Abstract
Plant NAPDH oxidase (NOX) gene family members are critical producers of ROS and play crucial roles in plant signaling, development, and stress responses. Opisthopappus taihangensis is a pivotal wild genetic resource in Asteraceae. To date, little knowledge exists about the functions of NOXs in O. taihangensis. In this study, seven typical NOXs and seven ferric reduction oxidases (FROs) were identified in O. taihangensis. Dispersed duplication might play a critical role in the expansion of the NOX/FRO gene family in O. taihangensis, and most of the NOX/FRO homologous pairs have undergone purifying selection. Although the results of the collinearity analysis show that these genes were relatively conserved, the gain and loss of members in this gene family occurred frequently during the evolution of Asteraceae. Phylogenetic analysis indicated that the FROs are relatively ancient, and the earliest diverged branch was revealed by the maximum likelihood (ML) tree. The Asteraceae NOXs were divided into six subgroups according to their relationship with Arabidopsis and rice members. The transcriptome profile unveiled tissue-specific expression patterns and complex response modes under drought and salt stresses. OtNOX6 and OtNOX7 could be recognized as important candidates to respond to drought and salt stress. Our results provide clues and references for further in-depth exploration of NOX/FRO function in O. taihangensis and other horticulture plants.
1. Introduction
Plants often face abiotic stresses, such as drought and salt, which can harm their growth and lead to significant agricultural losses worldwide. Therefore, it is important for modern breeders to understand how plants resist abiotic stress and utilize this knowledge to breed cultivars for drought tolerance [1]. The reactive oxygen species (ROS) signaling network is an evolutionary-conserved signal transduction key network in the plant kingdom [2]. Most abiotic stresses, including drought, salinity, flooding, and heat and cold stresses, disrupt the metabolic balance of cells, resulting in enhanced accumulation of ROS, such as hydrogen peroxide, superoxide anion, and nitric oxide [3,4]. The growing evidence shows that ROS, at a nontoxic level, plays important roles in plant development and stress response as signal transduction molecules [5].
Plasma membrane NADPH oxidases (NOXs), namely the respiratory burst oxidase homologs (RBOHs), are key enzymes with six transmembrane helixes for ROS production in plants, encoding a homolog of phagocyte gp91phox [6]. Generally, they serve as oxidoreductase to catalyze the generation of superoxide anions using cytoplasmic NAPDH as an electron donor [7]. The first plant NOX gene, OsrbohA, was isolated from Oryza sativa. Since then, NOX genes have been identified in many plants, such as Arabidopsis thaliana [8], Lycopersicon esculentum [9], Vitis vinifera [10], Malus domestica [11], and Zea mays [12]. To date, more than 150 protein members of the NOX family have been identified in various plants, including monocotyledons, dicotyledons, and lower plants [13]. The classical plant NOX proteins are NOX5-like homologs and generally contain the NADPH_Ox domain, several Ca2+-binding EF-hand motifs, the Ferric_reduct domain, and FAD- and NAD-binding motifs from the N to the C terminus. Furthermore, in addition to the typical NOXs, some ferric reduction oxidases (FROs), which are considered the isoforms of yeast ferric-chelate reductase (FRE), were also found in higher plants [14]. As the ancestral state of NOXs [15], these proteins are closely related but differ from typical plant NOXs. FROs also exhibit important roles, although they lack the NADPH_Ox domain and several Ca2+-binding EF-hand motifs compared to the typical NOXs [16].
Owing to the multiple functions of NOXs involved in plant growth, development, and stress responses, they are regarded as important molecular center “hubs” in ROS-mediated signaling transduction pathways, such as MAPK, CDPK, cGMP, and RACK [17,18]. Different NOX members may have different functions in plants. The NOX gene family has been most extensively characterized in Arabidopsis. In terms of growth and development, AtNOXB [19], AtNOXC [20], AtNOXD [21], and AtNOXE [22] are involved in seed germination, root hair formation, and endosperm and pollen development, respectively. AtNOXH and AtNOXJ play essential roles in pollen tube tip growth via Ca2+-activated ROS production [23]. AtNOXD and AtNOXF are not only involved in disease resistance and salt stress tolerance [22], but they also function in jasmonic acid (JA)-induced gene expression [24]. Compared to wild-type A. thaliana, mutants lacking these two genes generate less H2O2 and are more susceptible to pathogens than wild-type plants [25]. Furthermore, it has been reported that AtNOXD has significantly greater ROS-producing activity than AtNOXF. AtNOXD could mediate rapid and long-distance signaling triggered by diverse stimuli, including wounding, heat, cold, high-intensity light, and salt stresses [26]. It was also reported to be involved in lignin assembly [27], the survival of seeds under anoxic conditions [28], ABA-mediated ROS production, and stomatal closure [29].
Although NOXs have been identified and characterized in many plants, considering the multiple members in different plant species in which only a few NOXs were studied in detail, the potential functions of NOXs in ROS production during plant stress response remain to be investigated in depth. Being a perennial, endemic herb of the Taihang Mountains in China, Opisthopappus taihangensis (2n = 18) is a pivotal wild genetic resource in Asteraceae. It has significant medicinal [30] and horticultural values [31] and grows within the cracks of steep slopes and cliffs as a typical lithophyte. Due to habitat degradation and human exploitation, it has been listed as a second-class protected species in China [32]. In our previous observations, when transplanted into a common garden, O. taihangensis was more tolerant and survived more easily than its relatives, O. longilobus [33]. O. taihangensis has evolved adaptive traits such as cold and drought resistance to adapt to severe environmental conditions [1,33,34]. These characteristics make it an ideal germplasm for investigating the genetic basis of stress resistance in ornamental Asteraceae species or other horticulture herbs, for example, Chrysanthemum, one of the most valuable ornamental relatives of Opisthopappus, whose quality, productivity, and natural distribution are severely limited by drought stress [34].
In a previous study, the drought tolerance of O. taihangensis was illuminated in physiological and biochemical aspects. Under drought stress, a lower relative water content and chlorophyll content were observed in the leaves, while proline accumulation reached a high level [1]. The roots had a stronger water-retention capacity than the leaves. The accumulation of ABA in the roots promoted an increased content of proline and trehalose to maintain cell osmotic pressure, activated SOD and POD to scavenge excessive ROS to conserve the cell membrane structure, and induced suberin depositions to reduce moisture loss [34]. Transcriptome analysis further revealed that the upregulated genes are mainly involved in amino acid metabolism, TF regulation, the ABA signaling pathway, osmolyte metabolism, and antioxidant enzyme activity [1,34]. These findings highlight the physiological adaption and gene expression pattern landscape of O. taihangensis. However, in O. taihangensis, NOXs have never been identified at the whole genome level, and their underlying roles in drought or salt stress tolerance have not been sufficiently elucidated.
Thanks to the rapid development of sequencing technology, the sequencing of the high-quality reference genome of O. taihangensis has been completed [35,36], allowing us to easily access the genes of interest at the whole-genome level. Thus, in this study, we identified all the members of NOXs and FROs in O. taihangensis for the first time. Subsequently, comparative analyses were carried out to determine this gene family’s characteristics, evolution, and diversification. Furthermore, to explore the potential function of NOX/FRO genes during O. taihangensis development and stress responses, the transcriptome data of multi-tissues, drought, and salt stresses were utilized to generate the expression profiles, respectively. Finally, the expression patterns of NOXs/FROs were further validated with qRT-PCR. Our results not only provide clues and a reference for further in-depth exploration of NOX/FRO functions in O. taihangensis and other Asteraceae species but also broaden our understanding of the roles of NOXs/FROs in response to abiotic stress in horticultural plants.
