Abstract
This study was conducted to evaluate the Al3+ tolerance of sixteen camelina genotypes and to use melatonin or nano-selenium to alleviate Al3+-induced stress. A Petri dish study indicated seedling root length was suitable for describing the dose–response of seedling growth with increased Al3+ concentrations. Based on GR50 (Al3+ concentration causing a 50% reduction in the seedling root length), CamK6 (232.0 mg L−1) and CamK2 (97.0 mg L−1) were the most Al3+-tolerant and -sensitive genotypes, respectively. Under Al3+ stress, CamK6 and CamK2 treated by melatonin (50 μM) or nano-Se (0.4 mg L−1) showed a similar plant height and seed yield plant−1 (CamK6: 123.6 ± 9.8 cm and 0.562 ± 0.62 g; CamK2: 109.2 ± 8.7 cm and 0.49 ± 0.5 g) as the controls (CamK6: 121.1 ± 10.2 cm and 0.554 ± 0.4 g; CamK2: 110.0 ± 9.8 cm and 0.5 ± 0.4 g), and the values were greater than for the Al3+-treated plants (CamK6: 96.4 ± 9.2 cm and 0.48 ± 0.34 g; CamK2: 97.3 ± 8.1 cm and 0.42 ± 0.31 g). The results showed that melatonin or nano-Se through modulating biochemical reactions (e.g., antioxidant enzyme) can alleviate Al3+-induced growth inhibition in camelina. This study suggested melatonin or nano-Se can alleviate Al3+-induced growth inhibition by maintaining seed yield and improving oil quality in camelina.
1. Introduction
In soil, aluminum (Al) is solubilized into toxic forms of Al3+ ions [1]. Al3+ has been reported as a severe yield-restraining factor in acidic soils worldwide for crop production [2,3,4]. Upon entry into the root tip, Al3+ can trigger a series of physiological and biochemical reactions, including the overproduction of reactive oxygen species (ROS), plasma membrane disintegration, and an inhibition of root growth, thus affecting water and nutrient uptake. As a consequence, plant growth is severely suppressed and eventually causes significant seed yield reduction [4,5,6]. A possible approach for alleviating the adverse effect of Al3+-induced stress in plants could be achieved through agricultural managing strategies (e.g., application of biostimulants) that can potentially enhance plant resilience, and thus maintain productivity.
Camelina [Camelina sativa (L.) Crantz] is a relatively stress-resilient crop species potentially grown in marginal lands [7,8,9]. Camelina can not only be used as a healthy food but also has a range of industrial applications (e.g., cosmetics, pharmaceuticals, and biodiesel fuels) [7,10,11,12]. Previous studies have demonstrated the superior tolerance of camelina to drought [13,14], cold [15,16], diseases [17], and insect pests [18]. Nevertheless, camelina is highly sensitive to waterlogging [19] and heat stress [20]. Despite a basic understanding of camelina responses to biotic/abiotic stresses, to our knowledge, there is not enough information on camelina genotypes in response to Al3+-induced stress, although soil acidification resulting from Al3+ has been a global issue severely threatening crop production.
Studies have demonstrated an alleviation of abiotic stresses (e.g., saline, drought) by the application of various biostimulants (e.g., nanoparticles, bioactive fertilizers) in many crop species [21,22]. Brassinolide, through protecting photosynthetic organs and improving the carbon assimilation potential, enhanced the salt tolerance of rice seedlings [23]. Nano-selenium (nano-Se) as a biostimulant fertilizer has been applied in several crops, such as wheat (Triticum aestivum L.) [24] and strawberry (Fragaria × ananassa Duch.) [25], to enhance crop resilience. Melatonin has also been reported in many studies regarding its alleviative effect on salt [26], drought [27], heat [28], and heavy metal toxicity (e.g., Al3+) [29,30] in various plant species. In camelina, previous studies have also shown the potential of using regulators or fertilizers to reduce damage under stress [31,32]. By increasing antioxidant enzyme activities (e.g., superoxide dismutase, catalases), a foliar application of salicylic acid (SA) was effective in alleviating the injurious effect of drought stress in camelina [32]. Aghdasi et al. (2021) [33] reported that an exogenous application of boron (B) and 24-epibrasinolid not only improved oil quality but also mitigated late-season drought stress in camelina. Additionally, foliar-applied thiourea alleviated heat stress and improved the seed yield in camelina [20]. The application of Se or nano-Se effectively alleviated the adverse effect of drought and maintained the camelina seed yield [20,21]. Another study revealed that a foliar application of melatonin increased the salinity stress tolerance and improved the seed oil quality in camelina [34]. By contrast, denoting Al3+ as an important crop yield-restraining stress, few studies concerning its effect on camelina growth and productivity have been conducted thus far. Therefore, the objectives of this study were (i) to evaluate the Al3+ tolerance of a diverse camelina germplasm containing 16 different camelina genotypes and (ii) to test the alleviation potential by a foliar application of melatonin or nano-selenium (nano-Se) on Al3+-induced growth inhibition in camelina at the whole-plant level. We hypothesized the responses of the tested camelina to Al3+ concentrations would differ among the genotypes. A foliar application of melatonin or nano-Se with a suitable dose in Al3+-stressed camelina plants would potentially alleviate adverse effects, such as plant height reduction or seed yield loss through modulating the physiological and biochemical processes, and eventually enhance the camelina crop performance.
2. Materials and Methods
2.1. Seed Source and Reagents
In this study, sixteen camelina genotypes used in our previous studies [35] were tested. The detailed information concerning the camelina genotypes, accession number, seed origin, and seed accessibility is described in Supplementary Table S1. Among them, camelina ‘SO-40’ (a cultivar obtained from Sustainable Oils, Torrance, CA, USA) was compared with the other fifteen camelina genotypes. All these camelina genotypes are currently deposited in Yangzhou University, Jiangsu Province, China. Prior to the study, seed germination of the camelina genotypes showed >95%. Melatonin was purchased from Shanghai Macklin Biochemical Co., Ltd., Shanghai, China. Nano-se (Se0 nanoparticles; mean size: 50–78 nm) was purchased from Guilin Jiqi Group Co., Ltd., Guilin, China. Other chemicals and reagents that were used in this study were either purchased from Sigma-Aldrich (Shanghai, China) or the other chemical companies.
