Assessment of the Allelopathic Activity of Various Parts of Platycodon (Platycodon grandiflorus) and Its Mitigation by Activated Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Cultivation and Collection
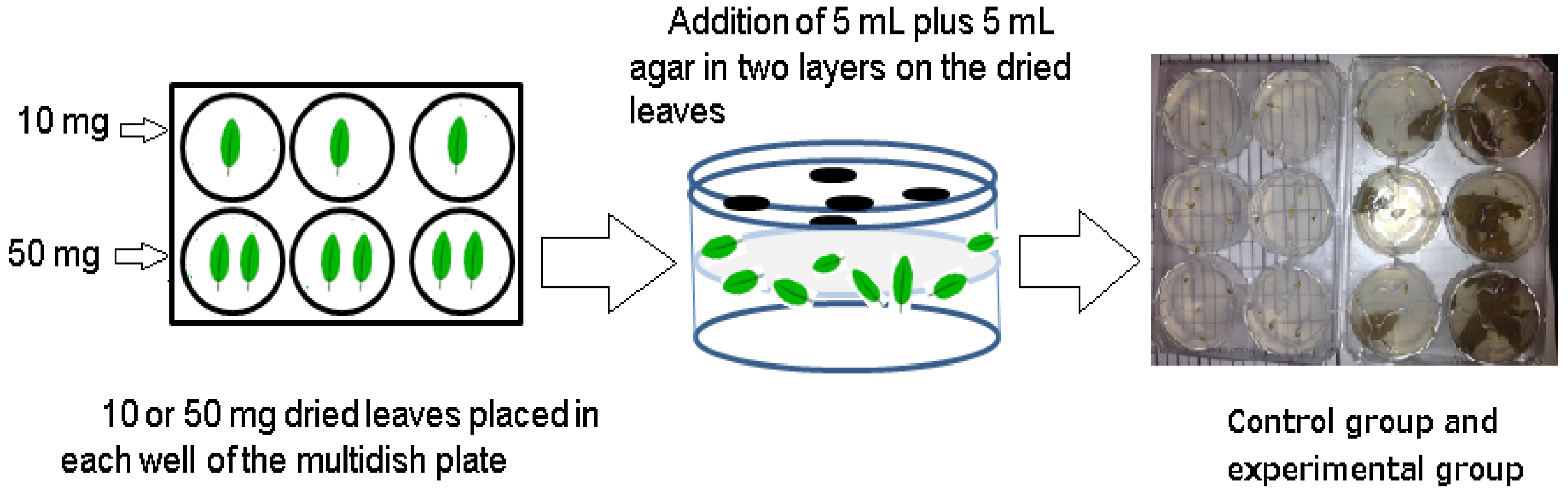
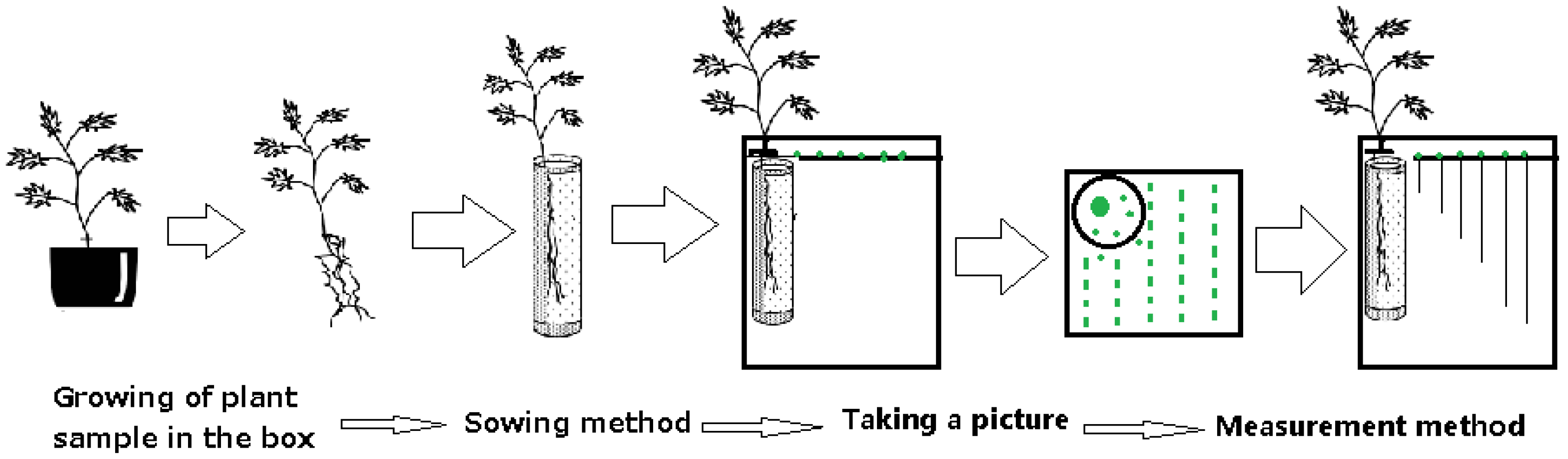
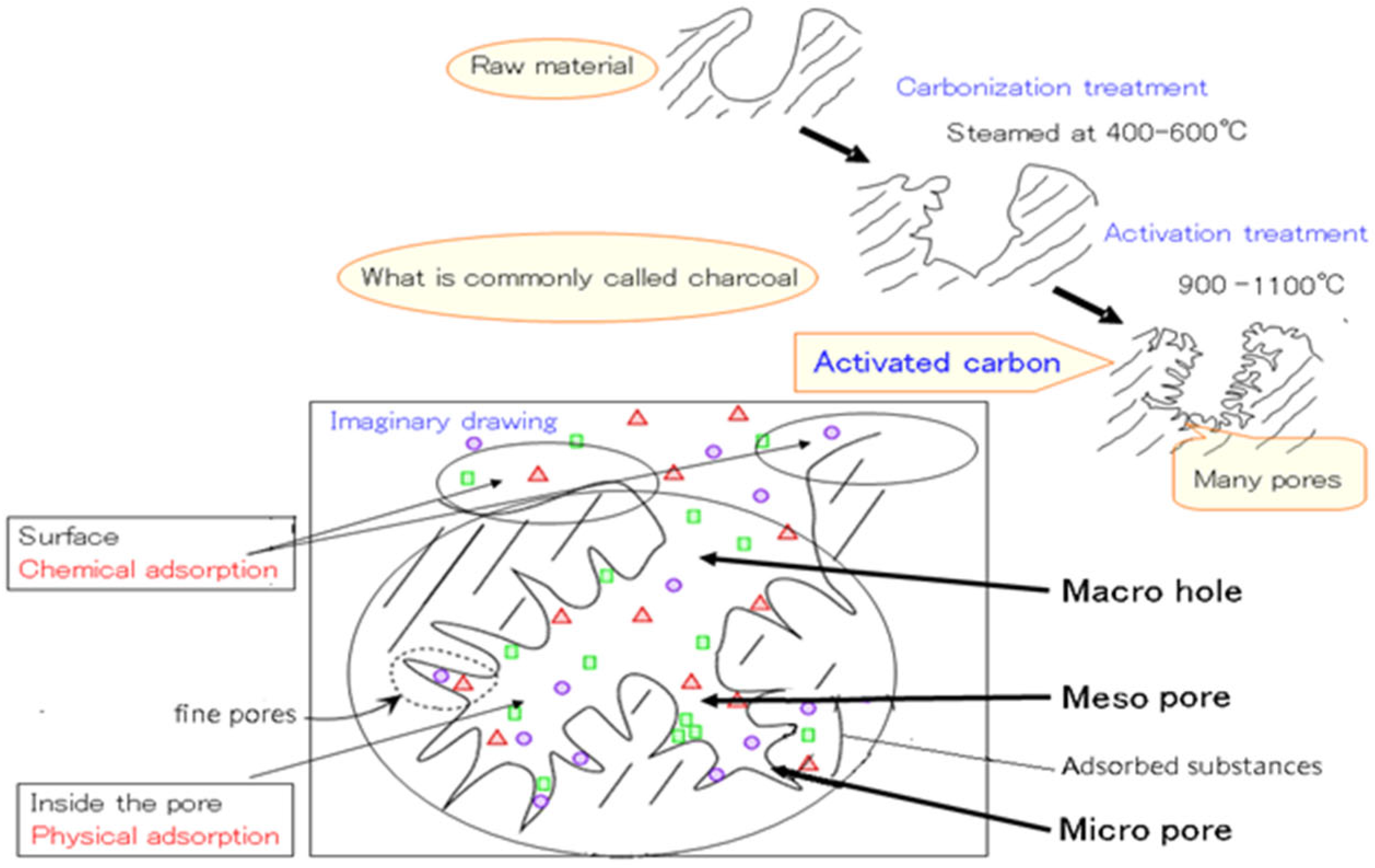