2. Materials and Methods
2.1. Identification of NOX/FRO Members in O. taihangensis
Using the protein sequences of Arabidopsis NOXs/FROs (https://www.arabidopsis.org/ (accessed on 10 February 2024)) as queries, BLASTP (E-value < 1 × 10−5) [37] was performed to initially identify the candidate NOX/FRO members in O. taihangensis (OtNOXs and OtFROs). Subsequently, for HMMER analysis, hidden Markov model (HMM) profiles of NADPH_Ox (pfam08414), EF-hand (pfam00036), Ferric_reduct (pfam01794), FAD_binding_8 (pfam08022), and NAD_binding_6 (pfam08030) obtained from Pfam (http://pfam.xfam.org/ (accessed on10 February 2024)) were used together to confirm the inclusion of the conserved domain within each candidate sequence. Finally, the candidates possessing all of the conserved domains were identified as OtNOXs, while those lacking NADPH_Ox (pfam08414) and EF-hand_1 (pfam00036) were identified as OtFROs.
2.2. Physicochemical Properties and Subcellular Localization Prediction
The protein physicochemical properties and subcellular localizations of the identified members were estimated using the online tools PROTPARAM [38] and PLANT-MPLOC [39], respectively. Prediction of the transmembrane helices was performed using the online tool TMHMM [40]. Additionally, according to the genomic annotation, the chromosomal location and gene structure of these identified NOXs/FROs were visualized using TBTOOLS 2.069 software [41]. The online tool MEME was used to detect the conserved motifs [42].
2.3. Phylogenetic Tree Construction
To comprehensively understand the phylogenic relationship of the NOXs/FROs, we also identified this gene family amongst 12 other Asteraceae species with the available genomic resources (Table S1). Then, together with the gene family members in A. thaliana and O. sativa, the putative protein sequences in the Asteraceae species were simultaneously aligned using MAFFT with the default parameters [43]. An ML tree was constructed using IQTREE 1.60 software with 1000 times ultrafast bootstraps, in which the JTT + F + R6 was chosen as the best-fit substation model according to the BIC value [44]. The online tool ITOL (https://itol.embl.de/ (accessed on 10 February 2024)) was used to visualize and edit the ML tree.
2.4. Collinearity Analysis and Selection Pressure Estimation
Using MCSCANX [45], an intraspecific and interspecies collinearity analysis of the NOX/FRO gene family was conducted, followed by visualization using TBTOOLS. To estimate the selection pressure of the NOXs/FROs, sequence alignment was firstly executed using MAFFT with the codon model. Then, we employed the YN00 script incorporated within the PAML package [46] to calculate the Ka (nonsynonymous substitution rate), Ks (synonymous substitution rate), and Ka/Ks ratio according to the method of Yang and Nielsen [47]. The gene pairs with a Ka/Ks ratio > 1 were considered under positive selection.
2.5. Gene Gain and Lost Analysis
The identification of the gene duplication mode was estimated using MCSCANX. To reveal the dynamics of expansion and construction of the NOX/FRO gene family during the evolution of Asteraceae, NOTUNG 2.9 software [48] was used to carry out the gene gain and loss analysis, in which A. thaliana and O. sativa were employed as the outgroup. For NOTUNG analysis, a species tree was obtained from the TIMETREE website (http://www.timetree.org/ (accessed on 10 February 2024)), while a gene tree was generated using IQTREE 1.6 software.
2.6. Cis-Acting Element Prediction and Gene Expression Analysis
Cis-acting element prediction was performed in the genomic region 2 kb upstream of the identified NOX/FRO genes using the online tool PLANTCARE [49].
The drought treatment transcriptome data of O. taihangensis were downloaded from the NCBI under the accession number PRJNA400848 (leaf tissues, 20% PEG6000 treatment for 0 h and 3.5 h with three replicates), PRJNA437359 (root tissues, 20% PEG6000 treatment for 0 h and 9 h with three replicates), and PRJNA526138 (leaf tissues, 5% and 25% PEG6000 treatment for 48 h with three replicates) [1,34].
Additionally, we collected healthy O. taihangensis samples from the common garden located at Shanxi Normal University (111°30′15″ E, 36°4′43″ N), which was followed by refrigeration in liquid nitrogen and storage at −80 °C prior to utilization. The voucher specimens were deposited in the herbarium of Shanxi Normal University under the accession number SNUP20230988. Different tissues, including the root, stem, leaf, bud, and flower, were separated for RNA extraction and sequencing. Furthermore, we used a mixed salt solution (a ratio of NaCl, Na2SO4, and NaHCO3 corresponding to 5:4:1) to treat the leaf tissues. For the time gradients, the leaf tissues were treated with 500 mM of the mixed salt solution for 0 h, 6 h, 24 h, and 48 h. For the concentration gradients, the leaf tissues were treated with 0 mM, 100 mM, 300 mM, and 500 mM of the mixed salt solution for 24 h. All treatments were implemented three times. The transcriptome data used in this study are listed in Table S2.
After RNA extraction and qualification, 150 bp paired-end reads were produced using the Illumina Hiseq platform. The raw data were filtered using FASTP 0.23.2 software [50] with default parameters. HASIT2 2.2.1 software [51] was used to map the clean data onto the high-quality reference genome. The gene expression profile was estimated using FEATURECOUNTS [52]. Differential expression analysis was conducted using the R package DESEQ2 [53], in which the genes with |Log2 Fold Change| > 1 and FDR < 0.05 were identified as differential expression genes (DEGs). An expression heatmap was generated using TBTOOLS.
2.7. Expression Validation by qRT-PCR
Finally, we performed qRT-PCR to validate the expression patterns of randomly selected NOX/FRO genes in leaf tissues. The primers were designed using PRIMER PREMIER 5.0 software [54] (Table S3). The qRT-PCR analysis was performed using the LightCycler Multiplex DNA Master Kit (Roche, Switzerland) with 10 μL of reaction mixture (5 μL of 5× reaction mix, 3 μL of distilled water, 1 μL of cDNA, and 0.5 μL of 10 mM primer each) and performed on the LightCycler 480 Real-Time PCR System (Roche, Switzerland) using the following program: 95 °C for 2 min, 45 cycles of 95 °C for 10 s, 56 °C for 15 s, and 72 °C for 10 s. Three biological replicates were performed for each of the selected genes, and all relative gene expression levels were calculated using 2−ΔΔCT.
3. Results
3.1. The Identification and Characterization of NOXs/FROs in O. taihangensis
The whole genomic sequence of O. taihangensis was used to identify its NOX/FRO members. Through genome scanning, we finally identified seven FROs and seven NOXs in O. taihangensis (Figure 1; Table S4). These genes are located on six chromosomes. Therein, the NOXs are located on five distinct chromosomes: 1, 2, 3, 4, and 9. The FROs are located on four chromosomes: 1, 2, 3, and 6. Chromosome 3 contained the most gene family members, including three NOXs and three FROs, followed by chromosomes 1 (two FROs and one NOX) and 2 (one FRO and one NOX). Chromosomes 4, 6, and 9 possess only one member, namely OtNOX6, OtFRO7, and OtNOX7, respectively (Figure 1). For these genes, we detected three types of duplication modes, including tandem duplication (TD), dispersed duplication (DD), and proximal duplication (PD) (Table S5). The DD mode (~57.14%) occurred most frequently in the NOX/FRO gene family in O. taihangensis, followed by the PD (~21.43) and TD (~14.29%), which is consistent with the chromosomal distribution pattern of FROs/NOXs in O. taihangensis (Figure 1).
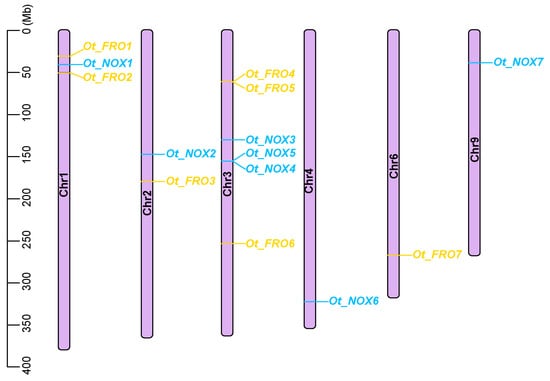
Figure 1.
