Vulnerability of Xylem Embolism in Maize Cultivars with Different Drought Tolerance under Water and Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Experimental Materials
2.3. Experimental Design
2.4. Sampling, Measurement, and Calculation Methods
2.4.1. Leaf Sample Collection
2.4.2. Stem Segment Sample Collection
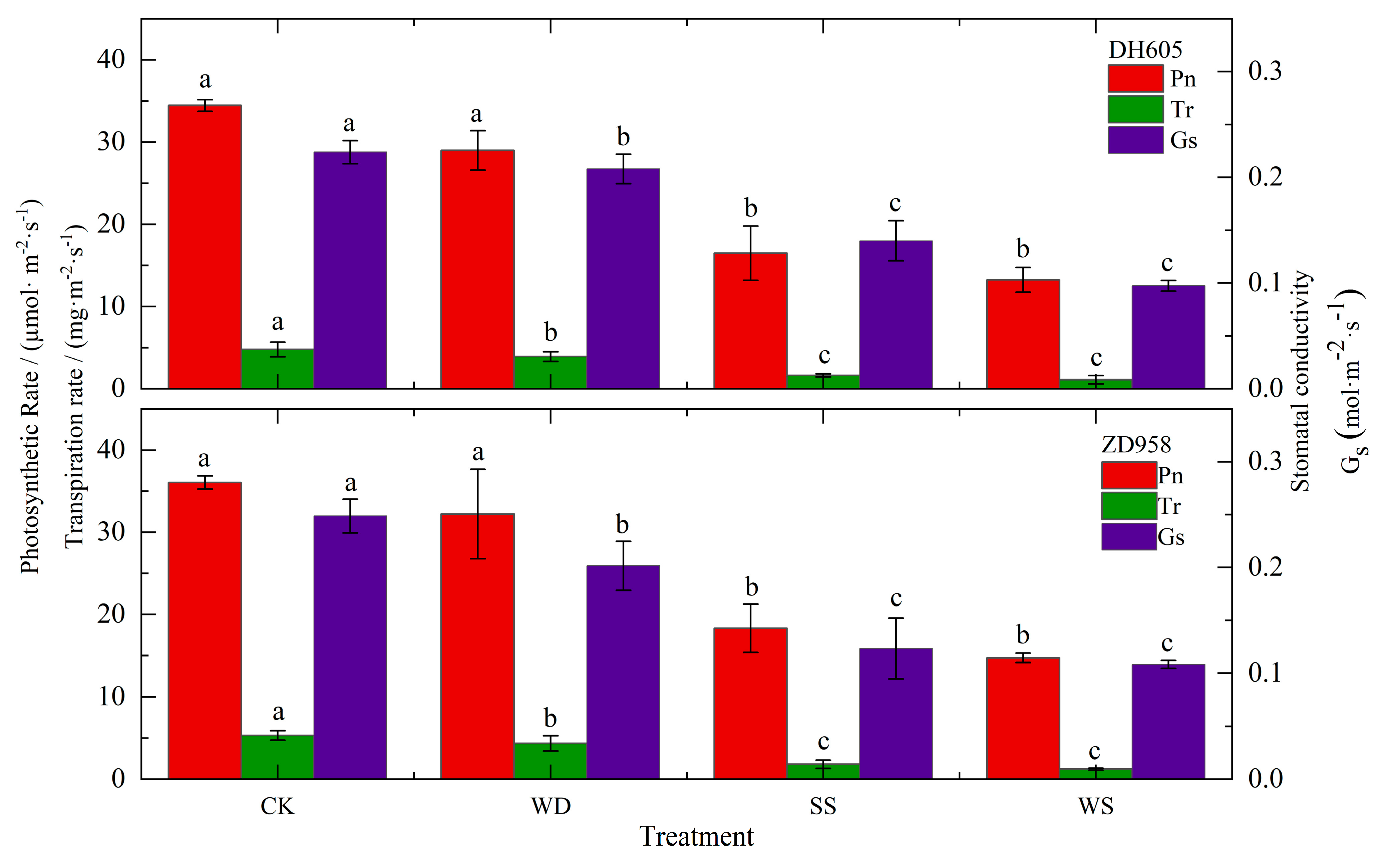
2.4.3. Leaf Stomatal Gas Exchange Parameters
2.4.4. Hydraulic Conductance of the Stem Xylem
2.4.5. Simulation of Specific Conductivity Curve