2.2. Study I: Evaluation of the Al3+ Tolerance of Camelina Genotypes
The Al3+ tolerance of the sixteen camelina genotypes was evaluated through a Petri dish-based method. Prior to the Al3+ concentration treatment, seeds of the tested camelina genotypes were surface sterilized with ethanol and NaClO. After that, 50 seeds from each genotype were evenly placed in Petri dishes contained filter papers which were pre-moistened with 5 mL of a range of Al3+ concentrations (0, 62.5, 125, 250, 500, 1000 mg L−1) (pH = 4.2–4.7 for the range of Al3+ aqueous solutions). This experiment was set up as a randomized complete block design (RCBD) with 3 replications of each genotype for each Al3+ concentration. The Petri dishes were covered with lids and incubated in a plant culture chamber maintained at 25 °C with a 10/14 h photoperiod. During the study period, all Petri dishes were monitored and 1 mL of distilled water was supplemented to maintain the moisture every two days. At 6 d after Al3+ treatment (DAT), the measured parameters including the percent germination, root, shoot, and seedling length of each camelina genotype of each treatment were determined from 15 randomly selected seedlings from each Petri dish (3 replicates). Root, shoot, and seedling length were determined using a micrometer (Yongkang Anguanlong Electromechanical Co., Ltd., Yongkang, Zhejiang province, China). The total chlorophyll (chlorophyll a and chlorophyll b) was extracted and determined using acetone incubation and spectroscopic methods [36], respectively. Subsequently, nonlinear regression analysis was applied to determine the suitable parameter for describing the dose–response of camelina seedling growth associated with the Al3+ concentration.
2.3. Study II: Effect of Melatonin or Nano-Se on Alleviation of the Al3+-Induced Stress in Camelina
While significant treatment effects (melatonin or nano-Se dose) were shown between the measured parameters and Al3+ concentrations, seedling root length was the most suitable parameter determined for describing the dose–response of seedling growth with the increased Al3+ concentrations. Based on the GR50 of root lengths estimated for the sixteen camelina genotypes, CamK6 and CamK2 were the most Al3+-tolerant and -sensitive genotypes, respectively, and used in this section of study accordingly.
2.3.1. Petri Dishes Study
Following the seed sterilization, 50 seeds each from CamK6 and CamK2 were treated with 5 mL of various concentration solutions as listed in Table 1. The Al3+ concentration of 97 mg L−1 (GR50 value of CamK2) (pH = 4.4) was single or co-applied with a range of concentrations of melatonin (50, 100, and 200 μM) (pH = 6.7–7.2) or nano-Se (0.4, 2, and 10 mg L−1) (pH = 6.6–6.8) (aqueous solution) to camelina seeds. The co-application of melatonin or nano-Se did not significantly affect the pH of Al3+ solution (4.3–4.6) after pH measurement. This experiment used the same design as above (n = 3). The Petri dishes were then maintained in a plant culture chamber with the same conditions as described above. At 6 DAT, the root length of the two camelina genotypes for each treatment (15 seedlings of each genotype from each Petri dish as a replicate, n = 3) was determined and subsequently compared for the potential effect of melatonin or nano-Se on the alleviation of the Al3+-induced root growth inhibition.

Table 1.
Summary of various aqueous solutions treatment in this study.
2.3.2. Whole-Plant Study
Firstly, young seedlings of CamK6 and CamK2 were grown and prepared in a greenhouse located at the Pratacultural Science Experimental Station of Yangzhou University (25 °C and 12/12 h photoperiod). When the seedlings reached to the 2–3-leaf stage (BBCH 11) [37], they were transplanted to bigger pots containing organic horticultural potting soil (Yiyuan Agriculture and Forestry Ltd., Suzhou, China) and maintained in the same greenhouse until the 5–6-leaf stage (BBCH 15) before treatment. The various treatments are described in Table 1. As the preliminary test showed the dose effect (97 mg L−1, GR50 value of CamK2) on CamK2 at the whole-plant level, the current Al3+ concentration (97 mg L−1) was applied to CamK6 and CamK2 plants in this section of study. Briefly, 25 mL of Al3+ solution concentration (97 mg L−1) was root-applied to the two camelina genotypes plants. At 8 h after Al3+ treatment (HAT), 30 mL of melatonin (50 μM) or nano-Se (0.4 mg L−1) was foliar-applied to those Al3+-treated camelina plants. Each treatment included individual three pots (each pot containing three camelina plants) for each genotype (each pot as a replicate, n = 3). The control groups included untreated control (distilled water), only Al3+ (97 mg L−1), melatonin (50 μM), or nano-Se (0.4 mg L−1)-treated camelina plants.
At 24 h after melatonin or nano-Se treatment, the antioxidant enzyme activities, including superoxide dismutase (SOD) (EC 1.15.1.1) and catalases (CAT) (EC 1.11.1.6), malondialdehyde (MDA) (a useful marker of oxidative stress), and soluble protein under each treatment were determined. Briefly, about 0.5 g of leaf sample from each treatment was collected and ground to powder in a mortar with liquid N and homogenized in 5 mL of 0.2 mM phosphate buffer (pH = 7.8). Then, the homogenate was centrifugated at 10,000× g for 20 min at 4 °C before measurement. The determination of those parameters followed Zou (2000) [38]. The plants were harvested at maturity (about 4 months’ cultivation) (BBCH 89) and accessed accordingly. The oil content of seeds resulted from each treatment was determined following the Soxhlet extraction method [35]. The oil fatty acid composition and concentration were analyzed by gas chromatography (GC) (GC9800, Shanghai Kechuang Chromatographic Instrument Co., Ltd., Shanghai, China) (flame ionization detector) fitted with a HP-88 capillary column (0.25 mm × 100 m × 0.2 μm, J & W, Folsom, CA, USA). Detailed information, such as that regarding the GC conditions and fatty acid methyl esters (FAMEs) sorting, was described in our previous study [35]. All the evaluations were carried out at the Laboratory of Grass Germplasm Resources Research and Utilization, Yangzhou University, China.
