Effect of Different Sowing Seasons, Growth Stages, Leaf Positions, and Soybean Varieties on the Growth of Clanis bilineata tsingtauica Mell Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Insects
2.2. Experimental Conditions and Materials
2.3. Evaluation of CBT-Rearing Factors under Summer Sowing Condition
2.4. Evaluation of CBT-Rearing Factors under Autumn Sowing Condition
2.5. Statistical Analyses
3. Results
3.1. Meteorological Conditions for the CBT Rearing of Summer and Autumn Sowing Seasons
3.2. Effects of Different Rearing Factors on the Growth of Different-Day-Old CBT Larvae
3.2.1. Effects of Different Leaf Positions and Soybean Varieties at V6 Stage in Different Sowing Seasons on the Growth of CBT Larvae
3.2.2. Effects of Different Leaf Positions, Soybean Growth Stage, and Soybean Varieties on the Growth of CBT Larvae in Autumn Sowing
3.2.3. Correlation between Larval Weight and Larval Survival Rate
3.3. Effects of Different Rearing Factors on the Weight of 21-Day-Old CBT Larvae
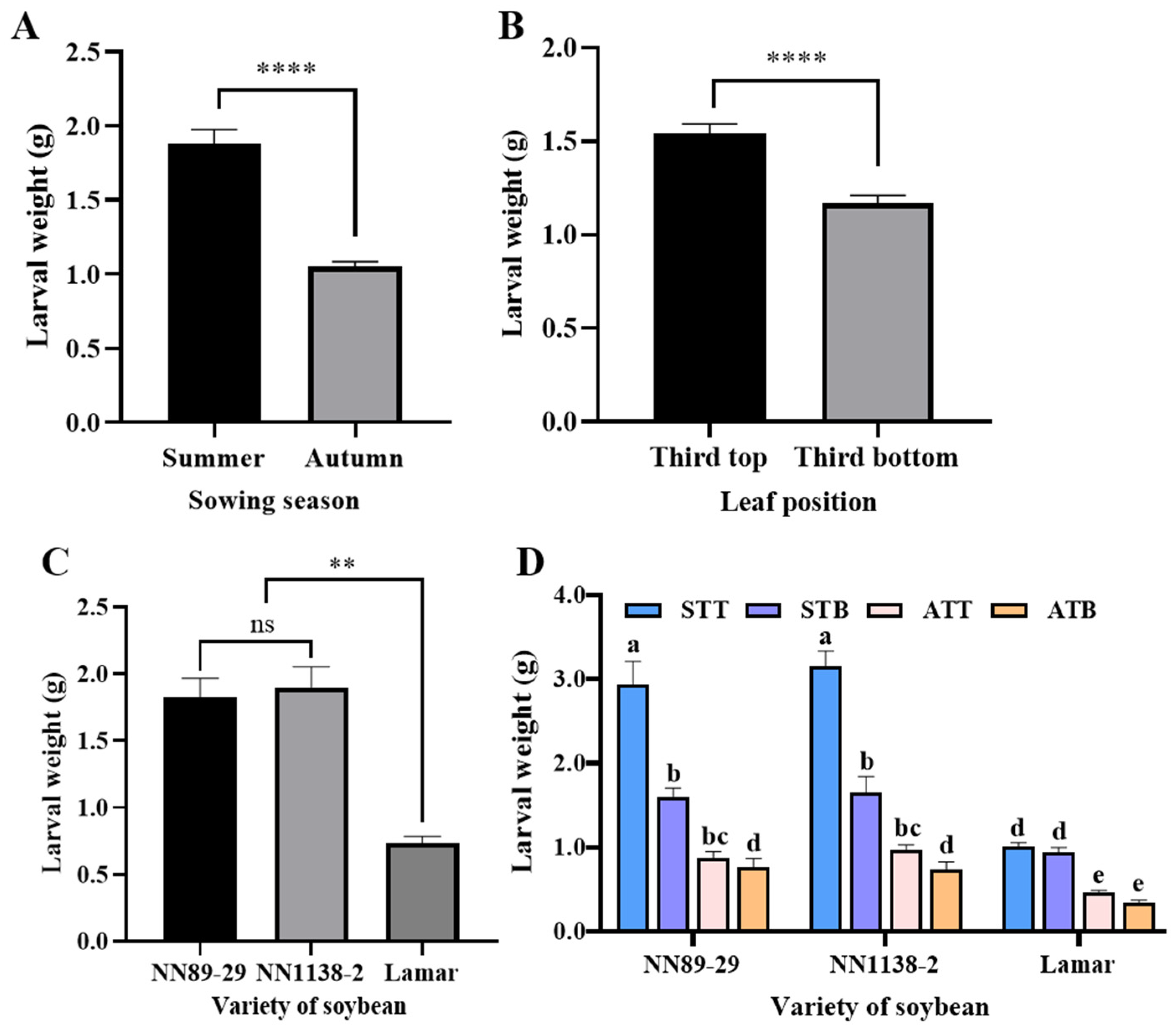
3.3.1. Effects of Different Sowing Seasons, Leaf Positions, and Soybean Varieties on 21-Day-Old CBT Larval Weight with Eggs Inoculated in Soybean V6 Stage
3.3.2. Effects of Different Growth Stages, Leaf Positions, and Soybean Varieties on 21-Day-Old CBT Larval Weight in Autumn Sowing
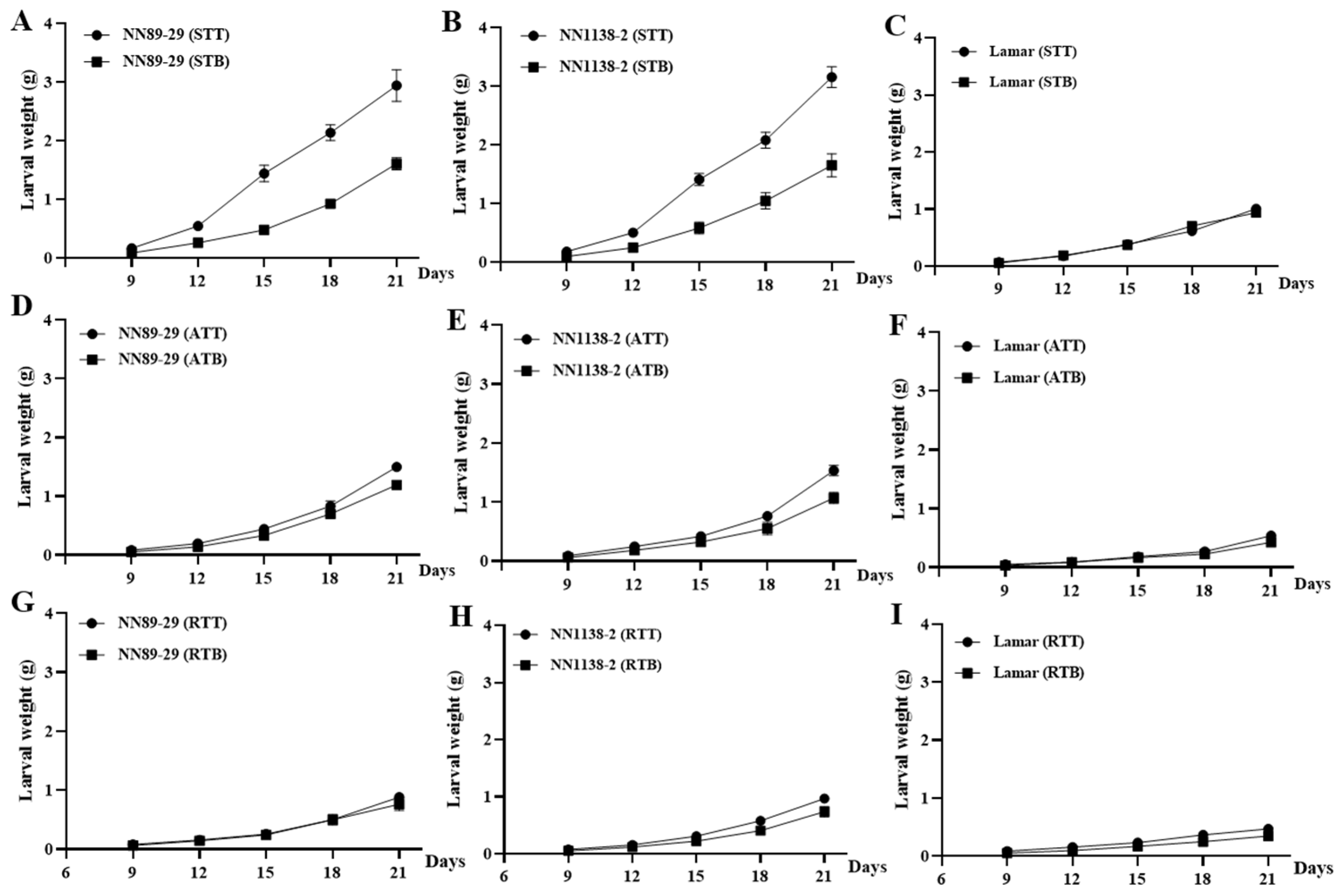
3.4. Trends of Different-Day-Old CBT Larval Weight of Each Treatment
3.5. Trends of Different Day-Old CBT Larval Survival Rate of Each Treatment
4. Discussion
4.1. Effect of Sowing Season on the Larval Growth of C. bilineata tsingtauica
4.2. Effect of Soybean Growth Stages on the Larval Growth of C. bilineata tsingtauica
4.3. Effect of Leaf Position on the Larval Growth of C. bilineata tsingtauica
4.4. Effect of Soybean Varieties on the Larval Growth of C. bilineata tsingtauica
4.5. Inspiration on the Evaluation of Soybean Varieties with Adaptability to C. bilineata tsingtauica