Chromosomal distribution of FROs/NOXs in O. taihangensis. Chromosome numbers were shown on each chromosome. Blue, NOX genes. Yellow, FRO genes.
Additionally, we estimated the physicochemical properties of these identified family members (Table 1). According to the subcellular localization prediction, all proteins were located at the cell membrane. The length of the NOXs/FROs ranged from 638 to 944, with an average of ~799.93. The molecular weight of these proteins ranged from 72,035.65 to 107,925.84, with an average value of 90,564.20. All of the NOX and FRO members were basic proteins with an inferred PI value lower than 7. A total of five proteins were unstable, with an instability index over 40, including OtFRO3, OtFRO6, OtFRO7, OtNOX1, and OtNOX3. Interestingly, we observed that all FROs were hydrophobic proteins, whereas all NOXs were hydrophilic proteins. The number of predicted transmembrane helices (TMHs) in the NOX members was consistently four, which was lower than that of the FROs (7–11).

Table 1.
Information of NOXs/FROs proteins identified in O. taihangensis.
We analyzed the protein domains and gene structures to reveal the sequence conservation and compared the differences among the identified NOXs/FROs (Figure 2). The OtFROs and OtNOXs were divided into two clades in the ML tree, indicating their inherent protein structure differences (Figure 2a). As expected, the identified members contained relatively conserved motifs, especially the NOXs. A total of 10 conserved motifs were detected. All of them were found in the NOXs except motif 6, which occurred only in the FROs (Figure 2b). Regarding the conserved domain (Figure 2c), the FROs lacked one NADPH_Ox and two EF hands, which all the NOXs possessed. To further explore whether these gene family members were also conserved at the level of gene structure, we performed gene structure analysis (Figure 2d; Table S6). The results showed that the gene structures of NOXs and FROs were highly distinct in O. taihangensis. The NOXs had a higher number of introns (10–13) and exons (11–14) than those of the FROs (introns: 1–9; exons: 8–10), as did the CDS. We also detected 5′ and 3′ UTRs in five genes, namely OtFRO3, OtFRO6, OtNOX3, OtNOX6, and OtNOX7, while only a short 3′ UTR was found in OtFRO1.
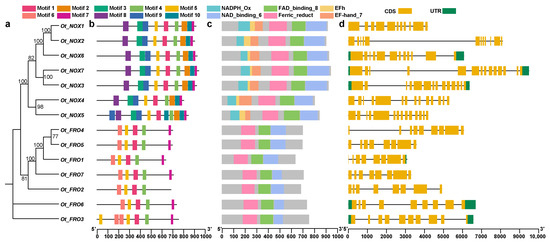
Figure 2.
The gene structures and protein domains of FROs/NOXs members. (a) Maximum likelihood (ML) phylogenetic tree of FROs/NOXs in O. taihangensis. (b) Conserved motifs of FROs/NOXs in O. taihangensis. Different motifs were represented by different colored boxes. (c) Protein domains of FROs/NOXs in O. taihangensis. Different domains were represented by different colored boxes. (d) Gene structures of FROs/NOXs in O. taihangensis.
3.2. The Phylogeny and Evolution of NOXs/FROs in Asteraceae
To comprehensively reveal the phylogenic relationship of NOXs/FROs in O. taihangensis and other Asteraceae species, we first constructed an ML tree (Figure 3) using multiple species, including A. thaliana, O. sativa, and 13 Asteraceae species. A total of 199 homologs were identified among these species for ML tree construction (Table S1). As the ancestral NOXs, the FROs were recognized as the earliest diverging branch in the ML tree. Furthermore, six subgroups (NOX Ⅰ to NOX Ⅵ) were identified within the NOXs, which is similar to the previous studies [10,17,18]. The O. taihangensis NOXs were distributed across all subgroups: OtNOX7 in subgroup NOX Ⅰ, OtNOX3 in subgroup NOX Ⅱ, OtNOX4 and OtNOX5 in subgroup NOX Ⅲ, OtNOX1 and OtNOX2 in subgroup NOX Ⅴ, and OtNOX6 in subgroup NOX Ⅵ. Interestingly, we found that subgroup NOX Ⅵ only contained the NOXs from Asteraceae without members from A. thaliana or O. sativa.
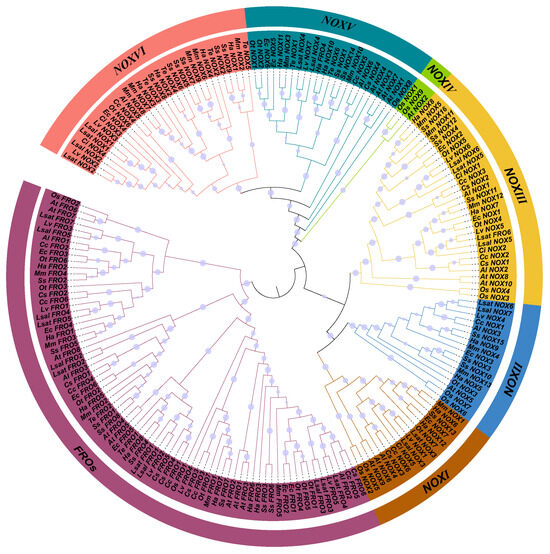
Figure 3.
The ML tree of FROs/NOXs proteins of Arabidopsis thaliana, Oryza sativa, and Asteraceae species. At, A. thaliana; Os, O. sativa; Al, Arctium lappa; Cs, Centaurea solstitialis; Ci, Cichorium intybus; Cc, Cynara cardunculus; Ec, Erigeron canadensis; Ha, Helianthus annuus; Lsal, Lactuca saligna; Lsat, Lactuca sativa; Lv, Lactuca virosa; Mm, Mikania micrantha; Ot, Opisthopappus taihangensis; Ss, Smallanthus sonchifolius; Te, Tagetes erecta. The size of the circles on each node presented the bootstrap value.
Meanwhile, collinearity analysis was performed to better understand the evolutionary relationship of the NOX/FRO gene family in O. taihangensis and Asteraceae. Within O. taihangensis, we found five OtFROs and five OtNOXs paralogous gene pairs (Figure 4a). OtFRO5 had higher collinearity with other OtFRO genes, forming paralogous pairs with OtFRO1, OtFRO2, OtFRO4, and OtFRO7. The OtNOX1, OtNOX6, and OtNOX7 each contained two collinear gene pairs with other OtNOX genes. A total of 11 collinear gene pairs between OtNOXs and OtFROs were also detected. OtNOX3 showed a collinearity relationship with most OtFROs, while OtFRO6 showed a collinearity relationship with most OtNOXs.
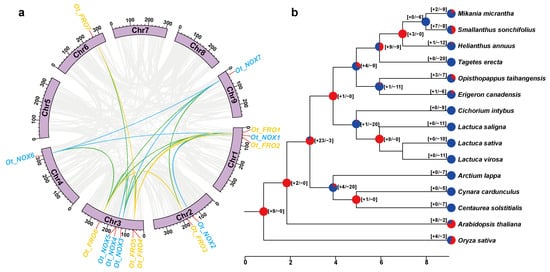
Figure 4.
Collinearity analysis and gene gain and lost inference for FROs/NOXs. (a) Genome-wide collinearity analysis for FROs/NOXs genes within O. taihangensis. The paralogous genes were mapped onto the chromosomes and linked to each other. Yellow line indicated the gene pairs between FROs while blue lines indicated the gene pairs between NOXs. The gene pairs with collinearity between FROs and NOXs were marked with green lines. (b) The landscape of gene gain and loss of FROs/NOXs during Asteraceae evolution. The numbers in parentheses on the node of branch represent the number of genes gained and lost, while the pie chart represents their proportion (red for gain and blue for loss).
