Seed Priming with Nanoencapsulated Gibberellic Acid Triggers Beneficial Morphophysiological and Biochemical Responses of Tomato Plants under Different Water Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Formulations
2.2. Plant Material and Experimental Design
2.2.1. Experiment 1
2.2.2. Experiment 2
2.3. Physiological Analyses
2.4. Morphological Analyses
2.5. Biochemical Analyses
2.6. Statistical Analysis
3. Results and Discussion
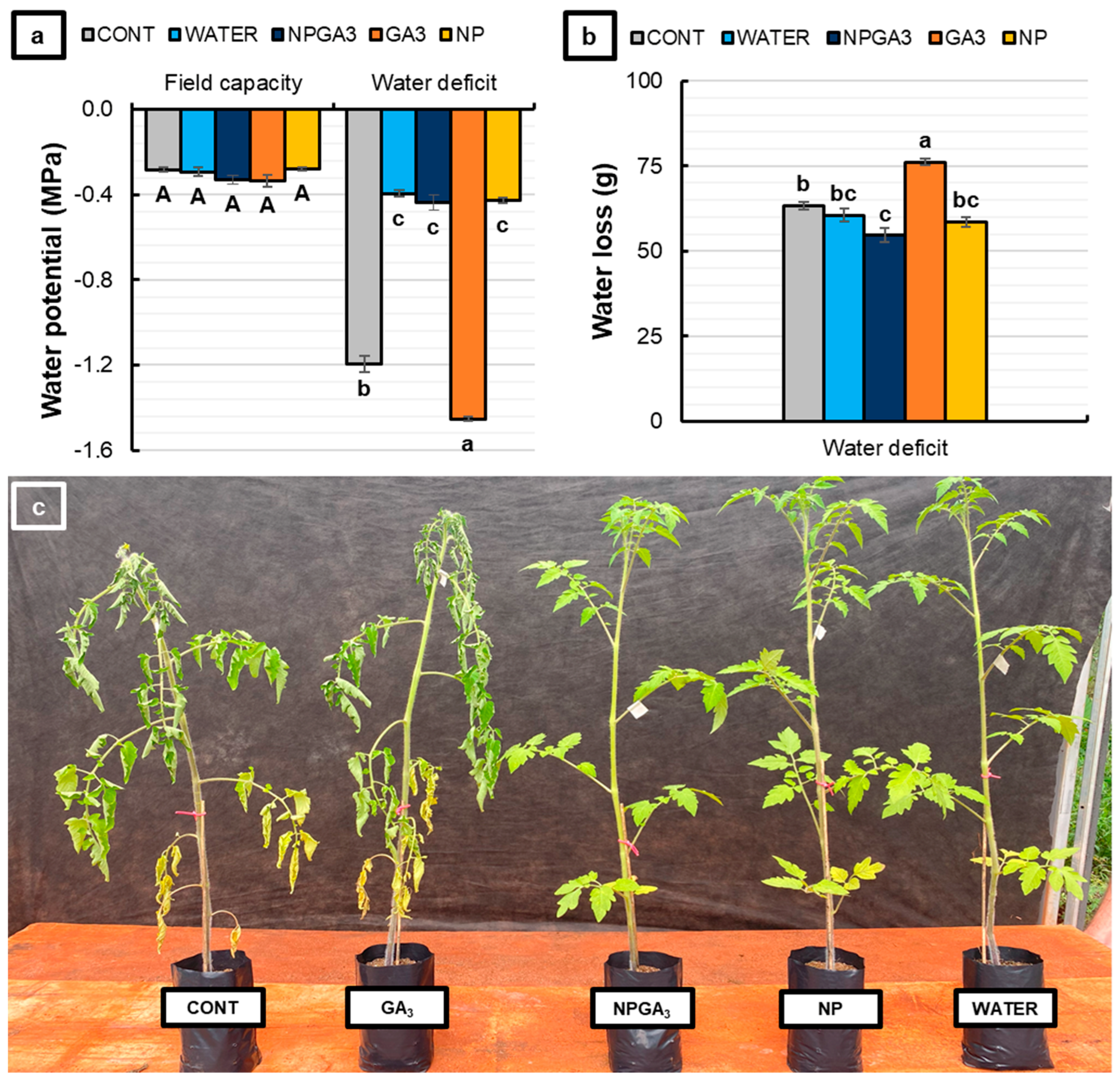
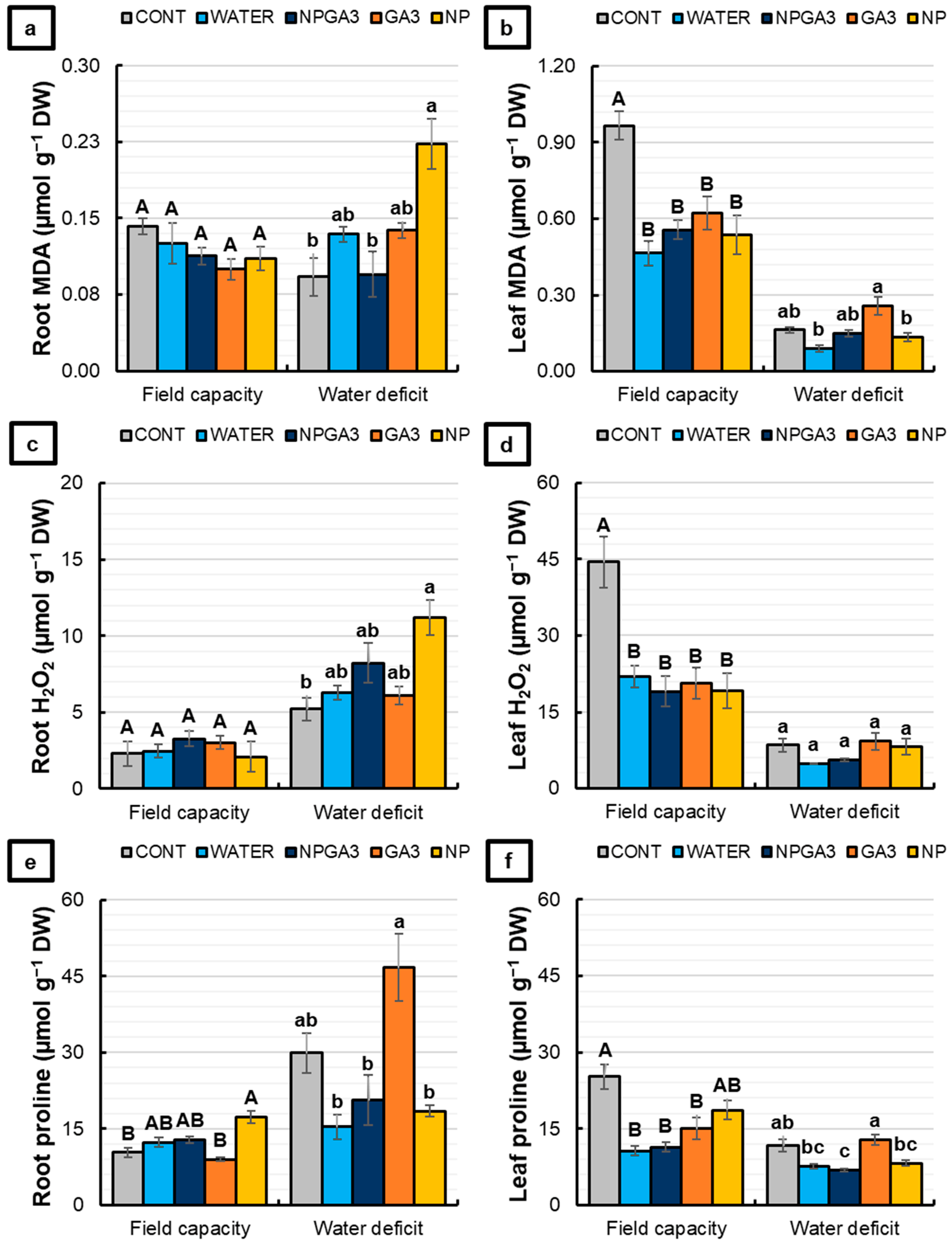
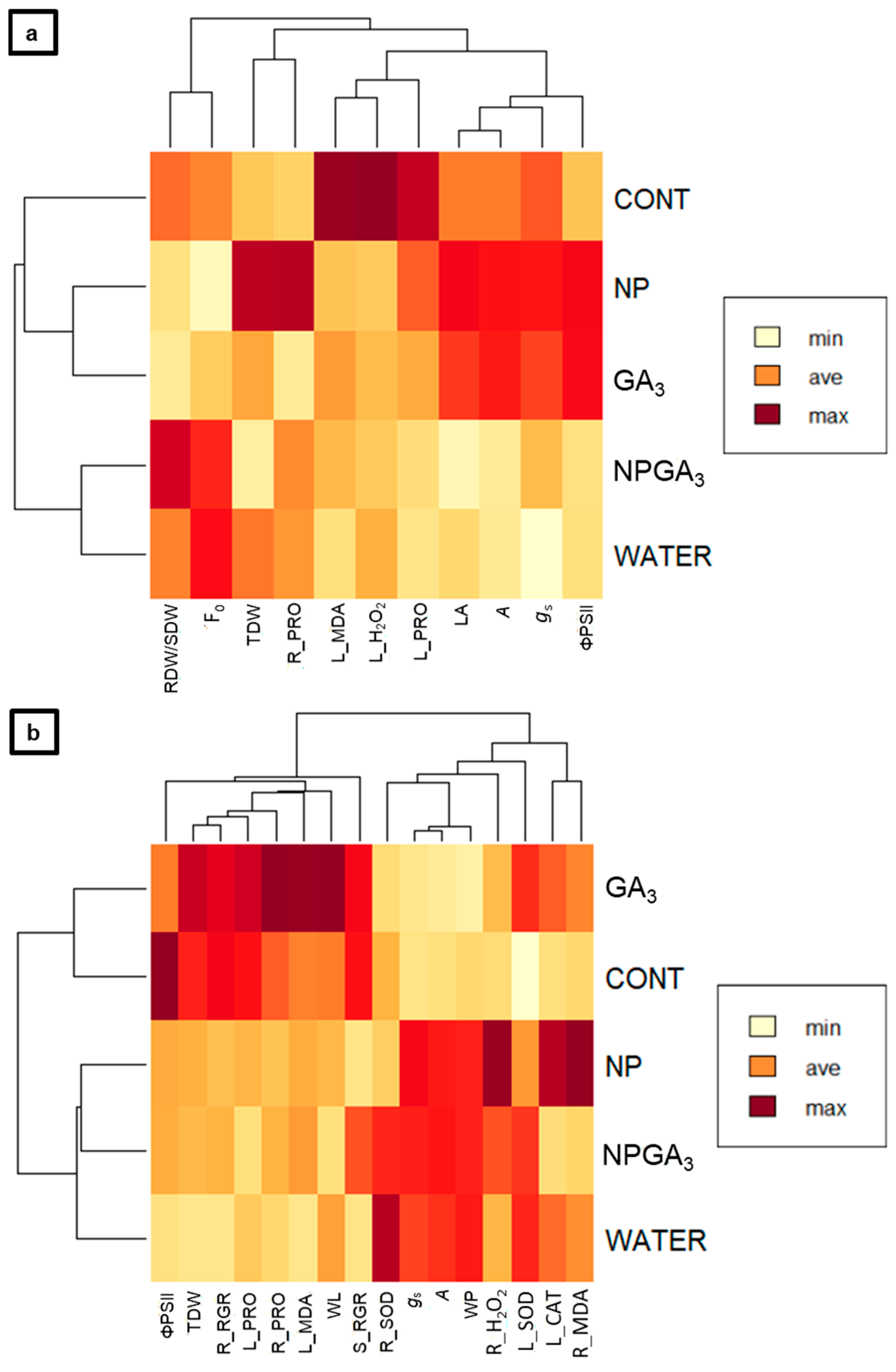
3.1. Experiment 1
3.2. Experiment 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tyczewska, A.; Woźniak, E.; Gracz, J.; Kuczyński, J.; Twardowski, T. Towards food security: Current state and future prospects of agrobiotechnology. Trends Biotechnol. 2018, 36, 1219–1229. [Google Scholar] [CrossRef]
- Wing, I.S.; De Cian, E.; Mistry, M.N. Global vulnerability of crop yields to climate change. J. Environ. Econ. Manag. 2021, 109, 102462. [Google Scholar] [CrossRef]
- Placide, R.; Hirut, G.B.; Stephan, N.; Fekadu, B. Assessment of drought stress tolerance in root and tuber crops. Afr. J. Plant Sci. 2014, 8, 214–224. [Google Scholar] [CrossRef][Green Version]
- Kebbas, S.; Lutts, S.; Aid, F. Effect of drought stress on the photosynthesis of Acacia tortilis subsp. raddiana at the young seedling stage. Photosynthetica 2015, 53, 288–298. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Campos, E.V.; do ESPereira, A.; Aleksieienko, I.; Do Carmo, G.C.; Gohari, G.; Santaella, C.; Oliveira, H.C. Encapsulated plant growth regulators and associative microorganisms: Nature-based solutions to mitigate the effects of climate change on plants. Plant Sci. 2023, 331, 111688. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, J.; Briones-Moreno, A.; Blázquez, M.A. Origin and evolution of gibberellin signaling and metabolism in plants. Semin. Cell Dev. Biol. 2021, 109, 46–54. [Google Scholar] [CrossRef]
