Abstract
The processes of occupation and exploitation in the Amazon have been increasing, and as a consequence, forest areas are being replaced by agroecosystems. As a consequence of this change, changes have been occurring in the soil attributes, and consequently, in the stability of aggregates in these environments. Thus, this work had two objectives: the first was to evaluate the impacts generated by the conversion of forests into agroecosystems on the soil attributes that are related to aggregates, in the southwestern region of the Amazon; the second objective was to evaluate the roles of pedophysical and pedochemical parameters on the stability of soil aggregates. The study was carried out on rural properties located in the southern part of Amazonas State, Brazil. Eight areas under different agroecosystems were selected: in the municipality of Canutama: (i) annatto, (ii) guarana, and (iii) cupuassu; in the municipality of Humaitá: (iv) cassava, (v) agroforestry, and (vi) sugarcane; and in the municipality of Manicoré: (vii) pasture and (viii) native forest. Unformed soil samples were collected from the 0.00–0.10 m layer and analyzed for aggregate stability, bulk density, soil organic carbon, and soil organic carbon stock. Univariate, bivariate, and multivariate analyses were performed. The largest soil aggregations occurred in the annatto, guarana, sugarcane, and pasture agroecosystems. We associate the greater aggregation capacity of soils with factors that are inherent to the cultivated species and soil properties. The first factor corresponds to the adaptability of the Amazonian and grass species and their ability to produce biomass. The second factor is related to the physical and chemical properties of Amazonian soils, largely influenced by the sand fraction, soil organic carbon, soil acidity, and availability of exchangeable Ca and Mg.
1. Introduction
Land use and land cover change data revealed that in the last 33 years, Brazil lost 71 Mha of natural vegetation mostly to cattle ranching and agricultural activities [1]. In this setting, recent studies on land use cover changes have shown that the Amazon native forest was reduced by 44.53 Mha, while pasture, agriculture, and planted forest areas increased over the 35 years (1985 to 2020) [2]. The Amazon tropical forest is the primary source of Neotropical biodiversity [3] and has an important and complex role governing the climate [4].
The processes of occupation and exploitation in the Amazon (e. g., road construction, agricultural advancement, and illegal timber trade) have increased deforestation in the recent decades [5]; as a consequence, forest areas have been mostly converted to agricultural and pasture mosaics (agroecosystems). These transformations affect various compartments of the ecosphere, in particular the biosphere and the pedosphere. Regarding the latter compartment, the soil attributes are modified in different ways and magnitudes [6], including the possibility that some agroecosystems can positively affect soil aggregation and carbon stocks, which often depend on the residue management cultural practices adopted in the area [7].
Soil aggregates, in their different classes and sizes, make up a basic unit of the soil structure, which influences and is influenced by many physical, chemical, and biological processes in the soil [8]. In this way, its management is essential for soil health, and studies have observed that aggregates have the ability to stabilize and protect soil organic matter from decomposition, improve water retention, soil infiltration, and hydraulic conductivity [9], and in addition, improve the soil’s resistance to water erosion [10]. Therefore, aggregates are directly related to efficient and sustainable agricultural production, mitigating climate change through the long-term sequestration of C in the soil [11].
The conversion of tropical forests into agroecosystems has consequences on the carbon cycle, impacting ecosystem services and interfering with the quality and maintenance of CO2 fluxes [12]. Previous studies have verified that the conversion of forest ecosystems to cultivated environments in the Amazon decreased the organic C content due to a reduction in the supply of C, losses as a result of erosion and decomposition of organic matter [13], losses of Ca2+ and Mg2+, and the acidification of soils.
Besides organic carbon, other soil attributes are also sensitive to the actions of use and management. The structure disruption, formation of compacted layers, and decrease in macro-pores and aggregate size are examples of these changes [14]. The water infiltration rate and higher root system penetration resistance and soil density are greatly affected by management [15]. In studies on the southern Amazonas, it was indicated that the use of inadequate agricultural practices directly affects the physical and chemical qualities of the soil, causing modifications in the organic matter content and the degradation of the soil attributes [16]. In addition, the weighted average diameter (WAD), geometric mean diameter (GAD), and different aggregate size class (e.g., >2, 1–2, and <1 mm) are key determinants of the soil quality and structure [17] and also play a central role in carbon accumulation [18,19].
Thus, knowledge about the changes in soil attributes caused by anthropic actions enables the adoption of management practices that allow for increased crop yields combined with environmental conservation [20,21]. The evaluation of soil attributes in natural and anthropized environments becomes necessary to seek subsidies and propose management techniques that aim to minimize and even inhibit possible changes [22].
Therefore, there is a need for studies that compare forest areas undergoing conversion to agroecosystems (agricultural areas and pastures), with the main hypothesis that the transformation of forest areas into agricultural areas and pastures changes the soil attributes, and consequently, soil aggregation. Thus, this work had two objectives: the first was to evaluate the impacts generated by the conversion of forests into agroecosystems on the soil attributes that are related to aggregates, in the southwestern region of the Amazon; the second objective was to evaluate the roles of pedophysical and pedochemical parameters on the stability of soil aggregates.
2. Materials and Methods
2.1. Location and Characterization of the Study Area
The study was carried out on rural properties located in the southern part of Amazonas State, in the municipalities of Canutama, Humaitá, and Manicoré, AM, Brazil. Eight areas with different agroecosystems were selected: three agroecosystems in the municipality of Canutama: (i) annatto (Bixa orellana L.), (ii) guarana (Paullinia cupana (Mart.) Ducke), and (iii) cupuassu (Theobroma grandiflorum (Willd. ex. Spreng) Schum); three agroecosystems in the municipality of Humaitá: (iv) cassava (Manihot esculenta), (v) agroforestry, and (vi) sugarcane (Saccharum officinarum); and two agroecosystems in the municipality of Manicoré: (vii) pasture (Brachiaria brizanta) and (viii) native forest (Figure 1 and Table 1).
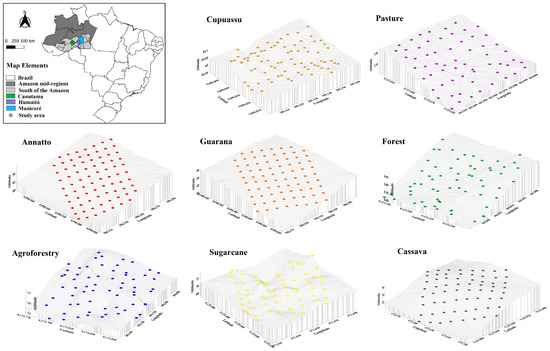
Figure 1.
Locations and sampling maps of areas with forest and agroecosystems in the southwestern Amazon region.

Table 1.
Descriptions of use and history of areas with forest and agroecosystems in the southern region of Amazonas.
The region has a tropical rainy climate, with an average rainfall between 2.250 and 2.750 mm/year, with a short dry period and a rainy period between October and June. The air humidity varies between 85 and 90% while the average annual temperature varies between 25 and 27 °C [23]. The rainy season in this region is from October to June, while the dry season is between June and August (Figure 2), according to data from the National Institute of Meteorology (INMET).
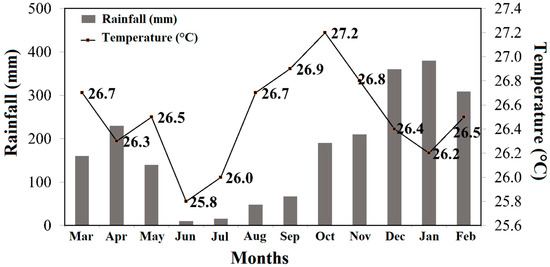
Figure 2.