Among the Asteraceae, different species presented relatively high collinearity (Figure S1). A total of 99 NOX and 47 FRO orthologous gene pairs were detected between different species pairs. However, we did not find collinearity between NOXs and FROs among any species pairs, indicating their stable differences in distinct species. O. taihangensis showed a closer relationship with Erigeron canadensis and Tagetes erecta. Between O. taihangensis and E. canadensis, we found three FRO orthologous and four NOX orthologous pairs. Between O. taihangensis and T. erecta, we also found two FRO and four NOX orthologous pairs (Figure S1).
Although significant collinearity of the FROs/NOXs was discovered (Figure 4a and Figure S1), gene gain and loss analysis showed that this gene family experienced dramatic dynamic changes with expansion and contraction in Asteraceae (Figure 4b and Figure S2). A total of 93 and 205 duplication and loss events occurred, respectively (Figure 4b and Figure S2). After differentiation from the A. thaliana and O. sativa outgroups, the common ancestor of Asteraceae FROs/NOXs experienced a significant duplication (+23). However, substantial loss events occurred during the subsequent evolution of Asteraceae, leading to the contraction of this gene family. Many Asteraceae species underwent only loss events without duplication, except for a few species with slight gene duplication. Interestingly, we noticed that O. taihangensis underwent three duplication events and seven loss events, and the other Asteraceae species that underwent duplication events always showed a closer relationship with O. taihangensis in the same clade in the species tree.
The relatively complex evolutionary dynamics urged us to explore the selection pressure of FROs/NOXs in Asteraceae. Within O. taihangensis, we found that all paralogous gene pairs were under purifying selection, with the value of Ka/Ks ranging from 0.1084 to 0.3278. As can be seen, when using O. taihangensis as a reference, all orthologous gene pairs also contained a Ka/Ks value less than 1, indicating purifying selection (Table S7).
3.3. Cis-Acting Elements Analysis and Gene Expression Pattern
The cis-acting elements in the upstream promoter region were analyzed to investigate the underlying function of FRO/NOX genes in O. taihangenesis (Figure 5). The results showed that a total of 46 kinds of identified elements could be divided into 6 large categories, including “Plant development and growth”, “Binding”, “Hormone responses”, “Light responses”, “Regulation”, and “Stress responses”. Totals of 296 and 275 cis-acting elements were identified in the FROs and NOXs, respectively. Interestingly, both the FROs and NOXs contained the highest numbers of cis-acting elements CAAT-box (208) (forming a transcription or a regulatory signal), which are related to abiotic stress, followed by ARE (34) (essential for anaerobic induction) and MBS (11) (binding site involved in drought inducibility), indicating they might play important roles in plant biotic stress response. In addition to abiotic stress, four types of plant hormone response elements were identified in large numbers, including 30 ABRE (involved in ABA-induced gene expression), 26 TGACG motifs (involved in MeJA-responsiveness), 22 CGTCA motifs (involved in MeJA-responsiveness), and 18 TCA elements (involved in SA responsiveness), suggesting the FROs/NOXs might be sensitively regulated by various phytohormones.
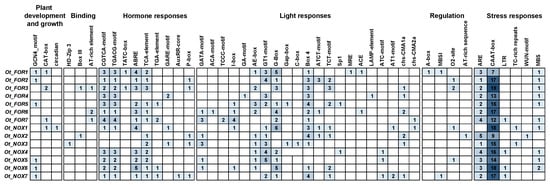
Figure 5.
Cis-acting elements in promoter regions of FROs/NOXs in O. taihangensis. The number in the colored box represented the number of cis-acting elements.
Furthermore, using the transcriptome data, we comprehensively explored the gene expression patterns of FROs/NOXs in O. taihangensis in both different tissues and treatments (Figure 6). The tissue-specific modes were found using the expression profile (Figure 6a). For example, OtNOX4 and OtNOX5 were predominantly expressed in the bud and flower, while OtNOX2 was expressed in the root. However, OtFRO 5 and 6 were significantly expressed in almost all tissues except the root. Additionally, OtFRO2, OtNOX1, and OtNOX6 showed a relatively lower expression pattern in the bud and flower. For all tissues, OtFRO3 always showed a relatively high expression level, followed by OtFRO1 and OtNOX7, which presented a moderate expression level. In contrast, OtFRO4 and OtFRO7 seemed not to be expressed in all the studied tissues.
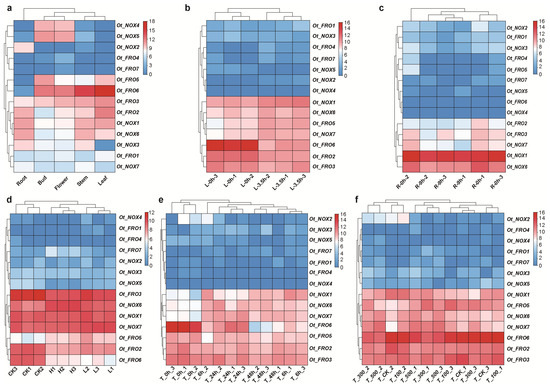
Figure 6.
Expression patterns of FROs/NOXs in O. taihangensis. (a) Expression patterns of putative FROs/NOXs in different tissues. Expression patterns of putative FROs/NOXs under abiotic stress treatment include (b) 20% PEG6000 for 3.5 h or 0 h in leaf; (c) 20% PEG6000 for 9 h or 0 h in root; (d) high (25% PEG6000), low (5% PEG6000), and control drought treatment in leaf for 48 h; (e) 500 mM mixed salt solution for 6 h, 24 h, and 48 h in leaf; and (f) 100 mM, 300 mM, and 500 mM mixed salt solution for 24 h.
To further investigate the roles of these genes under drought and salt abiotic stress, multiple comparisons of gene expression patterns among different treatments were conducted (Figure 6 and Figure S3; Table S2). Overall, we found that OtFROs2 and 3 and OtNOX1, 6, and 7 were highly expressed in both the roots (Figure 6c) and leaves (Figure 6b,d–f), while OtFRO5 and OtFRO6 were only highly expressed in the leaves, but not in the roots, which is consistent with the tissue-specific expression analysis (Figure 6a). In the comparison of L 3.5 h vs. L 0 h, we identified 8289 differentially expressed genes (DEGs), including 3839 upregulated and 4450 downregulated genes (Figures S3a and S4a). Therein, OtFRO5 (log2 FC = 1.95; FDR = 6.67 × 10−7) and OtNOX1 (log2 FC = 1.45; FDR = 2.96 × 10−9) were upregulated, while OtFRO2 (log2 FC = −1.69; FDR = 1.85 × 10−12) and OtFRO6 (log2 FC = −4.73; FDR = 4.85 × 10−46) were downregulated, respectively (Figure 6b and Figure S4b–d).
In addition to the comparison of L 3.5h vs. L 0h, we also found significantly different expression patterns of OtFROs/OtNOXs under other treatments, except for T_100 vs. T_CK and T_500 vs. T_300 (Figure S4a). Among these genes, 11 members underwent different expressions under drought or salt treatment (Figure S4; Table S8). OtNOX1 was upregulated in six comparison pairs, while OtNOX2, 3, and 5 were downregulated in two, one, and three comparison pairs, respectively (Figure S4c,d; Table S8). However, the other seven genes presented relatively complex regulated patterns. They showed upregulated or downregulated expressions in different comparison groups (Figure S4; Table S8). For example, OtFRO7 was upregulated in H vs. CK while downregulated in H vs. L. We also found downregulated expressions of OtFRO2, 3, and 6 in many comparisons, although they were simultaneously upregulated in comparisons of T_500 vs. T_CK (Figure S4c,d; Table S8). In terms of root tissues under drought treatment (Table S8), we found four genes that were downregulated, including OtNOX2, 3, 6, and 7.