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerland, P.; Raftery, A.; Sevcikova, H.; Li, N.; Gu, D.; Spoorenberg, T.; Alkema, L.; Fosdick, B.; Chumn, J.; Lalic, N.; et al. World population stabilization unlikely this century. Science 2014, 346, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Huis, A.V.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Pliantiangtam, N.; Chundang, P.; Kovitvadhi, A. Growth performance, waste reduction efficiency and nutritional composition of black soldier fly (Hermetia illucens) larvae and prepupae reared on coconut endosperm and soybean curd residue with or without supplementation. Insects 2021, 12, 682. [Google Scholar] [CrossRef]
- Halloran, A.; Roos, N.; Eilenberg, J.; Cerutti, A.; Bruun, S. Life cycle assessment of edible insects for food protein: A review. Agron. Sustain. Dev. 2016, 36, 57. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor Larvae) as an alternative protein source for monogastric animal: A review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Hawkey, K.J.; Lopez-Viso, C.; Brameld, J.M.; Parr, T.; Salter, A.M. Insects: A potential source of protein and other nutrients for feed and food. Annu. Rev. Anim. Biosci. 2021, 9, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D. Complete nutrient content of four species of feeder insects. Zoo Biol. 2013, 32, 27–36. [Google Scholar] [CrossRef]
- Zhang, S.; Qiu, K.; Tan, Z.; Zhu, F. Research progress of insect vitamin. HuaZhong Insect Res. 2020, 16, 126–131. [Google Scholar]
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Nakane, W.; Nakamura, H.; Nakazato, T.; Kaminaga, N.; Nakano, M.; Sakamoto, T.; Nishiko, M.; Bono, H.; Ogiwara, I.; Kitano, Y.; et al. Construction of TUATinsecta database that integrated plant and insect database for screening phytophagous insect metabolic products with medicinal potential. Sci. Rep. 2020, 10, 17509. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.; Wilkinson, K.A.; Treilhou, M.; Tene, N.; Castillo, D.; Sauvain, M. Prospecting peptides isolated from black soldier fly (Diptera: Stratiomyidae) with antimicrobial activity against Helicobacter pylori (Campylobacterales: Helicobacteraceae). J. Insect Sci. 2019, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, Y.M. Advances of integrated utilization on resource insect Clanis bilineata tsingtauica. Guizhou Agric. Sci. 2009, 37, 111–113. [Google Scholar]
- Zou, S.W. History of Chinese Entomology; Science Press: Beijing, China, 1981; pp. 1–186. [Google Scholar]