2.4. Statistical Analysis
Initially, the Shapiro–Wilk test (normality) on the data showed a normal distribution of residuals. For the data collected from study (I), ANOVA was conducted for the factors (Al3+ concentration and camelina genotype) and factors’ interactions effect on percent seed germination, root, shoot and seedling length, seedling fresh weight, and chlorophyll content. As the ANOVA showed a significant dose treatment effect (p < 0.05), the data were subjected to subsequent nonlinear regression analysis. The Al3+ concentrations causing a 50% reduction (GR50) in the parameters investigated of each genotype were respectively estimated by the log-logistic dose–response equation [39] (Equation (1)):
where y represents an estimate of percent germination, root, shoot, and seedling length, seedling fresh weight, and chlorophyll content of each genotype at x (Al3+ concentration). B is the slope of the dose–response curve through GR50. D and C are the upper and lower limit of the curve, respectively. When C = 0, the equation becomes a three-parameter model. Based on the p values calculated from the lack-of-fit test, the three- or four-parameter equations were used accordingly [39]. By comparing the model goodness of fit (R2) and root mean square deviation (RMSD), the most suitable parameter for describing the relationship between camelina growth inhibition and the Al3+ concentration was determined. The greatest value of GR50 was Al3+-tolerant, and lowest value was the Al3+-sensitive camelina genotype. The results collected from study (II) were presented as mean ± SE (standard error) of three replications. Fisher’s LSD test was applied to separate the mean values of treatment to determine the effect of melatonin or nano-Se on the alleviation of Al3+-induced stress in camelina genotypes. All statistical analyses were conducted using R 3.2.4 [40].
3. Results
3.1. Dose–Responses and Al3+ Tolerance of Tested Camelina Genotypes
Two-way ANOVA revealed that both the Al3+ concentration and the camelina genotype significantly affected the parameters investigated in each genotype (p < 0.05) (Table 2). Overall, the mean values of all of the aforementioned parameters (e.g., seedling root length) of each genotype consistently decreased with the increasing Al3+ concentration (ranges: 0–1000 mg L−1) (Figure 1), and the dose–responses for those tested genotypes were well described by a three-parameter log-logistic model with no evidence of a lack of fit shown (p > 0.05 for all models) (Table 3). While the Al3+ concentration significantly affected all those parameters (p < 0.05) (Table 2), among them, the root length was the most sensitive growth parameter to the Al3+ concentration, showing the GR50 values ranging from 97.0 to 232.0 mg L−1 across all genotypes compared with values of 463.4–896.0 mg L−1 for shoot length, 144.8–286.2 mg L−1 for seedling length, 311.8–1314.3 mg L−1 for fresh weight, 319.5–636.3 mg L−1 for chlorophyll content, and 848.0–20,661 mg L−1 for percent germination (Table 3). Additionally, the model generated for each genotype using root length showed the best goodness of fit and smallest root mean square (RMS) compared to the models generated with other parameters. These results demonstrated that the root length could be a reliable parameter to reflect the growth inhibition of camelina associated with the increased Al3+ concentration.

Table 2.
Summary of ANOVA for factors’ effect (Al3+ concentration and camelina genotype) and interactions on camelina percent germination, root, shoot, and seedling length, chlorophyll content, and fresh weight in the Petri dish test.
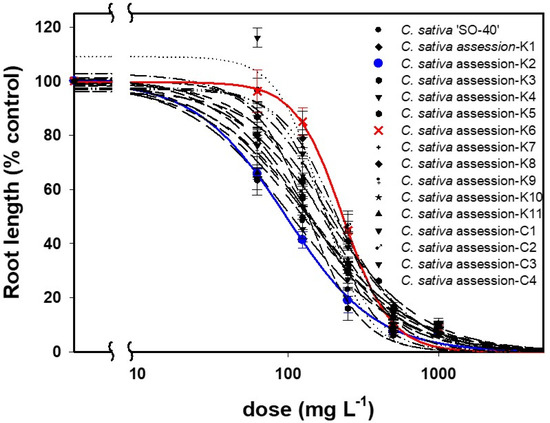
Figure 1.
Dose–responses of seedling root length for the tested sixteen camelina genotypes to a range of Al concentrations (0–1000 mg L−1) in the Petri dish study. The red and blue solid lines represent the most Al3+-resistant (CamK6, GR50 = 232.0 mg L−1) and -sensitive (CamK2, GR50 = 97.0 mg L−1) camelina genotypes determined based on the GR50 values estimated from the model (Equation (1)) and parameter estimates in Table 3, respectively.

Table 3.
Summary of the parameters estimated from the three-parameter log-logistic model a for root, shoot, and seedling length, fresh weight, chlorophyll content, and percent germination in the sixteen tested camelina genotypes under a range of Al3+ concentrations (0–1000 mg L−1) in the Petri dish test.
The Al3+ tolerance of the tested sixteen camelina genotypes was characterized using the GR50 values estimated using the root length from the model. The results showed that the most Al3+-tolerant and -sensitive genotypes were CamK6 and CamK2 with GR50 values of 232.0 and 97.0 mg L−1, respectively (Figure 1; Table 3). While the GR50 values differed when using different growth parameters, the models generated using other parameters (e.g., shoot length, seedling length) yielded similar results, with CamK6 showing the greatest GR50 values vs. CamK2 showing the smallest values among those genotypes (896.0 vs. 463.4 mg L−1 calculated using shoot length for CamK6 and CamK2, respectively; 286.2 vs. 144.8 mg L−1 calculated using seedling length; and 1314.3 vs. 87.3 mg L−1 calculated using seedling fresh weight) (Table 3).
3.2. Alleviation of Al3+-Induced Stress Using Melatonin and Nano-Se
3.2.1. Petri Dish Study
The effect of melatonin or nano-Se on the alleviation of Al3+-induced root growth inhibition was evaluated using camelina genotypes CamK6 and CamK2 determined in study (I). The single application of Al3+ at the concentration of 97.0 mg L−1 dramatically reduced the seedling root length of the two genotypes compared to untreated controls (Figure 2). For example, the seedling root lengths of CamK6 and CamK2 treated with Al3+ (97.0 mg L−1) were about 31.3 and 22.8 mm, respectively, which were significantly shorter than those of the untreated controls (CamK6 and CamK2: 58.5 and 56.3 mm, respectively). Exogenous application of melatonin at the concentration range of 50–200 µM effectively counterbalanced the Al3+-induced root growth inhibition, showing statistically similar root lengths (CamK6 and CamK2: 54.6–59.7 mm and 53.9–58.5 mm, respectively) to the untreated controls for both genotypes (Figure 2A,C). The additional promotion of root growth in the two camelina genotypes was observed by the single application of melatonin at the current dose range (CamK6 and CamK2: 66.2–68.8 mm and 59.4−64.7 mm, respectively) compared to the untreated controls. By contrast, for nano-Se, it only showed the effective alleviation of the Al3+-induced root growth inhibition at 0.4 mg L−1 for both genotypes (CamK6 and CamK2: 57.2 and 56.9 mm, respectively); when it was applied at higher doses, there was almost little/no alleviative effect observed (CamK6 and CamK2: 46.4 and 44.8 mm at 2 mg L−1; 35.3 and 34.2 mm at 10 mg L−1, respectively) (Figure 2B,D). There results indicated that melatonin applied at 50–200 μM positively improved camelina seedling root growth under Al3+-induced stress, whereas nano-Se counterbalanced the Al3+-induced root growth inhibition only at a lower dose (0.4 mg L−1).
