab (79.1) | 26.8 ± 2.71 c (71.6) | 33.6 ± 7.21 ab (82.8) | 34.6 ± 6.62 ab (86.9) | 31.9 ± 4.46 ab (86.8) | 25.9 ± 3.65 ab (83.9) | 36.2 ± 5.71 de (85.1) | 32.4 ± 5.87 b (78.4) | 34.9 ± 4.62 bc (82.7) | 26.4 ± 3.09 c (77.2) |
| 7 | 40.2 ± 4.28 abc (90.7) | 32.7 ± 2.49 b (76.4) | 42.1 ± 5.52 ab (88.2) | 29.3 ± 1.89 abc (78.3) | 35.2 ± 2.98 ab (86.6) | 36.0 ± 4.02 ab (90.6) | 34.7 ± 2.43 ab (94.3) | 27.7 ± 3.45 ab (89.6) | 37.7 ± 4.29 cde (88.7) | 34.4 ± 3.51 ab (83.3) | 38.3 ± 5.54 abc (90.7 | 28.5 ± 2.61 bc (83.3) |
| 8 | 40.6 ± 2.87 abc (91.6) | 33.5 ± 2.79 b (78.2) | 40.4 ± 5.45 ab (84.8) | 27.8 ± 2.06 bc (74.4) | 37.2 ± 3.09 ab (91.7) | 36.4 ± 4.87 ab (91.5) | 34.6 ± 5.79 ab (94.1) | 27.5 ± 2.21 ab (89.2) | 38.9 ± 3.24 cde (91.5) | 34.9 ± 3.89 ab (84.5) | 37.5 ± 5.96 abc (88.9) | 27.7 ± 1.91 bc (81.0) |
(1) Control, (2) 100 µM Vit PP soaking seeds, (3) 100 µM Vit PP foliar application, (4) 100 µM Vit PP soil application, (5) 1 mM Pb(NO3)2, (6) 1 mM Pb(NO3)2 + 100 µM Vit PP soaking seeds, (7) 1 mM Pb(NO3)2 + 100 µM Vit PP foliar application, (8) 1 mM Pb(NO3)2 + 100 µM Vit PP soil application; a–e—homogeneous groups.
4. Discussion
Lead is a significant environmental stressor that causes considerable damage to plant growth and development, hindering the advancement of sustainable agricultural practices. The World Health Organization recommends that the concentration of lead in soil should not exceed 85 mg kg−1 [53]. In this study, the dose of lead used, 1 mM Pb(NO3)2, equivalent to 207 mg kg−1 soil, significantly reduced only the length of roots and shoots in barley var. Eunova compared to the control. Differences in root growth are likely to be attributed to variations in lead uptake by cell walls and its accumulation in these organs, with lead accumulation in plant roots reaching as high as 95% [11,54,55].
4.1. Morphological Parameters
Literature reports indicate a decrease in morphological parameters under the influence of varying lead concentrations in wheat [34], beans [56], and tomatoes [57]. Our research [35,36] conducted on barley using the same lead dose showed a much more pronounced decrease in all investigated morphological parameters compared to this study. In studies conducted on MS media in vitro culture [35], there was a 46% decrease in root length, 59% decrease in seedling length, and 43% decrease in fresh weight compared to the control while, in experiments conducted on Petri dishes [36], the decreases were 81.8%, 36.6%, and 41.2%, respectively.
According to Hossain [58], plant responses and mechanisms to stress differ between hydroponic systems and field experiments.
Lead can bind to various soil components, such as clay minerals and organic matter, forming different complexes. However, only a small fraction of the lead in these complexes is available to plants [59].
Roots are the sole plant organs directly exposed to soil lead, likely contributing to the pronounced response observed in the barley var. Eunova studied in this experiment. The reduction in root growth may stem from disruptions in the physiological functions performed by roots, including the mobilization and circulation of elements from mineral and organic compounds in the soil, water uptake, and nutrient transport to other organs [5,60]. According to Aslam [14], lead ions can have variable effects on the absorption of important nutrients, potentially increasing the uptake of calcium and potassium while inhibiting magnesium. In addition, Dong et al. [61] suggest that elevated lead concentrations in soil substrates reduce the availability of other essential nutrients, such as iron, manganese, phosphorus, and zinc.
The reduced availability of these nutrients in cereal crops can have several adverse effects, including limiting the plants ability to produce ATP and adversely affecting the levels of enzymes responsible for all biochemical processes. Consequently, this could lead to reduced growth and yield [5].