2.4.6. Simulation of the Embolism Vulnerability Curve
2.4.7. Hydraulic Safety Margin (HSM)
2.5. Data Analysis
3. Results
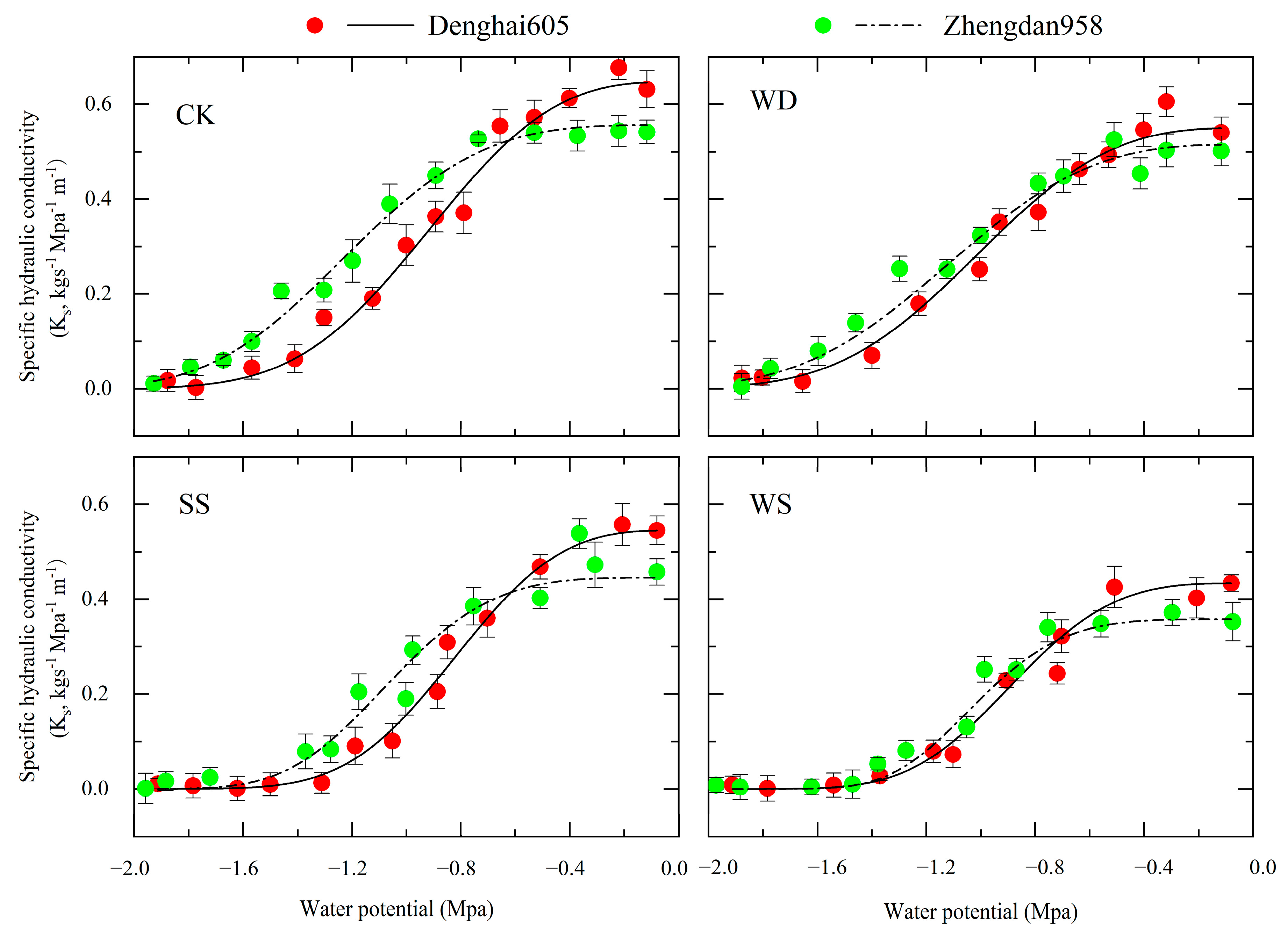
3.1. Xylem-Specific Hydraulic Conductivity Curve
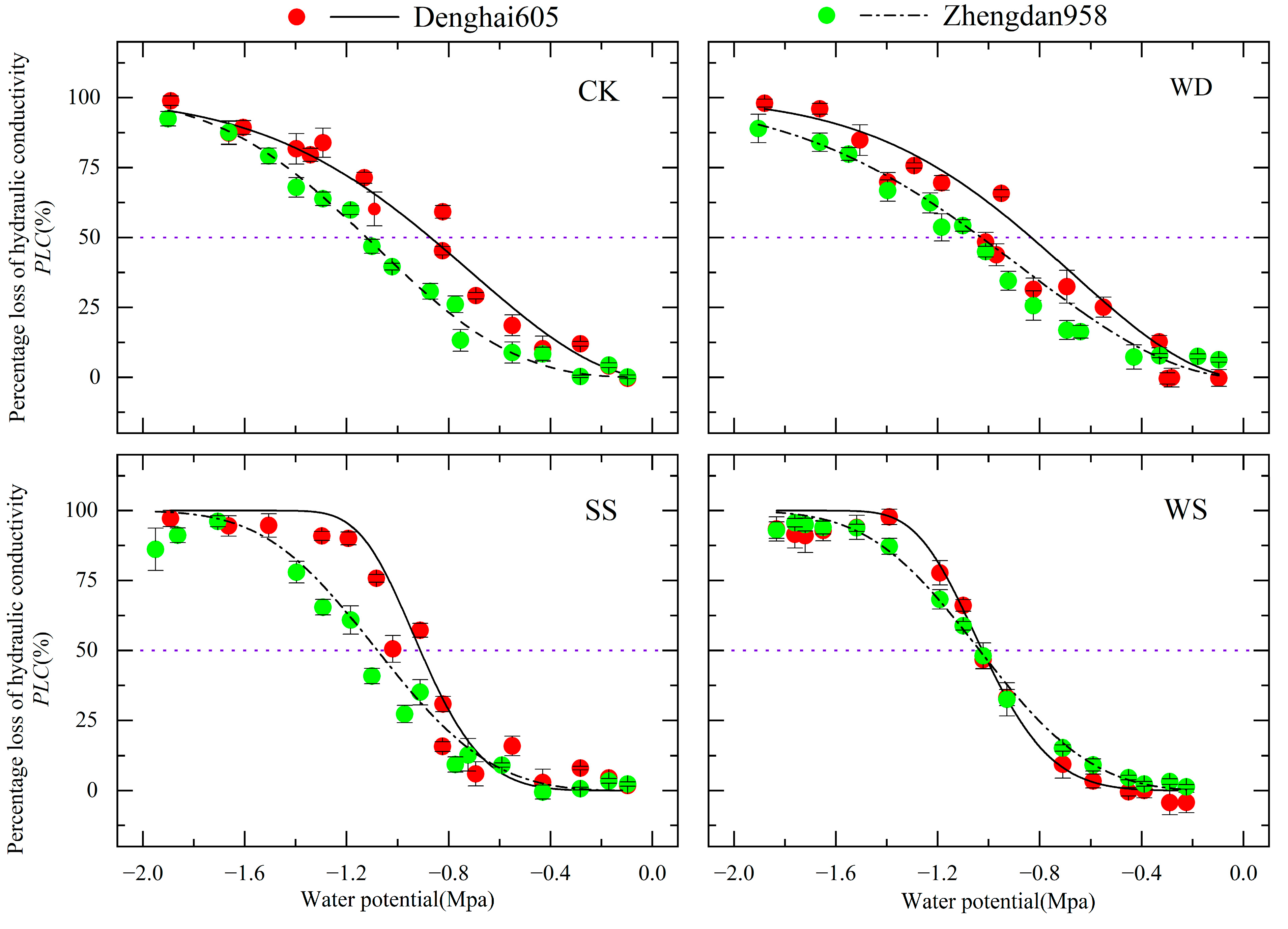
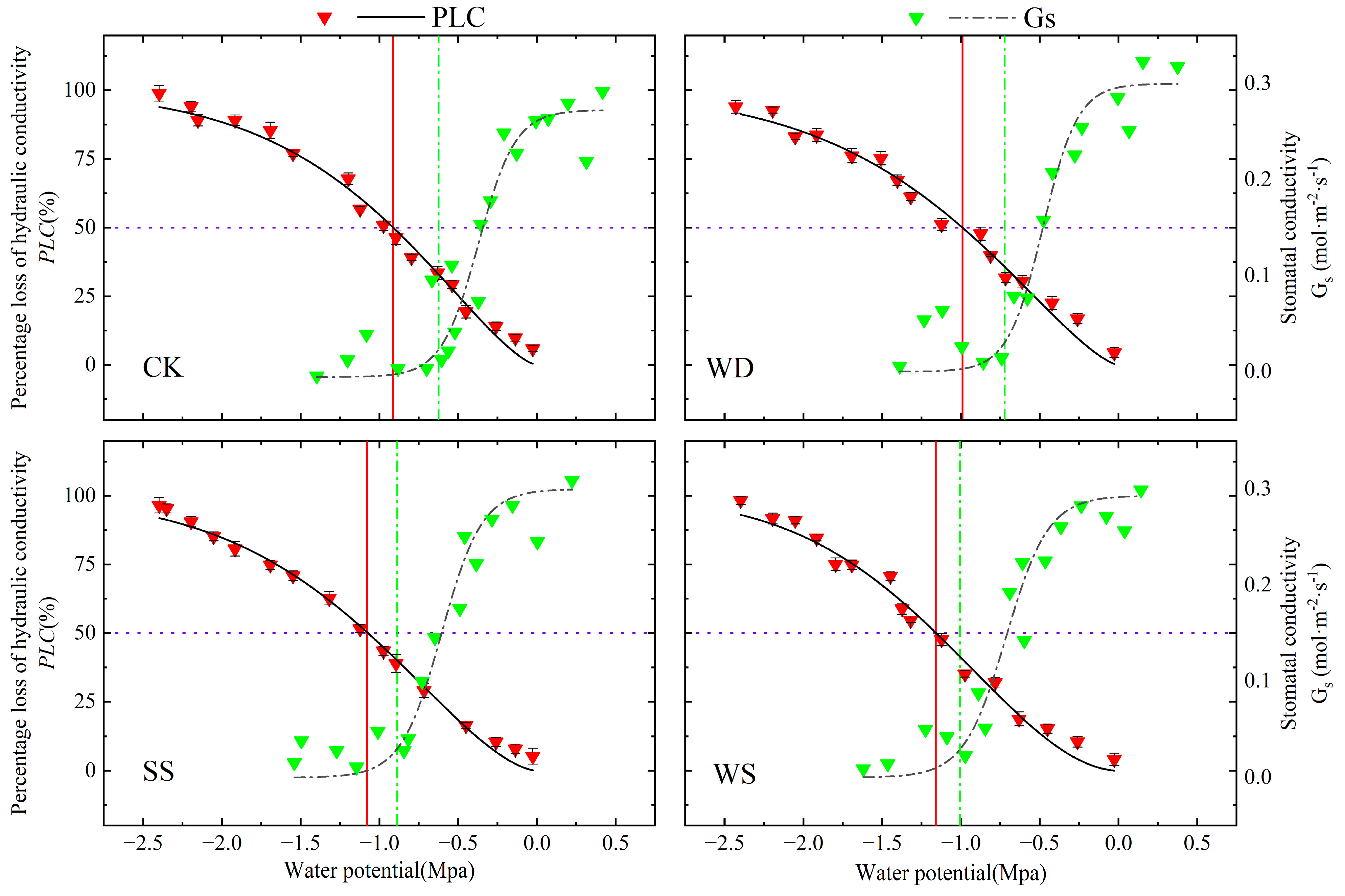
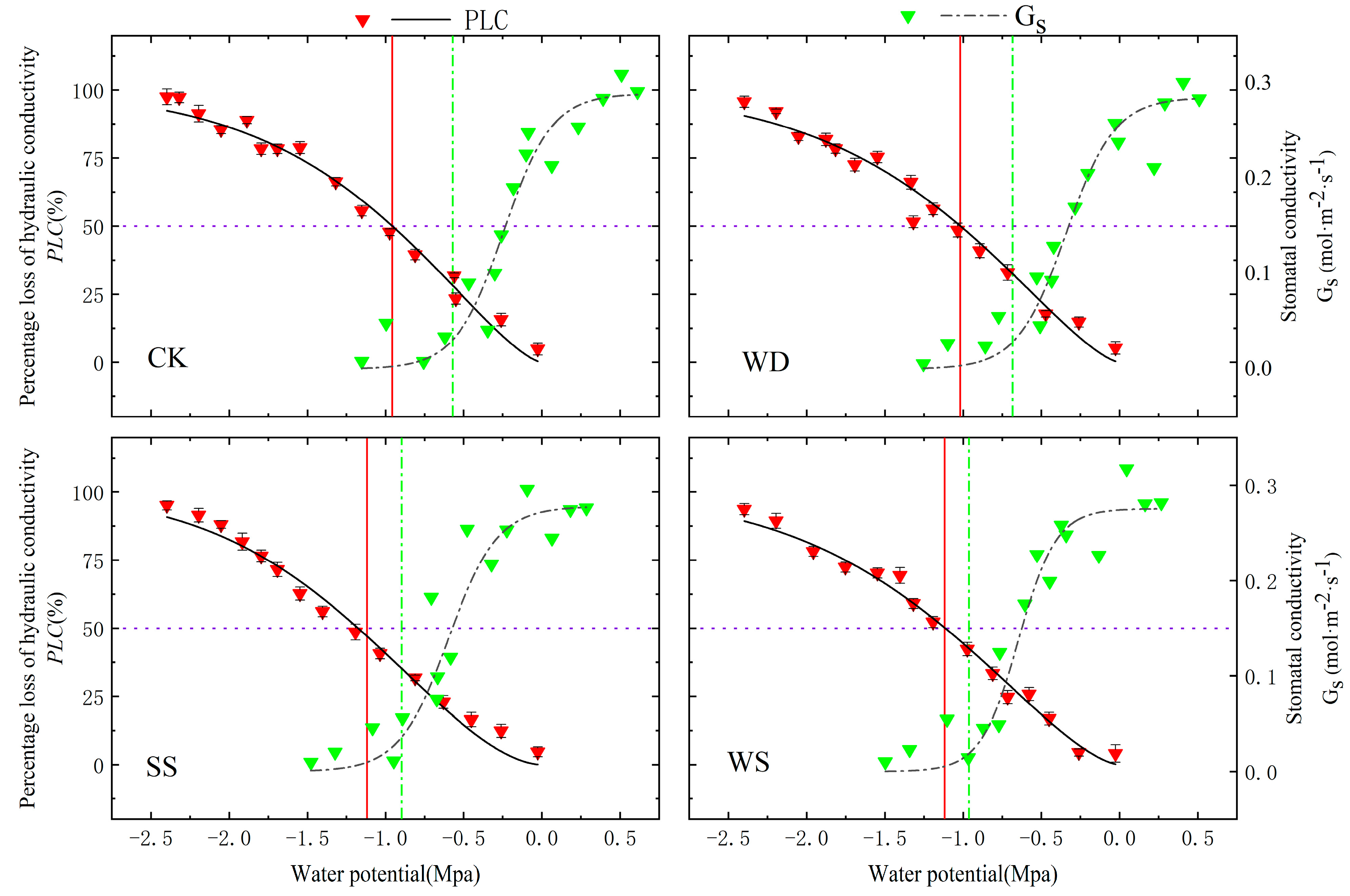
3.2. Vulnerability Curves for Stem Xylem Embolism
3.3. Hydraulic Safety Margins in Leaf Xylem
4. Discussion
5. Conclusions
- (1)
- In maize cultivars with varying levels of drought resistance, the drought-resistant ZD 958 showed lower water transport efficiency in the leaf xylem compared to the drought-sensitive DH 605. However, ZD 958 showed a stronger ability to resist cavitation in the xylem compared to DH 605. The drought resistance of maize cultivars showed no direct correlation with water transport efficiency but was closely associated with the ability to resist cavitation. Higher water transport efficiency might be more prone to inducing cavitation, suggesting that stronger drought resistance in a variety is associated with a greater ability to resist cavitation.
- (2)
- Water–salt stress increased the values and PLC in both the stem and leaf xylem, increasing the susceptibility of the xylem to cavitation. Overall, the severity of cavitation followed a gradient of WS > SS > WD > CK. Different stress conditions resulted in different degrees of cavitation, with salt stress inducing more severe cavitation compared to water stress. The degree of xylem cavitation was greater under salt stress.
