Effects of Nano-Silica and Multi-Walled Carbon Nanotubes on Grape Seedlings under Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Material
2.2. Methods
2.2.1. Determination of Germination Indexes of Grape Seeds
- (1)
- CK, deionized water;
- (2)
- S, 4 g/L NaCl solution;
- (3)
- S + Si40, 4 g/L NaCl solution + 40 μg/mL nano-silica suspension;
- (4)
- S + Si60, 4 g/L NaCl solution + 60 μg/mL nano-silica suspension;
- (5)
- S + Si80, 4 g/L NaCl solution + 80 μg/mL nano-silica suspension;
- (6)
- S + C90, 4 g/L NaCl solution + 90 μg/mL multi-walled carbon nanotube suspension;
- (7)
- S + Si40 + C90, 4 g/L NaCl solution + 40 μg/mL nano-silica + 90 μg/mL MWCNTs suspension.
2.2.2. Determination of Photosynthetic Indexes
2.2.3. Determination of Soluble Protein Content, Enzymatic Antioxidant Enzyme (SOD, POD, CAT) Activity, MDA Content, and DPPH· Free Radical Scavenging Activity
2.2.4. Determination of Related Enzyme Activities in the AsA-GSH Cycle
2.3. Data Analysis
3. Results
3.1. Effects of Two Nanomaterial Treatments on Grape Germination and Seedling Growth under Salt Stress
3.2. Effects of Two Nanomaterial Treatments on Photosynthetic Characteristics of Grape Seedling Leaves under Salt Stress
3.2.1. Effects of Two Nanomaterial Treatments on Leaf Stomatal Conductance (Gs) of Grape Seedlings under Salt Stress
3.2.2. Effects of Two Nanomaterial Treatments on Intercellular CO2 Concentration (Ci) in Grape Leaves under Salt Stress
3.2.3. Effects of Two Nanomaterial Treatments on Net Photosynthetic Rate (Pn) of Grape Seedlings under Salt Stress
3.2.4. Effects of Two Nanomaterial Treatments on Water Efficiency (We) of Grape Leaves under Salt Stress
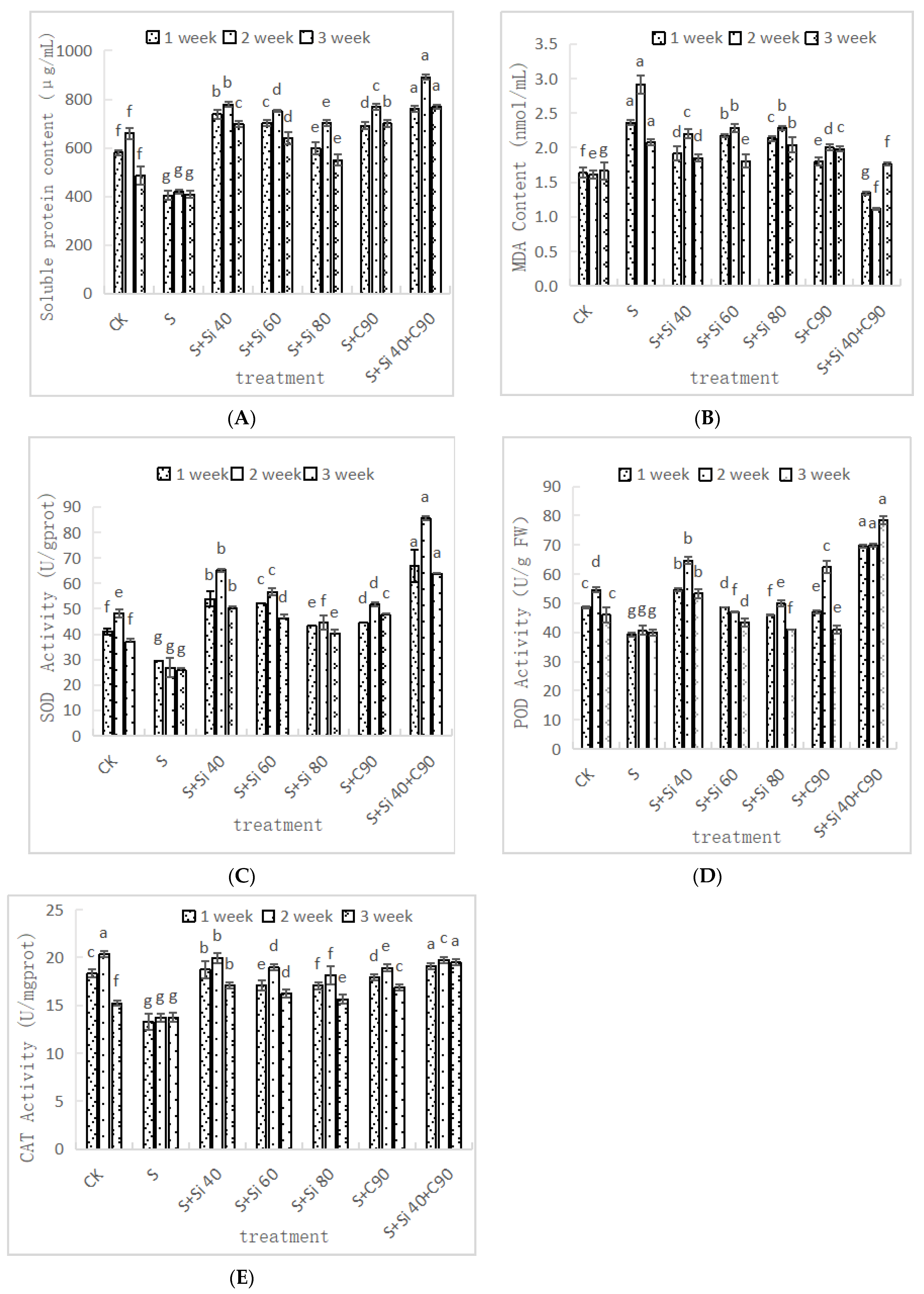
3.3. Effects of Two Nanomaterial Treatments on Soluble Protein Content and Antioxidant Enzyme Activities of Grape Seedlings under Salt Stress
3.3.1. Effects of Two Nanomaterial Treatments on Soluble Protein Content of Grape Seedlings under Salt Stress
3.3.2. Effects of Two Nanomaterial Treatments on Malondialdehyde (MDA) Content in Grape Seedlings under Salt Stress
3.3.3. Effects of Two Nanomaterial Treatments on Superoxide Dismutase (SOD) Activity of Grape Seedlings under Salt Stress
