Abstract
Heavy metal (HM) contamination poses a serious threat to safe crop production and human health, and different maize inbred lines respond differently to cadmium (Cd) stress. However, the morphological and physiological characteristics of maize inbred lines seedlings are not clear under Cd stress. In this study, we analyzed the agronomic traits and physiological and biochemical indices of inbred maize seedlings under Cd stress in the seedling stage using the inbred lines Kui3, CML118, Mo17, B73, and B77 as the materials. These five inbred maizes were treated with five different concentrations of Cd (0, 1, 3, 5, and 7 mg L−1, respectively) were applied and the indices of the maize seedlings determined on day 15. The aboveground and belowground biomass of five maize inbred lines seedlings showed a decreasing trend under Cd stress. Leaf relative water content and SPAD values also decreased, but the overall decrease in relative water content was small, and the differences were not significant. Surprisingly, Cd stress affected the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), leading to enhanced mem-brane lipid peroxidation. The cadmium content varied greatly between varieties under Cd stress, but all of them had lower Cd content above ground than below ground, and the varieties with the highest and lowest transfer coefficients were Mo17 (0.33–0.83) and B73 (0.06–0.44), respectively. Kui3 had the greatest difference in soluble protein content under Cd stress, which showed a de-creasing trend, and the soluble sugar content was significantly decreased in general compared to that of CK. The soluble sugar content was higher than CK under Cd treatment, and the proline content of the maize seedlings of all of the inbred lines showed an increasing trend compared to CK. Overall, there were significant genotypic differences in the Cd stress response to Cd toxicity in the maize inbred lines seedlings, and, in general, this study helps us to understand the mechanism of maize inbred lines seedlings response to Cd stress. It provides a theoretical basis for the se-lection and breeding of varieties, and food safety.
1. Introduction
Due to economic development and the irrational use of fertilizers in agriculture [1], heavy metal pollution has emerged as a serious and prominent issue [2]. Cd is considered to be one of the most hazardous heavy metals as it is high in biotoxins and can be easily transported [3,4]. On the one hand, Cd is easily dissolved in soil through surface or groundwater leaching, making it difficult to naturally degrade or manage. On the other hand, Cd is easily absorbed by plant roots and enters the human body through the food chain [5,6,7,8]. Although the amount of Cd absorbed by humans is not high, even very low concentrations can pose fatal risks to humans due to its long half-life [9]. For instance, Cd can lead to disorders of calcium metabolism in the human body, which can result in osteoporosis, cartilage disease, and bone pain [10]. It can also significantly increase the risk of developing kidney cancer, lung cancer, and prostate cancer [11,12].
Numerous studies have demonstrated that Cd exposure in plants triggers the production of substantial amounts of reactive oxygen species (ROS), and under Cd stress, the overaccumulation of ROS in plants leads to peroxidative damage [13,14,15], ultimately resulting in cell death. Plants have evolved antioxidant mechanisms to counteract oxidative stress, which include both non-enzymatic substances and antioxidant enzyme defenses. Non-enzymatic substances mainly include ascorbic acid (ABA) and glutathione (GSH) [16,17], whereas antioxidant enzyme defense systems mainly comprise SOD, POD, CAT, and ascorbate peroxidase (APX). SOD catalyzes the dismutations of O2− to O2 and H2O2, while CAT reduces hydrogen peroxide to water: 2H2O2 = O2 + 2H2O. Thus, the cell is protected from the H2O2 poison. SOD, POD, CAT, and other enzymes work together to defend against reactive oxygen species or other peroxide radicals that can harm the cell membrane system. Within a certain Cd concentration, these enzymes have a protective effect on the plant. However, high Cd concentrations can also harm these enzymes. Proline, soluble sugars, and soluble proteins in plants serve as osmoregulators and antioxidants, helping to mitigate heavy-metal-induced damage to plant cells [18,19]. These are crucial mechanisms for the adaptation of plants to abiotic stresses.
Cd enters the plant body and accumulates to a certain extent, inhibiting plant respiration and photosynthesis. This weakens the enzyme activity in the plant body and reduces the content of plant soluble protein and soluble sugar, resulting in slow growth, leaf yellowing, delayed climacteric period, etc. [20,21], which seriously affect crop yield, quality, and safety. When maize seedlings are subjected to Cd stress, plant growth is inhibited, leaves turn yellow and curl, aboveground biomass is significantly reduced, and Cd’s inhibition of the root is the most obvious [22,23]. The plant cannot carry out normal photosynthesis and transpiration, thus inhibiting the growth and development of the plant [24,25,26,27].
Maize is one of the world’s most important cereal crops. In recent years, the sown area has tended to stabilize, laying the foundation for the development of China’s plantations, animal husbandry, and industry. Continuous improvement in the yield and quality of maize is conducive to the stable development of the national economy. It has been found that maize itself has a strong enrichment effect on Cd, which makes the problem of the contamination of the maize production process by Cd increasingly prominent [28]. Most of the studies on the effects of Cd on maize have focused on biomass, enzyme activity, and chlorophyll content. However, the morphological and physiological characteristics of maize inbred lines seedlings remain unclear under Cd stress. The aim of this study was to simulate the effects of different Cd concentrations on the growth of maize inbred lines seedlings, to investigate the effect of different Cd concentrations on the growth of maize inbred lines seedlings. Therefore, the objectives of this study were: (1) to examine the Cd content in both aboveground and belowground maize seedlings; (2) to investigate differences in growth, photosynthetic capacity, and antioxidant properties in maize inbred lines under Cd stress in both aboveground and belowground maize seedlings; and (3) to gain a better understanding of maize inbred lines seedlings tolerance mechanisms in response to Cd stress. It provides a theoretical basis for the selection and breeding of varieties and food safety.
2. Materials and Methods
2.1. Maize Material Culture Conditions and Cd Treatment
Selection and breeding of the superior maize inbred lines Kui3, CML118, Mo17, B73, and B77 were used in this study. The seeds were soaked in 5% H2O2 for 15 min, then washed 3 times with distilled water. Then, they were sown on filter paper and kept at a constant temperature of 28 °C in light-avoiding environmental conditions. After 24 h, the dewy-white maize seeds were selected and sown in the maize seedling box. The box was set up to grow 100 seedlings. The day and night temperatures were set to (28 ± 1) and (22 ± 1) °C. Light intensity was set at 20,000 Lux light and dark times of 16 h and 8 h, respectively.