- Sun, Z.; Pan, J.; Wang, K.; Li, Q.; Guo, M.; Zhang, G.; Chen, F. Current status and prospects of rearing Clanisb ilineata tsingtauica Mell. Agric. Dev. Equip. 2019, 5, 64–67. [Google Scholar]
- Wu, S.; Meng, X.; Chen, S. The analysis and evaluation of main nutrient components for Herse bilineata tsingtauica. J. Huaihai Inst. Tech. 2000, 9, 58–61. [Google Scholar]
- Yan, M.; Lu, C.; Zhang, G.; Li, H.; Nie, X.; Zheng, D.; Zheng, X. Research advance in the exploitation and utilization of Clanisbilineata tsingtauica Mell. J. Anhui Agric. Sci. 2008, 36, 874–876. [Google Scholar]
- Gao, Y.; Zhao, Y.; Xu, M.; Shi, S. Soybean hawkmoth (Clanis bilineata tsingtauica) as food ingredients: A review. CyTA J. Food 2021, 19, 341–348. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.; Xu, M.; Shi, S. Clanis bilineata tsingtauica: A sustainable edible insect resource. Sustainability 2021, 13, 12533. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Zhao, H.; Zhang, Y. Rearing Clanis bilineata in soybean field and its profit. Entomol. Know. 2002, 39, 30–33. [Google Scholar]
- Wu, S.; Xia, Z. Hight-yield culture techniques for Clanis bilineata Walker and its benefits analysis. J. Anhui Agric. Sci. 2008, 36, 14135–14136. [Google Scholar]
- Freitas, M.M.; Souza, B.H.S.; Nogueira, L.; Bello, M.M.D.; Junior, A.L.B. Soybean defense induction to Spodoptera cosmioides herbivory is dependent on plant genotype and leaf position. Arth. Plant Interact. 2018, 12, 85–96. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Gomez, J.D.; Pinheiro, V.J.M.; Silva, J.C.; Romero, J.V.; Merino-Cabrera, Y.M.; Coutinho, F.S.; Lourencao, A.L.; Serrao, J.E.; Vital, C.E.; Fontes, E.P.B.; et al. Leaf metabolic profiles of two soybean genotypes differentially affect the survival and the digestibility of Anticarsia gemmatalis caterpillars. Plant Physiol. Biochem. 2020, 155, 196–212. [Google Scholar] [CrossRef]
- Cui, Z.; Gai, J.; Ji, D.; Ren, Z. Evaluation of soybean germplasm for resistance to leaf-feeding insects. Soybean Sci. 1997, 16, 93–102. [Google Scholar]
- Yang, Y.; Xing, G.; Gai, J. Evaluation of antibiosis to common cutworm (Spodoptera litura) and screening for resistance sources among wild soybeans (Glycine soja) in China. Soybean Sci. 2016, 35, 448–454. [Google Scholar]