- Shah, S.H.; Islam, S.; Mohammad, F.; Siddiqui, M.H. Gibberellic Acid: A Versatile Regulator of Plant Growth, Development and Stress Responses. J. Plant. Growth. Regul. 2023, 42, 7352–7373. [Google Scholar] [CrossRef]
- Elahi, N.N.; Raza, S.; Rizwan, M.S.; Albalawi, B.F.A.; Ishaq, M.Z.; Ahmed, H.M.; Mehmood, S.; Imtiaz, M.; Farooq, U.; Rashid, M.; et al. Foliar application of gibberellin alleviates adverse impacts of drought stress and improves growth, physiological and biochemical attributes of Canola (Brassica napus L.). Sustainability 2022, 15, 78. [Google Scholar] [CrossRef]
- Guo, T.; Gull, S.; Ali, M.M.; Yousef, A.F.; Ercisli, S.; Kalaji, M.K.; Telesiński, A.; Auriga, A.; Wróbel, J.; Rawan, N.S.; et al. Heat stress mitigation in tomato (Solanum lycopersicum L.) through foliar application of gibberellic acid. Sci. Rep. 2022, 12, 11324. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Shin, J.H.; Ahmad, B.; Shin, D.H.; Lee, I.J. Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J. Agric. Food Chem. 2010, 58, 7226–7232. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 2020, 10, 37. [Google Scholar] [CrossRef]
- Dawood, M.G. Stimulating plant tolerance against abiotic stress through seed priming. In Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer Nature: Singapore, 2018; pp. 147–183. [Google Scholar]
- Bhat, J.A.; Basit, F.; Alyemeni, M.N.; Mansoor, S.; Kaya, C.; Ahmad, P. Gibberellic acid mitigates nickel stress in soybean by cell wall fixation and regulating oxidative stress metabolism and glyoxalase system. Plant Physiol. Biochem. 2023, 198, 107678. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Hao, X.; Wang, T.; Li, H.; Shi, X.; Liu, Y.; Luo, H. Seed priming with gibberellin regulates the germination of cotton seeds under low-temperature conditions. J. Plant. Growth Regul. 2023, 42, 319–334. [Google Scholar] [CrossRef]