- (3)
- During stomatal closure, the water potential () of DH 605 and ZD 958 was higher than the water potential during severe cavitation (). Maize can maintain normal water transport by closing leaf stomata to halt transpiration, ensuring that xylem potential remains higher than the potential at which severe cavitation occurs (). We found that both cultivars adopt the same hydraulic regulation strategy, but ZD 958 showed higher hydraulic safety, with a broader safe water transport range, indicating stronger adaptability to arid and saline–alkali environments.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheikh, F.A.; Dar, Z.A.; Sofi, P.A.; Lone, A.A. Recent advances in breeding for abiotic stress (drought) tolerance in maize. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 2226–2243. [Google Scholar]
- Zhu, T.; Li, L.; Duan, Q.; Liu, X.; Chen, M. Progress in our understanding of plant responses to the stress of heavy metal cadmium. Plant Signal. Behav. 2021, 16, 1836884. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Wani, U.M.; Wani, Z.A.; Jan, N.; Kottakota, C.; Reddy, M.K.; Kaul, T.; John, R. Pyramiding ascorbate–glutathione pathway in Lycopersicum esculentum confers tolerance to drought and salinity stress. Plant Cell Rep. 2022, 41, 619–637. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, F.J.; Reineke, D.; Leventini, D.; Chen, C.C.L.; Barrett-Lennard, E.G.; Colmer, T.D.; Dodd, I.C.; Shabala, S.; Brown, P.; Bazihizina, N. Plant responses to heterogeneous salinity: Agronomic relevance and research priorities. Ann. Bot. 2022, 129, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Paredes, P.; Shi, H.; Ramos, T.B.; Dou, X.; Dai, L.; Pereira, L.S. Impacts of a shallow saline water table on maize evapotranspiration and groundwater contribution using static water table lysimeters and the dual Kc water balance model SIMDualKc. Agric. Water Manag. 2022, 273, 107887. [Google Scholar] [CrossRef]
- Wan, W.; Liu, Z.; Li, K.; Wang, G.; Wu, H.; Wang, Q. Drought monitoring of the maize planting areas in Northeast and North China Plain. Agric. Water Manag. 2021, 245, 106636. [Google Scholar] [CrossRef]
- Jacob, V.; Choat, B.; Churchill, A.C.; Zhang, H.; Barton, C.V.M.; Krishnananthaselvan, A.; Post, A.K.; Power, S.A.; Medlyn, B.E.; Tissue, D.T. High safety margins to drought-induced hydraulic failure found in five pasture grasses. Plant Cell Environ. 2022, 45, 1631–1646. [Google Scholar] [CrossRef]
- Tavares, J.V.; Oliveira, R.S.; Mencuccini, M.; Signori-Müller, C.; Pereira, L.; Diniz, F.C.; Gilpin, M.; Zevallos, M.J.M.; Yupayccana, C.A.S.; Acosta, M.; et al. Basin-wide variation in tree hydraulic safety margins predicts the carbon balance of Amazon forests. Nature 2023, 617, 111–117. [Google Scholar] [CrossRef]
- Tan, F.-S.; Song, H.-Q.; Fu, P.-L.; Chen, Y.-J.; Siddiq, Z.; Cao, K.-F.; Zhu, S.-D. Hydraulic safety margins of co-occurring woody plants in a tropical karst forest experiencing frequent extreme droughts. Agric. Forest Meteorol. 2020, 292, 108107. [Google Scholar] [CrossRef]
- Sakurai, G.; Miklavcic, S.J. On the efficacy of water transport in leaves. A coupled xylem-phloem model of water and solute transport. Front. Plant Sci. 2021, 12, 615457. [Google Scholar] [CrossRef]
- Johnson, D.M.; Katul, G.; Domec, J.C. Catastrophic hydraulic failure and tipping points in plants. Plant Cell Environ. 2022, 45, 2231–2266. [Google Scholar] [CrossRef]
- Li, D.; Yan, M.; Liang, H.; Li, Z.; Zhang, S. Exogenous Calcium Induces Different Hydraulic Strategies in Response to Osmotic Stress in Maize Seedlings. Plants 2023, 12, 1999. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.U.; Paul, A.; Chehri, A. Hydrotropism: Understanding the impact of water on plant movement and adaptation. Water 2023, 15, 567. [Google Scholar] [CrossRef]
- Ménard, D.; Blaschek, L.; Kriechbaum, K.; Lee, C.C.; Serk, H.; Zhu, C.; Lyubartsev, A.; Nuoendagula; Bacsik, Z.; Bergström, L. Plant biomechanics and resilience to environmental changes are controlled by specific lignin chemistries in each vascular cell type and morphotype. Plant Cell 2022, 34, 4877–4896. [Google Scholar] [CrossRef] [PubMed]
- Mantova, M.; Herbette, S.; Cochard, H.; Torres-Ruiz, J.M. Hydraulic failure and tree mortality: From correlation to causation. Trend Plant Sci. 2022, 27, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ding, R.; Kang, S.; Du, T.; Tong, L.; Zhang, Y.; Chen, J.; Shukla, M.K. Drought, salt, and combined stresses in plants: Effects, tolerance mechanisms, and strategies. Adv. Agron. 2023, 178, 107–163. [Google Scholar]