The exogenous application of nicotinamide (100 µM) had a positive impact on root and shoot length (Figure 2). These results are consistent with the findings of Vendruscolo et al. [39], where the exogenous application of nicotinamide at doses of 100–300 mg L−1, regardless of the application method (seed imbibition or foliar spraying), had a positive effect on the growth and yield of upland rice plants. In addition, a study by de Lima et al. [62] found that applying nicotinamide at concentrations ranging from 237.8 to 373.8 mg L−1 promoted growth and yield in soybean.
The improvement in plant growth characteristics following the exogenous application of this vitamin may result from increased energy reserves and nutrients, including carbohydrates [4,39,63,64], as reported by de Lima et al. [62], who reported that nicotinamide acts as a biostimulant. It serves as the primary precursor of nicotinamide adenine dinucleotide and its phosphate (NADH and NADPH), crucial coenzymes essential for ATP production and involved in oxidation-reduction reactions [65]. Niacin is vital for maintaining cellular metabolism, responsible for producing proteins, enzymes, carbohydrates, and lipids [66]. Furthermore, Hathout [67] demonstrated that soaking seeds in nicotinamide affected the growth of endogenous phytohormones, such as gibberellic acid and indoleacetic acid, which play a pivotal role in regulating plant growth and development.
The use of vitamin PP in this study, through seed soaking, foliar spraying, and watering, mitigated the deleterious effects of lead salts and improved root and shoot growth in combinations grown with lead. These findings align with those of other authors, who used vitamin PP to alleviate stress from factors other than lead, such as salt stress [4,65,68,69] drought [20], or water stress [21,70].
In this study, it was demonstrated that the most effective stress-relieving impact on morphological parameters was achieved through the application of vitamin PP via foliar sprays. Vendruscolo et al. [39] found that both methods of nicotinamide application (soaking and spraying) had similar effects on plant growth. However, in a drought stress experiment conducted by Khurshid et al. [70], two methods of applying vitamin PP were compared: spraying and fertigation. Different amounts of nicotinamide were used in both methods, with spraying ranging from 0.737 to 2.215 g L−1 and fertigation from 0.492 to 1.477 g L−1. Spraying proved more effective in alleviating drought stress on growth parameters.
The action of lead results in the generation of ROS. Their toxic effect involves excessive reactivity with various cellular components, such as proteins, sugars, lipids, and nucleic acids. As a result, various antioxidant systems (both enzymatic and nonenzymatic) are activated, leading to the production of substances indicating stress known as oxidative stress markers. CAT and POX are important antioxidant enzymes that function within cells to prevent the accumulation of excess ROS, specifically by detoxifying H2O2 [71]. ROS can induce lipid peroxidation in plants by oxidizing fatty acids present in cell membranes, leading to structural and functional damage. The extent of lipid peroxidation in plants can be evaluated through various methods, including the measurement of the volatile dialdehyde MDA content [72]. An increase in MDA within plant cells is an important indicator of oxidative stress [34,72,73]. In addition, Pro serves as a valuable indicator of stress intensity and acts as a nonenzymatic antioxidant, effectively counteracting the detrimental effects of ROS [71].
4.2. Biochemical Parameters
In this study, the biochemical response of barley var. Eunova was assessed by measuring the activity of antioxidant enzymes (CAT, POX), as well as the levels of Pro and MDA under abiotic stress conditions induced by the presence of 1 mM Pb(NO3)2 in the soil. The study revealed significant induction of enzymes such as CAT and POX, along with increased levels of Pro and MDA in plants growing in lead-contaminated soil compared to control plants. Similar results were reported by Dey et al. [74] for POX and by Jiang [75] for POX, Yang et al. [76] for CAT, POX, MDA, Alamri et al. [34] for CAT, MDA, Sędzik et al. [35] for CAT, POX, Pro, MDA, Cândido et al. [77] for CAT, MDA, Pirzadoh et al. [78] for Pro, Navabpaur et al. [73] for CAT, MDA, Khan et al. [13] for CAT, POX, Pro, Ahmad et al. [33] for Pro, Sędzik-Wójcikowska et al. [36] for CAT, POX, Pro, MDA. However, conflicting results were presented by Verma and Dubey [55], Dey et al. [74] for CAT, Sędzik et al. [35] for CAT at the highest lead dose, and Li et al. [79] for CAT and POX. However, conflicting results were presented by Verma and Dubey [55], Dey et al. [74] for CAT, Sędzik et al. [35] for CAT at the highest lead dose, and Li et al. [79] for CAT and POX. The varied responses of these enzymes under similar stress conditions reported by several authors may stem from not entirely identical experimental conditions. An increase in the activity of these enzymes indicates oxidative stress in cells. A significant decrease in CAT and POX activity in plants, as reported by Dey et al. [74], suggests a weakening of the systems for scavenging ROS generated in stressful situations. These authors propose that the decrease in enzyme activity could result from enzyme inhibition, given that these proteins are highly sensitive to a number of factors. The diverse responses in enzyme activity also depend on factors such as the plant species, the specific stress factor applied, its concentration, and the duration of exposure [80].