Cd stress was applied after it entered the one-leaf–one-heart stage, with the Cd2+ donor being Cd chloride (CdCl2 2.5H2O). In this experiment, a total of five inbred maize lines were tested, and five Cd treatment levels were set: 0, 1, 3, 5, and 7 mg L−1, with 0 being control (CK). After 15 d of stress, samples were taken from the control group and each treatment group, and the indices were determined.
2.2. Measurement Items and Methods
2.2.1. Growth Indicators
Plant height: Six seedlings were randomly selected and the height from the base of the stem to the growing point was measured using a straightedge with an index value of 1 mm and averaged.
Leaf area: The leaf length and maximum width of the 3rd unfolded leaf from the top of the maize seedling were measured, and the leaf area of a single leaf was calculated according to the following formula: length × width × 0.75.
Aboveground part of the seedling’s dry and fresh weight: The aboveground part of the seedling was placed on the balance and its fresh weight was measured; then, it was put into the oven at 105 °C to dehydrate for 0.5 h and dried at 75 °C until a constant weight was reached. Then, we measured its dry weight.
Relative water content: We took the 2nd leaf from the bottom of the maize seedling and measured its fresh weight. Then, we fully immersed it in deionized water, removed it after 2 h, and blotted the water on the surface of the leaf and then weighed it, which was recorded as the absorbed weight. Finally, we placed the leaf in the oven at 75 °C and dried it and measured its dry weight. Relative moisture content = (fresh weight − dry weight)/(absorbed weight − dry weight) × 100%.
2.2.2. Measurement of Physiological and Biochemical Indicators
The chlorophyll content (SPAD value) was measured using a handheld chlorophyll meter, the SPAD-502PLUS. For the determination of SPAD values, the meter was used to measure the upper, middle, and lower sections of the 2nd leaf from the bottom of the seedling, with an average taken from three replications for each treatment.
Antioxidant enzyme activity: Approximately 0.5 g of maize leaf and root samples was taken, to which was added a precooled phosphate buffer of 50 mmol L−1 PH 7.8. The samples were ground into homogenate in an ice bath and centrifuged at 10,000 r/min for 20 min at 4 °C. The supernatant was stored in a refrigerator for the determination of enzyme activity [29].
SOD activity: nitroblue tetrazolium (NBT) photochemical method [30]. The supernatant is the crude extract of SOD. We took a transparent test tube and added 0.1 mL of extraction solution, 1.5 mL of phosphate buffer (50 mmol L−1), 0.3 mL of EDTA-Na2 solution (0.1 mmol L−1), 0.3 mL of riboflavin solution (0.02 mmol L−1), 0.3 mL of nitroblue tetrazolium solution (0.75 mmol L−1), 0.3 mL of methionine solution (130 mmol L−1), and 0.5 mL of distilled water, which were mixed for the assay tube. The light control tube was prepared by adding 1.5 mL of phosphate buffer (50 mmol L−1), 0.3 mL of EDTA-Na2 solution (0.1 mmol L−1), 0.3 mL of methionine solution (130 mmol L−1), 0.3 mL of riboflavin solution (0.02 mmol L−1), 0.3 mL of nitroblue tetrazolium solution (0.75 mmol L−1), and 0.6 mL of distilled water. The dark control tube (with the same composition as the light control tube) was placed in the dark and protected from light, and the remaining test tubes were placed under 4000 Lux fluorescent lamps to develop the color reaction for 20 min. After the reaction, the dark control tube was used as a blank control to adjust to zero, and the absorbance of the measurement tube and the light control tube was measured at 560 nm.
POD activity: Guaiacol method [31]. The supernatant is the crude extract of POD. We took 3 mL of reaction solution and added the enzyme extract. We measured the absorbance at 470 nm, which was read every 30 s for a total of 5 times.
CAT activity: UV absorption method [31]. The supernatant is the crude extract of CAT. We took a new test tube and added 0.2 mL of enzyme solution and 0.3 mL of H2O2 (0.1 mol L−1). We measured the absorbance at 240 nm, which was read every 30 s for a total of 5 times.
MDA (Malondialdehyde) content: Thiobarbituric acid method. We pipetted 1 mL of supernatant and added 2 mL of 0.6% TBA solution, which was mixed well and then heated in a boiling water bath for 30 min. It was cooled quickly (ice bath) and then centrifuged for 10 min. We used the supernatant to determine the absorbance at 600, 532, and 450 nm wavelengths [32].
Proline content: Ninhydrin color development method [33]. We took 0.5 g of maize seedling leaf sample in a test tube, added 3% sulfosalicylic acid solution 5 mL, and heated in a boiling water bath for 10 min while shaking the test tube constantly. After cooling, it was filtered into a clean test tube. We pipetted 2 mL of proline extract into a new test tube and added acid ninhydrin reagent and glacial acetic acid, 2 mL each. It was heated in a boiling water bath for 30 min, the solution was cooled, and 4 mL of toluene was added. It was shaken for 30 s and then allowed to rest. Then, we added the upper layer of the solution to a 5 mL centrifugal tube, centrifuged it at 10,000 r/min for 5 min, and then took the solution to be measured in absorbance at 520 nm. Toluene solution was used as the blank control. The amount of proline was calculated from the standard linear equation, and the content of proline in the sample was calculated.
Soluble sugar content: Anthrone method. We accurately weighed 0.5 g of fresh maize seedling leaves into a large test tube, added 15 mL of distilled water, heated in a boiling water bath for 20 min, removed and cooled, filtered into a 50 mL volumetric flask, rinsed the residue with distilled water several times, and then condensed it to the scale line. We drew 1 mL of each sample from the volumetric flask, added 5 mL of anthrone reagent, shook them quickly, and heated the glucose for 10 min in a boiling water bath. We performed the same treatment for the test standard curve. We calculated the amount of soluble sugar from the standard linear equation and calculated the content of soluble sugar in the samples.
