QTL Mapping and Candidate Gene Mining for Stem Diameter Using Genetic Basis of Cultivated Soybean and Wild Soybean
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Genetic Mapping
2.2. Field Experiment and Plant Measurements
2.3. Statistical Analysis of Data
2.4. QTL Identification
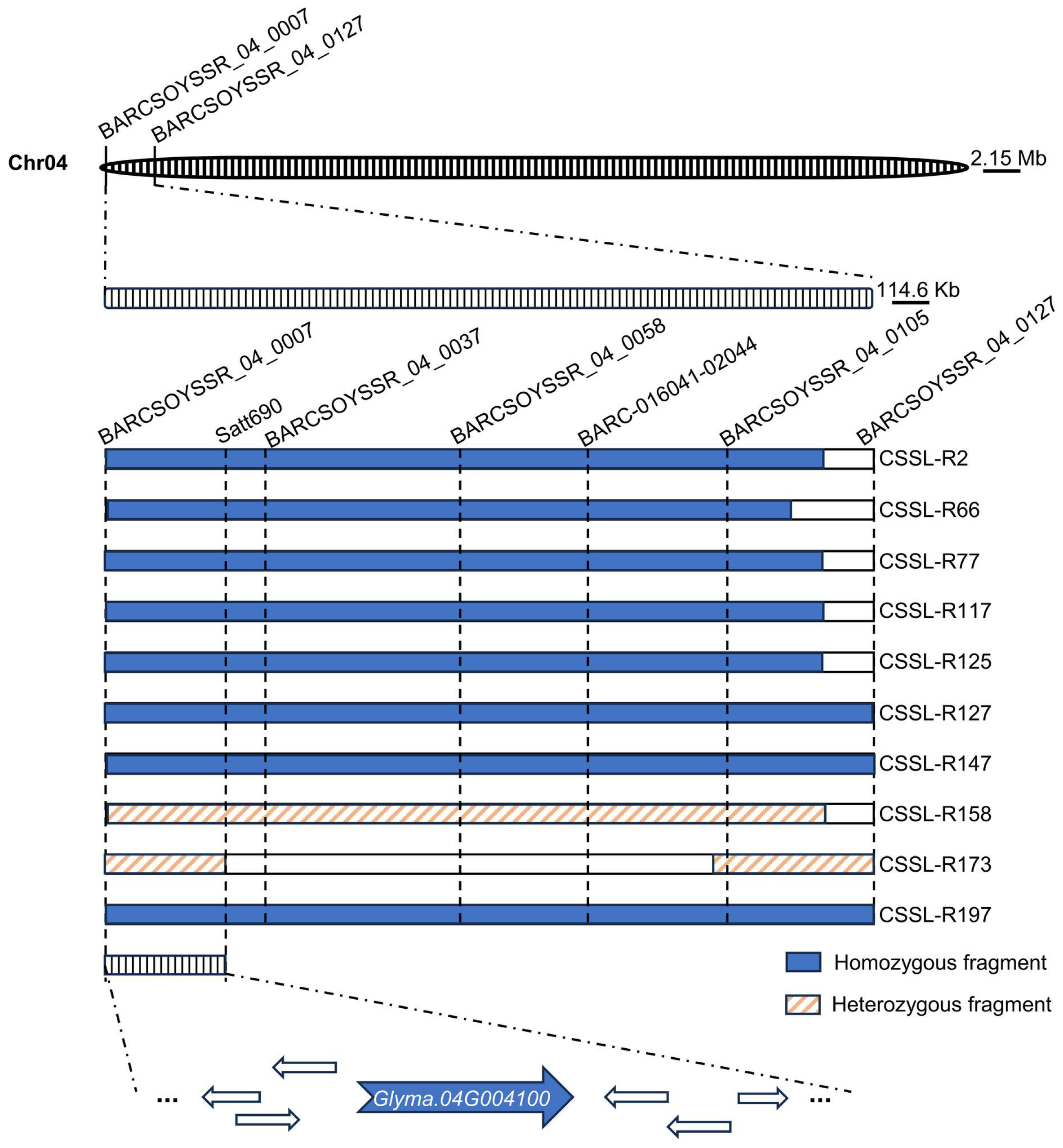
2.5. Chromosome Fragment Insertion Analysis
2.6. Candidate Gene Mining
2.7. Candidate Gene Single-Nucleotide Polymorphism (SNP) Analyses
2.8. RNA Sequencing and qPCR Verification
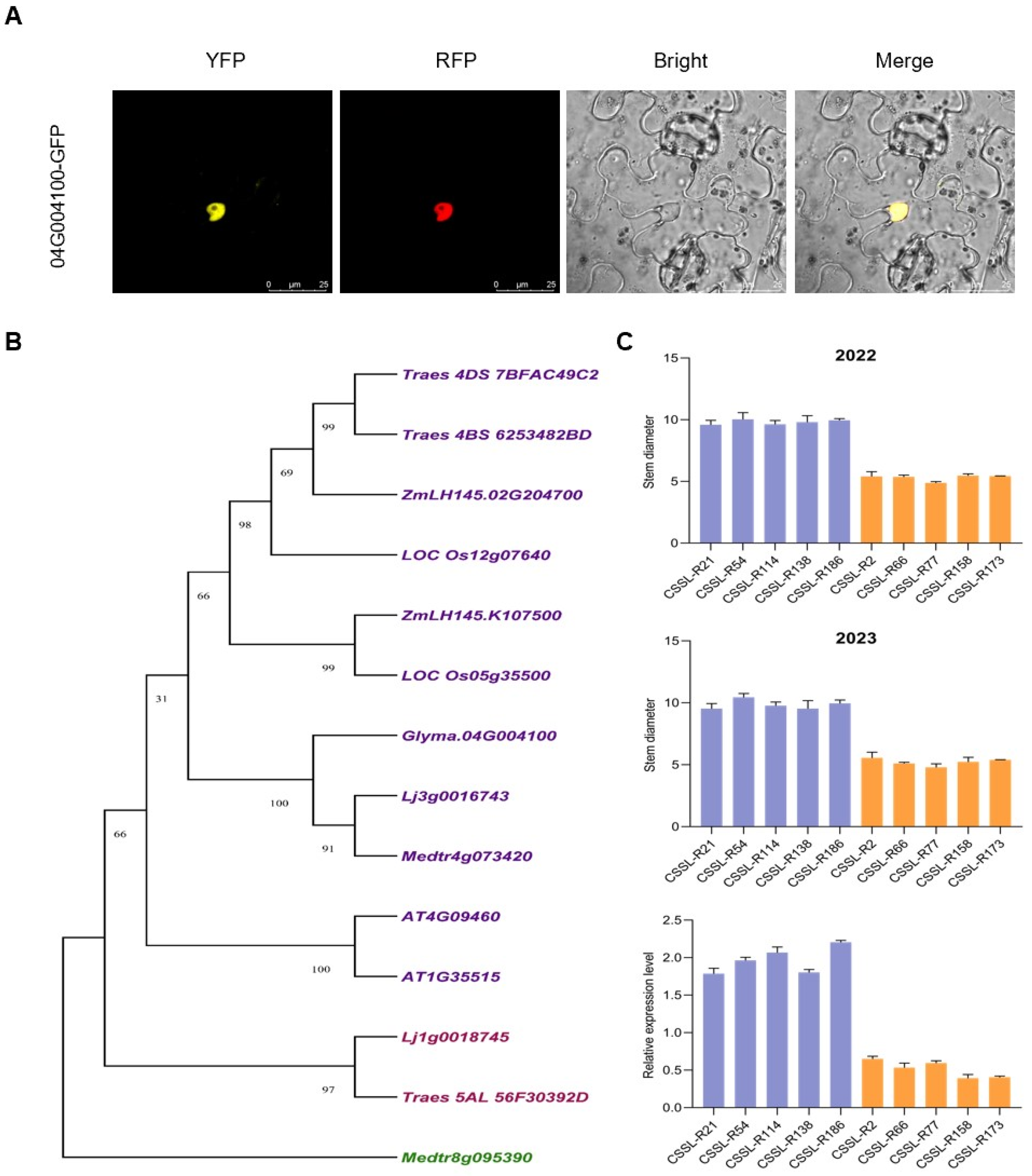
2.9. Subcellular Localization
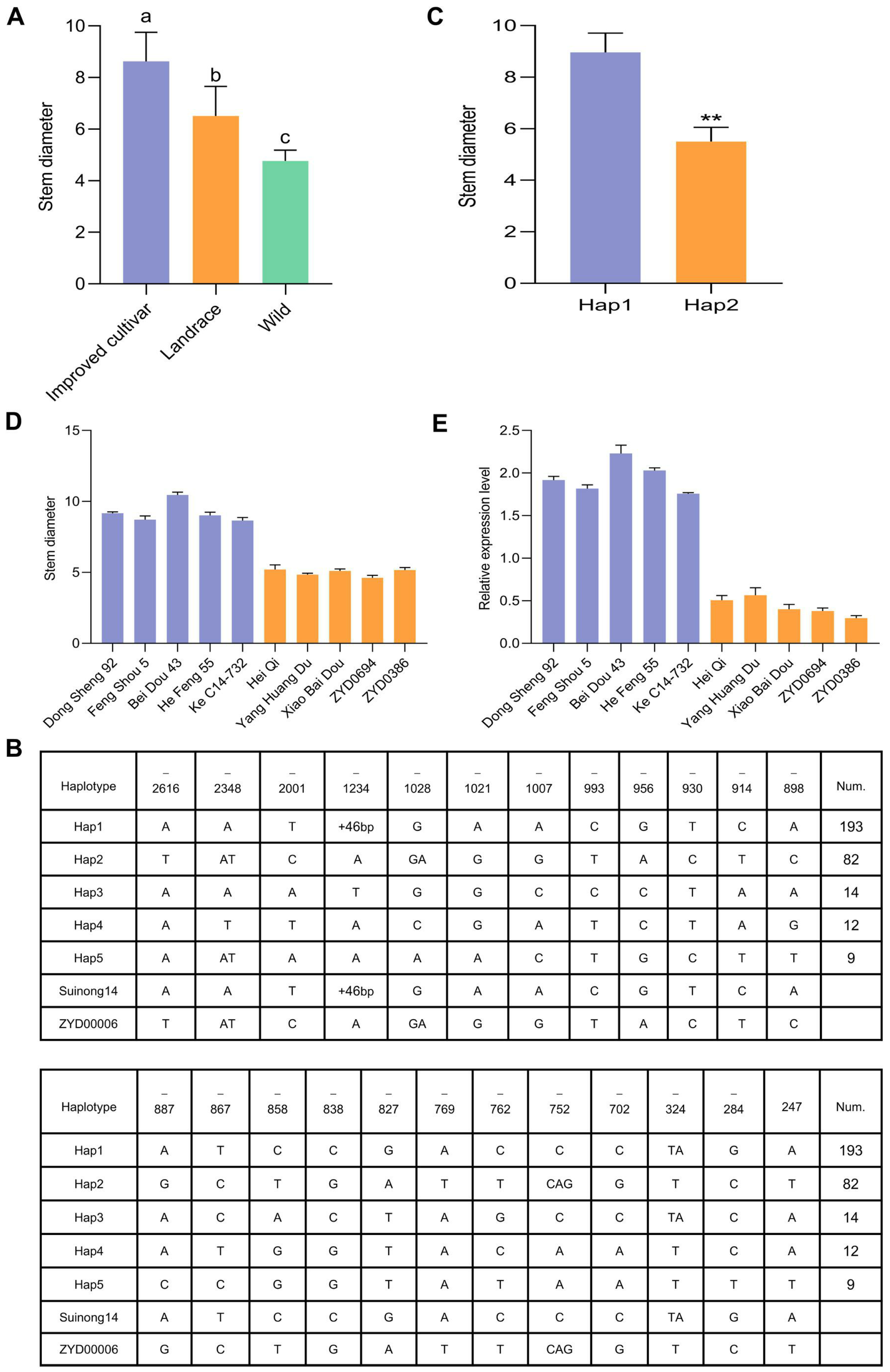
2.10. Haplotype Analyses
3. Results
3.1. The Suinong14 Soybean Variety Exhibits a Larger Stem Diameter than the Wild ZYD00006 Variety
3.2. SD Data Statistics and Associated QTL Identification in the Soybean CSSL Population
3.3. Candidate Interval Confirmation Based on Chromosome Fragment Insertion
3.4. RNA-seq Analyses of Suinong14 and ZYD00006 Plants during Stem Development
3.5. Identification of Stem Diameter-Related Candidate Genes Based on Single-Nucleotide Polymorphism Analysis
3.6. Expression Pattern Analysis of Glyma.04G004100 Encoding MYB4 Protein in CSSLs
3.7. Haplotype Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Song, Q.; Cregan, P.B.; Jiang, G.-L. Genome-wide association study, genomic prediction and marker-assisted selection for seed weight in soybean (Glycine max). Theor. Appl. Genet. 2016, 129, 117–130. [Google Scholar] [CrossRef]
- Dong, L.; Fang, C.; Cheng, Q.; Su, T.; Kou, K.; Kong, L.; Zhang, C.; Li, H.; Hou, Z.; Zhang, Y. Genetic basis and adaptation trajectory of soybean from its temperate origin to tropics. Nat. Commun. 2021, 12, 5445. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Chen, T.; Zhao, C.; Zhou, M. Lodging prevention in cereals: Morphological, biochemical, anatomical traits and their molecular mechanisms, management and breeding strategies. Field Crops Res. 2022, 289, 108733. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Li, N.; Pei, Y.; Peng, J.; Wang, Z. An integrated strategy coordinating endogenous and exogenous approaches to alleviate crop lodging. Plant Stress 2023, 9, 100197. [Google Scholar]