3.3.4. Effects of Two Nanomaterial Treatments on Peroxidase (POD) Activity of Grape Seedlings under Salt Stress
3.3.5. Effects of Two Nanomaterial Treatments on Catalase (CAT) Activity in Leaves of Grape Seedlings under Salt Stress
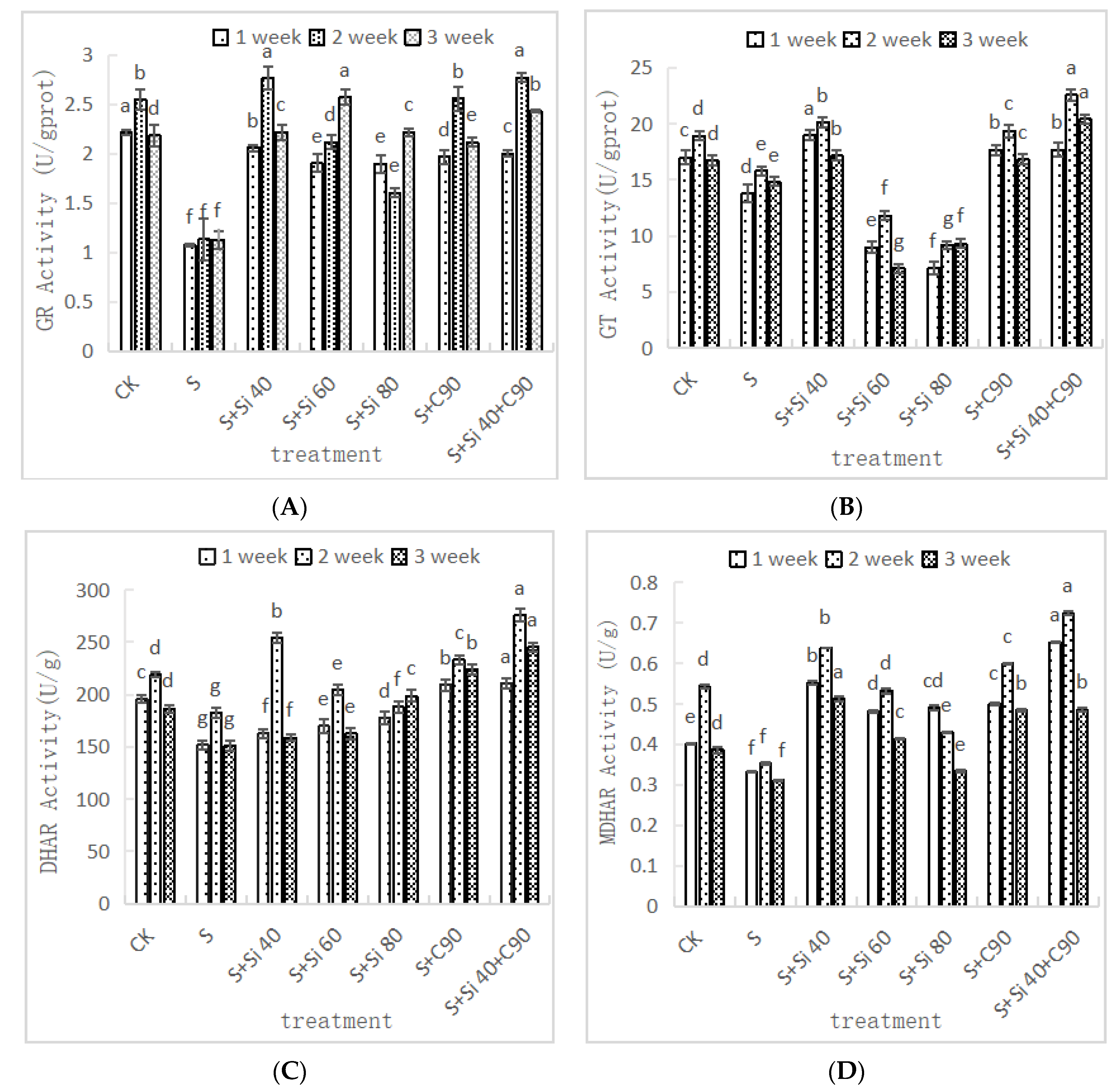
3.4. Effects of Two Nanomaterial Treatments on APX Activity and DPPH. Scavenging Activity of Grape Seedling Leaves under Salt Stress
3.5. Effects of Two Nanomaterial Treatments on the Activities of Related Enzymes in the AsA-GSH Cycle of Grape Seedling Leaves under Salt Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Huang, D.; Chen, C.; Zhu, S.; Gao, J. Regulation of ascorbate-glutathione cycle in peaches via nitric oxide treatment during cold storage. Sci. Hortic. 2019, 247, 400–406. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Yang, X.; Zhang, Y.; Hui, H.; Zhang, D.; Shu, J. Multi-walled carbon nanotubes enhanced the antioxidative system and alleviated salt stress in grape seedlings. Sci. Hortic. 2022, 293, 110698. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, X.; Sun, X.; Tan, Q.; Tang, Y.; Nie, Z.; Qu, C.; Chen, Z.; Hu, C. Antioxidant enzyme systems and the ascorbate–glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere 2015, 138, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; Kim, B.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Tiwari, S.; Parihar, P.; Singh, R.; Prasad, S.M. Carbon Nanotubes As Plant Growth regulators: Impacts On growth, Reproductive system, and Soil Microbial Community. In Nanomaterials in Plants, Algae and Microorganisms; Academic Press: Cambridge, MA, USA, 2019; Volume 2, pp. 23–42. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N.S.; Prabu, P. Influence of nanosilica powder on the growth of maize crop (Zea mays L.). Int. J. Green Nanotechnol. 2011, 3, 180–190. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Lee, I.-J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017, 8, 510. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Prochazkova, D.; Sairam, R.; Srivastava, G.; Singh, D. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Hodges, D.M.; Forney, C.F. The effects of ethylene, depressed oxygen and elevated carbon dioxide on antioxidant profiles of senescing spinach leaves. J. Exp. Bot. 2000, 51, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Zhang, Y.; Zhang, H. ABA participates in the regulation of vitamin C content in the fruit of strawberry using lanthanum nitrate. Sci. Hortic. 2018, 233, 455–459. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Kumar, G.N.M.; Knowles, N.R. Maturation and ripening of fruit of Amelanchier alnifolia Nutt. are accompanied by increasing oxidative stress. Ann. Bot. 1998, 81, 203–211. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Q. Novel hydrated graphene ribbon unexpectedly promotes aged seed germination and root differentiation. Sci. Rep. 2014, 4, 3782. [Google Scholar] [CrossRef]
- Andersen, C.P.; King, G.; Plocher, M.; Storm, M.; Pokhrel, L.R.; Johnson, M.G.; Rygiewicz, P.T. Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ. Toxicol. Chem. 2016, 35, 2223–2229. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; De Silva, K.; Biris, A.S.; Dervishi, E.; Villagarcia, H. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.C.; Zapata, L.; Chalbi, N.; Carvajal, M. Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J. Nanobiotechnol. 2016, 14, 42. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.C.; Chelbi, N.; Lopez-Zaplana, A.; Carvajal, M. Discerning the mechanism of the multiwalled carbon nanotubes effect on root cell water and nutrient transport. Plant Physiol. Biochem. 2020, 146, 23–30. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawani, M.; Elhawat, N.; Al-Otaibi, A. Exogenous nanosilica improves germination and growth of cucumber by maintaining K+/Na+ ratio under elevated Na+ stress. Plant Physiol. Biochem. 2018, 125, 164–171. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Gossett, D.R.; Banks, S.W.; Millhollon, E.P.; Lucas, M.C. Antioxidant response to NaCl stress in a control and an NaCl-tolerant cotton cell line grown in the presence of paraquat, buthionine sulfoximine, and exogenous glutathione. Plant Physiol. 1996, 112, 803–809. [Google Scholar] [CrossRef]