An increase in MDA levels indicates damage to cell membranes. This damage can result in reduced water absorption from the environment, decreased conductance, leading to reduced turgor, and a limitation of transpiration [81]. According to Dey et al. [74], the following membrane properties are altered when damaged: fluidity, permeability, rate, and selectivity of nutrient transport. Research conducted by Özturk and Demir [82] has shown that Pro plays an important role in plant responses to heavy metals, probably related to its antioxidant properties, metal-chelating function, and ability to protect enzymes such as CAT and POX. Pro is also involved in stabilizing membranes, proteins, and DNA [83].
The application of vitamin PP through seed soaking, foliar spraying, and irrigation in this study alleviated the deleterious effects of lead salts and improved the measured biochemical parameters in combinations growing with lead (Table 1 and Table 2). Exogenously applied vitamin PP reduced the activity of antioxidant enzymes (CAT and POX) and the levels of MDA and Pro in plants growing with lead. The most effective relief from lead stress was observed with foliar spraying and irrigation using vitamin PP. Our findings align with those of other authors, who used vitamin PP under stress conditions other than lead, such as salt stress [63], drought stress [20], and water stress [21]. In the study by El-Bassiouny et al. [63], the application of vitamin PP through seed imbibition and foliar spray reduced the effects of salinity stress by decreasing the Pro content in plants exposed to salinity stress, with the foliar spray having a greater effect on Pro. Meanwhile, in the study by El-Bassiouny et al. [21], the exogenous application of nicotinamide, in addition to mycorrhizal supplementation under water stress conditions, decreased the MDA content while increasing the activity of CAT and POX. The decrease in the activity of antioxidant enzymes (CAT and POX) and the content of MDA and Pro in lead-grown plants following the introduction of exogenous vitamin PP may be attributed to vitamin PP reducing ROS levels by enhancing both enzymatic and nonenzymatic antioxidant defense mechanisms, as well as the production of osmotically active substances [22].
4.3. Physiological Parameters
Chloroplasts are cell organelles susceptible to the production of ROS.
In this study, the physiological response of barley var. Eunova was determined by measuring the total chlorophyll and carotenoid content under abiotic stress conditions induced by the presence of 1 mM Pb(NO3)2 in the soil. The results demonstrated a decrease in pigment content in plants growing in lead-contaminated soil compared to control plants. Similarly, other studies have shown that lead decreases pigment content compared to controls [13,33,35,36,78,84]. The reduced chlorophyll content under the influence of lead may be attributed to its inhibition of magnesium availability. Magnesium occupies a central position in the chlorophyll molecule, and its presence is necessary for the proper functioning of this compound and the process of photosynthesis [14].
Exogenously applied vitamin PP, used in this study through irrigation, alleviated the harmful effects of lead salts and increased the pigment content in plants grown in the presence of lead. Similar outcomes have been reported by other authors using vitamin PP in response to stressors other than lead, such as salt stress [4,63,65,69] and drought stress [20]. The observed increase in chlorophyll content due to exogenously applied vitamin PP could be attributed to the activation of enzymes involved in regulating photosynthetic carbon reduction [85]. Vendruscolo and Seleguin [7] propose that this vitamin provides an antioxidant defense to the photosystem cells responsible for converting light energy into assimilates used in carboxylation processes, thereby influencing plant development and providing effective protection against stressors.