- Li, J.; Zhang, F.; Xing, G.; Gai, J. Influence of different defoliation rates at different growth stages to agronomic and quality traits of soybean cultivar NN99-6. Soybean Sci. 2018, 37, 715–722. [Google Scholar]
- Malabarba, J.; Meents, A.K.; Reichelt, M.; Scholz, S.S.; Peiter, E.; Rachowka, J.; Konopka-Postupolska, D.; Wilkins, K.A.; Davies, J.M.; Oelmuller, R.; et al. ANNEXIN1 mediates calcium-dependent systemic defense in Arabidopsis plants upon herbivory and wounding. New Phytol. 2021, 231, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Song, G. The Spatio-Tamporal Expression Difference of Bt Gene and Its Regulation in Different Leaf Postions of Bt Bollworm Resistant Cootton. Master’s Thesis, Northwest Sic-Tech University of Agriculture and Forestry, Yangling, China, 2003. [Google Scholar]
- Simmons, A.M.; Godfrey, L.D.; Yeargan, K.V. Ovipositional sites of the Potato Leafhopper (Homoptera: Cicadellidae) on vegetative stage soybean plants. Environ. Entomol. 1985, 14, 165–169. [Google Scholar] [CrossRef]
- Miyashita, T.; Kawanishi, K. Effects of rice leaf position and growth stage on larval development and survivorship of the rice leaf roller Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae). Jpn. J. Appl. Entomol. Zool. 2003, 47, 85–90. [Google Scholar] [CrossRef][Green Version]
- Rogge, S.A.; Meyhofer, R. Leaf age is important for assessment of resistance in chrysanthemum against Frankliniella occidentalis. J. Plant Dis. Prot. 2021, 128, 511–525. [Google Scholar] [CrossRef]
- Zhou, X.; Carter, N. Effects of temperature, feeding position and crop growth stage on the population dynamics of the rose grain aphid, Metopolophium-dirhodum (Hemiptera, Aphididae). Ann. Appl. Biol. 1992, 121, 27–37. [Google Scholar] [CrossRef]
- Terry, I.; Bradley, J.R., Jr.; van Duyn, J.W. Survival and development of Heliothis zea (Lepidoptera: Noctuidae) larvae on selected soybean growth stages. Environ. Entomol. 1987, 16, 441–445. [Google Scholar] [CrossRef]
- Xu, R.; Wang, C.; Li, W.; Zhang, L.; Xing, H. Advances in identification of the resistance of soybean to pests. Soybean Sci. 2007, 26, 771–774. [Google Scholar]