- Urano, J.; Nakagawa, T.; Maki, Y.; Masumura, T.; Tanaka, K.; Murata, N.; Ushimaru, T. Molecular cloning and characterization of a rice dehydroascorbate reductase. FEBS Lett. 2000, 466, 107–111. [Google Scholar] [CrossRef]
- Hossain, Z.; Mustafa, G.; Komatsu, S. Plant responses to nanoparticle stress. Int. J. Mol. Sci. 2015, 16, 26644–26653. [Google Scholar] [CrossRef]
- Kwon, S.-Y.; Choi, S.-M.; Ahn, Y.-O.; Lee, H.-S.; Lee, H.-B.; Park, Y.-M.; Kwak, S.-S. Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J. Plant Physiol. 2003, 160, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Bhandari, P. Interactive effects of silicon and arbuscular mycorrhiza in modulating ascorbate-glutathione cycle and antioxidant scavenging capacity in differentially salt-tolerant Cicer arietinum L. genotypes subjected to long-term salinity. Protoplasma 2016, 253, 1325–1345. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McLellan, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free. Radic. Res. 2009, 31, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Sohan, E.Y.; Khan, S.A.; Shinwari, Z.K. Silicon alleviates the adverse effects of salinity and drought stress on growth and endogenous plant growth hormones of soybean (Glycine max L.). Pakistan J. Bot. 2010, 42, 1713–1722. [Google Scholar]
- Jafari, M.; Haghighi, J.; Zare, H. Mulching impact on plant growth and production of rainfed fig orchards under drought conditions. J. Food Agric. Environ. 2012, 10, 428–433. [Google Scholar]
- Hossain, M.T.; Mori, R.; Soga, K.; Wakabayashi, K.; Kamisaka, S.; Fujii, S.; Yamamoto, R.; Hoson, T. Growth promotion and an increase in cell wall extensibility by silicon in rice and some other Poaceae seedlings. J. Plant Res. 2002, 115, 23–27. [Google Scholar] [CrossRef]
- Neu, S.; Schaller, J.; Dudel, E.G. Silicon availability modifies nutrient use efficiency and content, C:N:P stoichiometry, and productivity of winter wheat (Triticum aestivum L.). Sci. Rep. 2017, 7, 40829. [Google Scholar] [CrossRef] [PubMed]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Chen, K.; Chen, G.; Wang, S.; Zhang, C. Effects of silicon on growth of wheat under drought. J. Plant Nutr. 2003, 26, 1055–1063. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, W.; Chen, Q.; Liu, Y.; Ding, R. Effect of exogenous silicon (Si) on H+-ATPase activity, phospholipids and fluidity of plasma membrane in leaves of salt-stressed barley (Hordeum vulgare L.). Environ. Exp. Bot. 2006, 57, 212–219. [Google Scholar] [CrossRef]