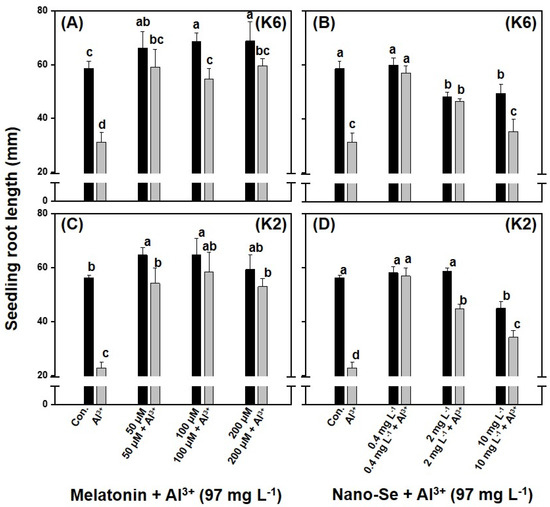
Figure 2.
The effects of various treatments on the seedling root length of CamK6 (A,B) and CamK2 (C,D) under Al3+-induced (97.0 mg L−1) seedling growth inhibition. Con.: untreated control; Al3+: 97.0 mg L−1; 50, 100, or 200 μM + Al3+: 50, 100, or 200 μM melatonin co-applied following 97.0 mg L−1 of Al3+ treatment; 0.4, 2, or 10 mg L−1 + Al3+: 0.4, 2, or 10 mg L−1 nano-Se co-applied following 97.0 mg L−1 of Al3+ treatment. Values are the means (n = 3), and bars denote the standard error of the mean. Different letters represent the significant different values determined by Fisher’s LSD test (p ≤ 0.05).
3.2.2. Whole-Plant Study
Further, the alleviative effect of melatonin or nano-Se on the Al3+-induced stress in camelina was re-confirmed using CamK6 and CamK2 at the whole-plant level. After 24 h of melatonin (97.0 mg L−1) or nano-Se (0.4 mg L−1) treatment, the two antioxidant enzyme activities (SOD and CAT) and MDA concentration (a marker of oxidative stress) were significantly increased under Al3+-induced stress in CamK6 (253.0 U g−1, 253.8 U min−1, and 16.1 µmol g−1 for SOD, CAT, and MDA, respectively) and CamK2 (292.9 U g−1, 267.0 U min−1, and 19.5 µmol g−1) genotypes compared with the untreated controls (CamK6: 133.4 U g−1, 166.6 U min−1, and 10.4 µmol g−1 for SOD, CAT, and MDA, respectively; CamK2: 215.5 U g−1, 171.8 U min−1, and 8.2 µmol g−1) (Figure 3A–F). Although the changing pattern of the three parameters varied differently in the two camelina genotypes, when melatonin or nano-Se was co-applied (Figure 3A–F), the higher values significantly decreased to a lower level similar to untreated controls (co-applied with melatonin: 140.3 U g−1, 169.2 U min−1, and 9.3 µmol g−1 for SOD, CAT, and MDA, respectively, in CamK6; 199.5 U g−1, 171.4 U min−1, and 9.4 µmol g−1, respectively, in CamK2; co-applied with nano-Se: 172.7 U g−1, 172.6 U min−1, and 7.1 µmol g−1 for SOD, CAT, and MDA, respectively, in CamK6; 158.6 U g−1, 144.3 U min−1, and 10.1 µmol g−1, respectively, in CamK2). Regarding the soluble protein, the Al3+ treatment significantly decreased its content in both camelina genotypes (21.4 and 20.7 mg g−1 in CamK6 and CamK2, respectively) compared to the untreated controls (32.4 and 31.3 mg g−1 in CamK6 and CamK2, respectively) (Figure 3G,H). The single application of melatonin showed no effect on improving the soluble protein content in either camelina genotype (30.8 and 27.6 mg g−1 in CamK6 and CamK2, respectively). The single application of nano-Se (0.4 mg L−1) showed no effect on improving the soluble protein in CamK6 compared to the untreated control (28.3 vs. 32.4 mg g−1), but significantly decreased the soluble protein in CamK2 compared to the control (23.6 vs. 31.3 mg g−1). The co-application of melatonin or nano-Se to the two camelina genotypes under Al3+ stress increased the soluble protein content (applied with melatonin: 28.9 and 27.7 mg g−1 in CamK6 and CamK2, respectively; applied with nano-Se: 35.6 and 28.5 mg g−1 in CamK6 and CamK2, respectively) to the normal level of untreated controls.
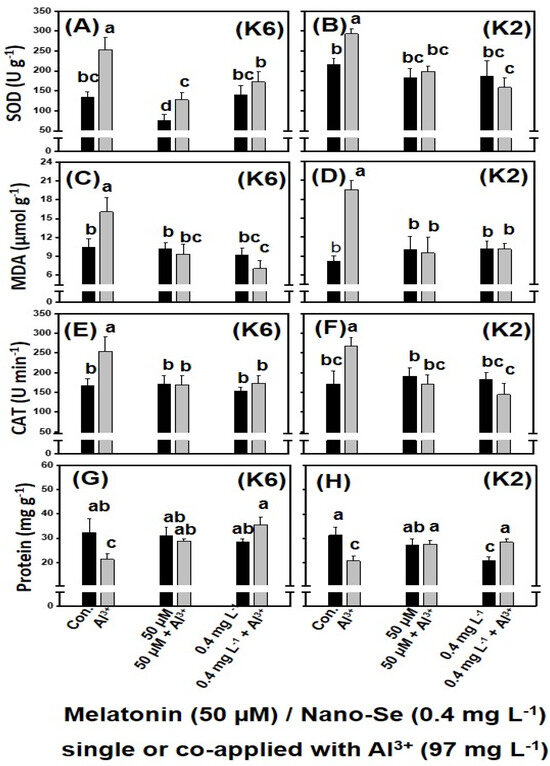
Figure 3.
The effects of various treatments on SOD (U g−1) (A,B), MDA (µmol g−1) (C,D), CAT (E,F), and soluble protein (mg g−1) (G,H) of CamK6 (A,C,E,G) and CamK2 (B,D,F,H) under Al3+-induced (97.0 mg L−1) plant growth inhibition at the whole-plant level. Con.: untreated control; Al3+: 97.0 mg L−1; 50 μM + Al3+: 50 μM melatonin co-applied following the root application of 97.0 mg L−1 Al3+ concentration; 0.4 mg L−1 + Al3+: 0.4 mg L−1 nano-Se co-applied following the root application of a 97.0 mg L−1 Al3+ concentration. Values are the means (n = 3), and bars denote the standard error of the mean. Different letters represent the significant different values determined by Fisher’s LSD test (p ≤ 0.05).