Cumulative temperature and precipitation for the period of March 2021 to February 2022 at the study site. Source: National Institute of Meteorology—INMET, website address http://www.inmet.gov.br (accessed on 28 July 2023).
The soil in the region was classified as Argissolo Vermelho-Amarelo Distrófico abrúptico, according to the Brazilian System of Soil Classification [24], and as Chromic Abruptic Acrisol (Clayic), according to the World Reference Base of Soils [25].
The agroecosystems situated in the municipality of Canutuma are located on the Amazonian Plain between the Purus and Madeira rivers, associated with ancient alluvial sediments from the Tertiary period, and characterized by large tabular reliefs, with very gentle relief and poor natural drainage [26]. The agroecosystems located in the region of Humaitá are founded on material originating from recent alluvial sediments, which chronologically originate from the Holocene. On the other hand, the agroecosystems located in the Manicoré region present soils developed from Rondonian granites originating from the Upper Precambrian [27].
2.2. Soil Sampling and Collection
To collect the soil, the litter layer present on the soil surface was removed. In all study environments, at each crossing point of the sampling grids, simple deformed and undeformed soil samples were collected at a depth of 0.00–0.10 m to determine the soil attributes.
Grids were established according to the size of the agroecosystem. In the area of the guarana, 90 × 70 m grids were established, with regular spacing between the sampling points of 10 × 10 m; for annatto, the established mesh was 90 × 56 m, with spacing between the sample points of 10 × 8 m; for the cupuassu area, the mesh had dimensions of 54 × 42 m, with regular spacing between the sample points of 6 × 6 m. In these areas, 80 sampling points were collected. In the cassava, sugarcane, agroforestry, pasture, and forest areas, 70 × 70 m grids were established, covering 0.49 ha, with 64 sampling points being collected in each area at the intersections of the grids, at regular spacings of 10 × 10 m.
2.3. Laboratory Analysis
The soil samples were dried in the shade, broken manually, and passed through a set of sieves with a mesh diameter of 9.51 mm, diameter of 4.76 mm, and diameter of 2.00 mm.
The separation and stability of the aggregates was determined according to Kemper and Chepil [28], with modifications in the following diameter classes: 4.76–2.00, 2.00–1.00, 1.00–0.50, 0.50–0.25, 0.25–0.125, and 0.125–0.063 mm. The stability of the soil aggregates was determined using the wet sieving method; after the samples were moistened by capillarity, they were subjected to vertical oscillations for 15 min at a frequency of 32 oscillations per minute using a Yoder vertical shaker [29]. The results were expressed as a percentage of the aggregates retained in each of the sieve classes for >2 mm, 1–2 mm, and <1 mm. The weighted average diameter (WAD) (according to Schaller and Stockinger, [30]) and the geometric mean diameter (GAD) [31] were obtained according to Equations (1) and (2), respectively:
where ni is the percentage of aggregates retained in a given sieve, Di is the average diameter of a given sieve, and N is the number of sieve classes.
The textural analysis of the soil was determined using the pipette method, with a 1 mol L−1 NaOH solution as the chemical dispersant and mechanical agitation using a Wagner-type agitator, in a slow rotation apparatus for 16 h at 50 rpm. The coarse and fine sand were separated by sieving, the clay was separated by sedimentation, and the silt was obtained from the difference.
The soil resistance to penetration (SRP), bulk density (BD), and total porosity (Tp) were determined in undeformed samples. The samples collected in volumetric rings were saturated by capillarity, up to about two thirds of the ring height, inside a plastic form. After reaching equilibrium at a matrix potential of −6 kPa, the samples were weighed again, and then the SRP was measured using a benchtop electronic penetrograph (MA-933, Marconi, SP, Brazil). Subsequently, the samples were taken to the oven at 105 °C for the determination of the BD and Tp [29].
The pH in water was determined potentiometrically using a pH meter at a 1:2.5 soil/water ratio. Calcium (Ca2+) and magnesium (Mg2+) were extracted using a 1 mol L−1 KCl solution and determined using atomic absorption spectrometry. The potential acidity (H + Al) was extracted with calcium acetate buffered at pH 7.00 and determined using tilulometry using NaOH at 0.025 mol L−1 [29]. Organic carbon (OC) was determined using the Walkley–Black method, as modified by Yeomans and Bremner [32]. The carbon stock (CS) was defined using Equation (3):
where CS is the carbon stock (t ha−1), BD is the bulk density (Mg m−3), h is the thickness of the soil layer sampled (cm), OC is the organic carbon content (g kg−1), and RM is the mass proportion of rock fragment content (dimensionless).
2.4. Statistical Analysis
Descriptive statistics were carried out to determine the average, median, and dispersion of the samples within each area, which are presented using boxplot graphics. Subsequently, an analysis of variance (ANOVA) was performed at a 5% probability to determine if there was a difference between the environments. After observing the significance, the Tukey test was performed at a 5% probability to discover which environments were different. The hypotheses of data normality within each environment were examined using the Kolmogorov–Smirnov (KS) test.
A bivariate statistical analysis was carried out, with the aim of verifying correlations between the variables studied in order to study the direct or antagonistic influences of physical and chemical taxes on soil aggregates. In the evaluation, the Pearson correlation test was used at a 5% probability.
A principal component factor analysis (PCA) was carried out, with the objective of finding the statistical significance of the sets of chemical and physical attributes in order to find which of the attributes that were reallocated to soil aggregates discriminated the environments the most.
The adequacy of the factorial analysis was performed using the Kaiser–Meyer–Olkin (KMO) measure, which evaluates the simple and partial corrections of the variables. Bartlett’s sphericity test was also performed to reject the equality between the correlation matrix and the identity. The extraction of factors was performed using the PCA, incorporating the variables that had commonalities equal to or greater > 5.0. The choice of the number of factors to be used was made using the Kaiser criterion (factors with eigenvalues greater than 1.0). In order to simplify the factorial analysis, an orthogonal rotation (varimax) was performed, and the variables and scores for the main components were represented in a factorial plane [33].
3. Results
3.1. Soil Aggregates in Different Agroecosystems
The WAD, GAD, and aggregate size distributions in different agroecosystems are shown in Figure 3. The MWD was significantly higher for annatto and pasture (3.13%; p < 0.05), although it did not differ significantly from the sugarcane area (3.08%; p < 0.05) (Figure 3). The GAD was significantly higher for pasture, forest, guarana, and annatto (2.83, 2.81, 2.79, 2.76%; p < 0.05). The MWD and GAD are significantly lower for agroforestry (2.76 and 2.04%, respectively; p < 0.05).
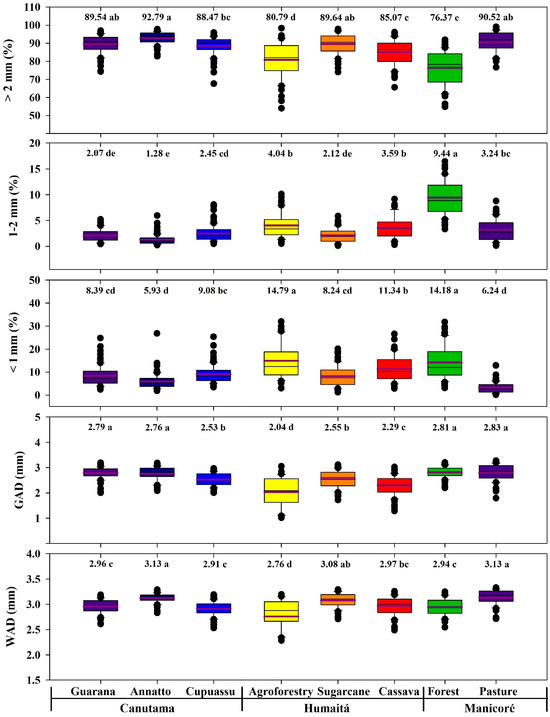
Figure 3.