- Liang, Y.C. Effects of Si on leaf ultrastructure, chlorophyll content and photosynthetic activity in barley under salt stress. Pedosphere 1998, 8, 289–296. [Google Scholar]


| Seedling | Root | |||||
|---|---|---|---|---|---|---|
| Treatment (T) | No. | GR | FW (g) | DW (g) | No. | Length (cm) |
| CK | 86 | 86% | 0.2162 ± 0.0044 b | 0.0422 ± 0.0036 a | 86 | 2.54 ± 0.14 d |
| S | 57 | 57% | 0.1477 ± 0.0049 e | 0.0211 ± 0.0031 e | 57 | 1.27 ± 0.18 f |
| S + Si40 | 90 | 90% | 0.2052 ± 0.0105 c | 0.0313 ± 0.0003 bc | 90 | 2.89 ± 0.27 b |
| S + Si60 | 80 | 80% | 0.1633 ± 0.0037 d | 0.0284 ± 0.0014 d | 80 | 2.41 ± 0.19 e |
| S + Si80 | 83 | 83% | 0.1393 ± 0.0032 f | 0.0280 ± 0.0009 d | 83 | 2.69 ± 0.16 c |
| S + C90 | 88 | 88% | 0.2059 ± 0.0035 c | 0.0311 ± 0.0013 bc | 88 | 2.84 ± 0.15 b |
| S + Si40 + C90 | 96 | 96% | 0.2455 ± 0.0233 a | 0.0316 ± 0.0014 b | 96 | 3.32 ± 0.33 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sheng, Y.; Shu, J.; Hao, S.; Wang, J.; Huang, Q.; He, K.; Qi, J.; Liu, J. Effects of Nano-Silica and Multi-Walled Carbon Nanotubes on Grape Seedlings under Salt Stress. Agronomy 2024, 14, 622. https://doi.org/10.3390/agronomy14030622
Li Y, Sheng Y, Shu J, Hao S, Wang J, Huang Q, He K, Qi J, Liu J. Effects of Nano-Silica and Multi-Walled Carbon Nanotubes on Grape Seedlings under Salt Stress. Agronomy. 2024; 14(3):622. https://doi.org/10.3390/agronomy14030622
Chicago/Turabian StyleLi, Yang, Yinsheng Sheng, Jing Shu, Shuqin Hao, Jinquan Wang, Qinglin Huang, Kailong He, Jiahui Qi, and Jin Liu. 2024. "Effects of Nano-Silica and Multi-Walled Carbon Nanotubes on Grape Seedlings under Salt Stress" Agronomy 14, no. 3: 622. https://doi.org/10.3390/agronomy14030622
APA StyleLi, Y., Sheng, Y., Shu, J., Hao, S., Wang, J., Huang, Q., He, K., Qi, J., & Liu, J. (2024). Effects of Nano-Silica and Multi-Walled Carbon Nanotubes on Grape Seedlings under Salt Stress. Agronomy, 14(3), 622. https://doi.org/10.3390/agronomy14030622