In the case of plant height, the co-application of melatonin or nano-Se at the current dose could effectively alleviate the Al3+-induced stress in the two camelina genotypes, significantly promoting the plants’ growth (Figure 4A,B). For example, in CamK6, the mean plant height under the Al3+-induced stress was 96.4 cm. When melatonin or nano-Se was co-applied, the mean plant height increased to a height (melatonin treatment: 123.6 cm; nano-Se treatment: 119.2 cm) that was comparable to the untreated control (121.1 cm). The seed yield plant−1 of the two camelina genotypes under the Al3+-induced stress also increased by the exogenous application of those chemicals (Figure 4C,D). As shown in Figure 4, the co-application of either melatonin or nano-Se effectively alleviated the Al3+-induced stress in CamK6 (Figure 4C) and CamK2 (Figure 4D) with a seed yield plant−1 obtained (CamK6: 0.562 and 0.558 g plant−1 for co-applied with melatonin and nano-Se, respectively; CamK2: 0.48 and 0.50 g plant−1 for co-applied with melatonin and nano-Se, respectively) comparable to the untreated controls (0.554 and 0.50 g plant−1 for CamK6 and CamK2, respectively). Regarding the oil content of the two camelina genotypes under those treatments, the single or co-applied melatonin or nano-Se with the Al3+ concentration completely counterbalanced the Al3+-induced stress on plants and increased the oil content (%) to the levels of untreated controls (Figure 4E,F).
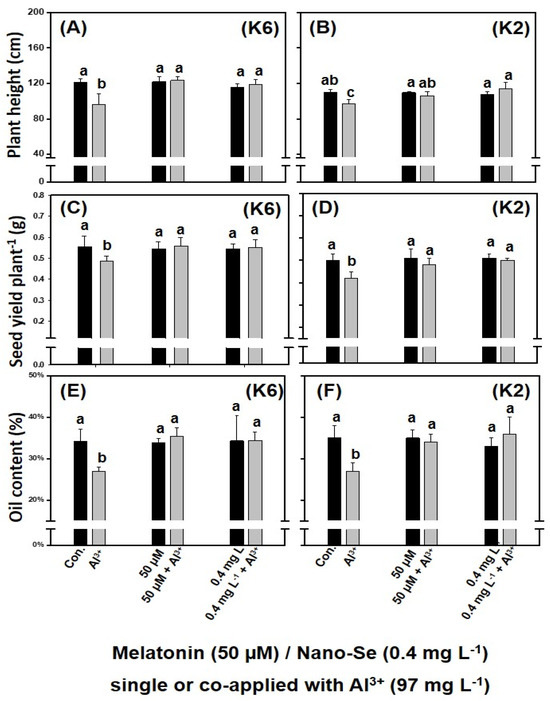
Figure 4.
The effects of various treatments on plant height (cm) (A,B), seed yield plant−1 (g) (C,D), and seed oil content (%) (E,F) of CamK6 (A,C,E) and CamK2 (B,D,F) under the Al3+-induced stress determined at harvest. Con.: untreated control; Al3+: 97.0 mg L−1; 50 μM + Al3+: 50 μM melatonin co-applied following the root application of 97.0 mg L−1 Al3+ concentration; 0.4 mg L−1 + Al3+: 0.4 mg L−1 nano-Se co-applied following the root application of 97.0 mg L−1 Al3+ concentration. Values are the means (n = 3), and bars denote the standard error of the mean. Different letters represent the significant different values determined by Fisher’s LSD test (p ≤ 0.05).
Supplementary Table S2 shows the major fatty acid composition and fatty acid groups of the two camelina genotypes under various treatments. While Fisher’s LSD test indicated differences in the mean values between the treatments, no consistent pattern regarding the impact of the various treatments on the principal fatty acids content was observed, even though the fatty acid contents, especially the three major UFA (C18:1, C18:2, and C18:3) in the two Al3+-treated camelina genotypes were lower than those of the untreated controls and the other treatments (Supplementary Table S2). For example, the contents of the three major UFA in Al3+-treated CamK6 were 12.05, 18.41, and 33.46%, which were significantly lower than the untreated controls (12.86, 21.78, and 35.74%, respectively) and the other treatments (ranges across different treatments: 12.86–13.2%, 20.81–22.28%, and 34.07–37.46%, respectively). A similar pattern of the changes in those fatty acids was also observed in CamK2. Compared with the lower contents of the principal fatty acids in the Al3+-treated camelina genotypes, the co-application of melatonin or nano-Se increased the contents of those fatty acids, indicating the alleviative effect on Al3+-induced stress in camelina genotypes.
4. Discussion
Al3+ toxicity is one of the major factors constraining plant growth and crop production worldwide [5]. In acid soil (pH < 5.5), aluminum is solubilized into the toxic form of Al3+ ions, which trigger signal disruption (e.g., ROS overproduction), resulting in oxidative damage and physiological changes (e.g., root growth inhibition), eventually reducing crop yields [41,42,43]. Melatonin, an indole compound, has been shown to exert multiple effects in plants, such as mediating root elongation and photosynthesis, enhancing the plant tolerance under biotic and abiotic stresses [3,30,44]. Nano-Se was used as a biostimulant fertilizer to enhance crop resilience under unfavorable environmental conditions [45,46]. The reported studies of using these chemicals on alleviating various stresses in several plant species, such as maize (Zea mays L.) and hickory (Carya cathayensis Sarg.) [2,3] may provide new possibilities for managing the abiotic stresses (e.g., Al3+) in camelina production in agricultural practice. The objective of this study was to evaluate the Al3+ tolerance of 16 camelina genotypes exposed to a range of Al3+ concentrations and, further, to test the potential for the application of melatonin or nano-Se in the alleviation of Al3+-induced stress. The results showed that although Al3+-induced stress adversely affected camelina plant physiological and biochemical processes, causing a final seed yield reduction in camelina, the co-application of melatonin or nano-Se could effectively alleviate the Al3+-induced growth inhibition and maintain a consistent camelina seed yield and oil quality.