5. Conclusions
The application of 1 mM Pb(NO3)2 resulted in a decrease in root and shoot length, along with increased catalase and peroxidase activity, as well as the content of malondialdehyde, proline, and assimilatory pigments during developmental stages in which spring barley var. Eunova was examined in a pot experiment. Exogenous vitamin PP had a significant and favorable effect on the morphological, biochemical, and physiological parameters studied, thereby reducing the toxicity of lead salts. The effect of the applied dose of vitamin PP depends on the form of application. The most effective reduction in lead stress was achieved through foliar spraying and watering with vitamin PP. This research suggests the potential use of vitamin PP to enhance plant resistance to lead stress. However, field studies are necessary to ensure the practical relevance of vitamin PP application methods in agriculture.
Author Contributions
Conceptualization, B.S.; Methodology, B.S.; Software, M.S.-W.; Formal analysis, B.S. and M.S.-W.; Investigation, M.S.-W.; Data curation, B.S.; Writing—original draft, B.S. and M.S.-W. All authors have read and agreed to the published version of the manuscript.
Funding
This research and APC was funded by the Subsidy of Polish Ministry of Science and Higher Education for West Pomeranian University of Technology in Szczecin.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abhilash, P.C.; Tripathi, V.; Edrisi, S.A.; Dubey, R.K.; Bakshi, M.; Dubey, P.K.; Singh, H.B.; Ebbs, S.D. Sustainability of crop production from polluted lands. Energ. Ecol. Environ. 2016, 1, 54–65. [Google Scholar] [CrossRef]
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The Challenge of Feeding the World. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef]
- Lambin, E.F.; Meyfroidt, P. Global land use change, economic globalization, and the looming land scarcity. Proc. Natl. Acad. Sci. USA 2011, 108, 3465–3472. [Google Scholar] [CrossRef]
- Vendruscolo, E.P.; Seleguin, A. Effects of vitamin pre-sowing treatment on sweet maize seedlings irrigated with saline water. Acta Agronómica 2020, 69, 20–25. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Stoleru, V.; Gavrilescu, M. Analysis of Heavy Metal Impacts on Cereal Crop Growth and Development in Contaminated Soils. Agriculture 2023, 13, 1983. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; Shreejana, K.C.; Das, A.; Anjum, N.; Poudel, A.; Suchi, A.K. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.; Nakanishi, H.; McBride, M.B.; Williams, M.A.; Yoshihara, T. Chemical speciation, plant uptake, and toxicity of heavy metals in agricultural soils. J. Agric. Food Chem. 2020, 68, 12856–12869. [Google Scholar] [CrossRef]
- Yan, X.; Gao, D.; Zhang, F.; Zeng, C.; Xiang, W.; Zhang, M. Relationships between Heavy Metal Concentrations in Roadside Topsoil and Distance to Road Edge Based on Field Observations in the Qinghai-Tibet Plateau, China. Int. J. Environ. Res. Public Health 2013, 10, 762–775. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Smith, S.R.; Alloway Carlton-Smithd, C.; Chambers, B.J. An Inventory of Heavy Metals Inputs to Agricultural Soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef]
- Gaya, U.; Ikechukwu, S. Heavy metal contamination of selected spices obtained from Nigeria. J. Appl. Sci. Environ. Manag. 2016, 20, 681–688. [Google Scholar] [CrossRef]
- Sharma, R.; Dubey, R. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Tiwari, S.; Tripathi, I.P.; Tiwari, H.L. Effects of Lead on Environment. Int. J. Emerg. Res. Manag. Technol. 2013, 2, 23–45. [Google Scholar]
- Khan, M.; Rolly, N.K.; Al Azzawi, T.N.I.; Imran, M.; Mun, B.-G.; Lee, I.-J.; Yun, B.-W. Lead (Pb)-Induced Oxidative Stress Alters the Morphological and Physio-Biochemical Properties of Rice (Oryza sativa L.). Agronomy 2021, 11, 409. [Google Scholar] [CrossRef]