- Zhuang, Y.; Li, X.; Hu, J.; Xu, R.; Zhang, D. Expanding the gene pool for soybean improvement with its wild relatives. Abiotech 2022, 3, 115–125. [Google Scholar] [CrossRef]
- Cristina da Silva Reis, K.; Savario, C.F.; Hoy, J.W. Effects of Pesticides on Red Rot of Planted Sugarcane. Plant Dis. 2023, 107, 3616–3622. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, T.; Zhao, C.; Zhou, M. Improving crop lodging resistance by adjusting plant height and stem strength. Agronomy 2021, 11, 2421. [Google Scholar] [CrossRef]
- Afza, R.; Hardarson, G.; Zapata, F.; Danso, S. Effects of delayed soil and foliar N fertilization on yield and N 2 fixation of soybean. Plant Soil 1987, 97, 361–368. [Google Scholar] [CrossRef]
- Khobra, R.; Sareen, S.; Meena, B.K.; Kumar, A.; Tiwari, V.; Singh, G. Exploring the traits for lodging tolerance in wheat genotypes: A review. Physiol. Mol. Biol. Plants 2019, 25, 589–600. [Google Scholar] [CrossRef]
- Zuber, U.; Winzeler, H.; Messmer, M.; Keller, M.; Keller, B.; Schmid, J.; Stamp, P. Morphological traits associated with lodging resistance of spring wheat (Triticum aestivum L.). J. Agron. Crop Sci. 1999, 182, 17–24. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, H.; Xie, D.; Li, J.; Yang, M.; Chang, T.; Wang, D.; Hu, L.; Xie, G.; Wang, J. Genome-wide association study in rice revealed a novel gene in determining plant height and stem development, by encoding a WRKY transcription factor. Int. J. Mol. Sci. 2021, 22, 8192. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.E.; Pasquariello, M.; Boden, S.A. TEOSINTE BRANCHED1 regulates height and stem internode length in bread wheat. J. Exp. Bot. 2020, 71, 4742–4750. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Togawa, E.; Hirotsu, N.; Ishimaru, K. Improvement of lodging resistance with QTLs for stem diameter in rice (Oryza sativa L.). Theor. Appl. Genet. 2008, 117, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Dan, D.; Yuan, Z.; Chen, Y.; Jin, J.; Yang, W.; Zhang, Z.; Li, N.; Li, S. Deciphering the genetic basis of lodging resistance in wild rice Oryza longistaminata. Front. Plant Sci. 2020, 11, 628. [Google Scholar] [CrossRef]