- Tsegay, B.A.; Andargie, M. Seed Priming with Gibberellic Acid (GA 3) Alleviates Salinity Induced Inhibition of Germination and Seedling Growth of Zea mays L., Pisum sativum Var. abyssinicum A. Braun and Lathyrus sativus L. J. Crop Sci. Biotechnol. 2018, 21, 261–267. [Google Scholar] [CrossRef]
- Li, Z.; Lu, G.Y.; Zhang, X.K.; Zou, C.S.; Cheng, Y.; Zheng, P.Y. Improving drought tolerance of germinating seeds by exogenous application of gibberellic acid (GA3) in rapeseed (Brassica napus L.). Seed Sci. Technol. 2010, 38, 432–440. [Google Scholar] [CrossRef]
- Akter, N.; Rafiqul Islam, M.; Abdul Karim, M.; Hossain, T. Alleviation of drought stress in maize by exogenous application of gibberellic acid and cytokinin. J. Crop Sci. Biotechnol. 2014, 17, 41–48. [Google Scholar] [CrossRef]
- Du, G.; Zhang, H.; Yang, Y.; Zhao, Y.; Tang, K.; Liu, F. Effects of Gibberellin Pre-Treatment on Seed Germination and Seedling Physiology Characteristics in Industrial Hemp under Drought Stress Condition. Life 2022, 12, 1907. [Google Scholar] [CrossRef] [PubMed]
- Sampedro-Guerrero, J.; Vives-Peris, V.; Gomez-Cadenas, A.; Clausell-Terol, C. Efficient strategies for controlled release of nanoencapsulated phytohormones to improve plant stress tolerance. Plant Methods 2023, 19, 47. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, H.; Zhou, J. The Applications of Nanotechnology in Crop Production. Molecules 2021, 26, 7070. [Google Scholar] [CrossRef]
- Periakaruppan, R.; Romanovski, V.; Thirumalaisamy, S.K.; Palanimuthu, V.; Sampath, M.P.; Anilkumar, A.; Sivaraj, D.K.; Ahamed NA, N.; Murugesan, S.; Chandrasekar, D.; et al. Innovations in Modern Nanotechnology for the Sustainable Production of Agriculture. ChemEngineering 2023, 7, 61. [Google Scholar] [CrossRef]