- Aslam, M.; Aslam, A.; Sheraz, M.; Ali, B.; Ulhassan, Z.; Najeeb, U.; Zhou, W.; Gill, R.A. Lead Toxicity in Cereals: Mechanistic Insight Into Toxicity, Mode of Action, and Management. Front. Plant Sci. 2021, 11, 587785. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Abd El Moneim, D.; Ahmad, P.; Chung, Y.S. Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, U.; Khan, S.M.; Khalid, N.; Ahmad, Z.; Jehangir, S.; Fatima Rizvi, Z.; Lho, L.H.; Han, H.; Raposo, A. Detoxifying the heavy metals: A multipronged study of tolerance strategies against heavy metals toxicity in plants. Front. Plant Sci. 2023, 12, 1154571. [Google Scholar] [CrossRef]
- Jorjani, S.; Pehlivan Karakaş, F. Physiological and Biochemical Responses to Heavy Metals Stress in Plants. Int. J. Second. Metab. 2024, 11, 169–190. [Google Scholar] [CrossRef]
- Sadak, M.S. Physiological role of yeast extract and nicotinamide on Pisum sativum L. plants under heat stress. Int. J. Pharm. Tech. Res. 2016, 9, 170–178. [Google Scholar]
- El-Bassiouny, H.M.S.; Abd El-Monem, A.A.; Abdallah, M.M.S.; Soliman, K.M. Role of arbuscular mycorrhiza, α-tocopherol and nicotinamide on the nitrogen containing compounds and adaptation of sunflower plant to water stress. Biosci. Res. 2018, 15, 2068–2088. [Google Scholar]
- Feng, D.; Wang, R.; Sun, X.; Liu, L.; Liu, P.; Tang, J.; Zhang, C.; Liu, H. Heavy metal stress in plants: Ways to alleviate with exogenous substances. Sci. Total Environ. 2023, 897, 165397. [Google Scholar] [CrossRef] [PubMed]
- Soares, T.F.S.N.; Dias, D.C.F.D.S.; Oliveira, A.M.S.; Ribeiro, D.M.; Dias, L.A.D.S. Exogenous brassinosteroids increase lead stress tolerance in seed germination and seedling growth of Brassica juncea L. Ecotoxicol. Environ. 2020, 193, 110296. [Google Scholar] [CrossRef] [PubMed]
- Guedes, F.; Maia, C.F.; Silva, B.; Batista, B.L.; Alyemeni, M.N.; Ahmad, P.; Lobato, A. Exogenous 24-epibrassinolide stimulates root protection, and leaf antioxidant enzymes in lead stressed rice plants: Central roles to minimize Pb content and oxidative stress. Environ. Pollut. 2021, 280, 116992. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ahmed, S.; Yasin, N.A.; Sardar, R.; Hussaan, M.; Gaafar, A.-R.Z.; Haider, F.U. 28-Homobrassinolide Primed Seed Improved Lead Stress Tolerance in Brassica rapa L. through Modulation of Physio-Biochemical Attributes and Nutrient Uptake. Plants 2023, 12, 3528. [Google Scholar] [CrossRef]
- Emamverdian, A.; Khalofah, A.; Pehlivan, N.; Zia-Ur-Rehman, M.; Li, Y.; Zargar, M. Exogenous application of jasmonates and brassinosteroids alleviates lead toxicity in bamboo by altering biochemical and physiological attributes. Environ. Sci. Pollut. Res. Int. 2024, 31, 7008–7026. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Kotowska, U.; Zambrzycka-Szelewa, E.; Sienkiewicz, A. Auxins and cytokinins regulate phytohormone homeostasis and thiol-mediated detoxification in the green alga Acutodesmus obliquus exposed to lead stress. Sci. Rep. 2020, 10, 10193. [Google Scholar] [CrossRef]
- Arshad, T.; Maqbool, N.; Javed, F.; Wahid, A.; Arshad, M.U. Enhancing the defensive mechanism of lead affected barley (Hordeum vulgare L.) genotypes by exogenously applied salicylic acid. J. Agric. Sci. 2017, 9, 139–146. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Matin, M.A.; Fardus, J.; Hasanuzzaman, M.; Hossain, M.S.; Parvin, K. Foliar application of salicylic acid improves growth and yield attributes by upregulating the antioxidant defense system in Brassica campestris plants grown in lead-amended soils. Acta Agrobot. 2019, 72, 1765. [Google Scholar] [CrossRef]
- Bali, S.; Kaur, P.; Kohli, S.K.; Ohri, P.; Thukral, A.K.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Jasmonic acid induced changes in physio-biochemical attributes and ascorbate-glutathione pathway in Lycopersicon esculentum under lead stress at different growth stages. Sci. Total Environ. 2018, 645, 1344–1360. [Google Scholar] [CrossRef]