Soluble protein content: Coomassie brilliant blue staining [34]. We took 0.5 g of maize seedling leaves, added 2 mL of phosphate buffer into a precooled mortar, ground it into a homogenous slurry with a small amount of quartz sand, transferred it to a centrifuge tube, and added buffer until the amount reached 5 mL, centrifuged it at 3000 r/min for 10 min. Then, we took 1 mL of soluble protein extract into a clean tube, added 5 mL of Coomassie brilliant blue solution, shook well, and left it for 2 min. The absorbance was measured by UV spectrophotometer at 595 nm. We calculated the amount of soluble protein from the standard linear equation and calculated the content of soluble protein in the sample.
2.2.3. Determination of Cd Content in Maize above Ground and below Ground under Different Treatments
After 15 d of Cd treatment, the lower aboveground and belowground parts were sampled. The belowground parts were soaked in a 20 mmol L−1 solution of EDTA-Na2 for 15 min and then rinsed with distilled water. They were then oven-dried at 105 °C for 30 min, together with the aboveground parts. The aboveground and belowground samples were dried at 75 °C until they reached constant weight, ground and crushed, and then, we weighed 0.25 g of the lower belowground part and 0.25 g of the lower aboveground part. Then, 0.25 g of the lower belowground and aboveground sample were weighed, digested in a mixture of HNO3-HClO4 (4:l, v:v) at 220 °C until the solution became transparent, and cooled to room temperature. Then, the digested solution was diluted with ultrapure water and filtered through 0.22 μm membranes. Finally, the measurements were performed using a PerkinElmer PinAAcle 900T atomic absorption (AA) spectrometer (PerkinElmer Inc., Shelton, CT, USA)
Translocation factor (TF) = Cd content aboveground in plant/Cd content belowground in plant.
Aboveground Cd accumulation = Aboveground dry weight × Aboveground Cd content.
Belowground Cd accumulation = dry weight of the lower root × Belowground Cd content.
2.2.4. Root Scanning
The overall root morphology was scanned by an EPSON scanner and then analyzed for total root length, total root surface area, total root volume, and mean root diameter by using Win Rhizo Rot Photo (version 2.0.2) analysis software.
2.3. Statistical Analysis
The results are presented as mean values ± standard errors/standard deviations. Data were processed and analyzed using Excel 2019, SPSS 25.0. One-way analysis of variance (ANOVA) and Duncan’s multiple comparison test (p < 0.05) were used for statistical treatment.
3. Results
3.1. Impact of Cd Stress on Maize Seedling Growth
After 15 d of incubation, compared with control maize seedlings, we observed that Cd had a significant inhibitory effect on the growth and development of the maize seedlings with increasing Cd concentrations (Figure 1). Cd-treated maize plants showed symptoms of wilting and marginal yellowing on the leaves, as well as dwarfing of plant growth, etc. The symptoms were aggravated with the Cd concentration, resulting in scorching, yellowing of the old leaves, and blackening of the root system.
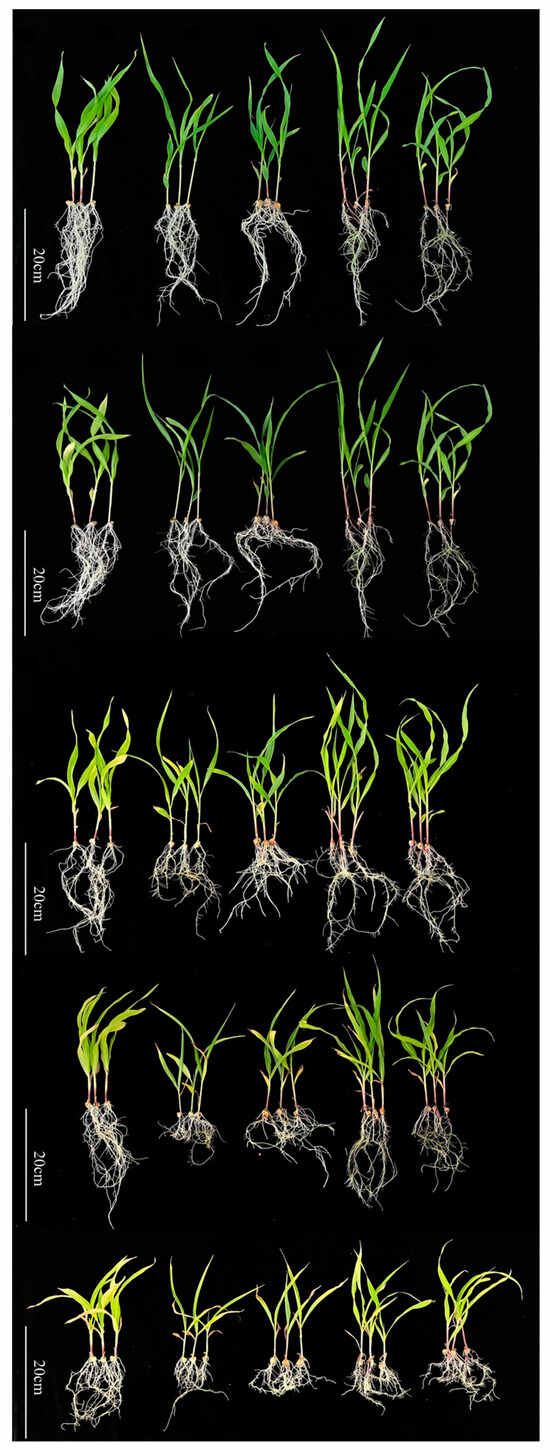
Figure 1.
Response of maize seedlings to exposure to 15 d Cd stress. From top to bottom are the different concentrations of Cd treatments. In order: 0 (CK), 1, 3, 5, and 7 mg L−1. From left to right are the different varieties. In order: Kui3, Mo17, CML118, B73, and B77. Maize seedlings under Cd stress treatment showed yellowing and curling of leaves and blackening of roots, as well as inhibition of seedling growth. Different varieties were not uniformly damaged under Cd stress.
