Mechanisms Underlying the Differential Sensitivity to Mesotrione in Sweet Corn
Abstract
1. Introduction
2. Materials and Methods
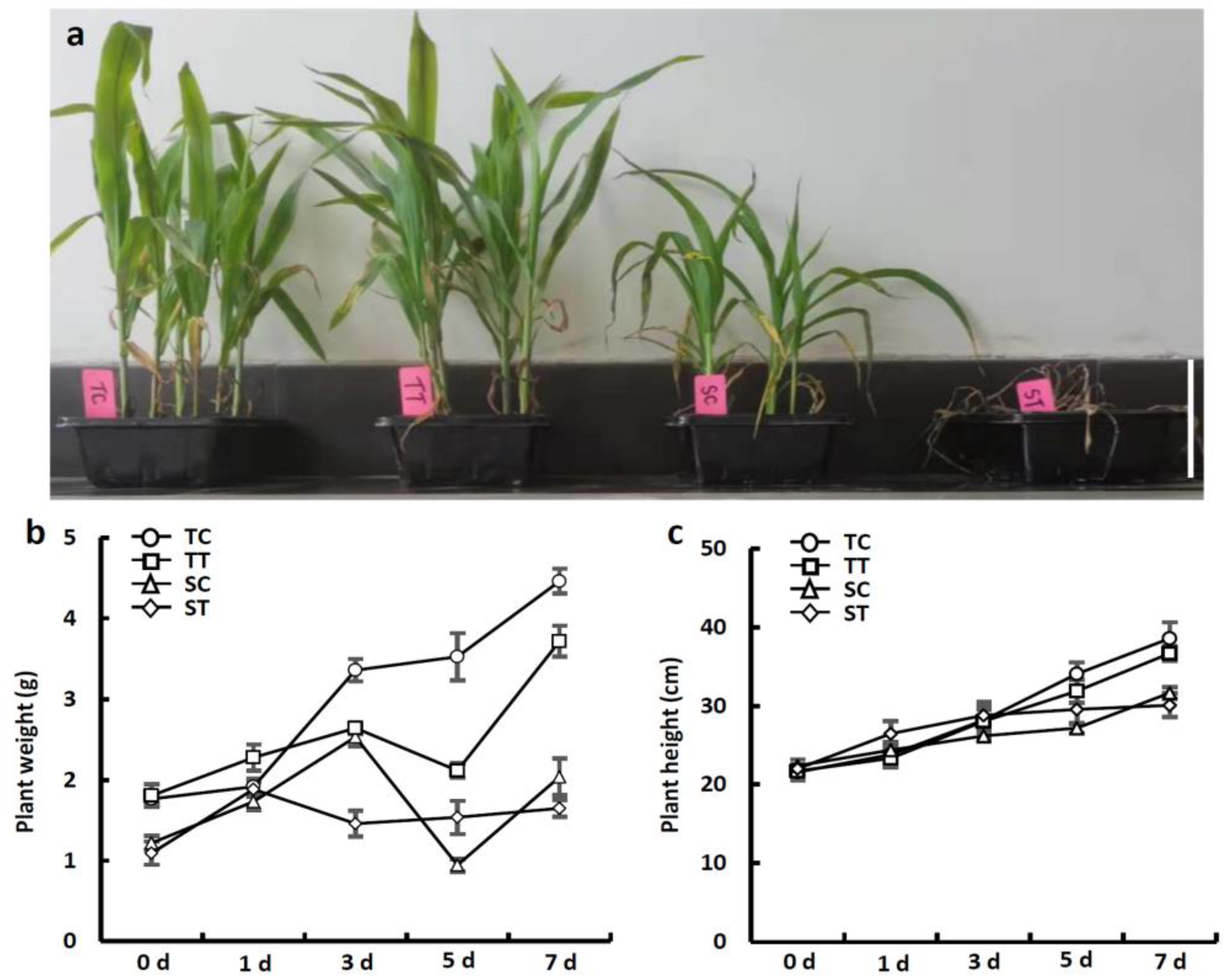
2.1. Plant Materials and Mesotrione Treatments
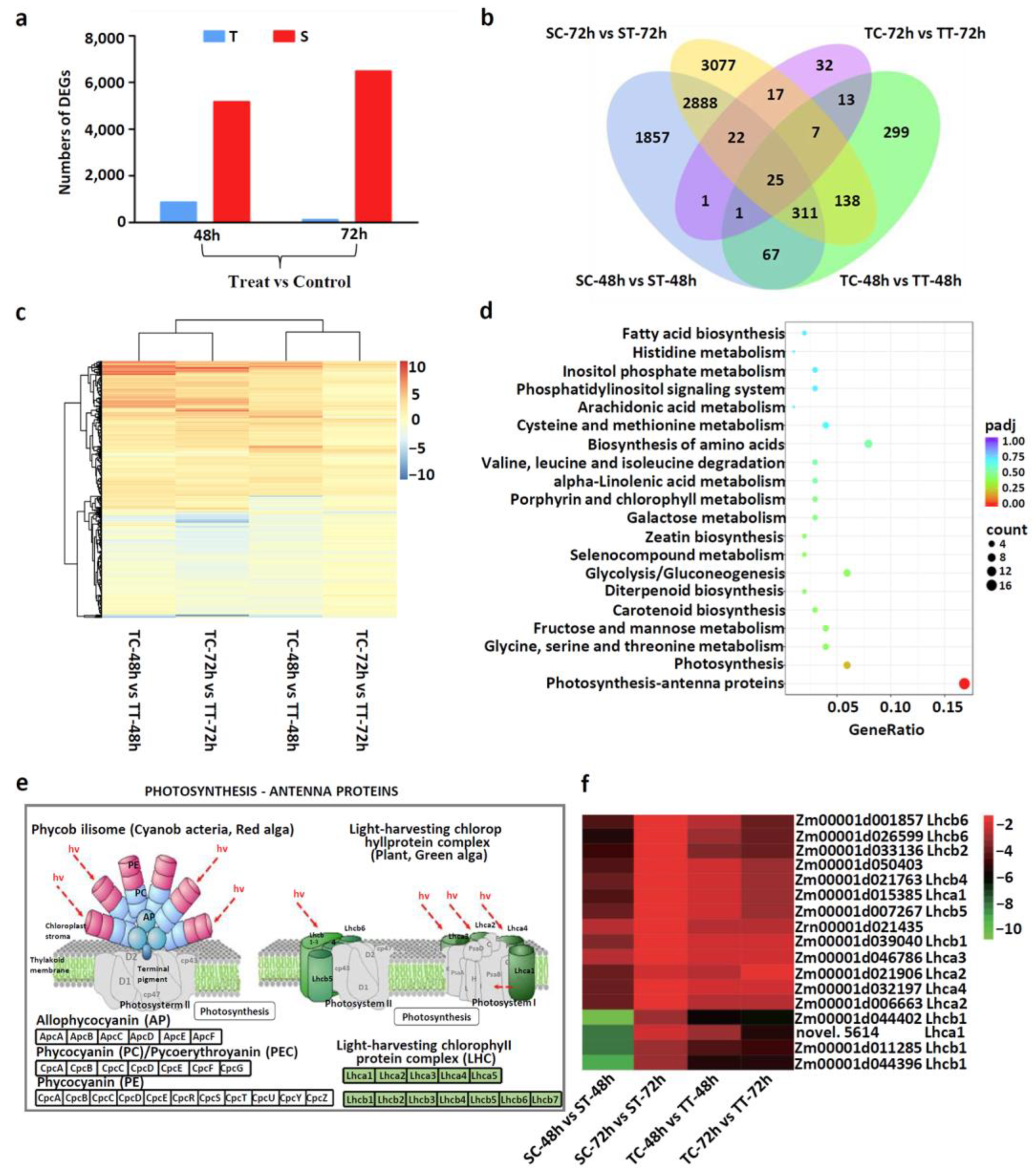
2.2. Transcription Sequencing and Data Analysis
2.3. Determination of Chlorophyll Fluorescence Parameters
2.4. C4 Pathway-Related Enzyme Activity Assay
2.5. Observation of Chloroplast Structure
2.6. Superoxide Anion Content and Hydrogen Peroxide Content Assay
2.7. Determination of Antioxidant Enzymatic Activity
2.8. Statistical Analysis
3. Results
3.1. Phenotypic Responses of Two Sweet Corn Inbred Lines to Mesotrione Stress
3.2. Transcriptome Profiles of the Two Sweet Corn Inbred Lines under Mesotrione Stress
3.3. Effect of Mesotrione on the Photosynthetic Efficiency
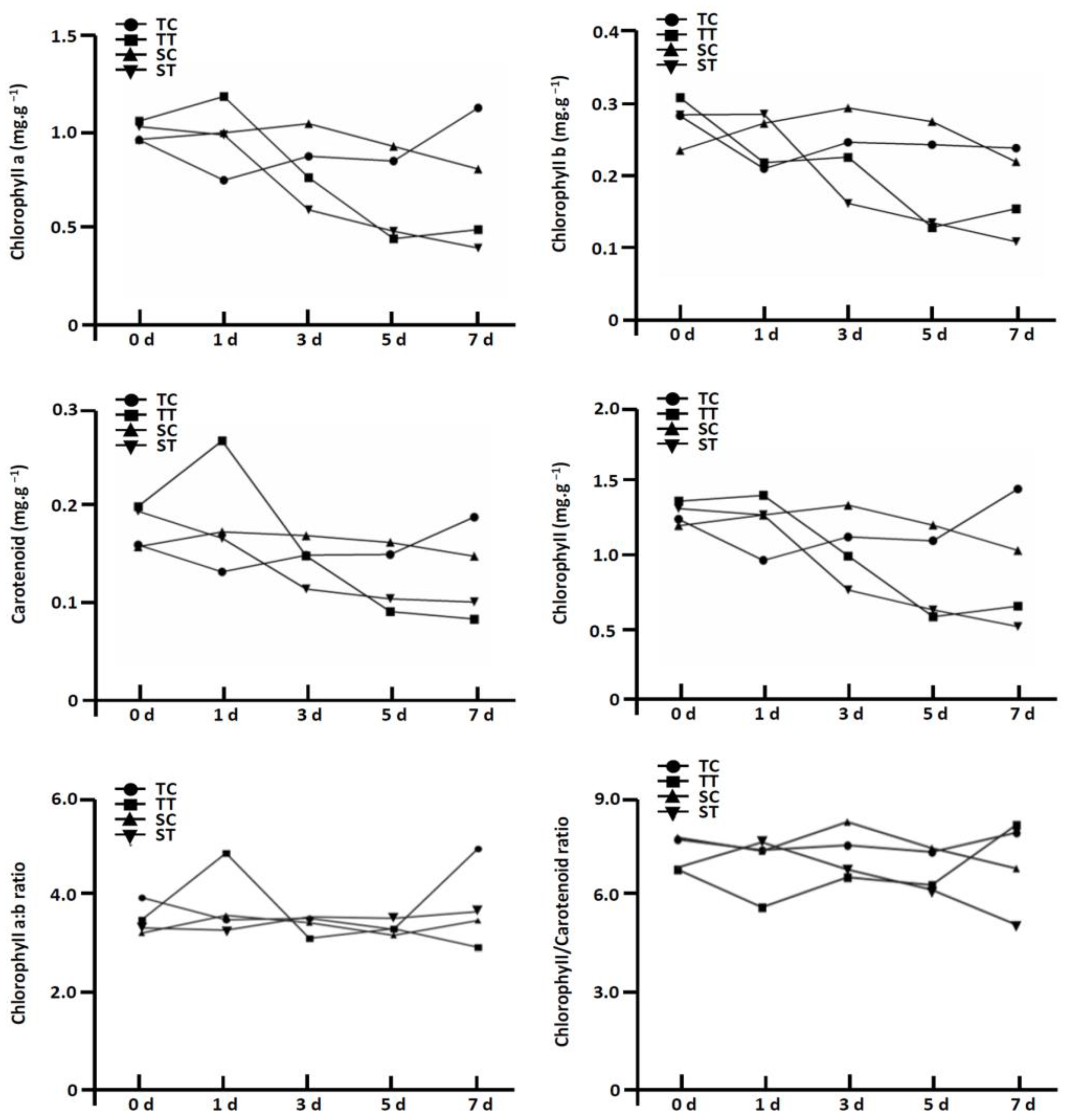
3.4. Effect of Mesotrione on Chloroplastic Development
3.5. Mesotrione Induces Oxidative Stress in Sweet Corn
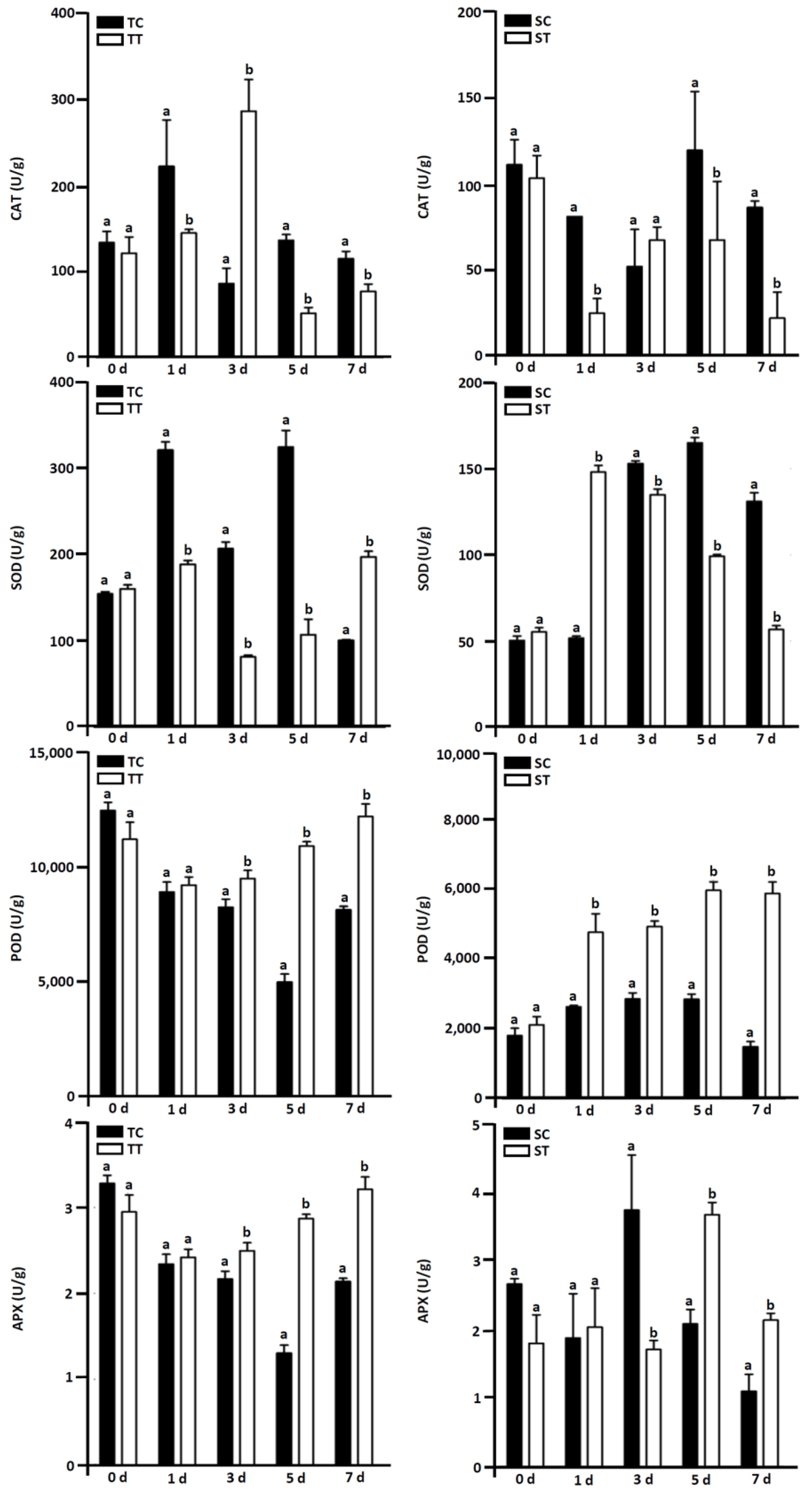
3.6. Effect of Mesotrione on Antioxidant Enzyme Activity
4. Discussion
4.1. Mesotrione Destroyed Chloroplast Structure and Affected the Rate of Photosynthesis in Sweet Corn
4.2. Mesotrione Disrupted the Balance of Redox Homeostasis in Sweet Corn
4.3. Mesotrione Affected the Structure of the Cell Walls in Sweet Corn