To validate the expression profile, we randomly selected six genes for qRT-PCR validation using gene-specific primers (Table S3) under the salt treatment (500 mM mixed salt solution) for six hours, including three FROs (OtFRO2, 3, and 6) and three NOXs (OtNOX2, 6, and 7). The expression patterns of these genes are consistent with the results of the transcriptome analysis (Figure 7). The significantly different leaf-specific expression of the NOX/FRO genes under salt stress indicates their role and involvement in leaf development and stress responses. After salt treatment for six hours, OtFRO2, OtFRO3, OtFRO6, and OtNOX2 showed significant downregulation expression, while OtNOX6 and OtNOX7 were significantly upregulated after treatment.
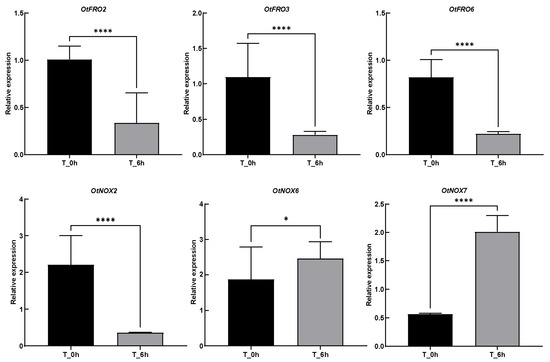
Figure 7.
Relative expression obtained from qRT-PCR experiments of some FROs (OtFRO2, 3, and 6) and NOXs (OtNOX2, 6, and 7) under salt treatment for six hours. The statistical significance using student’s t-test. ns = no significant different; * = p < 0.05, **** = p < 0.0001.
4. Discussion
Smart horticulture, an emerging industry stemming from traditional agriculture, uses advanced techniques like molecular breeding and genetic engineering to manage and develop food crops and ornamental plants. However, with global warming and environmental degradation, crop productivity and security are at risk from various abiotic stresses like drought, salt, extreme temperatures, and floods. Therefore, it was essential for horticulturists to understand how plants resist abiotic stresses in order to breed them for high stress tolerance [1]. O. taihangensis is one of the most important wild germplasm resources in Asteraceae. Previous studies have indicated that O. taihangensis contains high adaptability to abiotic stress, such as drought and cold, and, thus, could be regarded as an ideal material in Asteraceae species for molecular breeding research [1,33,34]. In this study, we first identified and characterized the typical NOXs and ancestral NOXs (FROs) in O. taihangensis. Our results not only lay a foundation for further investigating the biological functions of the family members in O. taihangensis, but they also provide a methodological reference for studying this gene family in other related ornamental species.
4.1. The Identification and Characterization of FROs/NOXs in O. taihangensis
In this study, we found that all identified OtNOX protein sequences had a higher molecular weight (F = 69.53, P = 2.45 × 10−6) and were much longer than the OtFROs (F = 64.74, P = 3.54 × 10−6) (Table 1). This might be because NOX genes contain more conserved domains than FROs [15,18]. Interestingly, all OtNOXs were predicted to be hydrophilic proteins with a GRAVY value lower than 0 and an aliphatic index lower than 100. In contrast, the OtFROs were predicted to be hydrophobin, with a GRAVY value greater than 0 and an aliphatic index greater than 100 (Table 1). We speculated that the distinction between OtNOXs and OtFROs is mainly caused by sequence differentiation. Previous studies also showed that the NOXs are hydrophilic in many species [55,56,57,58,59,60,61,62,63]. As expected, all identified proteins were predicted to possess multiple transmembrane helices (TMHs), and the predicted number of TMHs of OtFROs was higher than that of OtNOXs, which is also consistent with other studies [17]. The subcellular localization prediction showed all proteins were located at the cell membrane, which further indicates that these genes have a transmembrane structure, catalyzing NADPH in the cytoplasm while transferring electrons to generate ROS [59].
Additionally, when using specific antibodies against NOXs to screen different subcellular fractions, only the fractions containing the plasma membrane exhibited positive hybridization bands [64]. However, recent studies have indicated that the same members of NOXs were predicted to be located in the nucleus, mitochondria, chloroplast, or even thylakoid membrane, indicating different functions [10,59,65]. We speculated that different subcellular localizations were caused by distinct sources of ROS production in specific tissues. For example, mitochondrial ROS production is a significant source of oxidative stress damage in non-green tissues such as roots [3], while the limitation to the electron transport between photosystems also results in singlet oxygen in the thylakoid membranes [66].
4.2. The Evolution and Phylogeny of NOXs/FROs in Asteraceae
A total of seven OtFROs and seven OtNOXs were identified in O. taihangensis. In Asteraceae, the number of FROs ranged from 0 to 8, while the number of NOXs ranged from 4 to 16 (Table S1). It has been reported that no direct relationship exists between the genome size and the number of NOX genes [55,62]. However, researchers have found increasing numbers of NOX genes in higher plants; monocots especially accumulate more NOXs [61].
The number of gene family members is closely related to their replication mode. Generally, gene duplication occurs in five categories: singleton, dispersed, proximal, tandem, and whole genome duplication (WGD). WGDs are considered to be the major driving force for NOX duplication [55,61,63,67]. Nevertheless, our study found that most members arose from dispersed duplication rather than WGD (Table S5). Some dispersed duplication modes of NOXs were also observed in other species, such as Zea mays, Sorghum bicolor, and O. sativa [61]. Dispersed duplication has been reported to lead to divergence in NOX’ gene function [55].
The Asteraceae were estimated to have originated over 83 million years ago. A total of 41 WGD events have occurred within the Asteraceae family. In addition to the WGD, recent studies have pointed out that hexaploidization occurred approximately 70~78 Ma ago, which may have been shared by all existing Asteraceae plants [68,69,70]. The ancestral Asteraceae karyotype has nine paleochromosomes, while the most recent common ancestor of extant Asteraceae plants has fifteen basic chromosomes, revealing a highly flexible reshuffling of the Asteraceae paleogenome [68]. In this study, we found numbers of gain and loss events of NOXs/FROs during the evolution of Asteraceae (Figure 4b and Figure S2), which generally occurred at relatively ancient common ancestor nodes. We speculated that ancient polyploidization in Asteraceae events promoted the gain and expansion of the NOXs/FROs. After the genome duplication event, some functionally important gene copies were retained, whereas functionally redundant gene copies were lost or pseudogenized [71,72].
In the phylogenic analysis, we found all FROs clustered together and located at the base of the ML tree (Figure 3), indicating that the FROs are relatively ancient compared to the NOXs [13,15]. Typical NOXs have been identified only in terrestrial plants, which evolved from ancestral FROs by capturing the NADPH_Ox domain (Figure 3) [13,15]. The FROs in lower organisms are responsible for both ROS production and iron uptake, while in higher plants, NOX-mediated ROS production is mainly responsible for stress tolerance and development regulation, whereas FROs are mainly responsible for iron uptake and homeostasis [15,17]. These findings clearly show the functional divergence of FROs and NOXs in plantae during their long-term evolution history.
Furthermore, akin to other studies [6,10,17,18], it is noteworthy that many Asteraceae NOXs were mixed with members of A. thaliana and O. sativa, except subgroup NOX Ⅵ, which only included Asteraceae members. The divergence time between Asteraceae species and A. thaliana is estimated to be ~120.3 Ma (unpublished), a relatively ancient period. The formation and differentiation of Asteraceae NOX members in subgroup NOX VI might have occurred after the species divergence of Asteraceae spp. and A. thaliana.
4.3. The Cis-Acting Elements and Transcriptome Profile Revealed the Responding Pattern of FROs/NOXs under Abiotic Stresses
The cis-acting regulatory elements are key molecular controls for the transcriptional regulation of genes under stress conditions, which are induced through independent signal transduction pathways [60]. The upstream regulatory regions of FROs/NOXs in O. taihangensis contained different types of cis-acting regulatory elements, showing that these genes might distinctly respond to various stimuli, irrespective of their identical protein products (Figure 5). Similar observations were documented in A. thaliana, O. sativa, Capsicum annuum, Glycine max, and Triticum aestivum [56,63,73,74].