2.2. Experimental Methods
2.3. Statistical Analysis
3. Results
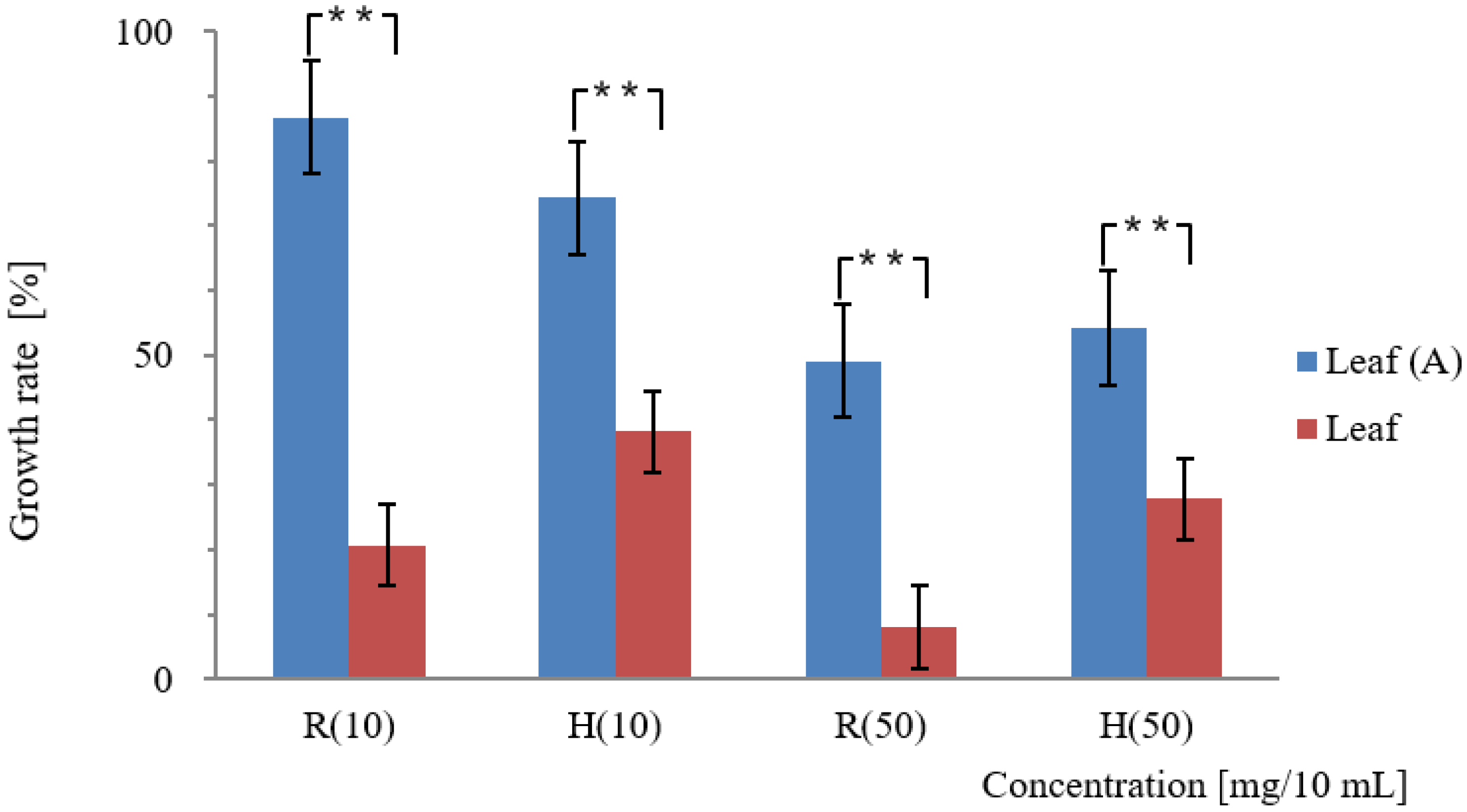
3.1. Bioassay Tests for Assessing the Allelopathic Activity of Platycodon Parts at Different Ages
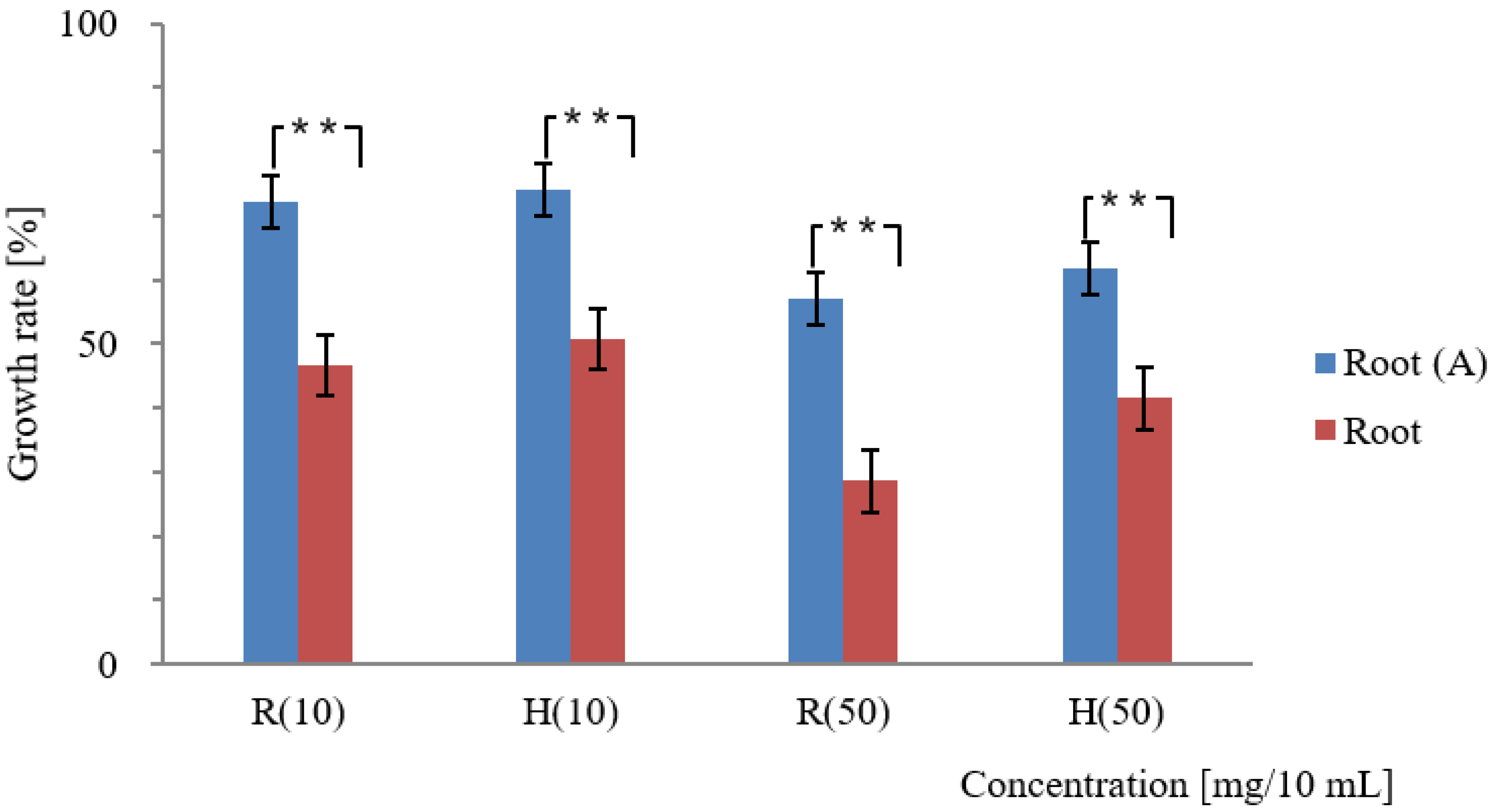
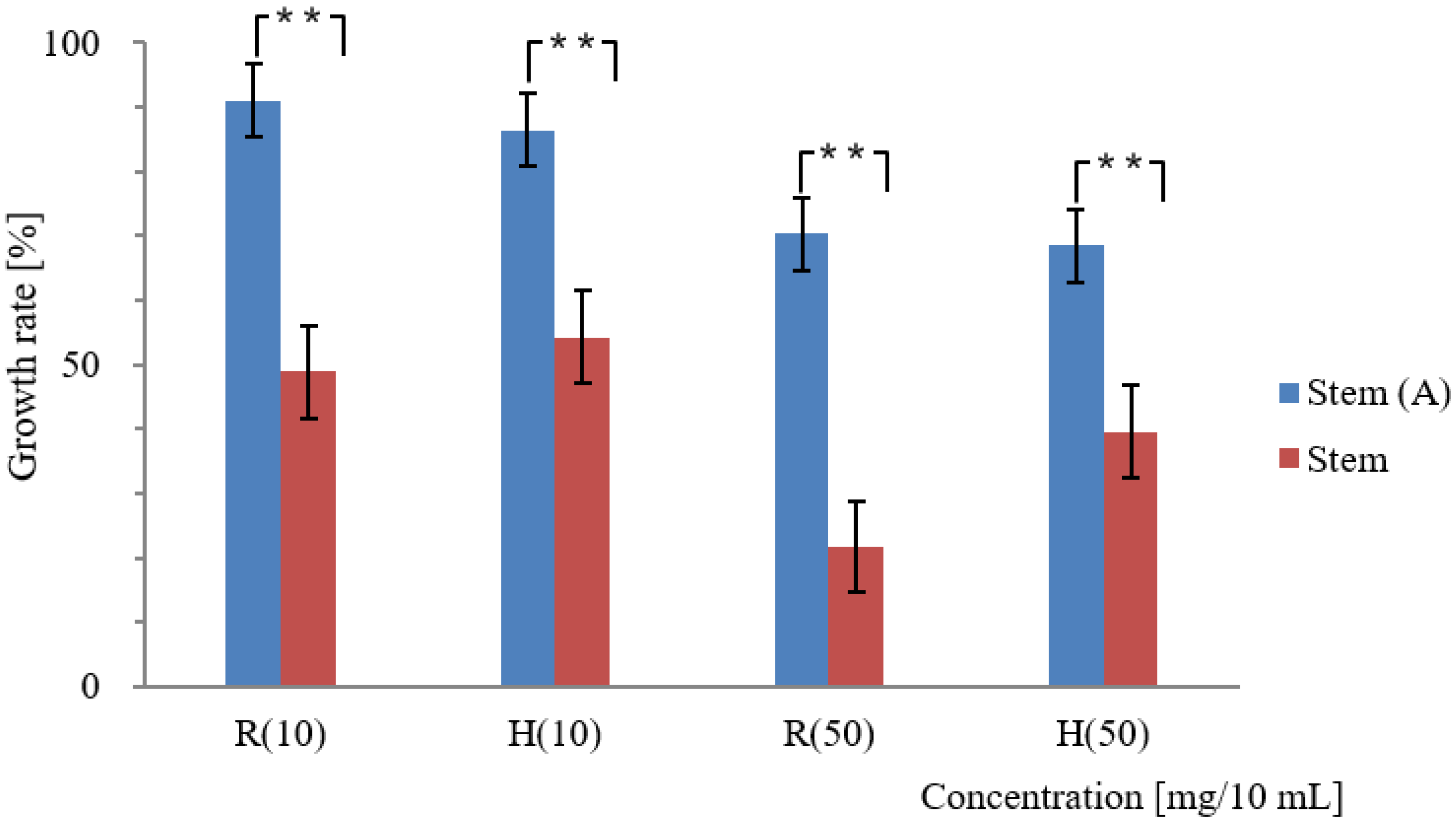