- Liu, X.; Yan, Y.; Liu, N.; Xu, Y.; Jiang, H.; Ye, Z.; Wang, H.; Gai, J.; Xing, G. Evaluation of rearing factors affecting Clanis bilineata tsingtauica Mell larvae fed by susceptible soybean variety NN89-29 in spring and autumn sowing. Insects 2023, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Villanuena, R.R.; Araneda, M.E.; Vela, M.; Seijo, J.C. Selecting stocking density in different climatic seasons: A decision theory approach to intensive aquaculture. Aquaculture 2013, 384, 25–34. [Google Scholar] [CrossRef]
- Guo, M.; Liao, H.; Deng, P.; Li, D.; Li, J.; Zhang, J.; Pan, J.; Chen, F. Effects of soybean varieties (lines) and planting density on survival and development of Clanis bilineata tsingtauica Mell larvae. J. Environ. Entomol. 2020, 42, 1401–1408. [Google Scholar]
- Xu, Y.; Ye, Z.; Xie, Z.; Zhang, D.; Liu, X.; Yan, Y.; Sun, L.; Chen, F.; Gai, J.; Xing, G. Comparison of trapping effects of different traps and monitoring the occurrence dynamics of Spodoptera litura in soybean fields of Dangtu, Anhui Province, China. Agrononmy 2023, 13, 47. [Google Scholar] [CrossRef]
- Xing, G.; Liu, K.; Gai, J. A high-throughput phenotyping procedure for evaluation of antixenosis against common cutworm at early seedling stage in soybean. Plant Methods 2017, 13, 66. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Fordonski, G.; Okorski, A.; Olszewski, A.; Dabrowska, J.; Pszczolkowska, A. The effect of sowing date on the growth and yield of soybeans cultivated in north-eastern poland. Agriculture 2023, 13, 2199. [Google Scholar] [CrossRef]
- Ludwig, D.; Anderson, J.M. Effects of different humidities, at various temperatures, on the early development of four saturniid moths (Platysamia cecropia linnaeus, Telea polyphemus cramer, Samia walkeri felder and felder, and Callosamia promethea drury), and on the weights and water contents of their larvae. Ecol. Soc. Am. 1942, 23, 259–274. [Google Scholar]
- Eberle, S.; Schaden, L.M.; Tintner, J.; Stauffer, C.; Schebeck, M. Effect of temperature and photoperiod on development, survival, and growth rate of mealworms, Tenebrio molitor. Insects 2022, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Nico, M.; Miralles, D.J.; Kantolic, A.G. Natural post-flowering photoperiod and photoperiod sensitivity: Roles in yield-determining processes in soybean. Field Crop. Res. 2019, 231, 141–152. [Google Scholar] [CrossRef]