- Pascoli, M.; Lopes-Oliveira, P.J.; Fraceto, L.F.; Seabra, A.B.; Oliveira, H.C. State of the art of polymeric nanoparticles as carrier systems with agricultural applications: A minireview. Energy Ecol. Environ. 2018, 3, 137–148. [Google Scholar] [CrossRef]
- Shelar, A.; Nile, S.H.; Singh, A.V.; Rothenstein, D.; Bill, J.; Xiao, J.; Chaskar, M.; Kai, G.; Patil, R. Recent advances in nano-enabled seed treatment strategies for sustainable agriculture: Challenges, risk assessment, and future perspectives. Nanomicro Lett. 2023, 15, 54. [Google Scholar] [CrossRef]
- Dutta, P. Seed priming: New vistas and contemporary perspectives. In Advances in Seed Priming; Rakshit, A., Singh, H., Eds.; Springer: Singapore, 2018; pp. 3–22. [Google Scholar]
- Oliveira, H.C.; Gomes, B.C.; Pelegrino, M.T.; Seabra, A.B. Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide 2016, 61, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Oliveira, P.J.; Gomes, D.G.; Pelegrino, M.T.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R.; Seabra, A.B.; Oliveira, H.C. Effects of nitric oxide-releasing nanoparticles on neotropical tree seedlings submitted to acclimation under full sun in the nursery. Sci. Rep. 2019, 9, 17371. [Google Scholar] [CrossRef]
- Silveira, N.M.; Prataviera, P.J.; Pieretti, J.C.; Seabra, A.B.; Almeida, R.L.; Machado, E.C.; Ribeiro, R.V. Chitosan-encapsulated nitric oxide donors enhance physiological recovery of sugarcane plants after water deficit. Environ. Exp. Bot. 2021, 190, 104593. [Google Scholar] [CrossRef]
- Silveira, N.M.; Seabra, A.B.; Marcos, F.C.C.; Pelegrino, M.T.; Machado, E.C.; Ribeiro, R.V. Encapsulation of S-nitrosoglutathione into chitosan nanoparticles improves drought tolerance of sugarcane plants. Nitric Oxide 2019, 84, 38–44. [Google Scholar] [CrossRef]