The accumulation of enzymatic antioxidants (e.g., SOD, CAT) is considered an effective strategy for plants under abiotic/biotic stresses to scavenge ROS and decrease oxidative damage [47,48]. The data from this study showed that under Al3+-induced stress, both the activities of SOD and CAT, the key enzymes involved in cell-defense processes, significantly increased as previous studies have reported in maize [27], oilseed rape (Brassica napus L.) [29], and wheat (Triticum aestivum) [30]. In contrast to the previous studies reporting an increase in SOD activity in Al3+-stressed oilseed rape after melatonin treatment [29] and drought-stressed tomato (Solanum lycopersicum L.) after Se treatment [49], in this study, the SOD and CAT activities of the camelina plants co-applied with melatonin or nano-Se were maintained at a normal level similar to untreated controls. Recently, our research team also observed a relatively stable SOD activity in drought-stressed camelina plants after nano-Se treatment [21]. Because the mechanism differences in melatonin-mediated Al3+ tolerance vary across different plant species [3] and the various defense mechanisms of plants possessed under various stresses (e.g., alteration of antioxidant enzyme activity, production of defensive pigments) [50], the increase in antioxidant enzyme activities after stress-alleviating chemical treatment may simplify the changing process of these enzyme activities under various stresses. In addition to the currently investigated antioxidants enzymes, there are other molecules related to the Al3+-induced responses in plant cells that might explain the stress-alleviating mechanism in camelina. Therefore, future studies on the analysis of transcriptomics, metabolomics, etc., may provide new information for better understanding the biochemical and molecular processes in this regard. The MDA level has been considered as a good proxy for the degree of lipid peroxidation in the cell membrane [48]. The higher accumulation of MDA content in the Al3+-treated camelina genotypes compared to the untreated controls demonstrated the occurrence of cell membrane damage due to Al3+ toxicity. The present study, through the co-application of melatonin or nano-Se to the Al3+-stressed camelina genotypes, significantly decreased its contents to a normal level. A decline in soluble protein content in the Al3+-treated camelina in this study was observed, which is in line with the results of previous studies reported in several crop species grown under abiotic stresses [28,51]. A plausible explanation regarding the soluble protein reduction can be related to the higher degradation of proteins under the Al3+-induced stress forming low-molecular-weight osmolytes to adjust the osmotic pressure [52]. The application of melatonin or nano-Se could potentially regulate/balance the contents of soluble protein in/outside of the cell, and thus alleviate the adverse effects of the Al3+-induced stress in camelina genotypes.
The co-application of melatonin or nano-Se to Al3+-stressed camelina plants could also alleviate the reduced plant height or seed yield associated with Al3+-induced stress. The root tip cell wall components, such as pectin and hemicellulose, were the primary location for Al3+ adsorption [53]. Upon entry into and accumulation of Al3+ in root tip cells, the higher concentration of Al3+ triggered various physiological and metabolic changes (e.g., disruption of carbohydrate metabolism, degradation of proteins, lipids peroxidation) and subsequent inhibition of root growth, eventually leading to seed yield reduction [5,54]. Under Al3+-induced stress, the application of melatonin altered the expression of synthetic genes of plants (e.g., synthesis and assembly of pectin and hemicellulose) [3,55], leading to the modification of the cell wall components and the final reduction in Al3+ content in cell wall, thus alleviating the Al3+-induced stress in camelina plants. As previously mentioned, plants possess various defense mechanisms under biotic or abiotic stresses, including the alteration of antioxidant activities and gene expression and the production of defensive pigments and substances. Studies are still needed to reveal the molecular mechanism of the alleviative effect of melatonin on Al3+-induced stress. In the case of nano-Se, to our knowledge, there are no studies regarding its application or alleviative mechanism on Al3+-induced stress in camelina. The reported studies in other crop species, such as strawberry (Fragaria × ananassa Duch.) [25] and wheat (Triticum aestivum L.) [24], showed that the exogenous application of nano-Se could potentially increase the contents of defense-related chemicals (i.e., flavonoid) in plants and thus enhance plant resilience under unfavorable conditions. Our recent study also showed the potential for using nano-Se to alleviate PEG-induced drought stress and maintain the seed yield and quality in camelina [21].
According to the results, the co-application of melatonin or nano-Se can not only improve the camelina seed yield under Al3+-induced stress, but also improve the quality of the oil extracted from the camelina seeds. For camelina, seed quality is mainly determined by the fatty acid components and the UFA contents [34]. A higher oil quality correlates with relatively greater UFA contents, such as C18:1~3. Various environmental adverse factors (e.g., drought, heat) have shown a negative effect on camelina seed oil content and fatty acid components [56]. The results in this study showed that a single application of melatonin or nano-Se to camelina plants increased the relative contents of UFA and reduced SFA in the seed oil, providing a new approach for improving the seed quality during camelina production.
5. Conclusions
This study was the first to evaluate the Al3+ tolerance of 16 camelina genotypes and test the potential of using melatonin or nano-Se to alleviate the adverse effect of the Al3+- induced stress in camelina at the whole-plant level. The Petri dish study demonstrated that root length could be a reliable parameter for describing the dose–response of growth inhibition in camelina associated with an increased Al3+ concentration. CamK6 and CamK2 were determined to be the most Al3+-tolerant and -sensitive genotypes based on the GR50 of root lengths estimated from the log-logistic model. At the whole-plant level, the foliar application of melatonin (50 μM) or nano-Se (0.4 mg L−1) not only effectively alleviated the reduced plant height or seed yield under the Al3+-induced stress (97.0 mg L−1), but also improved the seed oil quality by increasing the relative contents of UFA and reducing SFA. This study demonstrates that the foliar application of melatonin or nano-Se could be a potential way to manage the Al3+-associated abiotic stress in camelina, maintain the seed yield, and improve the seed oil quality. Additionally, the CamK6 genotype (GR50 = 232.0 mg L−1) would be a potential good germplasm source for breeding the Al3+-tolerant camelina genotype.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14030401/s1, Table S1: The information of camelina (Camelina sativa L.) seed sources used in this study; Table S2: Principal fatty acids composition and fatty acid groups (% of total fatty acids) and ratios of CamK2 and CamK6 under various treatments.