Boxplots and average tests for soil aggregates in areas with forest and agroecosystems in the southwestern Amazon region. The purple lines and the values above the graphs indicate the average values of the data. The different lowercase letters represent the significant differences for the environments studied, which were determined using the Tukey test at 5%.
The aggregates showed significant differences for the size classes >2.00, 2.00–1.00, and <1.00 mm (p < 0.05) (Figure 3). The distribution of the aggregate size class > 2 mm followed the order annatto > pasture > sugarcane > guarana > cupuassu > cassava > agroforestry > forest. These aggregate size classes were significantly higher for annatto (92.79%; p < 0.05). However, this aggregate class did not differ significantly between the annatto, pasture, and sugarcane areas (p < 0.05) (Figure 3). The content of aggregates > 2 mm is significantly lower in the forest area (76.37%, respectively; p < 0.05).
For the size class 2.00–1.00, the forest, agroforestry, and cassava environments showed the highest values compared to the other agroecosystems (p < 0.05). Thus, the distribution of the aggregate size class 1.00–2.00 mm followed the order forest > agroforestry > cassava > pasture > cupuassu > sugarcane > guarana > annatto, being statistically higher in the forest area (9.44%; p < 0.0 5). The size class 2.00–1.00 mm is also statistically higher in the forest, agroforestry, and cassava areas (4.04 and 3.59%, respectively; p < 0.05) compared to other agroecosystems. These aggregate sizes are statistically lower in the annatto area (1.28%; p < 0.05), although they did not differ significantly from sugarcane and guarana areas (2.12 and 2.07%, respectively; p < 0.05) (Figure 3).
The distribution of the aggregate size class < 1.00 mm followed the order agroforestry > forest > cassava > cupuassu > guarana > sugarcane > pasture > annatto, being statistically higher in the forest and agroforestry areas (14.79 and 14.18%, respectively; p < 0.05). These aggregate sizes are statistically lower for pasture and annatto (6.24 and 5.93%, respectively; p < 0.05), although they did not differ significantly from guarana and sugarcane areas (8.39 and 8.24%, respectively; p < 0.05) (Figure 3).
3.2. Physical and Chemical Attributes of Soil in Different Agroecosystems
The physical and chemical attributes of the soils are shown in Figure 4. Statistical differences were found for the BD, SRP, and Tp (p < 0.05). The BD was significantly higher for agroforestry, pasture, and sugarcane samples (1.31, 1.31, and 1.27 g cm−3, respectively; p < 0.05). The BD was significantly lower in the cupuassu and forest samples (0.92 and 0.88 g cm−3, respectively; p < 0.05). The SRP was significantly higher in cassava and agroforestry samples (4.56 and 4.48 MPa, respectively; p < 0.05), while those of cupuassu and forest presented the lowest BD values (0.56 MPa; p < 0.05). The Tp was significantly higher in the forest sample compared to other agroecosystems (48.42%; p < 0.05). Similar, the Tp for guarana and annatto are also significantly higher in relation to the other agroecosystems (45.86 and 45.53%, respectively; p < 0.05) (Figure 4). The sugarcane sample showed the lowest Tp contents (36.10%; p < 0.05).
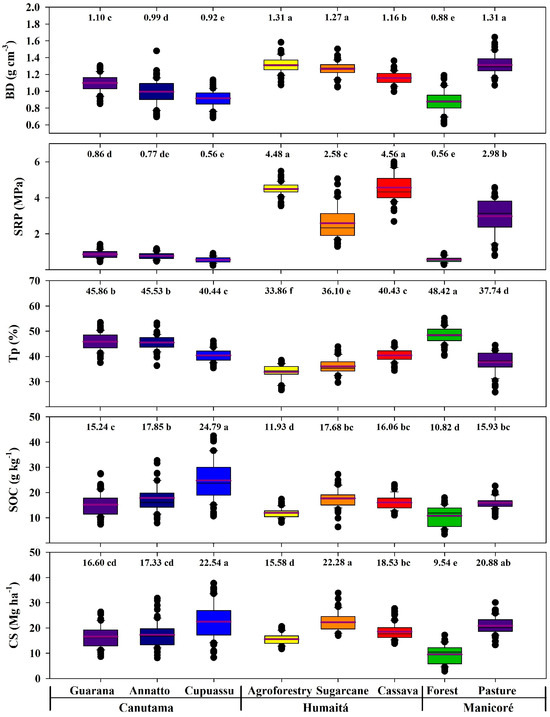
Figure 4.
Boxplots and tests for average density, resistance to penetration, porosity, organic carbon, and soil carbon stock in areas with forest and agroecosystems in southwestern Amazonia. The purple lines and the values above the graphs indicate the average values of the data. The different lowercase letters represent the significant differences of the environments studied, which were determined using the Tukey test at 5%.
The SOC was significantly higher for cupuassu (24.79 g kg−1; p < 0.05). The forest and agroforest areas showed the lowest SOC contents (11.93 and 10.82 g kg−1; p < 0.05). The CS contents were statistically higher in the cupuassu and sugarcane areas (22.54 and 22.28 Mg ha−1, respectively; p < 0.05), although they did not differ significantly from the pasture area (20.88 Mg ha−1; p < 0.05). The agroforestry area showed lower CS contents (15.58 Mg ha−1; p < 0.05), although this did not differ significantly from the annatto and guarana areas (17.33 and 16.60 Mg ha−1; p < 0.05) (Figure 4).
The sand contents were significantly higher in the pasture area (410 g kg−1; p < 0.05). Annatto and guarana areas presented similar sand contents (280 and 372, respectively; p < 0.05), with the same occurring in agroforestry, forest, and sugarcane areas (221, 241, and 241 g kg−1, respectively; p < 0.05). The lowest sand contents were observed in the cassava agroecosystem (159 g kg−1; p < 0.05). The silt levels showed significant differences between the agroecosystems, being highest in cassava (559 g kg−1; p < 0.05) and lowest in the pasture and agroforestry areas (228 and 230 g kg−1, respectively; p < 0.05). The clay contents were higher for agroforestry (549 g kg−1; p < 0.05), followed by pasture (362 g kg−1; p < 0.05), cupuassu, cassava, and sugarcane (272, 282 and 285 g kg−1, respectively; p < 0.05). The lowest clay contents occurred in the guarana and annatto areas (215 and 230 g kg−1, respectively; p < 0.05). Thus, the texture classes of the agroecosystems are clay (agroforestry), clay loam (pasture and sugarcane), silty clay loam (cassava), silt loam (forest), and loam (annatto, cupuassu, and guarana) (Figure 5).
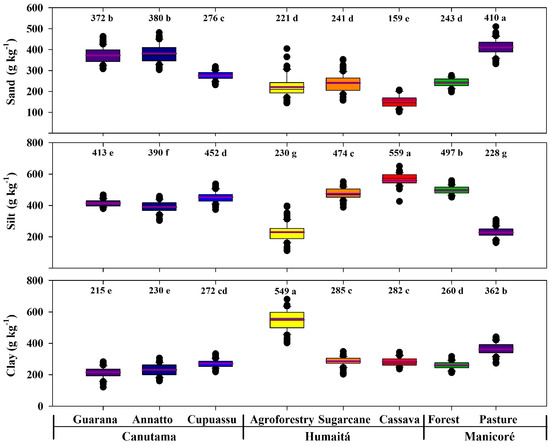
Figure 5.