- Shang, Q.; Zhang, D.; Li, R.; Wang, K.; Cheng, Z.; Zhou, Z.; Hao, Z.; Pan, J.; Li, X.; Shi, L. Mapping quantitative trait loci associated with stem-related traits in maize (Zea mays L.). Plant Mol. Biol. 2020, 104, 583–595. [Google Scholar] [CrossRef]
- Smitchger, J.; Weeden, N. Quantitative trait loci controlling lodging resistance and other important agronomic traits in dry field peas. Crop Sci. 2019, 59, 1442–1456. [Google Scholar] [CrossRef]
- Chen, H.; Shan, Z.; Sha, A.; Wu, B.; Yang, Z.; Chen, S.; Zhou, R.; Zhou, X. Quantitative trait loci analysis of stem strength and related traits in soybean. Euphytica 2011, 179, 485–497. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Yang, Y.-M.; Jia, L.; Liu, X.-Q.; Xu, H.-Q.; Lv, H.-Y.; Huang, Z.-W.; Zhang, D. QTL mapping of the genetic basis of stem diameter in soybean. Planta 2021, 253, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xu, X.; Zhang, L.; Yuan, S.; Chen, F.; Sun, S.; Jiang, B. Progress and future impacts on genomic dissection of soybean domestication and improvement. Crit. Rev. Plant Sci. 2024, 43, 116–130. [Google Scholar] [CrossRef]
- Zheng, H.; Hou, L.; Xie, J.; Cao, F.; Wei, R.; Yang, M.; Qi, Z.; Zhu, R.; Zhang, Z.; Xin, D. Construction of chromosome segment substitution lines and inheritance of seed-pod characteristics in wild soybean. Front. Plant Sci. 2022, 13, 869455. [Google Scholar] [CrossRef]
- Wang, J.; Feng, H.; Jia, X.; Ma, S.; Ma, C.; Wang, Y.; Pan, S.; Chen, Q.; Xin, D.; Liu, C. Identifications of QTLs and candidate genes associated with Pseudomonas syringae responses in cultivated soybean (Glycine max) and wild soybean (Glycine soja). Int. J. Mol. Sci. 2023, 24, 4618. [Google Scholar] [CrossRef]
- Xin, D.; Qi, Z.; Jiang, H.; Hu, Z.; Zhu, R.; Hu, J.; Han, H.; Hu, G.; Liu, C.; Chen, Q. QTL location and epistatic effect analysis of 100-seed weight using wild soybean (Glycine soja Sieb. & Zucc.) chromosome segment substitution lines. PLoS ONE 2016, 11, e0149380. [Google Scholar]