- Lin, P.A.; Paudel, S.; Afzal, A.; Shedd, N.L.; Felton, G.W. Changes in tolerance and resistance of a plant to insect herbivores under variable water availability. Environ. Exp. Bot. 2021, 183, 104334. [Google Scholar] [CrossRef]
- Xue, J.; Gou, L.; Zhao, Y.; Yao, M.; Yao, H.; Tian, J.; Zhang, W. Effects of light intensity within the canopy on maize lodging. Field Crop. Res. 2016, 188, 133–141. [Google Scholar] [CrossRef]
- Shan, F.; Sun, K.; Gong, S.; Wang, C.; Ma, C.; Zhang, R.; Yan, C. Effects of shading on the internode critical for soybean (Glycine max) lodging. Agronomy 2022, 12, 492. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, G.; Jin, Q.; Lv, B.; Peng, Z.; Jin, T.; Wen, H. Effect of temperature on developmental duration and feeding amount of Clanis bilineata tsingtauica. Chin. J. Trop. Crop. 2014, 35, 2442–2444. [Google Scholar]
- Hu, Z.; Xu, X.; Pan, L.; Li, M.; Zeng, J.; Muhammad, K.R.; Xing, G.; Gai, J. Resistance analyses of soybean organs to common cutworm (Spodoptera litura) at different reproductive stages. Soybean Sci. 2020, 39, 932–939. [Google Scholar]
- Yang, L.; Hu, X.P.; Golec, J.R.; Zeng, X. Effects of legume species and plant growth stage on attraction, fecundity, and development of the Kudzu Bug (Heteroptera: Plataspidae). J. Econ. Entomol. 2018, 111, 2217–2224. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, J.S.; Park, S.; Kim, Y.J.; Mani, V.; Lee, K.; Kwon, S.J.; Park, S.U.; Kim, J.K. Metabolite changes in soybean (Glycine max) leaves during the entire growth period. ACS Omega 2023, 8, 41718–41727. [Google Scholar] [CrossRef]
- Reynolds, G.W.; Smith, C.M. Effect of leaf position, leaf wounding and plant age of two soybean genotypes on soybean looper (Lepidoptera: Noctuidae) growth. Environ. Entomol. 1985, 14, 475–478. [Google Scholar] [CrossRef]
- Power, N.A.; Ganjisaffar, F.; Perring, T.M. Effect of temperature on the survival and developmental rate of immature Ooencyrtus mirus (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2020, 113, 1675–1684. [Google Scholar] [CrossRef]
- Wang, L.; Yi, J.; Zhu, S.; Li, B.; Chen, Y.; Shen, W.; Wang, W. Identification of a single-nucleocapsid baculovirus isolated from Clanis bilineata tsingtauica (Lepidoptera: Sphingidae). Arch. Virol. 2008, 153, 1557–1561. [Google Scholar] [CrossRef]