Boxplots and mean tests for soil textures in areas with forest and agroecosystems in southwestern Amazonia. The purple lines and the values above the graphs indicate the average values of the data. The different lowercase letters represent the significant differences of the environments studied, which were determined using the Tukey test at 5%.
The acidity and exchangeable cations (Ca2+ and Mg2+) are presented in Figure 6. The sugarcane sample showed a strong acid reaction (pH 4.42), which differed significantly from other agrosystems, which were very strongly acid (pH < 4.30). The H + Al content was statistically highest in the agroforestry area (18.95 cmolc kg−1; p < 0.05), followed by cassava (17.77 cmolc kg−1; p < 0.05). These contents were similar in the guarana, cupuassu, forest, and annatto areas (10.74, 10.29, 10.28, and 9.43 cmolc kg−1, respectively; p < 0.05), but significantly different for pasture (6.11 cmolc kg−1; p < 0.05). All agrosystems showed very low Ca2+ contents (<2 cmolc kg−1). These values were significantly higher in annatto and sugarcane (1.51 and 1.46 cmolc kg−1, respectively; p < 0.05), while the agroforestry and cassava areas exhibited the lowest Ca2+ contents (0.24 cmolc kg−1; p < 0.05). The Mg2+ contents are considered low in the agrosystems (<1.0 cmolc kg−1), being significantly higher in the sugarcane area (1.51 and 0.80 cmolc kg−1; p < 0.05) and lowest in the cassava and agroforestry areas (0.14 cmolc kg−1; p < 0.05).
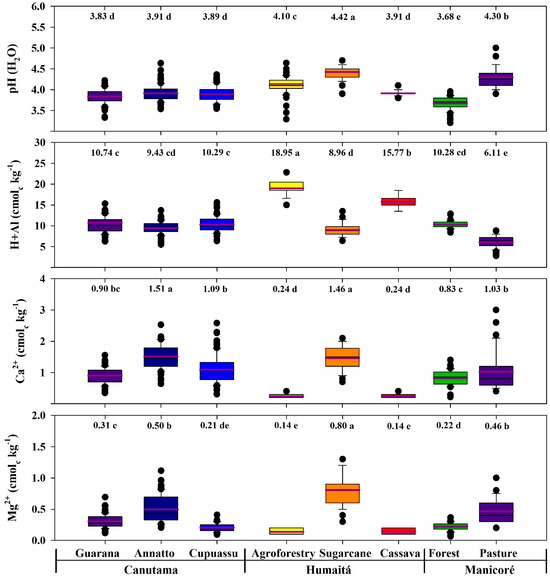
Figure 6.
Boxplots and mean tests for soil chemical attributes in areas with forest and agroecosystems in southwestern Amazonia. The purple lines and the values above the graphs indicate the average values of the data. The different lowercase letters represent the significant differences of the environments studied, which were determined using the Tukey test at 5%.
3.3. Interaction of Soil Attributes and Aggregates
We used Pearson’s correlation to evaluate the interactions in order to find the direct and antagonistic effects between the aggregates and the physical and chemical attributes of the soil (Table 2). The aggregate size class > 2 mm presented a negative correlation with the SRP (r = −0.11; p < 0.05), clay, and H + Al (r = −0.20; r = −0.23, respectively; p < 0.05) and a positive correlation (p < 0.05) with CS (r = 0.37), SOC (r = 0.32), sand (r = 0.31), exchangeable cations (r = 0.28), and pH (r = 0.19). A negative correlation (p < 0.05) was obtained between the aggregate size class 1–2 mm and CS (r = −0.43), SOC (r = −0.36), Mg2+ (r = −0.26), Ca2+ (r = −0.24), pH and sand (r = −0.25), and BD (r = −0.16). These aggregates also present a positive correlation with the Tp and silt (r = 0.17; r = 0.13, respectively; p < 0.05). In addition, the aggregate size class < 1 mm presented a negative correlation (p < 0.05) with CS and sand (r = −0.29), Ca2+ (r = −0.27), SOC (r = −0.26), Mg2+ (r = −0.25), and pH (r = −0.15) and a positive correlation with H + Al, clay, and SRP (r = 0.27; r = 22, and r = 0.15, respectively; p < 0.05).

Table 2.
Pearson’s correlation of soil attributes with aggregates in agroecosystems in southwestern Amazonia.
A negative correlation (p < 0.05) also was obtained between the GAD and H + Al, SRP (r = −0.40), and clay (r = −0.35), as well as between the WAD and SRP (r = −0.10; p < 0.01), H + Al, and clay (r = −0.27; r = −0.17, respectively; p < 0.05). Lastly, a positive correlation (p < 0.05) was obtained between the GAD and sand (r = 0.34), Tp (r = 0.33), Ca2+ (r = 0.28), and Mg2+ (r = 0.17) and between the WAD and Mg2+ (r = 0.27), Ca2+ (r = 0.25), sand (r = 0.18), CS (r = 0.16), SOC (r = 0.12), and pH (r = 0.13).
The factor analysis presented satisfactory results (KMO = 0.710; p < 0.05 for Bartlett’s test of sphericity) for the variables, showing suitability for the construction of a principal components analyses (PCAs). Two factors were responsible for explaining 55.93% of the variance in the distribution of households with eigenvalues greater than 1 (Figure 7).
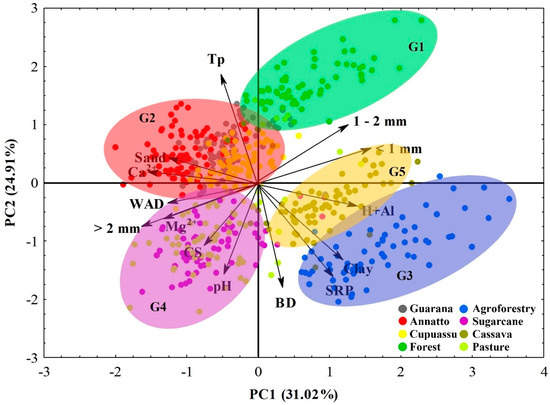
Figure 7.
Principal component analysis for areas with forest and agroecosystems in the southwestern Amazon region.
PCA 1 explained 31.02% of the data set’s total variance. This PCA shows a strong positive correlation with aggregates of 1–2 mm and <1 mm, H + Al, SRP, clay, and DB and a strong negative correlation with the Tp, sand, Ca2+, Mg2+, aggregates > 2 mm, WAD, CS, and pH. PCA 2 explained 24.91% of the variance in the data. This PCA shows a strong positive correlation with the Tp, sand, Ca2+, aggregates of 1–2 mm and <1 mm and a strong negative correlation with the WAD, aggregates > 2 mm, Mg2+, CS, pH, H + Al, clay, BD, and SRP. Thus, the aggregates of 1–2 mm and <1 mm and the Tp are mainly related to the forest area (Group 1); sand and Ca2+ are related to annatto, guarana, and cupuassu areas (Group 2); clay, H + Al, and SRP are related to agroforestry (Group 3); the WAD, aggregates > 2 mm, Mg2+, CS, and pH are related to sugarcane and pasture areas (Group 4); and finally, G5 is formed by the cassava area, which is close to the characteristics of the agroforestry area (Figure 7).