3.2. Effect of Activated Carbon on the Adsorption of Allelopathic Substances from Various Platycodon Components
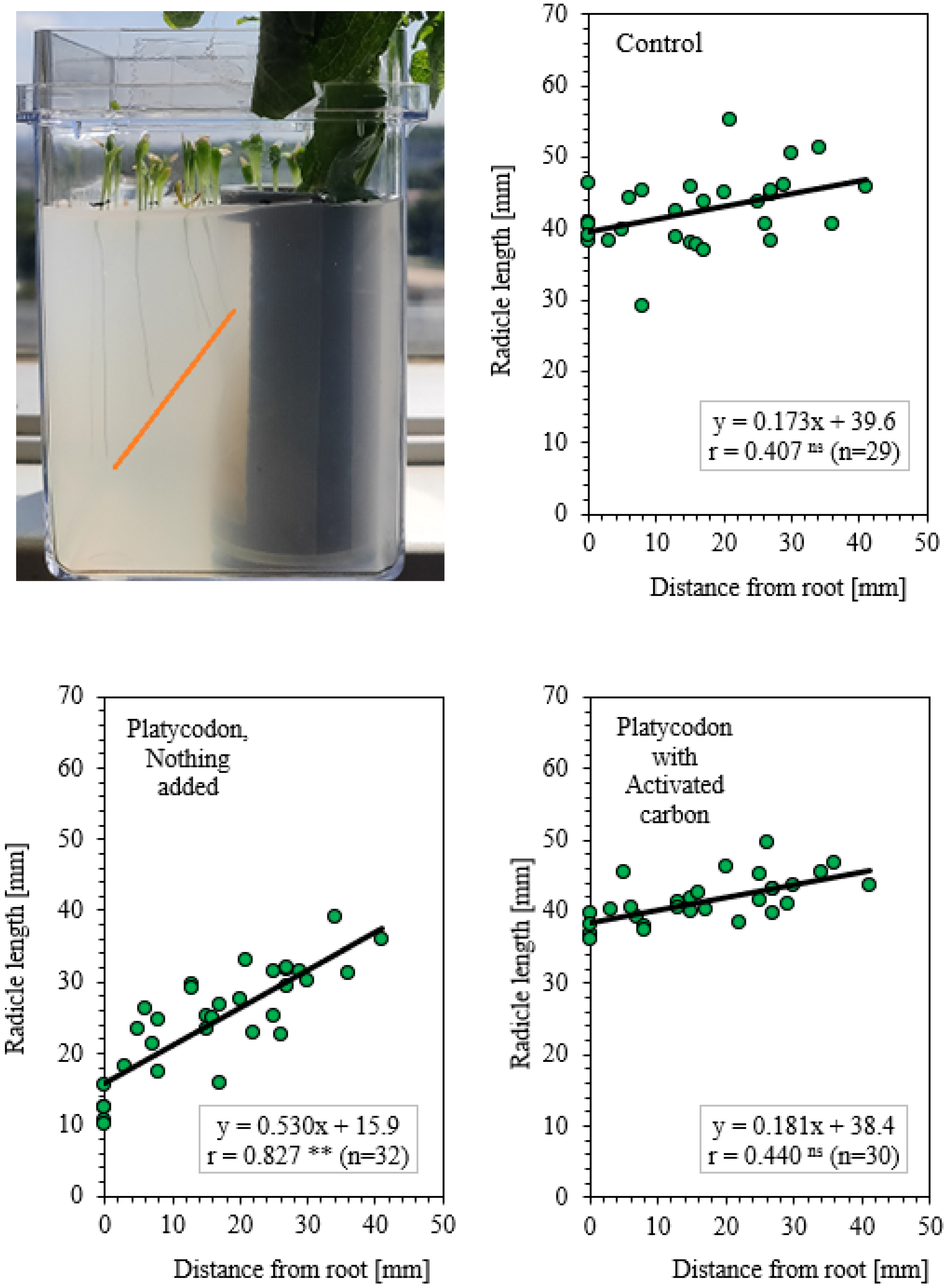
3.3. Plant Box Method: Allelopathic Activity of Root Secretions from Platycodon
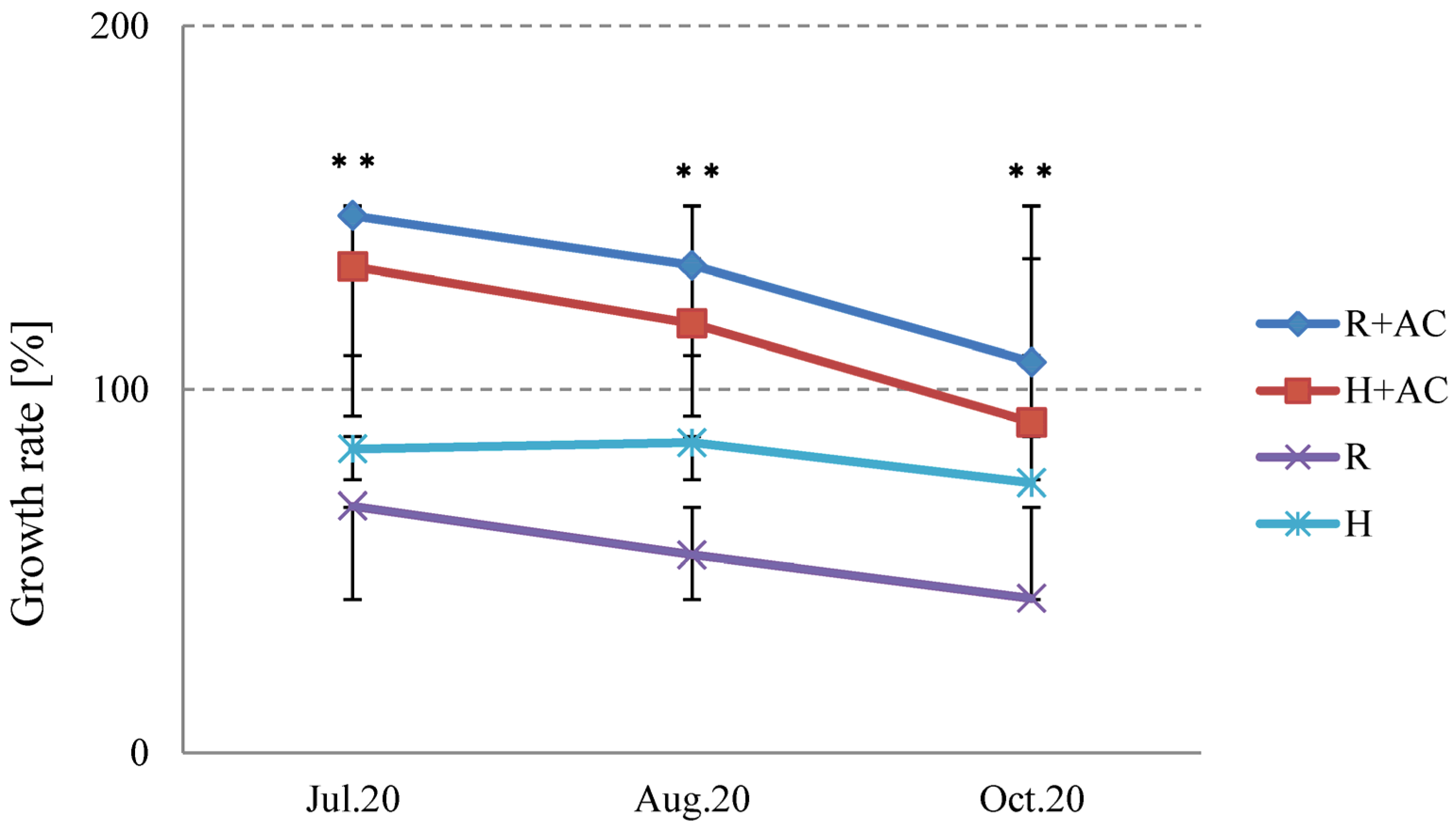
3.4. Effect of Activated Carbon on the Adsorption of Allelopathic Substances from Platycodon Roots
4. Discussion
4.1. Allelopathic Activity of Various Platycodon Components of Different Ages
4.2. Adsorption Effect of Activated Carbon and Its Impact on the Allelopathic Activity of Various Platycodon Components
4.3. Allelopathic Activity of Root Exudation of Platycodon and its Role as an Obstacle to Continuous Cropping
4.4. Adsorption Effect of Activated Carbon on the Root Secretion Allelopathy of Platycodon
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flora of China. 1983, Volume 73, p. 77. Available online: https://www.iplant.cn/info/Platycodon%20grandiflorus?t=z (accessed on 9 October 2023).