- Khan, I.; Iqbal, M.; Ashraf, M.Y.; Ashraf, M.A.; Ali, S. Organic chelants-mediated enhanced lead (Pb) uptake and accumulation is associated with higher activity of enzymatic antioxidants in spinach (Spinacea oleracea L.). J. Hazard. Mater. 2016, 317, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Saman, R.U.; Shahbaz, M.; Maqsood, M.F.; Lili, N.; Zulfiqar, U.; Haider, F.U.; Naz, N.; Shahzad, B. Foliar Application of Ethylenediamine Tetraacetic Acid (EDTA) Improves the Growth and Yield of Brown Mustard (Brassica juncea) by Modulating Photosynthetic Pigments, Antioxidant Defense, and Osmolyte Production under Lead (Pb) Stress. Plants 2023, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ahmed, S.; Yasin, N.A.; Wahid, A.; Sardar, R. Exogenous application of glutathione enhanced growth, nutritional orchestration and physiochemical characteristics of Brassica oleracea L. under lead stress. Physiol. Mol. Biol. Plants 2023, 29, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishany, M.Y.Y.; Khan, M.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A.; Al-Rabiah, H.; Mateen, M. Ascorbic acid improves the tolerance of wheat plants to lead toxicity. J. Plant Interact. 2018, 13, 409–419. [Google Scholar] [CrossRef]
- Sędzik-Wójcikowska, M.; Smolik, B.; Krupa-Małkiewicz, M. Effect of nicotinamide in alleviating stress caused by lead in spring barley seedling. J. Elem. 2019, 24, 281–291. [Google Scholar] [CrossRef]
- Sędzik-Wójcikowska, M.; Krupa-Małkiewicz, M.; Smolik, B. The Effect of Use of the Biologically Active Substances in Alleviating the Stress Caused by Lead in Barley Seedling on the Basis of Biochemical and Physiological Parameters. J. Ecol. Eng. 2023, 24, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.I.; Lee, T.G.; Lee, J.; Chae, M.J.; Lee, E.J.; Kim, M.S.; Jung, G.B.; Emmanuel, A.; Jeon, S.; Lee, B.R. Foliar-Applied Glutathione Mitigates Cadmium-Induced Oxidative Stress by Modulating Antioxidant-Scavenging, Redox-Regulating, and Hormone-Balancing Systems in Brassica napus. Front. Plant Sci. 2021, 12, 700413. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, M.; Terzi, H. Exogenous cysteine alleviates chromium stress via reducing its uptake and regulating proteome in roots of Brassica napus L. seedlings. S. Afr. J. Bot. 2021, 139, 114–121. [Google Scholar] [CrossRef]
- Vendruscolo, E.P.; Rodrigues, A.H.A.; Oliveira, P.R.; Leitão, R.A.; Campos, L.F.C.; Seleguini, A.; Lima, S.F. Exogenous application of vitamins in upland rice. Rev. Agric. Neotrop. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Gakière, B.; Hao, J.; de Bont, L.; Pétriacq, P.; Nunes-Nesi, A.; Fernie, A.R. NAD+ Biosynthesis and Signaling in Plants. Crit. Rev. Plant Sci. 2018, 37, 259–307. [Google Scholar] [CrossRef]
- Farooq, T.H.; Bukhari, M.A.; Irfan, M.S.; Rafay, M.; Shakoor, A.; Rashid, M.H.U.; Lin, Y.; Saqib, M.; Malik, Z.; Khurshid, N. Effect of Exogenous Application of Nicotinic Acid on Morpho-Physiological Characteristics of Hordeum vulgare L. under Water Stress. Plants 2022, 11, 2443. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, S.; Chen, X.; Liu, R.; Zhu, J.; Shi, L.; Ren, A.; Zhao, M.W. NAD+-dependent Glsirt1 has a key role on secondary metabolism in Ganoderma lucidum. Microbiol. Res. 2022, 258, 126992. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 28, 2690. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.A.; Javed, F.; Wahid, A.; Sadia, B. Alleviating effect of exogenous application of ascorbic acid on growth and mineral nutrients in cadmium stressed barley (Hordeum vulgare) seedlings. Int. J. Agric. Biol. 2016, 18, 73–79. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, A.; da Silva, J.A.T.; Fujita, M. Molecular Mechanism of Heavy Metal Toxicity and Tolerance in Plants: Central Role of Glutathione in Detoxification of Reactive Oxygen Species and Methylglyoxal and in Heavy Metal Chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Lück, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: New York, NY, USA, 1963. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Bates, L.S. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Sudhakar, C.; Lakshim, A.; Giridarakumar, S. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl Salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Arnon, D.J.; Allen, M.B.; Whatley, F. Photosynthesis by isolated chloroplast. Biochim. Biophys. Acta 1956, 20, 449–461. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and bof leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Hager, A.; Mayer-Berthenrath, T. Isolation and quantitative determination of carotenoids and chlorophylls of leaves, algaeand isolated chloroplasts using thin-layer chromatographic methods. Planta 1966, 69, 198–217. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Kumar, V.; Tripathi, S.; Sharma, P. Heavy metal phytoextraction potential of native weeds and grasses from endocrine-disrupting chemicals rich complex distillery sludge and their histological observations during in-situ phytoremediation. Ecol. Eng. 2018, 111, 143–156. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2023, 164, 645–655. [Google Scholar] [CrossRef]
- Hashem, H.A.; El-Sherif, N.A. Exogenous Jasmonic Acid Induces Lead Stress Tolerance in Kidney Bean (Phaseolus vulgaris L.) by Changing Amino Acid Profile and Stimulating Antioxidant Defense System. Jordan J. Biol. Sci. 2019, 12, 345–353. [Google Scholar]
- Afzaal, Z.; Hussain, I.; Ashraf, M.A.; Rasheed, R.; Javed, M.T.; Ansari, M.; Anwer, S.; Iqbal, M. Lead induced modulation in growth, chlorophyll pigment, nutrient uptake, antioxidant enzyme regulation, gene expression and fruit quality in two tomato cultivars. Int. J. Agric. Biol. 2020, 24, 1732–1744. [Google Scholar] [CrossRef]
- Hossain, B.; Akhtar, M. Growth and yield of barley (Hordeum vulgare L.) as affected by irrigation, sowing method and phosphorus level. Acad. J. Agric. Res. 2014, 2, 30–35. [Google Scholar]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Roumet, C.; Comas, L.H.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Bardgett, R.D.; De Deyn, G.B.; Johnson, D.; Klimešová, J.; et al. Root traits as drivers of plant and ecosystem functioning: Current understanding, pitfalls and future research needs. New Phytol. 2021, 232, 1123–1158. [Google Scholar] [CrossRef]
- Dong, D.; Zhao, X.; Hua, X.; Liu, J.; Gao, M. Investigation of the potential mobility of Pb, Cd and Cr (VI) from moderately contaminated farmland soil to groundwater in Northeast, China. J. Hazard. Mater. 2009, 162, 1261–1268. [Google Scholar] [CrossRef]
- de Lima, S.F.; Vendruscolo, E.P.; Alves, V.C.D.; Arguelho, J.C.; de Abreu Pião, J.; de Castro Seron, C.; Martins, M.B.; Witt, T.W.; Serafim, G.M.; Merquides, L.M. Nicotinamide as a biostimulant improves soybean growth and yield. Open Agric. 2024, 9, 20220259. [Google Scholar] [CrossRef]
- Bassuony, F.M.; Hassanein, R.A.; Baraka, D.M.; Khalil, R.R. Physiological Effects of Nicotinamide and Ascorbic Acid on Zea mays Plant Grown Under Salinity Stress II-Changes in Nitrogen Constituents, Protein Profiles, Protease Enzyme and Certain Inorganic Cations. Aust. J. Basic Appl. Sci. 2008, 2, 350–359. [Google Scholar]
- Mohamed, M.H.; Badr, E.A.; Sadak, M.S.; Khedr, H.H. Effect of garlic extract, ascorbic acid and nicotinamide on growth, some biochemical aspects, yield and its components of three faba bean (Vicia faba L.) cultivars under sandy soil conditions. Bull. Natl. Res. Cent. 2020, 44, 100. [Google Scholar] [CrossRef]
- Sadak, M.S.; Rady, M.; Badr, N.M.; Gaballah, M.S. Increasing sunflower salt tolerance using nicotinamide and α-tocopherol. Int. J. Acad. Res. 2010, 2, 263–270. [Google Scholar]
- Kirkland, J.B.; Meyer-Ficca, M.L. Niacin. Adv. Food Nutr. Res. 2018, 83, 83–149. [Google Scholar] [CrossRef] [PubMed]
- Hathout, T.A. Diverse effects of uniconazole and nicotinamide on germination, growth, endogenous hormones and some enzymic activities of peas. Egypt. J. Physiol. Sci. 1995, 19, 77–95. [Google Scholar]