- Peng, J.; Shi, X.; Sun, Y.; Li, D.; Liu, B.; Kong, F.; Yuan, X. QTLMiner: QTL database curation by mining tables in literature. Bioinformatics 2015, 31, 1689–1691. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiu, L.; Chang, R.; Liu, Z.; Guan, R.; Li, Y. Descriptors and Data Standard for Soybean (Glycine spp.); China Agriculture Press: Beijing, China, 2006. [Google Scholar]
- Wang, S.; Basten, C.; Zeng, Z. Windows QTL Cartographer Version 2.5. 2005. Available online: https://api.semanticscholar.org/CorpusID:67296679 (accessed on 20 November 2023).
- Wang, J.; Ma, C.; Ma, S.; Zheng, H.; Tian, H.; Wang, X.; Wang, Y.; Jiang, H.; Wang, J.; Zhang, Z. Genetic variation in GmCRP contributes to nodulation in soybean (Glycine max Merr.). Crop J. 2023, 11, 332–344. [Google Scholar] [CrossRef]
- Li, R.; Jiang, H.; Zhang, Z.; Zhao, Y.; Xie, J.; Wang, Q.; Zheng, H.; Hou, L.; Xiong, X.; Xin, D. Combined linkage mapping and BSA to identify QTL and candidate genes for plant height and the number of nodes on the main stem in soybean. Int. J. Mol. Sci. 2019, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Bell, S.M.; Chen, L.; Huang, S.; Wang, H.; Miao, J.; Qi, Y.; Sun, Y.; Liao, B.; Zeng, Y. Improving rice grain yield and reducing lodging risk simultaneously: A meta-analysis. Eur. J. Agron. 2023, 143, 126709. [Google Scholar] [CrossRef]
- Yuhui, C.; Xujun, C.; Ke, X.; Qikai, X.; Yawen, W.; Jing, L.; Caihong, D.; Zongxiu, S.; Zejian, G.; Jin-Song, Z. Dlf1, a WRKY Transcription Factor, Is Involved in the Control of Flowering Time and Plant Height in Rice. PLoS ONE 2014, 9, e102529. [Google Scholar]
- Guo, D.; Zhang, J.; Wang, X.; Han, X.; Wei, B.; Wang, J.; Li, B.; Yu, H.; Huang, Q.; Gu, H. The WRKY transcription factor WRKY71/EXB1 controls shoot branching by transcriptionally regulating RAX genes in Arabidopsis. Plant Cell 2015, 27, 3112–3127. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Jin, H.; Goff, V.H.; Auckland, S.A.; Rainville, L.K.; Paterson, A.H. Genetic analysis of stem diameter and water contents to improve sorghum bioenergy efficiency. G3. Genes Genomes Genet. 2020, 10, 3991–4000. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xiang, Y.; Xu, E.; Ge, X.; Li, Z. Major co-localized QTL for plant height, branch initiation height, stem diameter, and flowering time in an alien introgression derived Brassica napus DH population. Front. Plant Sci. 2018, 9, 332952. [Google Scholar] [CrossRef]