- Schenk, H.J.; Steppe, K.; Jansen, S. Nanobubbles: A new paradigm for air-seeding in xylem. Trend Plant Sci. 2015, 20, 199–205. [Google Scholar] [CrossRef]
- Johnson, K.M.; Lucani, C.; Brodribb, T.J. In vivo monitoring of drought-induced embolism in Callitris rhomboidea trees reveals wide variation in branchlet vulnerability and high resistance to tissue death. New Phytol. 2022, 233, 207–218. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Predicting long-term dynamics of soil salinity and sodicity on a global scale. Proc. Natl. Acad. Sci. USA 2020, 117, 33017–33027. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Li, X.; Xi, B.; Wu, X.; Choat, B.; Feng, J.; Jiang, M.; Tissue, D. Unlocking drought-induced tree mortality: Physiological mechanisms to modeling. Front. Plant Sci. 2022, 13, 835921. [Google Scholar] [CrossRef]
- Miyashita, K.; Tanakamaru, S.; T Maitani, K.K. Recovery responses of photosynthesis, transpiration, and stomatal conductance in kidney bean following drought stress. Environ. Exp. Bot. 2005, 53, 205–214. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Q.; Hu, S.; Wang, R.; Wang, H.; Zhang, K.; Zhao, H.; Ren, S.; Yang, Y.; Zhao, F. Effects of high temperature and drought stresses on growth and yield of summer maize during grain filling in North China. Agriculture 2022, 12, 1948. [Google Scholar] [CrossRef]
- Anami, S.; De Block, M.; Machuka, J.; Van Lijsebettens, M. Molecular improvement of tropical maize for drought stress tolerance in sub-Saharan Africa. Crit. Rev. Plant Sci. 2009, 28, 16–35. [Google Scholar] [CrossRef]
- Sperry, J.S.; Tyree, M.T.; Donnelly, J.R. Vulnerability of xylem to embolism in a mangrove vs an inland species of rhizophoraceae. Physiol. Plant. 1988, 74, 276–283. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M.H.; Tyree, M.T.; Zimmermann, M.H. Xylem dysfunction: When cohesion breaks down. In Xylem Structure and the Ascent of Sap; Springer: Berlin/Heidelberg, Germany, 2002; pp. 89–141. [Google Scholar]
- Venturas, M.D.; Sperry, J.S.; Hacke, U.G. Plant xylem hydraulics: What we understand, current research, and future challenges. J. Integr. Plant Biol. 2017, 59, 356–389. [Google Scholar] [CrossRef] [PubMed]
- Loepfe, L.; Martinez-Vilalta, J.; Piñol, J.; Mencuccini, M. The relevance of xylem network structure for plant hydraulic efficiency and safety. J. Theor. Biol. 2007, 247, 788–803. [Google Scholar] [CrossRef]
- Lens, F.; Gleason, S.M.; Bortolami, G.; Brodersen, C.; Delzon, S.; Jansen, S. Functional xylem characteristics associated with drought-induced embolism in angiosperms. New Phytol. 2022, 236, 2019–2036. [Google Scholar] [CrossRef]
- Creek, D.; Lamarque, L.J.; Torres-Ruiz, J.M.; Parise, C.; Burlett, R.; Tissue, D.T.; Delzon, S. Xylem embolism in leaves does not occur with open stomata: Evidence from direct observations using the optical visualization technique. J. Exp. Bot. 2020, 71, 1151–1159. [Google Scholar] [CrossRef]
- Yan, C.-L.; Ni, M.-Y.; Cao, K.-F.; Zhu, S.-D. Leaf hydraulic safety margin and safety–efficiency trade-off across angiosperm woody species. Biol. Lett. 2020, 16, 20200456. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Wan, X.; Liu, S. Strategies of tree species to adapt to drought from leaf stomatal regulation and stem embolism resistance to root properties. Front. Plant Sci. 2022, 13, 926535. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trend Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Zhu, S.D.; Jansen, S.; Cao, K.F. Topography strongly affects drought stress and xylem embolism resistance in woody plants from a karst forest in Southwest China. Funct. Ecol. 2021, 35, 566–577. [Google Scholar] [CrossRef]
- Chen, Y.J.; Maenpuen, P.; Zhang, Y.J.; Barai, K.; Katabuchi, M.; Gao, H.; Kaewkamol, S.; Tao, L.B.; Zhang, J.L. Quantifying vulnerability to embolism in tropical trees and lianas using five methods: Can discrepancies be explained by xylem structural traits? New Phytol. 2021, 229, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Y.; Oya, T.; Marcati, C.R.; Pereira, L.; Jansen, S. Root xylem in three woody angiosperm species is not more vulnerable to embolism than stem xylem. Plant Soil 2020, 450, 479–495. [Google Scholar] [CrossRef]