Author Contributions
Conceptualization, Z.-C.L., M.C. and C.-J.Z.; methodology, Z.-C.L., M.C. and C.-J.Z.; data curation, Z.-C.L., M.C. and C.-J.Z.; writing—original draft preparation, Z.-C.L., M.C. and C.-J.Z.; visualization, Z.-C.L., M.C., Y.T., Y.G. and H.-Z.W.; investigation, Z.-C.L., M.C., Y.T., Y.G. and H.-Z.W.; software, Z.-C.L., M.C. and C.-J.Z.; validation, Z.-C.L., M.C. and C.-J.Z.; writing—reviewing and editing, Z.-C.L., M.C., Y.T., Y.G., H.-Z.W., X.M., D.-S.K., X.Y., J.Y. and C.-J.Z. funding acquisition, C.-J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 32171670), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX23_3615), and High-Level Talents Program of Yangzhou University.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Rahman, R.; Upadhyaya, H. Aluminium toxicity its tolerance in plant: A review. J. Plant Biol. 2021, 64, 101–121. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.; Nagabovanalli, P.; Lux, A.; Vaculík, M. Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: A review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef]
- Zeng, H.; Li, Y.R.; Chen, W.J.; Yan, J.W.; Wu, J.S.; Lou, H.Q. Melatonin alleviates aluminum toxicity by regulating aluminum-responsive nonresponsive pathways in hickory. J. Hazard. Mater. 2023, 460, 132274. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.J. Crop production on acidic soils: Overcoming aluminum toxicity and phosphorus deficiency. Ann. Bot. 2010, 106, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V.; Hoekenga, O.A.; Pineros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pineros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina uses, genetics, genomics, production, and management. Ind. Crop Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- Li, X.; Mupondwa, E. Life cycle assessment of camelina oil derived biodiesel and jet fuel in the Canadian Prairies. Sci. Total Environ. 2014, 481, 17–26. [Google Scholar] [CrossRef]
- Zhang, C.J.; Gao, Y.; Jiang, C.; Liu, L.; Wang, Y.; Kim, D.S.; Yu, J.; Yu, L.; Li, F.; Fan, Y. Camelina seed yield and quality in different growing environments in northern China. Ind. Crop Prod. 2021, 172, 114071. [Google Scholar] [CrossRef]
- Aziza, A.E.; Quezada, N.; Cherian, G. Antioxidative effect of dietary camelina meal in fresh, stored, or cooked broiler chicken meat. Poultry Sci. 2010, 89, 2711–2718. [Google Scholar] [CrossRef]
- Betancor, M.B.; Sprague, M.; Usher, S.; Sayanova, O.; Campbell, P.J.; Napier, J.A.; Tocher, D.R. A nutritionally-enhanced oil from transgenic Camelina sativa effectively replaces fish oil as a source of eicosapentaenoic acid for fish. Sci. Rep. 2015, 5, 8104. [Google Scholar] [CrossRef]
- Sindelar, A.; Schmer, M.; Gesch, R.W.; Forcella, F.; Eberle, C.A.; Thom, M.; Archer, D.W. Winter oilseed production for biofuel in the, U.S. Corn Belt: Opportunities and limitations. GCB Bioenergy 2017, 9, 508–524. [Google Scholar] [CrossRef]
- George, N.; Levers, L.; Thompson, S.; Hollingsworth, J.; Kafika, S. Modeling identifies optimal fall planting times and irrigation requirements for canola and camelina at locations across California. Calif. Agric. 2017, 71, 214–220. [Google Scholar] [CrossRef]
- Schillinger, W.F. Camelina: Long-term cropping systems research in a dry Mediterranean climate. Field Crops Res. 2019, 235, 87–94. [Google Scholar] [CrossRef]
- Soorni, J.; Kazemitabar, S.K.; Kahrizi, D.; Ali, D.; Nadaliet, B. Genetic analysis of freezing tolerance in camelina [Camelina sativa (L.) Crantz] by diallel cross of winter and spring biotypes. Planta 2021, 253, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Auer, C. Over wintering assessment of camelina (Camelina sativa) cultivars and congeneric species in the northeastern US. Ind. Crops Prod. 2019, 139, 111532. [Google Scholar] [CrossRef]
- Séguin-Swartz, G.; Eynck, C.; Gugel, R.K.; Strelkov, S.E.; Olivier, C.Y.; Li, J.L.; Falk, K.C. Diseases of Camelina sativa (false flax). Can. J. Plant Pathol. 2009, 31, 375–386. [Google Scholar] [CrossRef]
- Soroka, J.; Olivier, C.; Grenkow, L.; Sguin-Swartz, G. Interactions between Camelina sativa (Brassicaceae) and insect pests of canola. Can. Entomol. 2015, 147, 193–214. [Google Scholar] [CrossRef]
- Stasnik, P.; Großkinsky, D.K.; Jonak, C. Physiological and phenotypic characterization of diverse Camelina sativa lines in response to waterlogging. Plant Physiol. Biochem. 2022, 183, 120–127. [Google Scholar] [CrossRef]
- Ahmad, M.; Waraich, E.A.; Tanveer, A.; Anwar-U-Hag, M. Foliar applied thiourea improved physiological traits yield of camelina canola under normal heat stress conditions, J. Soil Sci. Plant Nutr. 2021, 21, 1666–1678. [Google Scholar] [CrossRef]
- Wu, H.Z.; Gao, Y.; Zhang, Y.X.; Yu, J.L.; Kim, D.S.; Chen, M.; Wang, Y.W.; Fan, Y.; Zhang, H.X.; Yan, X.B.; et al. Exogenous application of multi-walled carbon nanotubes (MWCNTs) and nano-Selenium (Nano-Se) alleviated the PEG-induced water deficit stress and improved the crop performance of camelina. Agronomy 2023, 13, 979. [Google Scholar] [CrossRef]
- Zuo, Y.T.; Zeng, W.Z.; Ao, C.; Chen, H.R.; Huang, J.S. Effects of multiwalled carbon nanotube and Bacillus atrophaeus application on crop root zone thermal characteristics of saline farmland. Heliyon 2023, 9, e13510. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.W.; Feng, N.J.; Zheng, D.F.; Zhou, H.; Liu, L.; Chen, G.J.; Mu, B.M. Physiological mechanism of exogenous brassinolide alleviating salt stress injury in rice seedlings. Sci. Rep. 2022, 12, 20439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.R.; Li, D.; Shi, X.L.; Zhang, J.B.; An, O.S.; Wu, Y.L.; Kang, L.; Li, J.O.; Pan, C.P. Nanoselenium enhanced wheat resistance to aphids by regulating biosynthesis of, D.M.B.O.A.; volatile components. J. Agric. Food Chem. 2021, 69, 14103–14114. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.S.; Nie, X.J.; Zhang, T.; Li, S.; Wang, X.Y.; Du, X.H.; Tong, W.; Song, W.N. Melatonin: A small molecule but important for salt stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yang, X.; Ma, C.; Wang, Y.; Zhao, J. Melatonin enhances drought stress tolerance in maize through coordinated regulation of carbon and nitrogen assimilation. Plant Physiol. Biochem. 2021, 167, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Zhu, T.; Zhao, C.; Li, L.; Chen, M. The role of melatonin in salt stress responses. Int. J. Mol. Sci. 2019, 20, 1735. [Google Scholar] [CrossRef] [PubMed]