- Saeki, T.; Nikaido, T. Evaluations of saponin properties of HPLC analysis of Platycodon grandiforum. J. Pharm. Sci. 2003, 123, 431–441. [Google Scholar]
- Wei, J.H.; Tu, P.F.; Li, G.; Wang, W.Q.; Wang, W.Q.; Yang, C.M.; Sui, C. Situation and Trends in Development of Chinese Medicinal Agriculture in China. Mod. Chin. Med. 2015, 17, 94–104. [Google Scholar]
- Yong-Sook, K. Effects of Platycodon grandiflorum Feeding on Serum and liver Lipid Concentrations in Rats with Diet-induced Hyperlipidemia. J. Nutr. Sci. Vitaminol. 1995, 41, 489–491. [Google Scholar]
- Wei, S.Z. Comprehensive development and utilization of platycodon resources. J. Shaanxi Univ. Sci. Technol. 2005, 23, 146–148. [Google Scholar]
- Li, G.Q.; Bi, Y.W.; Chen, B.F.; Zhang, J.; Han, J.L.; Wang, Z.F. Research Advancesin Cultivationof Medicinal Plant Platycodon grandiflorum. J. Agric. 2016, 6, 55–59. [Google Scholar]
- Zhang, Z.Y.; Lin, W.X. The allelopathic autotoxicity and consecutive cropping obstacles of medicinal plants. J. Chin. Ecol. Agric. 2009, 17, 189–196. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Wang, W.Q. The formation mechanism and prevention of continuous cropping obstacles of medicinal plants. China Agric. Sci. Technol. Her. 2009, 11, 19–23. [Google Scholar]
- Sun, M.; Ye, L.Q.; Zhang, Z.L. Research progress on the causes and control of continuous cropping disorders of Panax Notoginseng. J. Mt. Agric. Biol. 2015, 34, 63–67. [Google Scholar]
- Zhu, L.X.; Huo, X.H.; Sun, H.X.; Bi, S.; Ma, W.W. Effects of continuous Platycodon grandiflorus cultivation on soil physical-chemical and biological properties. China J. Soil Water Conserv. 2013, 27, 177–181. [Google Scholar]
- Zhu, L.X.; Wang, J.H. Effects of controlled-release fertilizers on campanulaceae growth. China J. Appl. Ecol. 2010, 21, 2304. [Google Scholar]
- Bao, L.; Bai, M.R.; A, D.Q.; Fujii, Y. Screening of allelopathic activity of plants growing in the desert area of Inner Mongolia Autonomous Region and discovery of Sea Buckthorn and Sweet annie as highly active plants and estimation of their active substances. Jpn. J. Arid Land Stud. 2019, 29, 1–10. [Google Scholar]
- Molisch, H. Der Einfluss Einer Pflanze auf die Andere-Allelopathie; Fischer: Jena, Germany, 1937. [Google Scholar]
- Tukey, H.B., Jr. Implicattions of allelopathy in agricultural plant science. Bot. Rev. 1969, 35, 1–16. [Google Scholar] [CrossRef]
- Vives-peris, V.; De Ollas, C.; Gomez-cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Wu, L.K.; Lin, X.M.; Lin, W.X. Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates. Chin. J. Plant Ecol. 2014, 38, 298–310. [Google Scholar]
- Wu, H.M.; Lin, W.X. A commentary and development perspective on consecutive monoculture problems of medicinal plants. China J. Eco-Agric. 2020, 28, 775–793. [Google Scholar]
- Zang, A.H.; Gao, Y.; Xu, Y. Research progress on allelopathic effects of medicinal plants in China. China J. Chin. Herb. Med. 2011, 42, 1885–1890. [Google Scholar]
- Wang, R.; Dong, L.L.; Xu, J.; Chen, J.W.; Li, X.W.; Chen, S.L. Progress in improving continuous monoculture cropping issues in Panax ginseng by controlling soil-borne diseases. China J. Chin. Mater. Medica 2016, 41, 3890–3896. [Google Scholar]
- An, Y.; Yang, D.; Li, X.; Jin, X.J. Study on the effect and physiological mechanism of continuous cropping obstruction of Pinellia ternata. Acta Agric. Borealioccidentalis Sin. 2018, 27, 1017–1022. [Google Scholar]
- Dai, L.; Singh, S.K.; Gong, H.; Tang, Y.; Peng, Z.; Zhang, J.; Wu, D.; Zhang, H.; He, D. Rhizospheric microbial consortium of Lilium lancifolium Thunb. causes lily root rot under continuous cropping system. Front. Microbiol. 2022, 13, 981615. [Google Scholar] [CrossRef]
- Gu, L.; Niu, M.M.; Zheng, H.Y.; Wang, J.M.; Wu, L.K.; Li, Z.F.; Zhang, Z.Y. Effect of continuous cropping of Rehmannia on its morphological and physiological characteristics. J. Chin. Med. Mater. 2013, 36, 691–695. [Google Scholar]