4. Discussion
4.1. Effects of Management on Soil Attributes Related to Aggregates
The conversion of natural vegetation to agroecosystems has a direct impact on the soil attributes related to aggregation [18]. Studies have shown that the ability of agroecosystems to become more similar to natural environments is related to their capacity to incorporate carbon from the biomass of the aerial part [34] or the role of root growth in crop plants [35]. The largest classes of aggregates and diameters observed in environments with species of Amazonian origin (guarana and annatto) and grasses (sugarcane and pasture) (Figure 3) may be related to the adaptability of Amazonian species, the behaviors of grasses (fasciculated roots and dry mass production capacity), and the associated total organic carbon content, as seen in Figure 4.
The agroecosystems with cupuassu, pasture, and sugarcane had a higher capacity to store carbon than the other agroecosystems evaluated (Figure 4). These results are contrary to those reported in oxisols from agriculture and forest environments in the southern part of the Amazon, where the lowest SOC values were found in grassland areas compared to forest areas [36]. Some agroecosystems, especially those with native species, can present lower C losses, and therefore, higher organic carbon reserves when compared to forest environments, thus, not compromising the long-term sustainability of the systems in terms of the carbon balance [7]. In addition, we must also consider the high export of organic C by the forest in Amazonian environments due to the acidic nature of the soils, as well as the capacity and quality of the burlap formed [37]. Lastly, carbon is contained in the more labile fractions (free light fraction—FLL: 26–90% of the soil carbon) of the surface layers of soil under the Amazonian forest, which can easily be released from the soil through microbial activity due to the sensitivity of the FLL to management [38]. Another factor to be considered is that in most agricultural environments evaluated, producers leave residues of cultivated and invasive plants on the soil surface, favoring the increase in organic carbon in these environments [39].
The native forest conversion for crop plants in the Amazon region favors the process of soil erosion, reducing the clay content and increasing the sand content [14,40]. As a result, there is a reduction in the aggregate class > 2 mm and in aggregate diameters, favoring an increase in the aggregate classes 1–2 mm and <1 mm. Consequently, there is an increase in the soil density and soil resistance to penetration, resulting in a reduction in the total pore volume [40]. In contrast, in these studies, the areas with guarana and annatto presented higher values for the aggregate size class > 2 mm, GAD, and sand and the lowest clay contents (Figure 3, Figure 4 and Figure 5). This result is possibly related to a combination of factors, linked to the abundant development of the root system in terms of its depth, increased activity of soil macrofauna, and lack of soil preparation during the crop cycle [15]. Since the large macroaggregates (LMAs: >2 mm) are positively correlated with soil’s physicochemical and biological variables [41], the change in the proportion of these LMAs indicate that they can be used as a sensitive indicator to predict changes in the soil properties after natural forest conversion for agroecosystems in the Amazon. Areas with sugarcane and pasture also have high values of the aggregate class > 2 mm, GAD, WAD, BD, and SRP. These higher BD and SRP values in the pasture are due to trampling by large animals [40], while in the sugarcane area, they are due to soil preparation with heavy machines [15].
The management adopted for different agroecosystems has a direct impact on the soil chemistry, altering the active acidity (pH) and potential acidity (H + Al), which in turn have a direct effect on the availability of Ca2+ and Mg2+ for the soil solution, and consequently, for crop plants. This agrees with previous studies, showing that many soil properties are changed in the natural vegetation conversion to agricultural fields [42,43]. In our study, the environments cultivated with sugarcane had the highest pH values and lowest H + Al values, and consequently, the highest levels of Ca2+ and Mg2+. Previous studies also showed that sugarcane land use increases soil nutrient levels and reduces the soil acidity due to inputs of lime and fertilizers [44] (Figure 6). The soil pH has a strong effect on the concentrations of Ca2+ and Mg2+ in the solution, controlling the solubility of their respective metal hydroxides [45]. Thus, the content of these exchangeable cations, as well as Al3+ and Fe3+, has a great impact on soil aggregation, influencing the bonds between particles through particle coagulation and the strength of the interactions between the soil organic matter and particles. The strong correlation found between the aggregates size class > 2 mm and Ca2+ and Mg2+ confirms this evidence.
When evaluating the PCA, we observed the formation of five distinct groups. Group 1 was formed by the forest, characterized by having a higher proportion of aggregate of classes 1–2 mm and <1 mm and Tp. Higher values of these attributes in the forest area is due the accumulation of plant residues in the soil’s surface, constant renewal of the root system, high fauna and flora activity (pedobioturbation), and absence of anthropic changes. Similar results were obtained in others Amazon forest fragments [46]. Group 2 was formed by the guarana, annatto, and cupuassu areas and is characterized by sand and Ca2+, which may indicate a slight improvement in the chemical quality of the sandy soil surface, which is more susceptible to nutrient losses.
Group 3, formed by agroforestry, is characterized by SRP, clay and H + Al. Similarly, Group 5 is formed by cassava, which is close to the characteristics of agroforestry. This evidence confirms the role of clay in the retention of acid cations (cationic exchange complex) and chemical cementation, where the clay surfaces interact to form strong bonds that substantially increase soil adhesion, and consequently, increases the soil penetration resistance.
Group 4 (sugarcane and pasture) is characterized by higher aggregation (class > 2 mm, WAD), CS, pH, and Mg2+. The vigorous root system of grasses (e.g., Brachiaria genera) increase the aggregate stability and improve the soil quality, since (i) root systems increase carbon, which acts on the formation and stabilization of the soil structure (WAD x CS: r = 0.16; p < 0.05); (ii) roots release exudates that have a cementing effect, which influences the association of soil particles to stabilize macroaggregates [19]; and (iii) roots contribute to SOC and soil aggregation through the hosting of micro- and macroorganisms (SOC x > 2 mm: r = 0.32; SOC x WAD: r = 0.12; p < 0.05) [47].
Lastly, also is important to highlight that some aspects of implementing these systems under Amazonian conditions are advantageous; examples include soil water retention, a reduction in production costs, increased crop yields [48], and a reduction in runoff and sediment production [49]. The use of proper cultivation techniques associated with the plant species can cause the system to sequester C from the atmosphere, being an important strategy to offset CO2 emissions from fossil fuel consumption and mitigate climate change [50].
4.2. Soil Attributes Associated with Aggregate Formation
The interactions between the physical and chemical attributes involved in the formation of aggregates are highly complex and affected by a series of soil properties [46]. Our results indicate that aggregates in different agroecosystems of the Amazon biome are strongly influenced by the soil porosity (Tp); soil organic carbon contents and stock; texture, being mainly the particle size class, sand, and silt; soil acidity (pH and H + Al); and by the exchangeable cations (Ca2+ and Mg2+).
The attributes related to soil compaction (BD and SRP) showed a strong negative correlation with soil aggregation (Table 2). In general, the attributes related to macroaggregation and aggregates stability are positively correlated with SOC and negatively correlated with clay. This fact is due to the effect of the SOC on the physical properties of the soil, increasing aggregation (binding agent for soil aggregates and their stability), improving the soil porosity, and reducing the soil density [51]. Furthermore, previous studies have shown that in soils with a clay content greater than 42.46%, increasing the bulk density increased the clay content per unit volume, and consequently, increased soil-to-soil adsorption, which led to increased rates of adhesion and cohesion [52]. Thus, in an agroforestry system, the higher BD and clay contents contribute significantly to the increase of the SRP. Similar results were obtained in soils with different uses in the south Amazon region, where the positive correlation between the SRP and clay was justified by filling the spaces between the sand particles using the clay [16]. This higher soil compaction directly interferes with the soil porosity, which in turn is strongly influenced by the size of the aggregates, where the spaces between these aggregates are responsible for the formation of pores [46]. This assertation is confirmed by the correlation found between the Tp x aggregate 1–2 mm, Tp x GAD, and GAD x sand (p < 0.05), pointing toward the macroporosity formed by the stacking of grains (mainly grains) and aggregates with a higher GAD in soils with greater sand contents.