- Sami, A.; Shah, F.A.; Abdullah, M.; Yu, Z.X.; Jin, Z.K. Melatonin mitigates cadmium and aluminium toxicity through modulation of antioxidant potential in Brassica napus L. Plant Biol. 2020, 4, 679–690. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2020, 63, 126–145. [Google Scholar] [CrossRef]
- Bukhari, M.A.; Yousaf, M.; Ahmad, Z.; Rafay, M.; Shah, A.N.; Abbas, A.; Shah, A.A.; Javed, T.; Afzal, M.; Ali, S.; et al. Enhancing drought stress tolerance in camelina (Camelina sativa L.) through exogenous application of potassium. Physiol. Plantarum. 2022, 174, e13779. [Google Scholar] [CrossRef]
- Naderi, R.; Afranjeh, E.; Heidari, B.; Emam, Y.; Egan, T.P. Salicylic acid and superabsorbent polymers could alleviate water deficit stress in camelina (Camelina Sativa L.). Commun. Soil Sci. Plant Anal. 2023, 54, 2863–2873. [Google Scholar] [CrossRef]
- Aghdasi, S.; AghaAlikhani, M.; Modarres-Sanavy, S.A.M.; Kahrizi, D. Exogenously used boron and 24-epibrassinolide improved oil quality and mitigate late-season water deficit stress in camelina. Ind. Crop. Prod. 2021, 171, 113885. [Google Scholar] [CrossRef]
- Bakyani, M.R.F.; Alinia, M.; Kazemeini, S.A.; Abadía, J.; Dadkhodaie, A. Foliar application of melatonin improves the salt tolerance on and redox homeostasis and seed oil fatty acid profile in Camelina sativa. Plants 2022, 11, 3113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, J.; Gao, Y.; Li, Z.W.; Kim, D.-S.; Chen, M.; Fan, Y.; Zhang, H.; Yan, X.; Zhang, C.-J. Agronomic evaluation of shade tolerance of 16 spring Camelina sativa (L.) Crantz genotypes under different artificial shade levels using a modified membership function. Front. Plant Sci. 2022, 13, 978932. [Google Scholar] [CrossRef] [PubMed]
- Makeen, K.; Babu, G.S.; Lavanya, G.R.; Abraham, G. Studies of chlorophyll content by different methods in Black Gram (Vigna mungo L.). Int. J. Agric. Res. 2007, 2, 651–655. [Google Scholar]
- Martinelli, T.; Galasso, I. Phenological growth stages of Camelina sativa according to the extended BBCH scale. Ann. Appl. Biol. 2011, 158, 87–94. [Google Scholar] [CrossRef]
- Zou, Q. Plant physiology experimental guidance; Agriculture Press Publishing: Beijing, China, 2000. [Google Scholar]
- Knezevic, S.Z.; Streibig, J.C.; Ritz, C. Utilizing R software package for dose-response studies: The concept and data analysis. Weed Technol. 2007, 21, 840–848. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 3 January 2024).
- Dai, C.; Oiu, L.; Guo, L.; Jing, S.; Chen, X.; Cui, X.; Yang, Y. Salicylic acid alleviates aluminum-induced inhibition of biomass by enhancing photosynthesis and carbohydrate metabolism in Panax notoginseng. Plant Soil 2019, 445, 183–198. [Google Scholar] [CrossRef]
- Wang, L.; Fan, X.W.; Pan, J.L.; Huang, Z.B.; Li, Y.Z. Physiological characterization of maize tolerance to low dose of aluminum, highlighted by promoted by promoted leaf growth. Planta 2015, 242, 1391–1403. [Google Scholar] [CrossRef]
- Yan, L.; Riaz, M.; Liu, J.; Yu, M.; Cuncang, J. The aluminum tolerance and detoxification mechanisms in plants; recent advances and prospects. Crit Rev. Env. Sci. Tec. 2022, 52, 1491–1527. [Google Scholar] [CrossRef]
- Gao, T.; Liu, X.; Tan, K.; Zhang, D.; Zhu, B.; Ma, F.W.; Li, C. Introducing melatonin to the horticultural industry: Physiological roles, potential applications, and challenges. Hortic. Res. 2022, 22, uhac094. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Wu, Y.L.; Zhang, J.B.; An, Q.S.; Zhou, C.R.; Li, D.; Pan, C.P. Nano-selenium enhances the antioxidant capacity, organic acids and cucurbitacin B in melon (Cucumis melo L.) plants. Ecotoxicol. Environ. Saf. 2022, 241, 113777. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Pandey, P.; Upadhyay, T.K. Applications of nanotechnology-based agrochemicals in food security and sustainable agriculture: An overview. Agriculture 2022, 12, 1672. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S. Salinity effects on nitrogen metabolism in plants-focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene. 2019, 19, 100182. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semidaet, W.M. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Efeoglu, B.; Ekmekci, Y.; Cicek, N. Physiological responses of three maize cultivars to drought stress recovery. S. Afr. J. Bot. 2009, 75, 34–42. [Google Scholar] [CrossRef]
- Ahmed, Z.; Waraich, E.; Ahmad, R.; Shahbaz, M. Morpho-physiological and biochemical responses of camelina (Camelina sativa crantz) genotypes under drought stress. Int. J. Agric. Biol. 2017, 19, 1–7. [Google Scholar] [CrossRef]
- Nayyar, H.; Walia, D.P. Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol. Plantarum. 2003, 46, 275–279. [Google Scholar] [CrossRef]
- Yang, J.L.; Zhu, X.F.; Peng, Y.X.; Zheng, C.; Li, G.X.; Liu, Y.; Shi, Y.Z.; Zheng, S.J. Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol. 2011, 155, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.O.; Fan, W.; Jin, J.F.; Xu, J.M.; Chen, W.W.; Yang, J.L.; Zheng, S.J. A NAC-type transcription factor confers aluminium resistance by regulating cell wall-associated receptor kinase 1 and cell wall pectin. Plant Cell Environ. 2020, 43, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohyd. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Borzoo, S.; Mohsenzadeh, S.; Kahrizi, D. Water-deficit stress and genotype variation induced alteration in seed characteristics of Camelina sativa. Rhizosphere 2021, 20, 100427. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).