- Johnson, K.M.; Brodersen, C.; Carins-Murphy, M.R.; Choat, B.; Brodribb, T.J. Xylem embolism spreads by single-conduit events in three dry forest angiosperm stems. Plant Physiol. 2020, 184, 212–222. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, S.; Zhang, Y.; Luan, J.; Li, S.; Sun, P.; Wan, X.; Liu, S. Tradeoff between storage capacity and embolism resistance in the xylem of temperate broadleaf tree species. Tree Physiol. 2020, 40, 1029–1042. [Google Scholar] [CrossRef]
- Mohammadi Alagoz, S.; Zahra, N.; Hajiaghaei Kamrani, M.; Asgari Lajayer, B.; Nobaharan, K.; Astatkie, T.; Siddique, K.H.M.; Farooq, M. Role of root hydraulics in plant drought tolerance. J. Plant Growth Regul. 2023, 42, 6228–6243. [Google Scholar] [CrossRef]
- Carpýcý, E.B.; Celýk, N.; Bayram, G. Effects of salt stress on germination of some maize (Zea mays L.) cultivars. Afr. J. Biotechnol. 2009, 8, 4918–4922. [Google Scholar]
- Olayinka, B.U.; Abdulkareem, K.A.; Abdulbaki, A.S.; Alsamadany, H.; Alzahrani, Y.; Isiaka, K.; Ayinla, A.; Kolawole, O.S.; Idowu, A.O.; Odudu, F.U. Effects of priming on germination and biochemical attributes of three maize lines under nacl stress condition. Bioagro 2022, 34, 233–244. [Google Scholar] [CrossRef]
- Yu, J.R.; Wan, C.Y.; Zhu, S.D. Hydraulic vulnerability segmentation in woody plant species from tropical and subtropical karst forests. J. Plant Ecol. 2023, 47, 1576–1584. [Google Scholar]
- Gleason, S.M.; Wiggans, D.R.; Bliss, C.A.; Comas, L.H.; Cooper, M.; DeJonge, K.C.; Young, J.S.; Zhang, H. Coordinated decline in photosynthesis and hydraulic conductance during drought stress in Zea mays. Flora 2017, 227, 1–9. [Google Scholar] [CrossRef]
- Liu, J.; Kang, S.; Davies, W.J.; Ding, R. Elevated [CO2] alleviates the impacts of water deficit on xylem anatomy and hydraulic properties of maize stems. Plant Cell Environ. 2020, 43, 563–578. [Google Scholar] [CrossRef]
- Tyree, M.T.; Davis, S.D.; Cochard, H. Biophysical perspectives of xylem evolution: Is there a tradeoff of hydraulic effichÎncy for vulnerability to dysfunction? IAWA J. 1994, 15, 335–360. [Google Scholar] [CrossRef]
- De Guzman, M.E.; Santiago, L.S.; Schnitzer, S.A.; Álvarez-Cansino, L. Trade-offs between water transport capacity and drought resistance in neotropical canopy liana and tree species. Tree Physiol. 2017, 37, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Weithmann, G.; Link, R.M.; Banzragch, B.E.; Würzberg, L.; Leuschner, C.; Schuldt, B. Soil water availability and branch age explain variability in xylem safety of European beech in Central Europe. Oecologia 2022, 198, 629–644. [Google Scholar] [CrossRef]
- Zhao, L.M. Hydarulic Architecture Traits in Maize and Sorghum of Differing Drought Resistance. Master’s Thesis, Northwest A&F University, Xianyang, China, 2012. [Google Scholar]
- Ma, C.; Zhang, X.; Yao, Q.; Zhou, B.; Wang, Q.; Shao, M. Comparison of Xylem Anatomy and Hydraulic Properties in Black Locust Trees at Two Growth Stages in Semiarid China. Forests 2024, 15, 116. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Hochberg, U.; Rockwell, F.E.; Ponomarenko, A.; Chen, Y.J.; Manandhar, A.; Graham, A.C.; Holbrook, N.M. Xylem conduit deformation across vascular plants: An evolutionary spandrel or protective valve? New Phytol. 2023, 237, 1242–1255. [Google Scholar] [CrossRef]
- Cochard, H.; Coll, L.; Le Roux, X.; Ameglio, T. Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiol. 2002, 128, 282–290. [Google Scholar] [CrossRef]
- Alam, H.; Khattak, J.Z.K.; Ksiksi, T.S.; Saleem, M.H.; Fahad, S.; Sohail, H.; Ali, Q.; Zamin, M.; El-Esawi, M.A.; Saud, S. Negative impact of long-term exposure of salinity and drought stress on native Tetraena mandavillei L. Physiol. Plant. 2021, 172, 1336–1351. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Yabuta, S.; Ssenyonga, P.; Tamaru, S.; Sakagami, J.-I. Response of leaf water potential, stomatal conductance and chlorophyll content under different levels of soil water, air vapor pressure deficit and solar radiation in chili pepper (Capsicum chinense). Sci. Hortic. 2021, 281, 109943. [Google Scholar] [CrossRef]
- Kamatchi, K.A.M.; Anitha, K.; Kumar, K.A.; Senthil, A.; Kalarani, M.K.; Djanaguiraman, M. Impacts of combined drought and high-temperature stress on growth, physiology, and yield of crops. Plant Physiol. Rep. 2023, 1–9. [Google Scholar] [CrossRef]