- Swarup, S.; Cargill, E.J.; Crosby, K.; Flagel, L.; Kniskern, J.; Glenn, K.C. Genetic diversity is indispensable for plant breeding to improve crops. Crop Sci. 2021, 61, 839–852. [Google Scholar] [CrossRef]
- Satterlee, J.W.; Strable, J.; Scanlon, M.J. Plant stem-cell organization and differentiation at single-cell resolution. Proc. Natl. Acad. Sci. USA 2020, 117, 33689–33699. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Du, L.; Qu, Z.; Wang, H.; Dong, S.; Li, X.; Zhao, H. Integrated metabolomics and proteomics analysis to study the changes in Scutellaria baicalensis at different growth stages. Food Chem. 2023, 419, 136043. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Wang, H.; Yin, W.; Meng, W.; Xiao, Y.; Liu, D.; Zhang, X.; Dong, N.; Liu, J.; Yang, Y. Rice DWARF AND LOW-TILLERING and the homeodomain protein OSH15 interact to regulate internode elongation via orchestrating brassinosteroid signaling and metabolism. Plant Cell 2022, 34, 3754–3772. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Guo, T.; Guo, B.; Chen, Z.; An, X. Comprehensive analysis of the R2R3-MYB transcription factor gene family in Populus trichocarpa. Ind. Crops Prod. 2021, 168, 113614. [Google Scholar] [CrossRef]
- Thakur, S.; Vasudev, P.G. MYB transcription factors and their role in Medicinal plants. Mol. Biol. Rep. 2022, 49, 10995–11008. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lu, L.; Sheng, Z.; Zhao, D.; Tao, J. An R2R3-MYB network modulates stem strength by regulating lignin biosynthesis and secondary cell wall thickening in herbaceous peony. Plant J. 2023, 113, 1237–1258. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.E.; Barley, R.; Curtis, M.; Arroyo, J.M.; Dunham, M.; Hudson, A.; Martienssen, R.A. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 2000, 6815, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, X.; Thompson, A.; Guo, M.; Yoshida, S.; Asami, T.; Chory, J.; Yin, Y. Arabidopsis MYB30 is a direct target of BES1 and cooperates with BES1 to regulate brassinosteroid-induced gene expression. Plant J. 2010, 58, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Geisler, M.; Springer, P.S. LATERAL ORGAN FUSION1 and LATERAL ORGAN FUSION2 function in lateral organ separation and axillary meristem formation in Arabidopsis. Development 2009, 136, 2423. [Google Scholar] [CrossRef]
- Wang, X.C.; Wu, J.; Guan, M.L.; Zhao, C.H.; Geng, P.; Zhao, Q. Arabidopsis MYB4 plays dual roles in flavonoid biosynthesis. Plant J. 2020, 101, 637–652. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, N.; Zou, J.; Ran, J.; Chen, X. Rice MYB transcription factor OsMYB1R1 negatively regulates drought resistance. Plant Growth Regul. 2023, 99, 515–525. [Google Scholar] [CrossRef]
- Aydın, G. Effect of Osmyb4 gene expression on salinity tolerance of potato transformed with Oryza sativa Osmyb4 gene. Anadolu Tarim Bilim. Derg. 2020, 35, 115–123. [Google Scholar] [CrossRef][Green Version]
- Jin, H.; Cominelli, E.; Bailey, P.; Parr, A.; Mehrtens, F.; Jones, J.; Tonelli, C.; Weisshaar, B.; Martin, C. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J. 2000, 19, 6150–6161. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Man, Y.; Wang, Y. Light-and temperature-induced expression of an R2R3-MYB gene regulates anthocyanin biosynthesis in red-fleshed kiwifruit. Int. J. Mol. Sci. 2019, 20, 5228. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Shan, J.; Li, Y.; Zhang, Y.; Wang, Y.; Li, W.; Zhao, L. Overexpression of GmGAMYB accelerates the transition to flowering and increases plant height in soybean. Front. Plant Sci. 2021, 12, 667242. [Google Scholar] [CrossRef]
- Liao, Y.; Zou, H.-F.; Wang, H.-W.; Zhang, W.-K.; Ma, B.; Zhang, J.-S.; Chen, S.-Y. Soybean GmMYB76, GmMYB92, and GmMYB177 genes confer stress tolerance in transgenic Arabidopsis plants. Cell Res. 2008, 18, 1047–1060. [Google Scholar] [CrossRef]



| Trait | Year | Parents | CSSLs (n = 207) | |||
|---|---|---|---|---|---|---|