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, G.; Bartlett, D.W.; Fraser, T.E.M.; Hawkes, T.R.; Holt, D.C.; Townson, J.K.; Wichert, R.A. Mesotrione: A new selective herbicide for use in maize. Pest Manag. Sci. 2001, 57, 120–128. [Google Scholar] [CrossRef]
- Woodyard, A.J.; Bollero, G.A.; Riechers, D.E. Broadleaf weed management in corn utilizing synergistic postemergence herbicide combinations. Weed Technol. 2009, 23, 513–518. [Google Scholar] [CrossRef]
- Lee, D.L.; Prisbylla, M.P.; Cromartie, T.H.; Dagarin, D.P.; Howard, S.W.; Provan, W.M.; Ellis, M.K.; Fraser, T.; Mutter, L.C. The discovery and structural requirements of inhibitors of p-hydroxyphenylpyruvate dioxygenase. Weed Sci. 1997, 45, 601–609. [Google Scholar] [CrossRef]
- Tsegaye, Y.; Shintani, D.K.; DellaPenna, D. Overexpression of the enzyme p-hydroxyphenolpyruvate dioxygenase in Arabidopsis and its relation to tocopherol biosynthesis. Plant Physiol. Biochem. 2002, 40, 913–920. [Google Scholar] [CrossRef]
- Carol, P.; Kuntz, M. A plastid terminal oxidase comes to light: Implications for carotenoid biosynthesis and chlororespiration. Trends Plant Sci. 2001, 6, 31–36. [Google Scholar] [CrossRef]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.V.; Jugulam, M. Role of cytochrome P450 enzymes in plant stress response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef]