- do Carmo, G.C.; Iastrenski, L.F.; Debiasi, T.V.; da Silva, R.C.; Gomes, D.G.; Pelegrino, M.T.; Bianchini, E.; Stolf-Moreira, R.; Pimenta, J.A.; Seabra, A.B.; et al. Nanoencapsulation improves the protective effects of a nitric oxide donor on drought-stressed Heliocarpus popayanensis seedlings. Ecotoxicol. Environ. Saf. 2021, 225, 112713. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.G.; Debiasi, T.V.; Pelegrino, M.T.; Pereira, R.M.; Ondrasek, G.; Batista, B.L.; Oliveira, H.C. Soil Treatment with Nitric Oxide-Releasing Chitosan Nanoparticles Protects the Root System and Promotes the Growth of Soybean Plants under Copper Stress. Plants 2022, 11, 3245. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Joshi, A.; Kumari, S.; Kumaraswamy, R.V.; Saharan, V. Preparation of Cu-chitosan nanoparticle and its effect on growth and enzyme activity during seed germination in maize. J. Pharmacogn. Phytochem. 2017, 6, 669–673. [Google Scholar]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Saharan, V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754. [Google Scholar] [CrossRef]
- Gomes, D.G.; Pelegrino, M.T.; Ferreira, A.S.; Bazzo, J.H.; Zucareli, C.; Seabra, A.B.; Oliveira, H.C. Seed priming with copper-loaded chitosan nanoparticles promotes early growth and enzymatic antioxidant defense of maize (Zea mays L.) seedlings. J. Chem. Technol. Biotech. 2021, 96, 2176–2184. [Google Scholar] [CrossRef]
- Gomes, D.G.; Sanada, K.; Pieretti, J.C.; Shigueoka, L.H.; Sera, G.H.; Seabra, A.B.; Oliveira, H.C. Nanoencapsulation Boosts the Copper-Induced Defense Responses of a Susceptible Coffea arabica Cultivar against Hemileia vastatrix. Antibiotics 2023, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Kocięcka, J.; Liberacki, D. The potential of using chitosan on cereal crops in the face of climate change. Plants 2021, 10, 1160. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.E.S.; Silva, P.M.; Oliveira, J.L.; Oliveira, H.C.; Fraceto, L.F. Chitosan nanoparticles as carrier systems for the plant growth hormone gibberellic acid. Colloids Surf. B 2017, 150, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Pereira AD, E.S.; Oliveira, H.C.; Fraceto, L.F. Polymeric nanoparticles as an alternative for application of gibberellic acid in sustainable agriculture: A field study. Sci. Rep. 2019, 9, 7135. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef]
- Calvo, P.; Remunán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant. Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Camejo, G.; Wallin, B.; Enojärvi, M. Analyses of oxidation and antioxidants using microtiter plates. In Free Radical and Antioxidants Protocols; Amstrong, D., Ed.; Humana Press: Mölndal, Sweden, 1998; pp. 377–387. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Karalija, E.; Selovic, A. The effect of hydro and proline seed priming on growth, proline and sugar content, and antioxidant activity of maize under cadmium stress. Environ. Sci. Pollut. Res. 2018, 25, 33370–33380. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: Orlando, FL, USA, 1984; pp. 114–121. [Google Scholar]
- Peixoto PH, P.; Cambraia, J.; Sant’Anna, R.; Mosquim, P.R.; Moreira, M.A. Aluminum effects on lipid peroxidation and on the activities of enzymes of oxidative metabolism in sorghum. Braz. J. Plant. Physiol. 1999, 11, 137–143. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: II. Purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiol. 1977, 59, 315–318. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 16 June 2023).