The SOC and CS provided by the addition of organic matter in the different agroecosystems also directly influenced soil aggregation (>2 mm and WAG) (Table 2). This is due to the binding action provided by the components present in soil organic matter, where the products of its decomposition are used in the process of agglutinating soil particles, forming aggregates [34]. Our results showed that the SOM was important in promoting soil aggregation, especially in macroaggregates (aggregates size class < 2 mm). Previous studies also have reported a strong preferential accumulation of carbon in large macroaggregates [53,54]. Given the fact that microaggregates are bound together by persistent binding agents and then enmeshed (bound by temporary and transient binding agents to form macroaggregates [18]), our results point out that the increase in macroaggregation in Amazonian agroecosystems is strongly linked to soil carbon accumulation, where the carbon that is probably bound to microaggregates is further protected within macroaggregates. Although we did not evaluate soil microorganisms, studies have shown that with more carbon stored in large macroaggregates, more carbon is protected from microbial decomposition, being stored over a longer period, resulting in a net C sequestration into the soil rather than loss to the atmosphere as CO2 [55]. Thus, macroaggregates in agroecosytems in the Amazon can control the capacity of C soil stores.
Sand and clay were the particles that contributed most to the formation of aggregates. The sand fraction contributed to the formation of larger aggregates (>2 mm), GAD, and WAD, while the clay was involved in the formation mechanisms of smaller aggregate classes (1–2 mm and <1 mm) (Table 2). The soils studied are formed from the incomplete action of the ferralitization process, which results in a mineralogy consisting of 1:1 phyllosilicates (kaolinite) and iron oxides (goethite and hematite). Thus, the greater effect of sand on the formation of aggregates > 2 mm, GAD, and WAD is probably related to the presence of organic matter associated with oxides, which contribute to greater aggregation in these soils [56]. In this process, oxides act as a cementing agent through adsorption to the negative charges of organic colloids and can coat the surfaces of minerals, forming bridges (including other cations) between primary and secondary particles that constitute macroaggregates. The strong positive correlations found between the aggregates > 2 mm with sand, SOC, Ca2+, and Mg2+ confirm these assertions. These results corroborate studies that showed that around 50% of the Fe content in a wide variety of soils is associated with the silt and sand fractions, which as a result, are dominant fractions of water-stable macroaggregates [57,58].
Our data also indicate that soil acidification caused by the release of organic acids in root exudation and the decomposition of organic matter by microorganisms [59] contributes to the formation of macroaggregates and increases the WAD. Macroaggregates present a greater diversity of soil bacterial and fungal communities, where the SOC Tp influences the bacterial and soil pH, affecting the soil fungal community and leading to the increased negative control of the respiration of soil aggregates [60]. Fungal hyphae represent temporary and transient binding agents that bind microaggregates to form macroaggregates [18]. Thus, probably the most acidic soil conditions must protect the microbiological decomposition of organic carbon bound to mineral surfaces within large aggregates [61].
Soil chemical properties have a direct impact on crop development; however, little information is available on the effects of the soil structure, mainly related to soil aggregates. Our results showed a strong positive correlation between the soil acidity (pH and H + Al) and the exchangeable bases Ca2+ and Mg2+ with soil aggregates. Thus, the pH, Ca2+, and Mg2+ directly influenced the formation of aggregates > 2 mm, GAD, and WAD. With an increasing pH, there is greater availability of Ca2+ and Mg2+ in the soil solution due to the formation of cationic bridges between primary minerals covered by organic colloids and clay minerals [48]. Furthermore, we also observed an antagonistic effect of the pH on the formation of aggregates of 1–2 mm and <1 mm (Table 2). This fact can be explained by the increase in agglutination between particles and the formation of organomineral complexes, with a decrease in the pH due to the reduction in the repulsion of colloidal constituents with a negative surface charge [45].
5. Conclusions
The largest soil aggregations occurred in the annatto, guarana, sugarcane, and pasture agroecosystems when compared to other uses. To this, we add the importance of agroforestry with these trees and shrubs in order to minimize soil degradation, which can be achieved through the increase in the carbon content and the formation of macroaggregates in the soil.
We associate the greater aggregation capacity of soils with factors inherent to the cultivated species and soil properties. The first factor corresponds to the adaptability of Amazonian and grass species and their ability to produce biomass. The second factor is related to the physical and chemical properties of Amazonian soils, largely influenced by the sand fraction, organic carbon, soil acidity, and availability of Ca2+ and Mg2+.
The formation of macroaggregates is strong influenced by the SOC, while smaller aggregates of 1–2 mm and <1 mm are related to the acidification process in these environments. Thus, changes in the aggregates size class during natural forest conversion to crop plants are sensitive and useful early indicators of a changing soil quality and structure, which could potentially be applied in soil quality investigations in response to changes in field management.