- Ali, R.M. Effect of Nicotinic Acid and Nicotinamide Adenine Dinucleotide on Growth and Content of Oil, Glycerol and Ricinine Alkaloids of Salinity stressed Bicinus communis L. Phyton 2002, 42, 269–277. [Google Scholar]
- Abdelhamid, M.A.; Sadak Mervat, S.H.; Schmidhalter, U.; El-Saady, A.M. Interactive effects of salinity stress and nicotinamide on physiological and biochemical parameters of faba bean plant. Acta Biol. Colomb. 2013, 18, 499–510. [Google Scholar]
- Khurshid, N.; Bukhari, M.A.; Ahmad, T.; Ahmad, Z.; Jatoi, W.N.; Abbas, S.M.; Latif, A.; Raza, A.; Aurangzaib, M.; Hashem, A.; et al. Exogenously applied nicotinic acid alleviates drought stress by enhancing morpho-physiological traits and antioxidant defense mechanisms in wheat. Ecotoxicol. Environ. Saf. 2023, 263, 115350. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Rachoski, M.; Gazquez, A.; Calzadilla, P.; Bezus, R.; Rodriguez, A.; Ruiz, O.; Menendez, A.; Maiale, S. Chlorophyll fluorescence and lipid peroxidation changes in rice somaclonal lines subjected to salt stress. Acta Physiol. Plant. 2015, 37, 117. [Google Scholar] [CrossRef]
- Navabpour, S.; Yamchi, A.; Bagherikia, S.; Kafi, H. Lead-induced oxidative stress and role of antioxidant defense in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2020, 26, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Dey, J.; Patra, S.; Pothal, D. Changes in the antioxidative enzyme activities and lipid peroxidation in wheat seedlings exposed to cadmium and lead stress. Braz. J. Plant Physiol. 2007, 19, 53–60. [Google Scholar] [CrossRef]
- Jiang, N.; Luo, X.; Zeng, J.; Yang, Z.R.; Zheng, L.Y.; Wang, S. Lead toxicity induced growth and antioxidant responses in Luffa cylindrica seedlings. Int. J. Agric. Biol. 2010, 12, 205–210. [Google Scholar]
- Yang, Y.; Zhang, Y.; Wei, X.; You, J.; Wang, W.; Lu, J.; Shi, R. Comparative antioxidative responses and proline metabolism in two wheat cultivars under short term lead stress. Ecotoxicol. Environ. Saf. 2011, 74, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Cândido, G.S.; Lima, F.R.; Vasques, I.C.; Souza, K.R.; Martins, G.C.; Pereira, P.; Engelhardt, M.M.; Reis, R.H.C.; Marques, J.J. Lead effects on sorghum and soybean physiology in oxisols. Arch. Agron. Soil Sci. 2020, 67, 260–274. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Hakeem, K.R.; Alharby, H.F.; Rehman, R.U. Lead toxicity alters the antioxidant defense machinery and modulate the biomarkers in Tartary buckwheat plants. Int. Biodeterior. Biodegrad. 2020, 151, 104992. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.L.; Zhang, J.; Zheng, L.; Chen, D.; Wu, Z.; Shaheen, S.M.; Rinklebe, J.; Ok, Y.S.; Wang, H.; et al. Coconut-fiber biochar reduced the bioavailability of lead but increased its translocation rate in rice plants: Elucidation of immobilization mechanisms and significance of iron plaque barrier on roots using spectroscopic techniques. J. Hazard. Mater. 2020, 389, 122117. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, N. Oxidative stress and change in plant metabolism of maize (Zea mays L.) growing in contaminated soil with elemental sulfur and toxic effect of zinc. Plant Soil Environ. 2011, 57, 34–39. [Google Scholar] [CrossRef]
- Woźny, A.; Przybył, K. Komórki Roślinne w Warunkach Stresu; Wydawnictwo Naukowe Uniwersytetu im. Adama Mickiewicza w Poznaniu: Poznań, Poland, 2004. [Google Scholar]
- Özturk, L.; Demir, Y. In vivo and vitro protective role of proline. Plant Growth Regul. 2002, 38, 259–264. [Google Scholar] [CrossRef]
- Matysik, J.; Alia Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Rehman, M.Z.; Rizwan, M.; Ali, S.; Sabir, M.; Sohail, M.I. Contrasting effects of organic and inorganic amendments on reducing lead toxicity in wheat. Bull. Environ. Contam. Toxicol. 2017, 99, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.E.; Terry, N.; Huston, R.P. Limiting Factors in Photosynthesis. Plant Physiol. 1982, 10, 1541–1543. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).