- Nakka, S.; Thompson, C.R.; Peterson, D.E.; Jugulam, M. Target site–based and non–target site based resistance to ALS inhibitors in palmer amaranth (Amaranthus palmeri). Weed Sci. 2017, 65, 681–689. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Varanasi, V.K.; Bagavathiannan, M.; Brabham, C. Recurrent selection with sub-lethal doses of mesotrione reduces sensitivity in Amaranthus palmeri. Plants 2021, 10, 1293. [Google Scholar] [CrossRef]
- Revilla, P.; Anibas, C.M.; Tracy, W.F. Sweet corn research around the world 2015–2020. Agronomy 2021, 11, 534. [Google Scholar] [CrossRef]
- Pannacci, E.; Covarelli, G. Efficacy of mesotrione used at reduced doses for post-emergence weed control in maize (Zea mays L.). Crop Prot. 2009, 28, 57–61. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Zandstra, J.; Sikkema, P. Sweet corn (Zea mays) cultivar sensitivity to mesotrione. Weed Technol. 2002, 16, 421–425. [Google Scholar] [CrossRef]
- Pataky, J.K.; Williams, M.M.; Riechers, D.E.; Meyer, M.D. A common genetic basis for cross-sensitivity to mesotrione and nicosulfuron in sweet corn hybrid cultivars and inbreds grown throughout North America. J. Am. Soc. Hortic. Sci. 2009, 134, 252–260. [Google Scholar] [CrossRef]