Author Contributions
Conceptualization, A.F.L.d.L., M.C.C.C. and B.C.M.; methodology, J.d.B.S. and F.G.d.S.; software, R.M.B.; validation, A.F.L.d.L., M.C.C.C. and D.M.P.d.S.; formal analysis, R.S.M., W.d.O.A. and F.P.d.O.; investigation, A.F.L.d.L.; resources, M.C.C.C., D.M.P.d.S. and R.M.B.; data curation, F.G.d.S.; writing—original draft preparation, A.F.L.d.L. and M.C.C.C.; writing—review and editing, J.d.B.S., W.d.O.A. and F.P.d.O.; supervision, M.C.C.C.; project administration, M.C.C.C., D.M.P.d.S. and F.G.d.S.; funding acquisition, M.C.C.C., D.M.P.d.S. and R.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the first author (Alan Ferreira Leite de Lima).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Souza, C.M., Jr.; Shimbo, J.Z.; Rosa, M.R.; Parente, L.L.; Alencar, A.A.; Rudorff, B.F.T.; Hasenack, H.; Matsumoto, M.; Ferreira, L.G.; Souza-Filho, P.W.M.; et al. Reconstructing three decades of land use and land cover changes in Brazilian biomes with Landsat archive and Earth Engine. Remote Sens. 2020, 12, 2735. [Google Scholar] [CrossRef]
- Silveira, J.G.; Oliveira Neto, S.N.; Canto, A.C.B.; Leite, F.F.G.D.; Cordeiro, F.R.; Assad, L.T.; Silva, G.C.C.; Marques, R.O.; Dalarme, M.S.L.; Ferreira, I.G.M.; et al. Land use, land cover change and sustainable intensification of agriculture and livestock in the Amazon and the Atlantic Forest in Brazil. Sustainability 2022, 14, 2563. [Google Scholar] [CrossRef]
- Antonelli, A.; Zizka, A.; Carvalho, F.A.; Scharn, R.; Bacon, C.D.; Silvestro, D.; Condamine, F.L. Amazonia is the primary source of Neotropical biodiversity. Proc. Natl. Acad. Sci. USA 2018, 115, 6034–6039. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.C.A.; Spracklen, D.V. Climate benefits of intact Amazon forests and the biophysical consequences of disturbance. Front. For. Glob. Chang. 2019, 2, 47. [Google Scholar] [CrossRef]
- West, T.A.P.; Fearnside, P.M. Brazil’s conservation reform and the reduction of deforestation in Amazonia. Land Use Policy 2021, 100, 105072. [Google Scholar] [CrossRef]
- Locatelli, J.L.; de Lima, R.P.; Santos, R.S.; Cherubin, M.R.; Creamer, R.E.; Cerri, C.E.P. Soil Strength and Structural Stability Are Mediated by Soil Organic Matter Composition in Agricultural Expansion Areas of the Brazilian Cerrado Biome. Agronomy 2023, 13, 71. [Google Scholar] [CrossRef]
- Wen, S.; Hao, J.; Wang, J.; Xiong, S.; Jiang, Y.; Zhu, Y.; Jiao, Y.; Yang, J.; Zhu, J.; Tian, X. Enhancing Soil Aggregation and Organic Carbon Retention in Greenhouse Vegetable Production through Reductive Soil Disinfestation with Straw and Fertiliser: A Comprehensive Study. Agronomy 2024, 14, 179. [Google Scholar] [CrossRef]
- Peng, X.; Yan, X.; Zhou, H.; Zhang, Y.Z.; Sun, H. Assessing the contributions of sesquioxides and soil organic matter to aggregation in an Ultisol under long-term fertilization. Soil Tillage Res. 2015, 146, 89–98. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Liang, G.; Zheng, F.; Zhang, M.; Li, S. A global meta-analysis of the impacts of no-tillage on soil aggregation and aggregate-associated organic carbon. Land Degrad. Dev. 2021, 32, 5292–5305. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Yu, X.; Jia, G. Soil microorganism regulated aggregate stability and rill erosion resistance under different land uses. Catena 2023, 228, 107176. [Google Scholar] [CrossRef]
- Zhao, S.X.; Ta, N.; Wang, X.D. Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Tang, X.; Alhaj Hamoud, Y.; Shaghaleh, H.; Zhao, J.; Wang, H.; Wang, J.; Zhao, T.; Li, B.; Lu, Y. Responses of Soil Labile Organic Carbon on Aggregate Stability across Different Collapsing-Gully Erosion Positions from Acric Ferralsols of South China. Agronomy 2023, 13, 1869. [Google Scholar] [CrossRef]
- Noronha, R.L.; Souza, Z.M.; Soares, M.D.R.; Campos, M.C.C.; Farhate, C.V.V.; Oliveira, S.R.M. Soil carbon stock in archaeological black earth under different land use systems in the Brazilian Amazon. Agron. J. 2020, 112, 12–22. [Google Scholar] [CrossRef]
- Souza, F.G.; Campos, M.C.C.; Pinheiro, E.N.; Lima, A.F.L.; Brito Filho, E.G.; Cunha, J.M.; Santos, E.A.N.; Brito, W.B.M. Aggregate stability and carbon stocks in Forest conversion to different cropping systems in Southern Amazonas, Brazil. Carbon Manag. 2020, 11, 81–96. [Google Scholar] [CrossRef]
- Silva, L.I.; Campos, M.C.C.; Wadt, P.G.S.; Cunha, J.M.; Oliveira, I.A.; Freitas, L.; Santos, E.A.N.; Brito Filho, E.G. Resistência à penetração de um Latossolo Vermelho-Amarelo Distrófico sob diferentes manejos e métodos. R. Dep. De Geogr. 2020, 40, 40–48. [Google Scholar] [CrossRef]
- Frozzi, J.C.; Cunha, J.M.; Campos, M.C.C.; Bergamin, A.C.; Brito, W.B.M.; Fraciscon, U.; Silva, D.M.P.; Lima, A.F.L.; Brito Filho, E.G. Physical attributes and organic carbon in soils under natural and anthropogenic environments in the South Amazon region. Environ. Earth. Sci. 2020, 79, 251–266. [Google Scholar] [CrossRef]
- Churchman, G.J. The philosophical status of soil science. Geoderma 2010, 157, 214–221. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro) aggregates soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Silva, J.F.d.; Gontijo Neto, M.M.; Silva, G.F.d.; Borghi, E.; Calonego, J.C. Soil Organic Matter and Aggregate Stability in Soybean, Maize and Urochloa Production Systems in a Very Clayey Soil of the Brazilian Savanna. Agronomy 2022, 12, 1652. [Google Scholar] [CrossRef]
- Ye, L.; Ji, L.; Chen, H.; Chen, X.; Tan, W. Spatial Contribution of Environmental Factors to Soil Aggregate Stability in a Small Catchment of the Loess Plateau, China. Agronomy 2022, 12, 2557. [Google Scholar] [CrossRef]
- Kumar, D.; Purakayastha, T.J.; Das, R.; Yadav, R.K.; Shivay, Y.S.; Jha, P.K.; Singh, S.; Aditi, K.; Prasad, P.V.V. Long-Term Effects of Organic Amendments on Carbon Stability in Clay–Organic Complex and Its Role in Soil Aggregation. Agronomy 2023, 13, 39. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteoro. Zeitschrift. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.E.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- IUSS Working Group WRB World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Maia, M.A.M.; Marmos, J.L. Geodiversidade do Estado do Amazonas; CPRM: Manaus, Brazil, 2010; pp. 73–77. Available online: https://rigeo.sgb.gov.br/handle/doc/16624 (accessed on 21 March 2024).
- Radam Brasil. Levantamento de Recursos Naturais; Ministério das Minas e Energia v. 27: Rio de Janeiro, Brazil, 1982; p. 561.