- Tan, Y.; Cui, Y.; Li, H.; Kuang, A.; Li, X.; Wei, Y.; Ji, X. Rhizospheric soil and root endogenous fungal diversity and composition in response to continuous Panax notoginseng cropping practices. Microbiol. Res. 2017, 194, 10–19. [Google Scholar] [CrossRef]
- Liu, H.; Niu, M.; Zhu, S.; Zhang, F.; Liu, Q.; Liu, Y.; Liu, R.H.; Zhang, Y.P. Effect study of continuous monoculture on the quality of Salvia miltiorrhiza Bge roots. BioMed Res. Int. 2020, 2020, 4284385. [Google Scholar] [CrossRef]
- Wu, H.; Wu, L.; Zhu, Q.; Wang, J.; Qin, X.; Xu, J.; Kong, L.; Chen, J.; Lin, S.; Umar, K.M.; et al. The role of organic acids on microbial deterioration in the Radix pseudostellariae rhizosphere under continuous monoculture regimes. Sci. Rep. 2017, 7, 3497. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, S.; Qin, J.; Dai, J.; Zhao, F.; Gao, L.; Lian, X.; Shang, W.; Xu, X.; Hu, X. Changes in the microbiome in the soil of an American ginseng continuous plantation. Front. Plant Sci. 2020, 11, 572199. [Google Scholar] [CrossRef]
- Nishio, M. The current situation and causes of continuous cropping disorders. Jpn. J. Chem. Biol. 1985, 23, 582–589. [Google Scholar]
- Zhang, Y.Y.; Zhu, D.W.; Wang, X.; Zhang, L.Z.; Shi, G.Y.; Zhou, J.; Liu, W. Properties of plant root exudates and obstacles for continuous cropping of medicinal plants. China J. Hubei Agric. Sci. 2014, 53, 1441–1444. [Google Scholar]
- Qu, Y.T.; Zhang, Q.Q.; Yu, Y.F.; Duyxanale, S.; Cai, L.L.; Zhang, S.J.; Li, Y.F.; Li, Y.C. Research advances on mechanisms and preventions of the medicinal plants’ continuous cropping obstacles from the perspective of rhizosphere microecology. J. Zhejiang Univ. (Agric. Life Sci.) 2022, 48, 403–414. [Google Scholar]
- Huang, Y.F.; Zhang, E.H.; Zhang, X.H.; Wang, H.Z.; Wang, Q.; Liu, Q.L.; Cui, J.J.; Xu, S.R. Autotoxicity of water extracts from continuous cropping soil of Lilium davidii var. unicolor Salisb. J. Northwest AF Univ. (Nat. Sci. Ed.) 2020, 48, 84–93. [Google Scholar]
- Liu, R.X.; Li, S.B.; Zhang, R.E.; Nie, C.P.; Li, Y.Y. Allelopathic effects of Platycodon aqueous extract on wheat seedlings. China J. Biol. 2014, 31, 28–32. [Google Scholar]
- Gao, Z.R.; Zang, G.P.; Su, C.C.; Wang, Z.L.; Shi, Z.G.; Gong, Y.F.; Wei, Y.J. Research progress on the formation mechanism and mitigation measures of continuous cropping obstacles in medicinal plants. China J. Anhui Agric. Sci. 2023, 51, 21–25. [Google Scholar]
- Nishihara, E.; Motoki, S. Agricultural use of activated carbon—New technology for soil purification, Nature and Science and Technology Series. Jpn. Rural Cult. Assoc. 2009, 10–11. [Google Scholar]
- Asao, T.; Hasegawa, Y.; Sueda, K.; Tomita, K.; Taniguchi, T. Autotoxicity of root exudates from taro. Jpn. J. Sci. Hortic. 2003, 97, 389–396. [Google Scholar] [CrossRef]
- Asao, T.; Ohba, T.; Tomita, K.; Ota, K.; Hosoki, T. Effects of adding activated carbon to culture media with different dissolved oxygen concentrations for hydroponically grown cucumbers on plant growth and the number of harvested fruits. Jpn. J. Hortic. Soc. 1999, 68, 1194–1196. [Google Scholar] [CrossRef]
- Motoki, S.; Nishihara, E.; Kitazawa, H.; Kyutoku, Y.; Uehara, T.; Yagasaki, K.; Sakai, H.; Shigemori, I. Effects of the application of activated carbon on soybean growth and yield in continuous legume fields. Jpn. J. Crop Sci. Soc. 2012, 81, 77–82. [Google Scholar] [CrossRef]
- Nishihara, E.; Takahashi, S.; Hirata, T.; Nakano, T. Utilization of activated carbon for allelopathy avoidance technology in fava beans. Kindergart. Sch. Misc. 2006, 75, 569. [Google Scholar]
- Fujii, Y. Establishment of an allelopathy assay method and the function of the active substance L-DOPA contained in Mucuna. Jpn. Agric. Environ. Res. Inst. Rep. 1994, 10, 115–218. [Google Scholar]