In terms of biomass, for all Cd treatments, the addition of Cd treatment suppressed aboveground dry fresh weight and belowground dry fresh weight of maize in all lines compared to the control. Moreover, the root–crown ratio increased significantly with increasing Cd stress concentration in all lines (Figure 1; Table 1), with the strongest increase in root–crown ratio observed in B77.

Table 1.
Effect of different Cd treatments on growth.
3.2. Impact of Cd Stress on Maize Blades
Cd stress induces obvious external morphological changes, the most intuitive manifestation of which is the difference in leaf area as an external characteristic. In this study, the leaf area of all maize lines decreased with increasing concentration of Cd, reaching a significant level compared to the control (Figure 2). Especially in the 7 mg L−1 treatment, the leaf area of seedlings decreased abruptly, with the largest decrease in Mo17 (−75.97%), followed by CML118 (−75.65%). The relative water content of plant leaves is one of the indicators of the plant’s own water retention capacity, and all of the maize seedlings under Cd stress treatments reduced the relative water content of leaves compared with the control, although the magnitude was smaller (Figure 3).
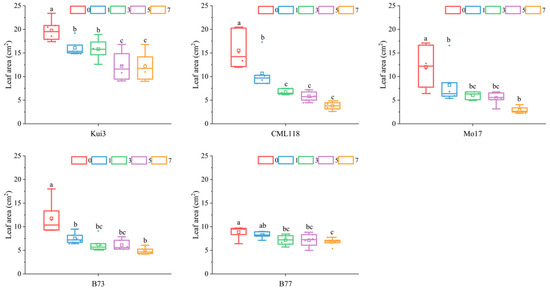
Figure 2.
Effects of Cd stress on leaf area of maize seedlings. Note: Different lowercase letters indicate significant differences between treatments (p ≤ 0.05).
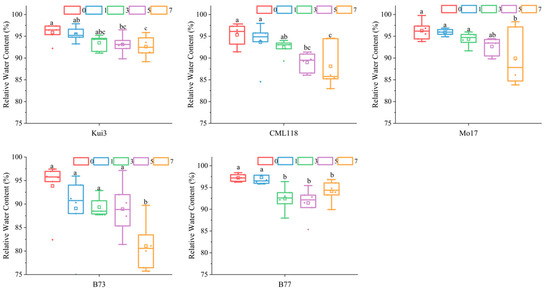
Figure 3.
Effects of Cd stress on relative water content of maize seedlings. Note: Different lowercase letters indicate significant differences between treatments (p ≤ 0.05).
3.3. Effect of Cd on Root Morphological Traits
The root morphology of control (0 mg L−1) and Cd-treated plants was investigated by using a root scanner. It was found that the inhibitory effect was further enhanced with the increase in Cd stress concentration, resulting in an abnormal root system of maize seedlings Kui3, CML118, and Mol7. It appeared that low concentrations promote the growth of total root length, but the total root length of CML118 was inhibited to the highest extent. The high concentration of Cd treatment caused structural abnormalities in the maize root system, roughly reducing the total root length, average diameter, root surface area, and root volume of the seedling root system of five maize varieties. This impeded growth and development (Figure 4; Table 2). Surface area, root volume of the seedling root systems of the five maize cultivars, and growth and development were also impeded (Figure 4; Table 2).
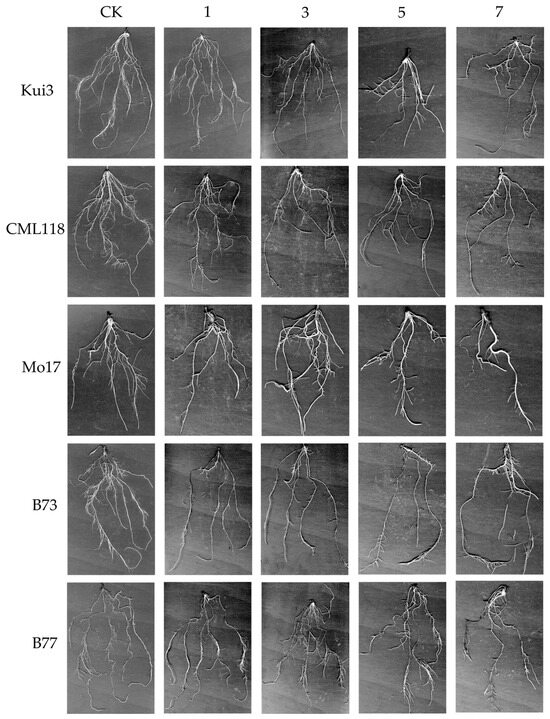
Figure 4.
Root scan map. From top to bottom are the different inbred lines. In order: Kui3, Mo17, CML118, B73, and B77. From left to right are the different concentrations of Cd treatments. In order: 0 (CK), 1, 3, 5, and 7 mg L−1.

Table 2.
Effects of different concentrations of Cd on root structure of maize.
3.4. Effect of Cd Stress on SPAD Value
Cd stress treatment significantly inhibited maize leaf chlorophyll synthesis and photosynthesis, and leaf SPAD values were lower than those of CK after Cd treatment (Figure 5). Except for Mo17, which showed an upward trend compared with CK under 1 mg L−1 treatment, the others all exhibited a downward trend, with Mol7 experiencing the smallest overall decrease and Kui3 experiencing the largest overall decrease under Cd stress and Cd concentration treatments, respectively, followed by B77, although the difference was not significant.
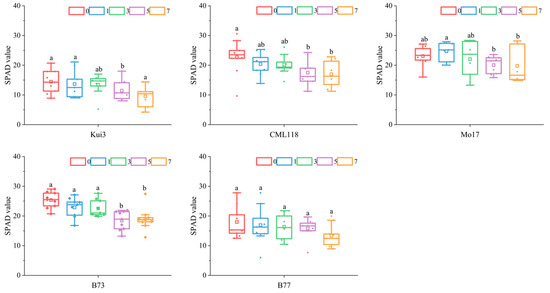
Figure 5.