- Kemper, W.D.; Rosenau, R.C. Size distribution of aggregates. In Methods of Soil Analysis; Part 1. Physical and Mineralogical Methods. Ed 9.; American Society of Agronomy: Madison, NY, USA, 1986; pp. 425–442. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; EMBRAPA: Brasília, Brazil, 2017; 573p. [Google Scholar]
- Schaller, F.W.; Stockinger, K.R. A comparison of five methods for expressing aggregation data1. Soil Sci. Soc. Am. J. 1953, 17, 310–313. [Google Scholar] [CrossRef]
- Alvarenga, C.C.; Mello, C.R.D.; Mello, J.M.D.; Silva, A.M.D.; Curi, N. Índice de qualidade do solo associado à recarga de água subterrânea (IQSRA) na bacia hidrográfica do Alto Rio Grande, MG. Rev. Bras. Cienc. Solo 1986, 36, 1608–1619. [Google Scholar] [CrossRef][Green Version]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Burak, D.L.; Passos, R.R.; Sarnaglia, A.S. Utilização da análise muitivariada na avaliação de parâmetros geomorfológicos e atributos físicos do solo. Enciclopédia Biosf. 2010, 6, 1–11. [Google Scholar]
- Zhao, Z.; Mao, Y.; Gao, S.; Lu, C.; Pan, C.; Li, X. Organic carbon accumulation and aggregate formation in soils under organic and inorganic fertilizer management practices in a rice–wheat cropping system. Sci. Rep. 2023, 13, 3665. [Google Scholar] [CrossRef]
- Zhong, W.; Shuai, Q.; Zeng, P.; Guo, Z.; Hu, K.; Wang, X.; Zeng, F.; Zhu, J.; Feng, X.; Lin, S.; et al. Effect of Ecologically Restored Vegetation Roots on the Stability of Shallow Aggregates in Ionic Rare Earth Tailings Piles. Agronomy 2023, 13, 993. [Google Scholar] [CrossRef]
- Petter, F.A.; Lima, L.B.; Morais, L.A.; Tavanti, R.F.R.; Nunes, M.E.; Freddia, O.S.; Marimon, B.H., Jr. Carbon stocks in oxisols under agriculture and forestin the southern Amazon of Brazil. Geoderma Reg. 2017, 11, 53–61. [Google Scholar] [CrossRef]
- Enck, B.F.; Campos, M.C.C.; Pereira, M.G.; Souza, F.G.; Santos, O.A.Q.; Diniz, Y.V.D.F.G.; Martins, T.S.; Cunha, J.M.; Lima, A.F.L.; Souza, T.A.F.D. Forest–Fruticulture Conversion Alters Soil Traits and Soil Organic Matter Compartments. Plants 2022, 11, 2917. [Google Scholar] [CrossRef]
- Marques, J.D.O.; Luizão, F.J.; Teixeira, W.G.; Nogueira, E.M.; Fearnside, P.M.; Sarrazin, M. Soil carbon stocks under Amazonian forest: Distribution in the soil fractions and vulnerability to emission. Open J. For. 2017, 7, 121–142. [Google Scholar] [CrossRef][Green Version]
- Assunção, S.A.; Pereira, M.G.; Rosset, J.S.; Berbara, R.L.L.; García, A.C. Carbon input and the structural quality of soil organic matter as a function of agricultural management in a tropical climate region of Brazil. Sci. Total Environ. 2019, 658, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.F.L.; Campos, M.C.C.; Enck, B.F.; Simões, W.S.; Araújo, R.M.; Santos, L.A.C.; Cunha, J.M. Physical soil attributes in areas under forest/pasture conversion in northern Rondônia. Brazil. Environ. Monit. Assess. 2022, 194, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yuan, H.; Yu, M.; Cheng, X. Large macroaggregate properties are sensitive to the conversion of pure plantation to uneven-aged mixed plantations. Catena 2020, 194, 104724. [Google Scholar] [CrossRef]
- Melo, V.F.; Orrutéa, A.G.; Motta, A.C.V.; Testoni, S.A. Land use and changes in soil morpgology and physical-chemical properties in southern Amazon. Rev. Bras. Cienc. Solo 2017, 41, e0170034. [Google Scholar] [CrossRef]
- Moura, E.G.; Sousa, R.M.; Campos, L.S.; Cardoso-Silva, A.J.; Mooney, S.J.; Aguiar, A.C.F. Could more efficient utilization of ecosystem services improve soil quality indicators to allow sustainable intensification of Amazonian family farming. Ecol. Indic. 2021, 127, 107723. [Google Scholar] [CrossRef] [PubMed]
- Cherubin, M.R.; Franco, A.L.C.; Cerri, C.E.P.; Oliveira, D.M.S.; Davies, C.A.; Cerri, C.C. Sugarcane expansion in Brazilian tropical soils—Effects of land use change on soil chemical atributes. Agric. Ecosyst. Environ. 2015, 211, 173–184. [Google Scholar] [CrossRef]
- Regelink, I.C.; Stoof, C.R.; Rousseva, S.; Weng, L.; Lair, G.J.; Kram, P.; Nikolaidis, N.P.; Kercheva, M.; Banwart, S.; Comans, R.N. Linkages between aggregate formation, porosity and soil chemical properties. Geoderma 2015, 247, 24–37. [Google Scholar] [CrossRef]
- Soares, M.D.R.; Souza, Z.M.; Campos, M.C.C.; Cooper, M.; Tavares, R.L.M.; Lovera, L.H.; Farhate, C.V.V.; Cunha, J.M. Physical quality and posority aspects of Amazon Anthropogenic soils under different management systems. Appl. Environ. Soil Sci. 2022, 2022, 6132322. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Rao, C.A.R.; Kareemulla, K.; Nagasree, K.B.; Venkateswarlu, S.K. Estimation of economic returns to soil and water conservation research-an ex ante analysis. Agric. Resour. Econ. Rev. 2010, 23, 41–46. [Google Scholar] [CrossRef]
- Ngetich, K.F.; Diels, J.; Shisanya, C.A.; Mugwe, J.N.; Mucheru-muna, M.; Mugendi, D.N. Effects of selected soil and water conservation techniques on runoff. sediment yield and maize productivity under sub-humid and semi-arid conditions in Kenya. Catena 2014, 121, 288–296. [Google Scholar] [CrossRef]
- Oliveira, I.A.; Campos, M.C.C.; Freitas, L.; Aquino, R.E.; Cunha, J.M.; Soare, M.D.R.; Silva, L.S.; Fonseca, J.S.; Silva, D.M.P.; Souza, Z.M.; et al. Carbon stock variability and aggregate stability in soils of Amazon, Brazil. Aust. J. Crop Sci. 2018, 12, 922–930. [Google Scholar] [CrossRef]
- Jiao, S.; Li, J.; Li, Y.; Xu, Z.; Kong, B.; Li, Y.; Shen, Y. Variation of soil organic carbon and physical properties in relation to land uses in the Yellow River Delta, China. Sci. Rep. 2020, 10, 0317. [Google Scholar] [CrossRef]
- Zheng, K.; Cheng, J.; Xia, J.; Liu, G.; Xu, L. Effects of soil bulk density and moisture content on the physico-mechanical propertiesof paddy soil in plough layer. Water 2021, 13, 2290. [Google Scholar] [CrossRef]
- Puerta, V.L.; Pereira, E.I.P.; Wittwer, R.; Heijden, M.V.D.; Six, J. Improvement of soil structure through organic crop management, conservation tillage and grass-clover ley. Soil Tillage Res. 2018, 180, 1–9. [Google Scholar] [CrossRef]
- Guest, E.J.; Palfreeman, L.J.; Holden, J.; Chapman, P.J.; Firbank, L.G.; Lappage, M.G.; Helgason, T.; Leake, J.R. Soil macroaggregation drives sequestration of organic carbon and nitrogen with three-year grass-clover leys in arable rotations. Sci. Total Environ. 2022, 852, 158358. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Rasmussen, C.; Heckman, K.; Wieder, W.R.; Keiluweit, M.; Lawrence, C.R.; Berhe, A.A.; Blankinship, J.C.; Crow, S.E.; Druhan, J.L.; Hicks Pries, C.E.; et al. Beyond clay: Towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 2018, 137, 297–306. [Google Scholar] [CrossRef]
- Barberis, E.; Ajmore-Marsan, F.; Boero, V.; Arduino, E. Aggregation of soil particles by iron oxides in various size fractions of soil B horizons. J. Soil Sci. 1991, 42, 525–542. [Google Scholar] [CrossRef]
- Puget, P.; Chenu, C.; Balesdent, J. Dynamics of soil organic matter associated with particle-size fractions of water-stable aggregates. Eur. J. Soil Sci. 2000, 51, 595–605. [Google Scholar] [CrossRef]
- Perelomov, L.V. The role of interactions between bacteria and clay minerals in pedochemical processes. Geochem. Int. 2023, 61, 1026–1035. [Google Scholar] [CrossRef]
- Yang, C.; Liu, N.; Zhang, Y. Soil aggregates regulate the impact of soil bacterial and fungal communities on soil respiration. Geoderma 2019, 337, 444–452. [Google Scholar] [CrossRef]
- Yu, H.; Ding, W.; Chen, Z.; Zhang, H.; Luo, J.; Bolan, N. Accumulation of organic C components in soil and aggregates. Sci. Rep. 2015, 5, 13804. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).