- Souza, T.D.; Fernandes, F.O.; Sanches, A.C.; Nascimenyo, J.D.; Pinto, A.A.; Rpolanczyk, R.A. Relation between Helicoverpa armigera (Hubner) (Lepidoptera/Noctuidae) mortality and entomopathogenic fungi persistence in soybean leaflets. Egypt. J. Biol. Pest Control 2022, 32, 10. [Google Scholar] [CrossRef]
- Adie, M.M.; Krisnawati, A.; Baliadi, Y. Evaluation for soybean resistance to armyworm Spodoptera litura (Lepidoptera: Noctuidae). IOP Conf. Ser. Earth Environ. Sci. 2020, 484, 012020. [Google Scholar] [CrossRef]
- Smith, C.M.; Gilman, D.F. Comparative resistance of multiple insect-resistant soybean genotypes to the soybean looper. J. Econ. Entomol. 1981, 74, 400–403. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B.; Gui, F.; Qin, Y.; Deng, P.; Liao, H. Nutritional and feeding adaptability of Clanis bilineata tsingtauica larvae to different cultivars of soybean, (Glycine max). Foods 2023, 12, 1721. [Google Scholar] [CrossRef] [PubMed]




| Source of Variation | DF | LW9 | LW12 | LW15 | LW18 | LW21 | LSR12 | LSR15 | LSR18 | LSR21 |
|---|---|---|---|---|---|---|---|---|---|---|
| SS | 1 | 37.2 ** | 178.4 ** | 126.3 ** | 169.7 ** | 119.3 ** | 2.5 | 1.8 | 5.8 * | 0.2 |
| LP | 1 | 34.2 ** | 28.1 ** | 66.6 ** | 63.1 ** | 67.9 ** | 9.8 * | 11.7 * | 7.0 * | 4.9 * |
| V | 2 | 18.5 ** | 16.6 ** | 42.1 ** | 73.9 ** | 93.3 ** | 3.5 * | 0.8 | 0.7 | 1.7 |
| SS × LP | 1 | 5.3 * | 15.3 * | 41.2 ** | 32.9 ** | 19.7 ** | 6.2 * | 13.5 * | 13.3 * | 9 * |
| SS × V | 2 | 2.5 | 9.5 * | 10.1 * | 7.5 * | 5.6 * | 1.5 | 0.7 | 0.2 | 0.9 |
| LP × V | 2 | 3.3 * | 6.9 * | 15.8 ** | 18.2 ** | 12.9 ** | 0.2 | 1.7 | 0.2 | 2.8 |
| SS × LP × V | 2 | 1.2 | 5.5 * | 10.9 ** | 12.6 ** | 5.6 * | 1.1 | 3.4 * | 4.9 * | 1.9 |
| Error | 24 |
| Source of Variation | DF | LW9 | LW12 | LW15 | LW18 | LW21 | LSR12 | LSR15 | LSR18 | LSR21 |
|---|---|---|---|---|---|---|---|---|---|---|
| GS | 1 | 2.9 | 4.8 * | 19.9 ** | 13.5 * | 81.6 ** | 3.1 | 3.6 | 1.8 | 6.6 * |
| LP | 1 | 36.4 ** | 17.2 ** | 14.7 * | 10.4 * | 34.4 ** | 13.3 * | 25.2 ** | 16.9 ** | 15.9 * |
| V | 2 | 5.5 * | 20.1 ** | 28.3 ** | 41.3 ** | 119.6 ** | 1.6 | 0.6 | 0.2 | 0.3 |
| GS × LP | 1 | 0.08 | 0.1 | 0.3 | 0.1 | 3.1 | 2.6 | 1.9 | 1.3 | 0.6 |
| GS × V | 2 | 7.3 * | 12.6 ** | 9.6 * | 7.9 * | 13.4 ** | 1.3 | 0.4 | 0.3 | 1.3 |