Effects of Cd stress on SPAD value of maize seedlings. Note: different lowercase letters indicate significant differences between treatments (p ≤ 0.05).
3.5. Effects of the Protective Enzyme System and MDA Content under Cd Stress
The protective enzymes and MDA in the maize seedlings were affected by Cd stress treatment (Figure 6a–d). POD activity was higher than CK in all cases compared with the control (Figure 6a). SOD varied with the Cd stress concentration (Figure 6b). Kui3 had lower SOD activity than CK under the 1 and 3 mg L−1 treatments, and SOD in the leaves of all other varieties had an increasing trend compared with CK. CAT activity of Kui3 had a decreasing, then increasing, and then decreasing trend under cadmium concentration treatments (Figure 6c). The trend was the same in CML118 and B77, which were decreasing and then increasing, and in Mo17, which was first increasing and then decreasing, while B73 was a stepwise increase and reached the highest value at 7 mg L−1 concentration treatment. MDA is a product of cell membrane lipid peroxidation. Its content can be used as a measure of the damage of membrane lipid peroxidation. The aboveground MDA content of B73 was lower than CK under the treatment of Cd stress (Figure 6d), indicating that B73 has a low degree of membrane lipid peroxidation. Therefore, it has a strong ability to tolerate Cd, which helps to maintain normal physiological activities.
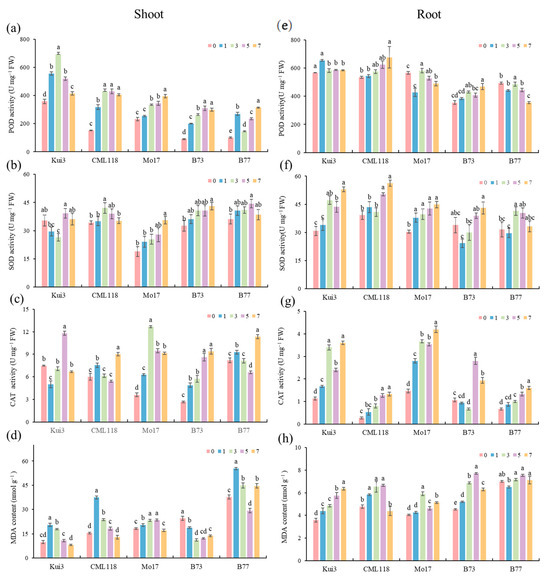
Figure 6.
Effect of Cd stress on activities of (a,e) POD, (b,f) SOD, (c,g) CAT, and (d,h) MDA in maize. Aboveground on the left, belowground on the right. Different letters indicate significant differences at p < 0.05.
The trend of the effects of protective enzymes and MDA in the belowground part of the maize seedlings under Cd stress treatment was not consistent with that in the aboveground part (Figure 6e–h). Compared to the control, the overall POD activity of all varieties except B77 was higher than that of Mo17 (Figure 6e). With the increase in Cd concentration, the seedlings produced a stress response, and the POD activity was enhanced to remove a large amount of H2O2 produced by the seedling body. The SOD activity of B73 was lower than that of CK under Cd treatments at concentrations of 1 mg L−1 and 3 mg L−1. The SOD activity of B77 was lower than that of CK at a concentration of 1 mg L−1. The SOD activity of all other varieties was higher than that of CK under stress (Figure 6f). This may be because reactive oxygen species cannot be removed in time to inhibit enzyme activity, thus damaging the cells. Under Cd stress conditions, the CAT content in the maize seedlings increased substantially to reach the maximum value and there was a significant difference (p < 0.05). The seedlings’ own CAT enzyme activity increased significantly to improve Cd tolerance. However, the fact that the activity of B73 at a concentration of 1–3 mg L−1 decreased (Figure 6g) may be due to the deepening of the degree of oxidation of membrane lipids, resulting in cellular damage and inhibition of the activity of CAT. B77 in the Cd stress concentration treatments showed no significant differences, while the other inbred maize lines for testing had significant differences (p < 0.05) under different Cd concentrations. The MDA content of CML118 and B77 was lower than that of CK under the treatment of 7 mg L−1 Cd concentration, which inhibited the MDA content.
3.6. Effects of Osmoregulatory Capacity under Cd Stress
The level of soluble protein responds to the strength of cellular metabolic activities under Cd stress conditions. Maize seedlings will produce anti-heavy-metal proteins due to the stress response to reduce their damage (Figure 7a). The increase in soluble protein content is conducive to the cells’ ability to carry out normal physiological activities. In the Cd stress treatment, the expression of cellular genes in Kui3 was destroyed, resulting in the blockage of protein synthesis and processing, destruction of the corresponding enzyme system, and a reduction in soluble protein in B77 under Cd stress conditions. It may be that the number of enzymes involved in metabolic activities in the stress response in the seedlings increased, producing a significant increase in the content of soluble protein (p < 0.05). The difference between the treatments and the control was significant, which may be due to the stress response of the maize seedlings to the low concentration of heavy metal stress.
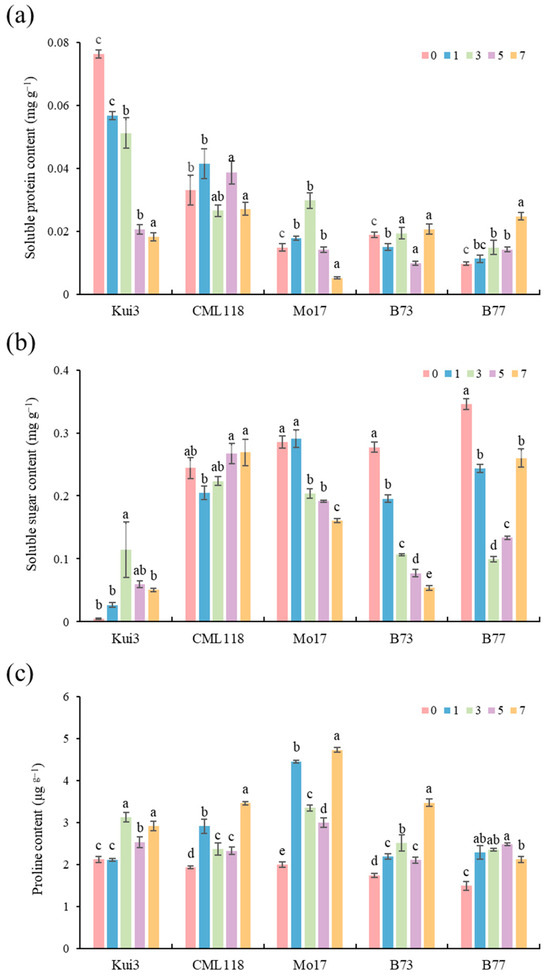
Figure 7.