- Maxwell, K. Resistance is useful: Diurnal patterns of photosynthesis in C3 and crassulacean acid metabolism epiphytic bromeliads. Funct. Plant Biol. 2002, 29, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.X.; Xu, N.W.; Yang, M.; Li, X.L.; Han, J.L.; Lin, X.H.; Yang, Q.; Lv, G.H.; Wang, J. Responses of photosynthesis, antioxidant enzymes, and related gene expression to nicosulfuron stress in sweet maize (Zea mays L.). Environ. Sci. Pollut. Res. 2022, 29, 37248–37265. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, H.; Guo, Z.; Meng, Y.; Yang, M.; Li, X.; Yang, Q. Adaptation responses in C4 photosynthesis of sweet maize (Zea mays L.) exposed to nicosulfuron. Ecotoxicol. Environ. Saf. 2021, 214, 112096. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Zaccaro, M.L.d.M. Chlorophyll fluorescence as a marker for herbicide mechanisms of action. Pestic. Biochem. Physiol. 2012, 102, 189–197. [Google Scholar] [CrossRef]
- Singh, J.; Garai, S.; Das, S.; Thakur, J.K.; Tripathy, B.C. Role of C4 photosynthetic enzyme isoforms in C3 plants and their potential applications in improving agronomic traits in crops. Photosynth. Res. 2022, 154, 233–258. [Google Scholar] [CrossRef]
- Breeden, G.K.; Brosnan, J.T.; Elmore, M.T.; Kopsell, D.A.; Mueller, T.C. Response of hybrid bermudagrass (Cynodon dactylon × C. transvaalensis) to three HPPD-inhibitors. Weed Sci. 2011, 59, 458–463. [Google Scholar]