We noticed that elements related to phytohormones and abiotic stresses, such as CAAT-box, were frequently identified in O. taihangensis (Figure 5). Previous studies have shown that ROS signaling pathways are closely related to phytohormone regulatory pathways. For example, ABA can induce the expression of the NOX gene and improve tomato tolerance [75]. As important stress signaling molecules, JAs regulate the plant’s antioxidant enzyme system and also participate in the regulation of NOX activity [76]. Additionally, three NOXs were associated with auxin-mediated root hair development in O. sativa [77].
The tissue-specific expression pattern of NOXs has been illuminated in many species [10,63,74,77,78,79], which regularly reflect the crosstalk and distinctions in the functions of family members. In this study, we found that the FROs and NOXs existed in tissue-specific expression patterns (Figure 6a and Figure 7), indicating that these genes play specific roles in different tissues and organs in O. taihangensis. The transcriptome profile under drought and salt treatment demonstrated relatively complex dynamics of these genes responding to stress (Figure 6, Figure 7, Figures S3 and S4). Among the different comparisons under treatment, eleven genes were identified as DEGs, and seven of them exhibited both upregulation and downregulation (Figure S4 and Table S8). A similar phenomenon has been observed in other species such as C. annuum [63]. O. sativa [18], Triticum aestivum [17,74,79], Nicotiana tabacum [60], Zea mays [61], Solanum melongena [59], and Vitis vinifera [10].
These studies have consistently shown that the response patterns of the NOX gene family to drought and salt stress are diverse and complex in various organisms. Interestingly, Wang et al. [6] found that the expression patterns of Ghrboh genes in response to salinity were largely opposite to those obtained in response to drought stress in Gossypium hirsutum. Genes upregulated under salt stress tended to be downregulated under drought stress, and vice versa. Similar results were found in our study; for example, OtFRO5 (Table S8) was upregulated under drought while downregulated under salt.
It has been proposed that proteins classified in the same phylogenetic groups may have similar functions [60]. In Arabidopsis, AtNOX4/6 (AtRBOHD/F) produced ROS that function as signal molecules to regulate Na+/K+ balance under salt stress. The double mutant of AtNOX4/6 showed decreased salt tolerance owing to the ability to absorb K+ less efficiently, selectively [25,80,81,82]. In O. sativa, the overexpression of OsNOX1 and OsNOX2 enhances plant drought tolerance. Mutants of OsNOX2 not only show reduced ROS production but also lead to decreased drought resistance. In this study, we found that putative OtNOX7 was clustered in the same clade (subgroup NOX Ⅰ) with AtNOX4, AtNOX6, and OsNOX2 (Figure 3), presenting highly variable expression patterns under drought and salt treatment. This indicates its important role in regulating the stress response in O. taihangensis, which was also confirmed by the qRT-PCR (Figure 7). In addition to OtNOX7, we found that OtNOX6, a member belonging to the unique branch of the Asteraceae (subgroup NOX Ⅵ, Figure 3), exhibited variable expression patterns under different treatments.
Interestingly, these two genes presented downregulation under drought treatment in root tissues (Figure 6 and Figure S4; Table S8). Between O. taihangensis and A. thaliana, homology analysis showed that OtNOX6 and OtNOX7 are one-to-one orthologous genes of AtNOX4 and AtNOX6, respectively (Table S7). In a previous study, we found that these two genes underwent exon-skipping under salt stress [36]. Studies have revealed that alternative splicing events frequently occur in response to external stimuli. To summarize, OtNOX6 and OtNOX7 could be recognized as important candidates in response to abiotic stress, such as salt and drought, in O. taihangensis, although further in-depth investigations are needed.
5. Conclusions
In this study, we first identified the NOXs/FROs gene family in O. taihangensis and other Asteraceae species. The physicochemical properties and protein domains were distinct between the FROs and NOXs. Evolutionary analysis showed that these putative members were divided into seven groups, while subgroup NOX Ⅵ was unique to Asteraceae. Dispersed duplication was the main mode of NOXs/FROs duplication in O. taihangenesis. During the evolution of Asteraceae, the number of NOXs/FROs changed dramatically, which might be due to genome duplication. The transcriptome profile revealed the tissue-specific expression patterns and complex dynamics of NOXs/FROs under drought and salt stresses, which were further validated by qRT-PCR. OtNOX6 and OtNOX7 could be recognized as important candidates for responding to abiotic stresses in O. taihangensis.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy14040653/s1, Figure S1: The collinearity analysis between A. thaliana, O. sativa and 13 Asteraceae species. Blue lines represented the orthologous FROs/NOXs gene pairs among these species; Figure S2: The reconstruction of the gene tree to show the gene gain and loss of FROs/NOXs in Asteraceae. The nodes marked by blue color indicating gene gain while the branches marked by red indicating gene loss. The number around the nodes represented bootstrap value with 1000 times replications; Figure S3: The transcriptome differentially express analysis of (a) L-3.5h vs. L-0h, (b) R-9h vs. R-0h, (c) H vs. CK, (d) L vs. CK, (e) H vs. L, (f) T_6h vs. T_0h, (g) T_24h vs. T_0h, (h) T_28h vs. T_0h, (i) T_24h vs. T_6h, (j) T_48h vs. T_6h, (k) T_48h vs. T_24h, (l) T_100 vs. T_CK, (m) T_300 vs. T_CK, (n) T_500 vs. T_CK, (o) T_300 vs. T_100, (p) T_500 vs. T_100, and (q) T_500 vs. T_300; Figure S4: The summary of FROS/NOXS expression in O. taihangensis. (a) The number of differently expressed genes (DEGs) in whole transcriptome and FROs/NOXs, respectively. The UpSet plot of (b) all differently expressed FROs/NOXs, (c) upregulated and (d) downregulated members in different compassion, respectively; Table S1: Summary of genome resources used in this study; Table S2: Summary of the RNA-seq used in this study; Table S3: Primers for the qRT-PCR experiment; Table S4: Protein sequences of the identified NOXs/FROs genes in O. taihangensis; Table S5: Duplication types of NOXs/FROs genes in O. taihangensis; Table S6: Summary of the gene structure of identified NOXs/FROs in O. taihangensis; Table S7: The ka/ks value of one-to-one orthologous FROs/NOXs gene pairs in O. taihangensis and other species; Table S8: Summary of differently expressed analysis of FROs/NOXs in O. taihangensis.
Author Contributions
Conceptualization, H.Z. and Y.W.; methodology, H.Y.; software, H.Y.; validation, H.Y., H.L. (Hengzhao Liu) and M.H.; formal analysis, X.F.; investigation, N.Z.; resources, T.G.; data curation, D.L., H.L. (Haochen Li) and Z.G.; writing—original draft, H.Y., H.L. (Hengzhao Liu) and M.H.; writing—review and editing, H.Y., H.Z., and Y.W.; visualization, Y.S. and M.C.; supervision, H.Z. and Y.W.; project administration, H.Z. and Y.W.; funding acquisition, H.Z. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31970358), the Research Project of Shanxi Scholarship Council (2020-090), Natural Science Foundation of Shanxi Province of China (20210302124501), the Innovation Project of Postgraduate Education of Shanxi Province (2022Y493), the Postgraduate Innovation Project of Shanxi Normal University (2022XSY001), the Science and Technology Program of Shaanxi Academy of Science (2023K-26 and 2019K-06), Shaanxi Forestry Science and Technology Innovation Key Project (SXLK2023-02-20), and Qinling Hundred Talents Project of Shaanxi Academy of Science (2023K-49).
Data Availability Statement
The transcriptome data can be obtained from the SRA database under the accession number PRJNA400848, PRJNA437359 and PRJNA526138.