Effects of Cd stress on osmotic adjustment ability of maize seedlings: (a) soluble protein; (b) soluble sugar; (c) proline. Different letters indicate significant differences at p < 0.05.
Soluble sugars are considered to be one of the important osmotic regulators of plant adaptation to adverse conditions, and the maize seedlings in this study showed that a certain concentration of Cd stress could increase the soluble sugar content. However, above a certain threshold, this was reflected as an inhibitory effect, and the soluble sugar content of the plant body decreased due to Cd stress (Figure 7b).
Proline is an important osmoregulatory substance in plants, and the increase in proline content in plants is an adaptive response to adversity stress. Higher Cd concentration significantly increased the proline content, and the greater the increase in proline content, the greater the tolerance to Cd stress, and the proline content was significantly increased among treatments compared to the control (Figure 7c).
3.7. Cd Uptake and Transport in Different Maize Varieties
In order to study Cd uptake in inbred maize lines, Cd content in aboveground and belowground parts of the seedlings was examined. Significant changes in Cd content were observed under different Cd concentration stresses and varieties (p < 0.05; Figure 8a,b). With increasing Cd stress, its concentration significantly increased in all maize lines, especially in the roots of five inbred maize lines. Although all Cd concentrations increased Cd in the maize seedlings, this increase was more pronounced when Cd concentrations were used at dosages of 5 or 7 mg L−1 and all Cd levels were greater below ground than above ground (Figure 8a,b). The overall Cd content of CML118 above ground was lower than that of other varieties at all Cd concentrations. However, the accumulation of Mol7 below ground was more stable under Cd stress, and the root content of all lines reached the highest value at 7 mg L−1 (Figure 8b). The Cd content of Kui3 was higher than that of other lines in both the aboveground and the belowground parts under Cd treatment at 7 mg L−1.
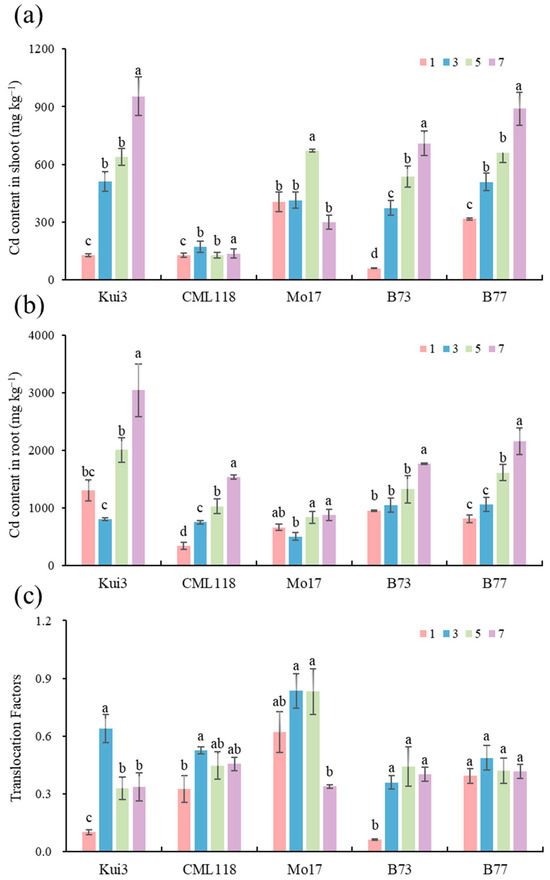
Figure 8.
Cd accumulation and cadmium transport in maize seedlings under Cd stress treatment: (a) Cd accumulation aboveground; (b) Cd accumulation in root; (c) translocation factor. Different letters indicate significant differences at p < 0.05.
The transfer coefficient Indicates the ability of heavy metals to be transported and distributed from roots to the aboveground parts. The higher the transport coefficient, the stronger the ability of heavy metals to be transported from roots to the aboveground parts. The overall transport coefficients of the maize seedlings were all <1 (Figure 8c). The Cd transport coefficients of most of the test lines reached their maximum values at 3 mg L−l, and all of them first increased and then decreased. Mo17 and B73 were the highest and lowest transfer coefficients, respectively, with the transfer coefficients ranging from 0.33 to 0.83 for Mo17 and from 0.06 to 0.44 for B73, indicating that the Mol7 has the strongest ability to transfer Cd from the belowground part to the aboveground part. B73 has the weakest Cd-transferring ability from below ground to above ground. The reason for the stronger Cd accumulation capacity in the belowground part and the weaker capacity in the aboveground part may be that there is less transfer from below ground to above ground.