- Quan, L.-J.; Zhang, B.; Shi, W.-W.; Li, H.-Y. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Ślesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kuźniak, E.; Urbanek, H. The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiol. Plant. 2000, 22, 195–203. [Google Scholar] [CrossRef]
- Dan Hess, F. Light-dependent herbicides: An overview. Weed Sci. 2000, 48, 160–170. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Rosenqvist, E.; van Kooten, O. Chlorophyll fluorescence: A general description and nomenclature. In Practical Applications of Chlorophyll Fluorescence in Plant Biology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 31–77. [Google Scholar]
- Ostroumov, E.E.; Khan, Y.R.; Scholes, G.D.; Govindjee. Photophysics of photosynthetic pigment-protein complexes. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Springer: Berlin/Heidelberg, Germany, 2014; pp. 97–128. [Google Scholar]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.; Collier, C.J.; Mercurio, P.; Negri, A.P. Phytotoxicity of four photosystem II herbicides to tropical seagrasses. PLoS ONE 2013, 8, e75798. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, W.; Zhao, K.; Yu, H.; Zhang, J.; Wang, J. Evaluation of weed control efficacy and crop safety of the new HPPD-inhibiting herbicide-QYR301. Sci. Rep. 2018, 8, 7910. [Google Scholar] [CrossRef]