- Xue, F.; Liu, W.; Cao, H.; Song, L.; Ji, S.; Tong, L.; Ding, R. Stomatal conductance of tomato leaves is regulated by both abscisic acid and leaf water potential under combined water and salt stress. Physiol. Plant. 2021, 172, 2070–2078. [Google Scholar] [CrossRef]
- Knipfer, T.; Bambach, N.; Hernandez, M.I.; Bartlett, M.K.; Sinclair, G.; Duong, F.; Kluepfel, D.A.; McElrone, A.J. Predicting stomatal closure and turgor loss in woody plants using predawn and midday water potential. Plant Physiol. 2020, 184, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.-Y.; Dang, Q.-L.; Xu, C.-Y.; Jiang, C.-D.; Zhang, W.-F.; Xu, X.-W.; Yang, X.-F.; Zhang, S.-R. Stomatal sensitivity to vapor pressure deficit and the loss of hydraulic conductivity are coordinated in Populus euphratica, a desert phreatophyte species. Front. Plant Sci. 2020, 11, 1248. [Google Scholar] [CrossRef]
- Dayer, S.; Herrera, J.C.; Dai, Z.; Burlett, R.; Lamarque, L.J.; Delzon, S.; Bortolami, G.; Cochard, H.; Gambetta, G.A. The sequence and thresholds of leaf hydraulic traits underlying grapevine varietal differences in drought tolerance. J. Exp. Bot. 2020, 71, 4333–4344. [Google Scholar] [CrossRef]
| d/cm | ω/(mg·kg−1) | PH | EC/(μS·cm−1) | |||
|---|---|---|---|---|---|---|
| Organic Matter | Available Nitrogen | Available Phosphorus | Available Potassium | |||
| 0–20 | 1.64 | 42.63 | 8.82 | 203.92 | 8.47 | 229.0 |
| 20–40 | 0.78 | 19.92 | 1.64 | 170.07 | 8.80 | 214.0 |
| Cultivar | Treatment | Soil Moisture | Nacl Concentration/(mmol·L−1) |
|---|---|---|---|
| ZD958 | CK958 | 70~75 | 0 |
| WD958 | 55~60 | 0 | |
| SS958 | 70~75 | 50 | |
| WS958 | 55~60 | 50 | |
| DH605 | CK605 | 70~75 | 0 |
| WD605 | 55~60 | 0 | |
| SS605 | 70~75 | 50 | |
| WS605 | 55~60 | 50 |
| Indicator | Cultivar | Treatment | Weibull Function Parameters | (MPa) | ||
|---|---|---|---|---|---|---|
| (kg Mpa−1 m−1) | b (Mpa) | c | ||||
| ZD958 | CK | 0.56 ± 0.015 b | 1.36± 0.010 a | 3.61 ± 0.125 c | -- | |
| WD | 0.51 ± 0.006 d | 1.27 ± 0.011 b | 3.13 ± 0.130 e | -- | ||
| SS | 0.45 ± 0.054 e | 1.16± 0.010 cd | 4.16 ± 0.239 b | -- | ||
| WS | 0.36 ± 0.039 g | 1.11 ± 0.007 bc | 4.72 ± 0.157 a | -- | ||
| DH605 | CK | 0.65 ± 0.014 a | 1.16 ± 0.005 de | 2.93 ± 0.066 g | -- | |
| WD | 0.55 ± 0.019 c | 1.06 ± 0.005 ef | 3.04 ± 0.051 f | -- | ||
| SS | 0.53 ± 0.009 c | 0.99 ± 0.005 f | 3.13 ± 0.166 e | -- | ||
| WS | 0.43 ± 0.034 f | 0.93 ± 0.012 g | 3.53 ± 0.076 d | -- | ||
| PLC | ZD958 | CK | -- | 1.28 ± 0.022 a | 2.85 ± 0.215 d | −1.17 ± 0.02 g |
| WD | -- | 1.24 ± 0.014 a | 2.32 ± 0.095 e | −1.13 ± 0.05 f | ||
| SS | -- | 1.20 ± 0.027 b | 3.58 ± 0.296 c | −1.08 ± 0.05 e | ||
| WS | -- | 1.15± 0.012 b | 3.47 ± 0.132 c | −1.03 ± 0.08 d | ||
| DH605 | CK | -- | 1.06 ± 0.037 c | 1.91 ± 0.144 f | −0.88 ± 0.09 ab | |
| WD | -- | 1.02 ± 0.029 cd | 1.99 ± 0.211 f | −0.91 ± 0.07 b | ||
| SS | -- | 0.97 ± 0.008 d | 4.67 ± 0.703 b | −0.91 ± 0.08 a | ||
| WS | -- | 0.89 ± 0.027 f | 5.66 ± 0.533 a | −0.91 ± 0.09 c | ||
| Treatment | Cultivar | |||
|---|---|---|---|---|
| CK | DH605 | −0.625 | −0.914 | 0.289 |
| ZD958 | −0.571 | −0.957 | 0.386 | |
| WD | DH605 | −0.722 | −0.99 | 0.268 |
| ZD958 | −0.684 | −1.019 | 0.335 | |
| SS | DH605 | −0.886 | −1.078 | 0.192 |
| ZD958 | −0.896 | −1.119 | 0.223 | |
| WS | DH605 | −1.007 | −1.159 | 0.152 |
| ZD958 | −0.962 | −1.176 | 0.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Q.; Gao, S.; Han, Y.; Li, H. Vulnerability of Xylem Embolism in Maize Cultivars with Different Drought Tolerance under Water and Salt Stress. Agronomy 2024, 14, 438. https://doi.org/10.3390/agronomy14030438
Li Y, Wang Q, Gao S, Han Y, Li H. Vulnerability of Xylem Embolism in Maize Cultivars with Different Drought Tolerance under Water and Salt Stress. Agronomy. 2024; 14(3):438. https://doi.org/10.3390/agronomy14030438
Chicago/Turabian StyleLi, Yanbin, Qian Wang, Shikai Gao, Yuhang Han, and Hongxing Li. 2024. "Vulnerability of Xylem Embolism in Maize Cultivars with Different Drought Tolerance under Water and Salt Stress" Agronomy 14, no. 3: 438. https://doi.org/10.3390/agronomy14030438
APA StyleLi, Y., Wang, Q., Gao, S., Han, Y., & Li, H. (2024). Vulnerability of Xylem Embolism in Maize Cultivars with Different Drought Tolerance under Water and Salt Stress. Agronomy, 14(3), 438. https://doi.org/10.3390/agronomy14030438