4. Discussion
4.1. Changes in Biomass under Cd Treatment
Cd stress affects plant growth and morphology [35,36] and causes different changes in plant tissues, including inhibition of total root length growth [37], necrosis of stems and leaves [38], and reduction in dry matter mass [39]. The root system has a certain plasticity that enables plants to survive in adverse environments, and it is an important organ for absorbing and transporting nutrients. The length of the root system to a certain extent determines the plant’s ability to absorb water, synthesize organic matter, and transport inorganic salts. The root morphology of pepper decreased the RL, SA, and increased RD under Cd stress [40]. In this experiment, it was also found that Cd stress affected the total root length, average diameter, root surface area, and root volume of the root systems of the five lines, and increased RD (Table 2; Figure 4). The total root length of CML118 decreased the most under Cd stress treatment, indicating a higher degree of inhibition. When plants are faced with adversity, metabolites are distributed unbalanced among the root tubes, usually preferentially to the root system, leading to an increase in the root-crown ratio of each species, which in turn favors a better uptake of water and nutrients by the root system to supply aboveground growth. For example, Corylus heterophylla root–shoot ratio increased with increasing concentration under adversity salt stress [41]. The root–crown ratio of maize seedlings also increased with the increase of Cd concentration. It should be that the root systems can adapt to unfavorable environments by changing their shape and distribution under adversity [42]. At the same time, Cd concentrations had a remarkable inhibitory effect on the fresh weight, dry weight, and shoot length of maize plants [43]. This inhibition of plant growth by Cd has been observed in other plants, such as tobacco [17] and cucumber [44]. Under these experimental conditions, the effects of different Cd concentrations (1, 3, 5, and 7 mg L−1) on the fresh and dry weights of maize seedling roots were significant (Table 1). The fresh and dry weights of the maize seedlings showed a decreasing tendency, especially in the case of concentrations of 5 and 7 mg L−1, where the weight was significantly reduced compared with to control. This may be the root in order to prevent the entry of Cd2+, epidermal cell wall thickening intercellular space reduction, and the production of lignin deposition. It is difficult to achieve lignification of xylem nutrient elements and water with xylem and phloem [45]. There are not enough nutrient elements and water in the aboveground part of the transport, causing growth to stall and causing biomass reduction [46].
4.2. Cd Affects Relative Leaf Water Content and SPAD Values of Seedlings
Cd stress reduces the relative water content of foliage in maize inbred lines seedlings. The relative water content of the leaf surface of the maize inbred lines seedlings showed a decreasing trend with the increase in Cd concentration (Figure 3). When Cd enters the plant and accumulates to a certain extent, it destroys chlorophyll synthesis and inhibits photosynthesis. Nada et al reported that the chlorophyll content of apricot seedlings decreased under Cd stress [17]. In this experiment, the SPAD values of inbred maize seedlings also decreased with increasing Cd concentrations (Figure 5). Although the difference was not significant compared with CK, photosynthesis directly affects the growth of maize seedlings and can also indirectly affect the growth by inhibiting photosynthesis, providing the material basis for growth.
4.3. Antioxidant Defense System Stress Response
Under normal conditions of production, the production and removal of free radicals in plant cells are in a state of dynamic equilibrium. However, as Cd stress is prolonged and its concentration increases, the balance of free radical metabolism in plants suffers damage, resulting in the accumulation of a large number of free radicals. These further damage the cell membrane system and disrupt the normal intracellular metabolism [17]. In plant bodies, each protective enzyme plays distinct roles: SOD mainly converts O2− to H2O2 and O2, while POD and CAT mainly convert H2O2 to non-toxic and harmless H2O and O2. Some studies have claimed that under Cd stress, the activities of SOD, POD, and CAT increased significantly [47,48,49]. Javed et al., who had new experimental conclusions, pointed out that Cd stress significantly increased the activities of SOD, POD, and CAT, while belowground shows a decreasing trend. [50]. The results of our experiment were not quite the same as those of the abovementioned studies (Figure 6). SOD activity was generally higher than that of CK under Cd stress treatment, However, the SOD content of Kui3 was lower than that of the control under the 1 and 3 mg L−1 treatments in our results, which might be due to the differences in the intensity and duration of Cd stress and crop species. CAT activity showed different trends among the varieties. POD activity in the treatment groups of Kui3, CML118, and B73 in the lower part of the ground was significantly higher under Cd stress, whereas Mo17 and B77 decreased after being subjected to Cd stress. SOD activity had an overall upward trend under Cd stress, and the SOD content of B73 decreased under the 1 and 3 mg L−1 treatments. CAT was lower than that of the control under the Cd stress treatments. CAT content under treatment decreased, possibly because Cd stress destroyed the variety’s own antioxidant enzyme system [51]. SOD will catalyze the generation of superoxide radicals O2 to generate H2O2. Although the main function of CAT is as a scavenger of H2O2, its high concentration and more oxidative H2O2 inhibited the activity of CAT. It was hypothesized that under a certain range of Cd stress, seedlings would increase their resistance to Cd stress by increasing the activity of antioxidant enzymes. However, when the Cd concentration exceeded the tolerance range of the maize seedlings, the balance of the reactive oxygen species scavenging system was affected, the cell structure was destroyed, and the activity of the peroxidase enzyme was reduced [52,53].
4.4. Cd Treatment Triggers Oxidative Damage to Lipid Membranes
The MDA, as a product of lipid peroxidation, reflected the strength of the degree of peroxidation of membrane lipids in the plant, and the higher the content of MDA [54], the greater the degree of damage to the plant. In this experiment, the degree of lipid peroxidation in the lipid membrane of maize seedlings from different inbred lines varies with cadmium treatment. In the aboveground maize varieties Kui3 and B77, the MDA content was significantly reduced under Cd stress, indicating that the peroxide damage to plant tissues was reduced, promoting growth and development (Figure 6d). The trend of MDA changes in the belowground part was also different (Figure 6h). The overall trend of the treatments was increasing compared with CK. The MDA content of maize variety B77 changed less under Cd stress, indicating that the impact of Cd stress in maize variety B77 is smaller and more resistant. Kui3 had the largest increase in MDA, indicating that the concentration of Cd stress increases and the degree of peroxidation of cell membrane lipids is aggravated. Li et al. observed a significant increase in MDA content in maize seedlings with the extension of Cd treatment time [55]. Strawberry plants showed a significant increase in MDA production when treated with Cd applications. Moreover, in strawberry, leaf had higher MDA content than root [56]. The experimental results were not consistent with those of the predecessors. This may be due to differences in the intensity and duration of Cd stress as well as crop species.