- Nemat Alla, M.M.; Hassan, N.M. Changes of antioxidants and GSH-associated enzymes in isoproturon-treated maize. Acta Physiol. Plant. 2007, 29, 247–258. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Mersey, B.G.; Hall, J.C.; Anderson, D.M.; Swanton, C.J. Factors affecting the herbicidal activity of glufosinate-ammonium: Absorption, translocation, and metabolism in barley and green foxtail. Pestic. Biochem. Physiol. 1990, 37, 90–98. [Google Scholar] [CrossRef]
- Sterling, T.M. Mechanisms of herbicide absorption across plant membranes and accumulation in plant cells. Weed Sci. 1994, 42, 263–276. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, G.; Li, X.; Wang, T.; Wu, Z.; Fang, R.; Chen, J. Mechanisms Underlying the Differential Sensitivity to Mesotrione in Sweet Corn. Agronomy 2024, 14, 555. https://doi.org/10.3390/agronomy14030555
Lv G, Li X, Wang T, Wu Z, Fang R, Chen J. Mechanisms Underlying the Differential Sensitivity to Mesotrione in Sweet Corn. Agronomy. 2024; 14(3):555. https://doi.org/10.3390/agronomy14030555
Chicago/Turabian StyleLv, Guihua, Xiangnan Li, Tingzhen Wang, Zhenxing Wu, Ruiqiu Fang, and Jianjian Chen. 2024. "Mechanisms Underlying the Differential Sensitivity to Mesotrione in Sweet Corn" Agronomy 14, no. 3: 555. https://doi.org/10.3390/agronomy14030555
APA StyleLv, G., Li, X., Wang, T., Wu, Z., Fang, R., & Chen, J. (2024). Mechanisms Underlying the Differential Sensitivity to Mesotrione in Sweet Corn. Agronomy, 14(3), 555. https://doi.org/10.3390/agronomy14030555