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Ahmad, A.; Hashmi, S.S.; Palma, J.M.; Corpas, F.J. Influence of metallic, metallic oxide, and organic nanoparticles on plant physiology. Chemosphere 2022, 290, 133329. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Avellan, A.; Yun, J.; Morais, B.P.; Clement, E.T.; Rodrigues, S.M.; Lowry, G.V. Critical review: Role of inorganic nanoparticle properties on their foliar uptake and in planta translocation. Environ. Sci. Technol. 2021, 55, 13417–13431. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Mellidou, I.; Moustakas, M. Harnessing chlorophyll fluorescence for phenotyping analysis of wild and cultivated tomato for high photochemical efficiency under water deficit for climate change resilience. Climate 2021, 9, 154. [Google Scholar] [CrossRef]
- Urban, L.; Aarrouf, J.; Bidel, L.P. Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yang, F.; Liu, Z.; Li, Y.; Di, X.; Wang, J.; Lin, J. Uniform water potential induced by salt, alkali, and drought stresses has different impacts on the seedling of Hordeum jubatum: From growth, photosynthesis, and chlorophyll fluorescence. Front. Plant Sci. 2021, 12, 733236. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Li, R.; Chen, Z.H.; Wang, Y. Stomata at the crossroad of molecular interaction between biotic and abiotic stress responses in plants. Front. Plant Sci. 2022, 13, 1031891. [Google Scholar] [CrossRef]
- Dow, G.J.; Berry, J.A.; Bergmann, D.C. The physiological importance of developmental mechanisms that enforce proper stomatal spacing in a Rabidopsis thaliana. New Phytol. 2014, 201, 1205–1217. [Google Scholar] [CrossRef]
- Nemeskéri, E.; Neményi, A.; Bőcs, A.; Pék, Z.; Helyes, L. Physiological factors and their relationship with the productivity of processing tomato under different water supplies. Water 2019, 11, 586. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Zhou, D.; Zhang, Y.; Song, R.; Li, C.; Li, J.; Gao, J. The Roles of Gibberellins in Regulating Leaf Development. Plants 2023, 12, 1243. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Han, W.; Kang, J. Responses of root system architecture to water stress at multiple levels: A meta-analysis of trials under controlled conditions. Front. Plant Sci. 2022, 13, 1085409. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.Y.; Zhao, D.D.; Ning, Q.R.; Wei, J.P.; Li, Y.; Wang, M.M.; Liu, X.; Jiang, C.; Liang, Z.W. A multi-year beneficial effect of seed priming with gibberellic acid-3 (GA3) on plant growth and production in a perennial grass, Leymus chinensis. Sci. Rep. 2018, 8, 13214. [Google Scholar] [CrossRef]
- Liu, X.; Quan, W.; Bartels, D. Stress memory responses and seed priming correlate with drought tolerance in plants: An overview. Planta 2022, 255, 45. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Dezfuli, P.M.; Sharifzadeh, F. Seed invigoration techniques to improve germination and early growth of inbred line of maize under salinity and drought stress. Gen. Appl. Plant Physiol. 2008, 34, 215–226. [Google Scholar]
- Nasir, M.W.; Yasmeen, A.; Imran, M.; Zoltan, T. Seed priming to alleviate drought stress in cotton. J. Agric. Environ. Sci. 2019, 21, 14–22. [Google Scholar]