Acknowledgments
Thanks to Peng Zhao from Northwest University for his professional assistance in data analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gu, H.; Yang, Y.; Xing, M.; Yue, C.; Wei, F.; Zhang, Y.; Zhao, W.; Huang, J. Physiological and transcriptome analyses of Opisthopappus taihangensis in response to drought stress. Cell Biosci. 2019, 9, 56. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2013, 65, 1241–1257. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.; Liu, D.; Cheng, Y.; Zhang, X.; Song, L.; Hu, M.; Dong, J.; Shen, F. Comprehensive analysis of the Gossypium hirsutum L. respiratory burst oxidase homolog (Ghrboh) gene family. BMC Genom. 2020, 21, 91. [Google Scholar] [CrossRef]
- Bedard, K.; Lardy, B.; Krause, K.-H. NOX family NADPH oxidases: Not just in mammals. Biochimie 2007, 89, 1107–1112. [Google Scholar] [CrossRef]
- Torres, M.A.; Onouchi, H.; Hamada, S.; Machida, C.; Hammond-Kosack, K.E.; Jones, J.D.G. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J. 1998, 14, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Davydov, O.; Orazova, S.; Yesbergenova, Z.; Ophir, R.; Stratmann, J.W.; Fluhr, R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum [W]. Plant Cell 2004, 16, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Xu, X.; Gao, M.; Li, J.; Guo, C.; Song, J.; Wang, X. Genome-wide analysis of respiratory burst oxidase homologs in grape (Vitis vinifera L.). Int. J. Mol. Sci. 2013, 14, 24169–24186. [Google Scholar] [CrossRef] [PubMed]
- Cepauskas, D.; Miliute, I.; Staniene, G.; Gelvonauskiene, D.; Stanys, V.; Jesaitis, A.J.; Baniulis, D. Characterization of apple NADPH oxidase genes and their expression associated with oxidative stress in shoot culture in vitro. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 124, 621–633. [Google Scholar] [CrossRef]
- Nestler, J.; Liu, S.; Wen, T.-J.; Paschold, A.; Marcon, C.; Tang, H.M.; Li, D.; Li, L.; Meeley, R.B.; Sakai, H.; et al. Roothairless5, which functions in maize (Zea mays L.) root hair initiation and elongation encodes a monocot-specific NADPH oxidase. Plant J. 2014, 79, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-H.; Wang, P.-Q.; Zhang, P.-P.; Nie, X.-M.; Li, B.-B.; Tai, L.; Liu, W.-T.; Li, W.-Q.; Chen, K.-M. NADPH oxidases: The vital performers and center hubs during plant growth and signaling. Cells 2020, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-L.; Li, W.-Y.; Miao, H.; Yang, S.-Q.; Li, R.; Wang, X.; Li, W.-Q.; Chen, K.-M. Comprehensive genomic analysis and expression profiling of the NOX gene families under abiotic stresses and hormones in plants. Genome Biol. Evol. 2016, 8, 791–810. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, L.; Du, J.; Yuan, Y.; Cheng, X.; Ling, H.-Q. Molecular and biochemical characterization of the Fe (III) chelate reductase gene family in Arabidopsis thaliana. Plant Cell Physiol. 2005, 46, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-H.; Wei, X.-Y.; Yuan, B.; Yao, L.-B.; Ma, T.-T.; Zhang, P.-P.; Wang, X.; Wang, P.-Q.; Liu, W.-T.; Li, W.-Q.; et al. Genome-wide identification and functional analysis of NADPH oxidase family genes in wheat during development and environmental stress responses. Front. Plant Sci. 2018, 9, 906. [Google Scholar] [CrossRef]
- Wang, G.-F.; Li, W.-Q.; Li, W.-Y.; Wu, G.-L.; Zhou, C.-Y.; Chen, K.-M. Characterization of rice NADPH oxidase genes and their expression under various environmental conditions. Int. J. Mol. Sci. 2013, 14, 9440–9458. [Google Scholar] [CrossRef]
- Müller, K.; Carstens, A.C.; Linkies, A.; Torres, M.A.; Leubner-Metzger, G. The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. N. Phytol. 2009, 184, 885–897. [Google Scholar] [CrossRef]
- Takeda, S.; Gapper, C.; Kaya, H.; Bell, E.; Kuchitsu, K.; Dolan, L. Local positive feedback regulation determines cell shape in root hair cells. Science 2008, 319, 1241–1244. [Google Scholar] [CrossRef]
- Penfield, S.; Li, Y.; Gilday, A.D.; Graham, S.; Graham, I.A. Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell 2006, 18, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-J.; Xu, S.; Han, B.; Wu, M.-Z.; Yuan, X.-X.; Han, Y.; Gu, Q.; Xu, D.-K.; Yang, Q.; Shen, W.-B. Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. Plant J. 2011, 66, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T.; et al. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 2014, 26, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Maruta, T.; Inoue, T.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Arabidopsis NADPH oxidases, AtrbohD and AtrbohF, are essential for jasmonic acid-induced expression of genes regulated by MYC2 transcription factor. Plant Sci. 2011, 180, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Dangl, J.L.; Jones, J.D.G. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 517–522. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef] [PubMed]
- Denness, L.; McKenna, J.F.; Segonzac, C.; Wormit, A.; Madhou, P.; Bennett, M.; Mansfield, J.; Zipfel, C.; Hamann, T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 2011, 156, 1364–1374. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Zhang, Q.; Li, M.; Yan, M.; Wang, R.; Wang, L.; Welti, R.; Zhang, W.; Wang, X. Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 2009, 21, 2357–2377. [Google Scholar] [CrossRef]
- Ge, S.; Chen, J.; Liu, N.; Bu, R. Antioxidant activity and stability of total flavonoids from Opisthopappus taihangensis. Food Sci. Technol. 2019, 44, 241–245. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Tian, Z. Biology Characteristic and protection and exploitation of Opisthopapus taihangensis Shih. Hubei Agric. Sci. 2012, 51, 3775–3776. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, Y.; Ye, H.; Feng, X.; Qie, Q.; Liu, L.; Chai, M. Reproductive biology and breeding systems of two Opisthopappus endemic and endangered species on the taihang mountains. Plants 2023, 12, 1954. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Zang, E.; Liu, Z.-X.; Lan, Y.-F.; Hao, W.-L.; He, S.; Fan, X.; Sun, G.-L.; Wang, Y.-L. Adaptation insights from comparative transcriptome analysis of two Opisthopappus species in the Taihang mountains. BMC Genom. 2022, 23, 466. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y.; Zhong, J.; Zhang, T.; Li, D.; Ba, T.; Xu, T.; Chang, L.; Zhang, Q.; Sun, M. Root physiological traits and transcriptome analyses reveal that root zone water retention confers drought tolerance to Opisthopappus taihangensis. Sci. Rep. 2020, 10, 2627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zang, E.; Zhang, H.; Liu, Z.; Lan, Y.; He, S.; Hao, W.; Cao, Y. Genome sizes and characteristics of cliff plants Opisthopappus taihangensis and O. longilobus on Taihang Mountains. Guihaia 2022, 42, 1582–1589. [Google Scholar]
- Han, M.; Niu, M.; Gao, T.; Shen, Y.; Zhou, X.; Zhang, Y.; Liu, L.; Chai, M.; Sun, G.; Wang, Y. Responsive alternative splicing events of Opisthopappus species against salt stress. Int. J. Mol. Sci. 2024, 25, 1227. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.-C.; Shen, H.-B. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef]
- Möller, S.; Croning, M.D.R.; Apweiler, R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nielsen, R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol. Biol. Evol. 2000, 17, 32–43. [Google Scholar] [CrossRef]