- Fujii, Y.; Shibuya, T. Establishment of an activity evaluation method specific to allelopathy—Search for candidate plants by a mixed planting test in agar medium using a plant box. Jpn. J. Weed Res. 1992, 37, 156–157. [Google Scholar]
- Motoki, S.; Nishihara, E.; Takahashi, N.; Limbers, H.; Shinohara, Y. Effects of astivated carbon to reduce allelopathy during raising the seedling stage. Jpn. J. Hortic. Res. 2007, 6, 603–609. [Google Scholar]
- Zhai, Z.G. Yellow pines become green, chestnut production increases, etc. Research progress on plant root exudates. Jiangxi J. Agric. 2012, 24, 28–32. [Google Scholar]
- Zhu, L.X.; Bi, S.; Liu, Y. Preliminary study on the autotoxicity of Platycodon grandiflorum. China J. Lishizhen Med. Med. Res. 2013, 24, 1992–1994. [Google Scholar]
- Wang, Y.P.; Wang, H.T. Allelopathic substances secreted by plant roots and their environment in soil Behavior. J. Soil Bull. 2010, 41, 502–507. [Google Scholar]
| 10 mg | 50 mg | ||||||
|---|---|---|---|---|---|---|---|
| Date | Part | R | H | G | R | H | G |
| 20 July | Leaf | 26.1 ± 1.3 | 43.8 ± 1.4 | 100 ± 0.0 | 11.9 ± 1.0 | 38.9 ± 1.7 | 86.7 ± 11.5 |
| Root | 58.8 ± 0.7 | 56.9 ± 0.3 | 100 ± 0.0 | 29.1 ± 0.5 | 47.4 ± 0.3 | 100 ± 0.0 | |
| Stem | 77.3 ± 2.2 | 94.6 ± 2.5 | 100 ± 0.0 | 22.8 ± 1.3 | 45.8 ± 1.4 | 93.3 ± 8.9 | |
| 20 August | Leaf | 20.2 ± 1.3 | 34.9 ± 1.6 | 93.3 ± 8.9 | 7.6 ± 1.4 | 31.4 ± 2.3 | 86.7 ± 10.9 |
| Root | 57.4 ± 1.4 | 64.1 ± 1.1 | 100 ± 0.0 | 48.9 ± 0.7 | 60.0 ± 0.6 | 100 ± 0.0 | |
| Stem | 53.1 ± 1.7 | 67.6 ± 1.9 | 100 ± 0.0 | 22.5 ± 1.5 | 40.0 ± 2.0 | 100 ± 0.0 | |
| 20 October | Leaf | 15.6 ± 1.6 | 35.8 ± 2.1 | 100 ± 0.0 | 4.6 ± 0.5 | 13.1 ± 0.7 | 93.3 ± 8.9 |
| Root | 23.6 ± 3.5 | 31.0 ± 2.6 | 93.3 ± 8.9 | 7.7 ± 0.7 | 17.0 ± 0.9 | 100 ± 0.0 | |
| Stem | 30.5 ± 3.4 | 45.2 ± 3.1 | 100 ± 0.0 | 19.9 ± 2.5 | 33.1 ± 2.5 | 100 ± 0.0 | |
| AIR | Leaf | 79.4 | 61.8 | 0 | 91.9 | 72.2 | 6.7 |
| Root | 53.4 | 49.3 | 2.2 | 71.4 | 58.5 | 4.4 | |
| Stem | 46.3 | 30.8 | 2.2 | 78.3 | 60.4 | 2.2 | |
| 10 mg | 50 mg | ||||||
|---|---|---|---|---|---|---|---|
| Date | Part | R | H | G | R | H | G |
| 20 August | Leaf | 90.2 ± 2.7 | 80.9 ± 1.8 | 100 ± 0.0 | 54.5 ± 3.1 | 62.6 ± 3.2 | 100 ± 0.0 |
| Root | 64.3 ± 2.8 | 67.9 ± 1.8 | 100 ± 0.0 | 75.6 ± 2.5 | 72.8 ± 1.4 | 100 ± 0.0 | |
| Stem | 118.9 ± 3.6 | 106.2 ± 2.4 | 100 ± 0.0 | 68.5 ± 2.1 | 71.5 ± 1.6 | 93.3 ± 8.9 | |
| 10 October | Leaf | 83.2 ± 1.3 | 67.7 ± 0.9 | 100 ± 0.0 | 43.6 ± 1.6 | 45.9 ± 2.2 | 100 ± 0.0 |
| Root | 80.1 ± 4.2 | 83.4 ± 3.4 | 100 ± 0.0 | 38.4 ± 1.7 | 50.9 ± 0.9 | 100 ± 0.0 | |
| Stem | 62.9 ± 1.8 | 66.6 ± 1.5 | 100 ± 0.0 | 71.9 ± 2.0 | 65.3 ± 1.8 | 100 ± 0.0 | |
| AIR | Leaf | 13.3 | 25.7 | 0 | 50.9 | 45.8 | 0 |
| Root | 27.8 | 24.3 | 0 | 43.0 | 38.1 | 0 | |
| Stem | 9.1 | 13.6 | 0 | 29.7 | 31.6 | 2.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, L.; Zhao, X.; Kang, G.; Suzuki, K.; Ismail, T.; Fujii, Y.; Motoki, S. Assessment of the Allelopathic Activity of Various Parts of Platycodon (Platycodon grandiflorus) and Its Mitigation by Activated Carbon. Agronomy 2024, 14, 385. https://doi.org/10.3390/agronomy14020385
Bao L, Zhao X, Kang G, Suzuki K, Ismail T, Fujii Y, Motoki S. Assessment of the Allelopathic Activity of Various Parts of Platycodon (Platycodon grandiflorus) and Its Mitigation by Activated Carbon. Agronomy. 2024; 14(2):385. https://doi.org/10.3390/agronomy14020385
Chicago/Turabian StyleBao, Long, Xuemei Zhao, Gaowa Kang, Kaito Suzuki, Tamer Ismail, Yoshiharu Fujii, and Satoru Motoki. 2024. "Assessment of the Allelopathic Activity of Various Parts of Platycodon (Platycodon grandiflorus) and Its Mitigation by Activated Carbon" Agronomy 14, no. 2: 385. https://doi.org/10.3390/agronomy14020385
APA StyleBao, L., Zhao, X., Kang, G., Suzuki, K., Ismail, T., Fujii, Y., & Motoki, S. (2024). Assessment of the Allelopathic Activity of Various Parts of Platycodon (Platycodon grandiflorus) and Its Mitigation by Activated Carbon. Agronomy, 14(2), 385. https://doi.org/10.3390/agronomy14020385