| ZYD00006 | Suinong14 | Mean ± SD | Kurtosis | Skewness | ||
| SD | 2022 | 4.97 ** | 8.73 | 7.75 ± 1.09 | 0.35 | −0.54 |
| 2023 | 4.85 ** | 8.25 | 7.78 ± 1.13 | 0.53 | −0.40 | |
| Trait | Year | Chr/LG | QTL | Position (Mb) | LOD | R2 | ADD | Previous Studies |
|---|---|---|---|---|---|---|---|---|
| SD | 2022 | Chr06/C2 | qSD-22-06 | 24.0 | 3.74 | 4 | 2.12 | qSS-C2-3 [17] |
| Chr19/L | qSD-22-19 | 41.4 | 3.25 | 5 | −0.78 | |||
| Chr04/C1 | qSD-22-04 | 1.3 | 6.40 | 3 | −1.20 | |||
| Chr09/K | qSD-22-09 | 44.3 | 4.42 | 6 | 0.08 | |||
| 2023 | Chr11/B1 | qSD-23-11 | 32.5 | 6.12 | 2 | 0.42 | q11SD2018_DW [18] | |
| Chr04/C1 | qSD-23-04 | 1.3 | 7.29 | 5 | −0.75 | |||
| Chr02/D1b | qSD-23-02 | 15.1 | 3.08 | 3 | 1.32 | |||
| Chr07/M | qSD-23-07 | 36.7 | 3.30 | 4 | 1.76 | qPH-m-2 [27] | ||
| Chr20/I | qSD-23-20 | 17.1 | 5.52 | 4 | −0.56 | |||
| Chr03/N | qSD-23-03 | 42.9 | 5.83 | 2 | −0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Li, F.; Li, L.; Ma, S.; Yu, L.; Tang, C.; Zhao, K.; Song, Z.; Liu, C.; Chen, Q.; et al. QTL Mapping and Candidate Gene Mining for Stem Diameter Using Genetic Basis of Cultivated Soybean and Wild Soybean. Agronomy 2024, 14, 1019. https://doi.org/10.3390/agronomy14051019
Chen L, Li F, Li L, Ma S, Yu L, Tang C, Zhao K, Song Z, Liu C, Chen Q, et al. QTL Mapping and Candidate Gene Mining for Stem Diameter Using Genetic Basis of Cultivated Soybean and Wild Soybean. Agronomy. 2024; 14(5):1019. https://doi.org/10.3390/agronomy14051019
Chicago/Turabian StyleChen, Lin, Fuxin Li, Lanxin Li, Shengnan Ma, Lin Yu, Chunshuang Tang, Kuangyu Zhao, Zhen Song, Chunyan Liu, Qingshan Chen, and et al. 2024. "QTL Mapping and Candidate Gene Mining for Stem Diameter Using Genetic Basis of Cultivated Soybean and Wild Soybean" Agronomy 14, no. 5: 1019. https://doi.org/10.3390/agronomy14051019
APA StyleChen, L., Li, F., Li, L., Ma, S., Yu, L., Tang, C., Zhao, K., Song, Z., Liu, C., Chen, Q., & Wang, J. (2024). QTL Mapping and Candidate Gene Mining for Stem Diameter Using Genetic Basis of Cultivated Soybean and Wild Soybean. Agronomy, 14(5), 1019. https://doi.org/10.3390/agronomy14051019