- Chen, K.; Durand, D.; Farach-Colton, M. NOTUNG: A program for dating gene duplications and optimizing gene family trees. J. Comput. Biol. J. Comput. Mol. Cell Biol. 2000, 7, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Singh, V.K.; Mangalam, A.K.; Dwivedi, S.; Naik, S. Primer Premier: Program for design of degenerate primers from a protein sequence. BioTechniques 1998, 24, 318–319. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, M.; Zhang, Y.; Si, W.; Cheng, B.; Li, X. Systematic analysis of the Rboh gene family in seven gramineous plants and its roles in response to arbuscular mycorrhizal fungi in maize. BMC Plant Biol. 2023, 23, 603. [Google Scholar] [CrossRef]
- Liu, J.; Lu, H.; Wan, Q.; Qi, W.; Shao, H. Genome-wide analysis and expression profiling of respiratory burst oxidase homologue gene family in Glycine max. Environ. Exp. Bot. 2019, 161, 344–356. [Google Scholar] [CrossRef]
- Chang, Y.; Li, B.; Shi, Q.; Geng, R.; Geng, S.; Liu, J.; Zhang, Y.; Cai, Y. Comprehensive analysis of respiratory burst oxidase homologs (Rboh) gene family and function of GbRboh5/18 on Verticillium Wilt resistance in Gossypium Barbadense. Front. Genet. 2020, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Gui, T.-Y.; Gao, D.-H.; Ding, H.-C.; Yan, X.-H. Identification of respiratory burst oxidase homolog (Rboh) family genes from pyropia yezoensis and their correlation with Archeospore Release. Front. Plant Sci. 2022, 13, 929299. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Jiang, Z.; Zhou, Y.; Shen, L.; He, J.; Xia, X.; Zhang, L.; Yang, X. Genome-wide identification and expression analysis of respiratory burst oxidase homolog (RBOH) gene family in eggplant (Solanum melongena L.) under abiotic and biotic stress. Genes 2023, 14, 1665. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Kakar, K.U.; Yang, Z.; Nawaz, Z.; Lin, S.; Guo, Y.; Ren, X.-l.; Baloch, A.A.; Han, D. Systematic study of the stress-responsive Rboh gene family in Nicotiana tabacum: Genome-wide identification, evolution and role in disease resistance. Genomics 2020, 112, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Yan, A.; Deng, J.; Xie, Y.; Liu, S.; Liu, D.; He, L.; Weng, J.; Xu, J. Evolutionary analysis of respiratory burst oxidase homolog (RBOH) genes in plants and characterization of ZmRBOHs. Int. J. Mol. Sci. 2023, 24, 3858. [Google Scholar] [CrossRef]
- Himanen, K.I.H.; Cheng, C.; Che, Q.; Su, S.; Liu, Y.; Wang, Y.; Xu, X. Genome-wide identification and characterization of respiratory burst oxidase homolog genes in six Rosaceae species and an analysis of their effects on adventitious rooting in apple. PLoS ONE 2020, 15, e0239705. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Y.; Ali, B.; Ahmed, W.; Tang, Y.; Li, H. Genome-wide identification, classification, evolutionary expansion and expression of Rboh family genes in pepper (Capsicum annuum L.). Trop. Plant Biol. 2021, 14, 251–266. [Google Scholar] [CrossRef]
- Simon-Plas, F.o.; Elmayan, T.; Blein, J.P. The plasma membrane oxidase NtrbohD is responsible for AOS production in elicited tobacco cells. Plant J. Cell Mol. Biol. 2002, 31, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; He, Y.; Hu, W.; Zhang, Y.; Wang, X.; Tang, H. Identification of NADPH oxidase family members associated with cold stress in strawberry. FEBS Open Bio 2018, 8, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P. Production of reactive oxygen species by photosystem II. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2009, 1787, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, D.; Li, S.; Dai, Y.; Cao, Y. Evolutionary and functional analysis of the plant-specific NADPH oxidase gene family in Brassica rapa L. R. Soc. Open Sci. 2019, 6, 181727. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, Y.; Wang, Z.; Bao, S.; Feng, Y.; Wang, J.; Yu, Z.; Long, F.; Xiao, Z.; Hao, Y.; et al. Two-step model of paleohexaploidy, ancestral genome reshuffling and plasticity of heat shock response in Asteraceae. Hortic. Res. 2023, 10, uhad073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, C.-H.; Liu, M.; Hu, Y.; Panero, J.L.; Luebert, F.; Gao, T.; Ma, H. Phylotranscriptomic insights into Asteraceae diversity, polyploidy, and morphological innovation. J. Integr. Plant Biol. 2021, 63, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Su, J.; Wang, H.; Zhang, Z.; Zhang, X.; Van de Peer, Y.; Chen, F.; Fang, W.; Guan, Z.; Zhang, F.; et al. Analyses of a chromosome-scale genome assembly reveal the origin and evolution of cultivated chrysanthemum. Nat. Commun. 2023, 14, 2021. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Song, X.; Liu, T.; Huang, Z.; Ren, J.; Hou, X.; Du, J.; Li, Y. Patterns of evolutionary conservation of ascorbic acid-related genes following whole-genome triplication in Brassica rapa. Genome Biol. Evol. 2014, 7, 299–313. [Google Scholar] [CrossRef]
- Liang, Y.; Xiong, Z.; Zheng, J.; Xu, D.; Zhu, Z.; Xiang, J.; Gan, J.; Raboanatahiry, N.; Yin, Y.; Li, M. Genome-wide identification, structural analysis and new insights into late embryogenesis abundant (LEA) gene family formation pattern in Brassica napus. Sci. Rep. 2016, 6, 24265. [Google Scholar] [CrossRef]
- Kaur, G.; Pati, P.K. Analysis of cis-acting regulatory elements of respiratory burst oxidase homolog (Rboh) gene families in Arabidopsis and rice provides clues for their diverse functions. Comput. Biol. Chem. 2016, 62, 104–118. [Google Scholar] [CrossRef]
- Sharma, Y.; Ishu; Shumayla; Dixit, S.; Singh, K.; Upadhyay, S.K. Decoding the features and potential roles of respiratory burst oxidase homologs in bread wheat. Curr. Plant Biol. 2024, 37, 100315. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Chen, Z.; Yu, J.-Q. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef]
- Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Exogenous jasmonic acid modulates the physiology, antioxidant defense and glyoxalase systems in imparting drought stress tolerance in different Brassica species. Plant Biotechnol. Rep. 2014, 8, 279–293. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kim, Y.-J.; Hong, W.-J.; Lee, C.; Jeon, J.-S.; Jung, K.-H. Genome-wide analysis of root hair preferred RBOH genes suggests that three RBOH genes are associated with auxin-mediated root hair development in rice. J. Plant Biol. 2019, 62, 229–238. [Google Scholar] [CrossRef]
- Ranjan, A.; Jayaraman, D.; Grau, C.; Hill, J.H.; Whitham, S.A.; Ané, J.-M.; Smith, D.L.; Kabbage, M. The pathogenic development of Sclerotinia sclerotiorum in soybean requires specific host NADPH oxidases. Mol. Plant Pathol. 2018, 19, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Navathe, S.; Singh, S.; Singh, V.K.; Chand, R.; Mishra, V.K.; Joshi, A.K. Genome-wide mining of respiratory burst homologs and its expression in response to biotic and abiotic stresses in Triticum aestivum. Genes Genom. 2019, 41, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Kurusu, T.; Kuchitsu, K.; Tada, Y. Plant signaling networks involving Ca2+ and Rboh/Nox-mediated ROS production under salinity stress. Front. Plant Sci. 2015, 6, 427. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, H.; Sun, L.; Jiao, Y.; Zhang, G.; Miao, C.; Hao, F. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J. Exp. Bot. 2011, 63, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Ben Rejeb, K.; Benzarti, M.; Debez, A.; Bailly, C.; Savouré, A.; Abdelly, C. NADPH oxidase-dependent H2O2 production is required for salt-induced antioxidant defense in Arabidopsis thaliana. J. Plant Physiol. 2015, 174, 5–15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).