| LP × V | 2 | 0.03 | 0.3 | 0.7 | 1.2 | 3.1 | 1.4 | 1.5 | 0.5 | 0.6 |
| GS × LP × V | 2 | 1.6 | 2.9 | 1.7 | 0.7 | 0.9 | 0.2 | 1.3 | 2.6 | 3.7 * |
| Error | 24 |
| Indicator | LW9 | LW12 | LW15 | LW18 | LW21 | LSR12 | LSR15 | LSR18 | LSR21 |
|---|---|---|---|---|---|---|---|---|---|
| LW9 | 0.64 ** | 0.57 ** | 0.42 ** | 0.39 ** | 0.29 * | 0.33 * | 0.32 * | 0.30 * | |
| LW12 | 0.74 ** | 0.77 ** | 0.67 ** | 0.65 ** | 0.01 | 0.13 | 0.14 | 0.14 | |
| LW15 | 0.79 ** | 0.86 ** | 0.78 ** | 0.75 ** | 0.15 | 0.04 | 0.06 | 0.08 | |
| LW18 | 0.76 ** | 0.86 ** | 0.92 ** | 0.85 ** | 0.04 | −0.04 | −0.03 | −0.01 | |
| LW21 | 0.71 ** | 0.77 ** | 0.84 ** | 0.92 ** | 0.12 | 0.01 | 0.01 | −0.01 | |
| LSR12 | 0.19 * | 0.20 * | 0.25 * | 0.23 * | 0.25 * | 0.73 ** | 0.73 ** | 0.54 ** | |
| LSR15 | 0.15 | 0.16 | 0.21 * | 0.17 | 0.19 * | 0.70 ** | 0.81 ** | 0.75 ** | |
| LSR18 | 0.12 | 0.16 | 0.17 | 0.14 | 0.15 | 0.54 ** | 0.83 ** | 0.90 ** | |
| LSR21 | 0.11 | 0.11 | 0.15 | 0.13 | 0.13 | 0.47 ** | 0.68 ** | 0.72 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Yan, Y.; Yang, L.; Xu, Y.; Jiang, H.; Ye, Z.; Wang, H.; Gai, J.; Xing, G. Effect of Different Sowing Seasons, Growth Stages, Leaf Positions, and Soybean Varieties on the Growth of Clanis bilineata tsingtauica Mell Larvae. Agronomy 2024, 14, 397. https://doi.org/10.3390/agronomy14020397
Liu N, Yan Y, Yang L, Xu Y, Jiang H, Ye Z, Wang H, Gai J, Xing G. Effect of Different Sowing Seasons, Growth Stages, Leaf Positions, and Soybean Varieties on the Growth of Clanis bilineata tsingtauica Mell Larvae. Agronomy. 2024; 14(2):397. https://doi.org/10.3390/agronomy14020397
Chicago/Turabian StyleLiu, Nan, Yulu Yan, Longwei Yang, Yufei Xu, Huiyan Jiang, Zhihao Ye, Hao Wang, Junyi Gai, and Guangnan Xing. 2024. "Effect of Different Sowing Seasons, Growth Stages, Leaf Positions, and Soybean Varieties on the Growth of Clanis bilineata tsingtauica Mell Larvae" Agronomy 14, no. 2: 397. https://doi.org/10.3390/agronomy14020397
APA StyleLiu, N., Yan, Y., Yang, L., Xu, Y., Jiang, H., Ye, Z., Wang, H., Gai, J., & Xing, G. (2024). Effect of Different Sowing Seasons, Growth Stages, Leaf Positions, and Soybean Varieties on the Growth of Clanis bilineata tsingtauica Mell Larvae. Agronomy, 14(2), 397. https://doi.org/10.3390/agronomy14020397