- Tao, Q.; Lv, Y.; Mo, Q.; Bai, M.; Han, Y.; Wang, Y. Impacts of priming on seed germination and seedling emergence of Cleistogenes songorica under drought stress. Seed Sci. Technol. 2018, 46, 239–257. [Google Scholar] [CrossRef]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.; Gilbertson, L.M.; Giraldo, J.P.; Kinsella, J.M.; Landry, M.P.; et al. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 2020, 1, 416–425. [Google Scholar] [CrossRef]
- Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Alian, A.; Altman, A.; Heuer, B. Genotypic difference in salinity and water stress tolerance of fresh market tomato cultivars. Plant Sci. 2000, 152, 59–65. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Imtiaz, H.; Shiraz, M.; Mir, A.R.; Siddiqui, H.; Hayat, S. Nano-Priming Techniques for Plant Physio-Biochemistry and Stress Tolerance. J. Plant Growth Regul. 2023, 42, 6870–6890. [Google Scholar] [CrossRef]






| Treatments | Formulation | GA3 (µg mL−1) |
|---|---|---|
| WATER | Deionized water (control)) | 0.0 |
| ALG/CS_GA3_50 | Alginate/chitosan containing GA3 | 50.0 |
| ALG/CS_GA3_5 | Alginate/chitosan containing 10× diluted GA3 | 5.0 |
| ALG/CS_GA3_0.5 | Alginate/chitosan containing 100× diluted GA3 | 0.5 |
| CS/TPP_GA3_50 | Chitosan/tripoliphosphate containing GA3 | 50.0 |
| CS/TPP_GA3_5 | Chitosan/tripoliphosphate containing 10× diluted GA3 | 5.0 |
| CS/TPP_GA3_0.5 | Chitosan/tripoliphosphate containing 100× diluted GA3 | 0.5 |
| Treatments | Seed Priming | GA3 (µg mL−1) |
|---|---|---|
| CONT | Seeds not subjected to priming (control) | 0.0 |
| WATER | Deionized water (control)) | 0.0 |
| NPGA3 | Alginate/chitosan nanoparticles containing GA3 | 5.0 |
| GA3 | Gibberellic acid (GA3) | 5.0 |
| NP | Alginate/chitosan nanoparticles | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fregonezi, B.F.; Pereira, A.E.S.; Ferreira, J.M.; Fraceto, L.F.; Gomes, D.G.; Oliveira, H.C. Seed Priming with Nanoencapsulated Gibberellic Acid Triggers Beneficial Morphophysiological and Biochemical Responses of Tomato Plants under Different Water Conditions. Agronomy 2024, 14, 588. https://doi.org/10.3390/agronomy14030588
Fregonezi BF, Pereira AES, Ferreira JM, Fraceto LF, Gomes DG, Oliveira HC. Seed Priming with Nanoencapsulated Gibberellic Acid Triggers Beneficial Morphophysiological and Biochemical Responses of Tomato Plants under Different Water Conditions. Agronomy. 2024; 14(3):588. https://doi.org/10.3390/agronomy14030588
Chicago/Turabian StyleFregonezi, Bruno F., Anderson E. S. Pereira, Josué M. Ferreira, Leonardo F. Fraceto, Diego G. Gomes, and Halley C. Oliveira. 2024. "Seed Priming with Nanoencapsulated Gibberellic Acid Triggers Beneficial Morphophysiological and Biochemical Responses of Tomato Plants under Different Water Conditions" Agronomy 14, no. 3: 588. https://doi.org/10.3390/agronomy14030588
APA StyleFregonezi, B. F., Pereira, A. E. S., Ferreira, J. M., Fraceto, L. F., Gomes, D. G., & Oliveira, H. C. (2024). Seed Priming with Nanoencapsulated Gibberellic Acid Triggers Beneficial Morphophysiological and Biochemical Responses of Tomato Plants under Different Water Conditions. Agronomy, 14(3), 588. https://doi.org/10.3390/agronomy14030588