4.5. Changes in Soluble Protein Content
As the concentration of Cd stress increased, the metabolic activities of antioxidant enzymes in the plant increased, resulting in higher protein synthesis. Hence, the soluble protein content increased, which was beneficial in allowing the cells to carry out normal physiological activities. Soluble proteins reflect the strength of metabolic activity within the cell. Haque et al. investigated the beet plants’ ability to cope with Cd toxicity at the cellular level. It was found that Cd stress resulted in a significant decrease in the soluble protein content of sugar beets [57]. However, this is not the case in this study, although it might be possible that the adaptive mechanisms for coping with Cd are genotype-dependent in inbred maize. The increase in soluble protein content of B77 under cadmium stress indicates that B77 was more metabolically active and responsive to the stress (Figure 7a). Therefore, it exhibited higher tolerance to cadmium stress. In contrast, Kui3 displayed the greatest decrease, indicating the weakest resistance. The mechanistic basis of Cd toxicity is crucial for introducing any form of crop improvement initiative. Therefore, these results serve as valuable research for a better understanding of how inbred maize plants cannot overcome this obstacle with Cd.
4.6. Regulation of Soluble Sugars under Cd Stress
The osmotic regulation of soluble sugars allows plants to have sufficient carbohydrate reserves to support basal metabolism in unfavorable conditions. Under heavy metal stress, the carbohydrate metabolism in seedlings is disturbed, and the change in soluble sugar content in plants depends on the concentration of Cd and the plant species. In other words, the low-concentration treatment might increase the soluble sugar in plants [51], while Cd stress reduces the soluble sugar content under high-concentration conditions [52]. In particular, the soluble sugar content of Kui3 increased the most under Cd treatment, making it more sensitive to Cd stress (Figure 7b). To maintain normal growth and development, Kui3 required more osmoregulatory substances. The decrease in soluble sugar content might be related to the destruction of the photosynthetic system [58].
4.7. Cd Stress Induced High Accumulation of Proline
Plants accumulate various osmoprotective substances as an additional physiological response [53]. The proteinogenic amino acid proline functions as anosmolyte, radical scavenger, electron sink, stabilizer ofmacromolecules, and a cell wall component [59]. The higher the proline content, the stronger the ability to withstand osmotic stress. The proline content in Lactucasativa increased proportionally with an increase in Cd concentration [60]. Proline content was enhanced in maize seedlings under Cd stress. Seedlings regulate osmotic balance and protect the integrity of their cell structure and function by increasing proline content. With the increase in Cd concentration treatment, the proline content of Mol7 increased significantly, showing a certain degree of Cd tolerance (Figure 7c). The smallest change was Kui3. Cd stress affected the various organs of B77 with varying degrees of damage. A 1–5 mg L−1 concentration of Cd stress promoted the accumulation of proline in B77, while a 7 mg L−1 concentration of Cd stress had an inhibitory effect on proline accumulation. This suggests that maize can maintain its metabolic balance through osmoregulatory substances under a certain degree of Cd stress [54].
4.8. Cd Accumulation and Transport
By measurement of Cd content in the upper and lower parts of the ground of the five lines revealed the accumulation and distribution of Cd in different parts of the maize seedlings (Figure 8). Cd is usually accumulated in the roots, because this is the first organ exposed to heavy metal and it is also translocated into the shoots. The Cd content in the lower part of the ground was higher than that in the upper part of the ground [55,56]. This may be because the roots were the first organ that came into contact with Cd. Cd is mainly enriched in roots, with only a small fraction transferred to the ground, and the above-ground part of the plant is more sensitive to Cd, and even low concentrations of Cd will also cause damage to it, and to avoid this damage, plants will retain Cd in the roots through various mechanisms [61].
5. Conclusions
This work illustrates the physiological consequences, and the redox status of Cd-exposed maize inbred seedings. The Cd stress treatments investigated in this study caused significant peroxidative damage to maize seedlings, which formed an antioxidant enzyme response system to scavenge ROS and reduce Cd toxicity by increasing the activities of SOD, POD, and CAT. Cd stress may inhibit the growth indices of maize seedlings, such as plant height, leaf area, and the relative water content of leaves, and it may also inhibit photosynthesis and reduce the chlorophyll content of maize seedlings. As the stress level increased, the chlorophyll content of the maize seedlings decreased. Cd treatment also altered Cd accumulation among the five varieties, with the Cd content below ground being higher than that above ground among all varieties. As a result, the root system tended to be one of the most severely affected organs. Cd stress also disrupted the expression of cellular genes, leading to the blockage of protein synthesis and processing in the plant body. Ultimately, this resulted in a decrease in the soluble protein content and a decrease in the content of soluble sugars under Cd stress treatments, while proline increased in the stressed seedlings in response to Cd stress. The results of this study showed that the toxic effects of Cd stress on maize seedlings increased with increasing levels of Cd treatment, but there were significant genotypic differences in the response of maize seedlings to Cd toxicity. These findings can be used to gain a better understanding of inbred maize line seedlings’ tolerance mechanisms in response to Cd stress and can aid in developing Cd-toxicity resistant lines and the regulation of Cd toxicity, resulting in better inbred maize genotypes for Cd-enriched soil.
Author Contributions
M.D. (Min Deng) and M.D. (Meijuan Duan) designed and supervised this study. S.D., Q.Z. and A.Z. performed the experiment. S.D. and Y.W. analyzed the data. M.D. (Min Deng) and S.D. prepared the manuscript. M.D. (Min Deng), S.D. and M.D. (Meijuan Duan) revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32101700), China Postdoctoral Science Foundation (2 022M711122), and the Science and Technology Innovation Program of Hunan Province (2021RC2082).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank Yueying Wu, Qingping Zeng, and Aoni Zhang for their help.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Tao, S. The Challenges and Solutions for Cadmium-contaminated Rice in China: A Critical Review. Environ Int. 2016, 92, 515–532. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Environmental Health Criteria 134: Cadmium; WHO: Geneva, Switzerland, 1992. [Google Scholar]
- Qin, P.; Wang, L.; Liu, K.; Mao, S.; Li, Z.; Gao, S.; Shi, H.; Liu, Y. Genomewide association study of Aegilops tauschii traits under seedling-stage cadmium stress. Crop J. 2015, 3, 405–415. [Google Scholar] [CrossRef]
- Sandalio, L.; Dalurzo, H.; Gómez, M.; Romero-Puertas, M.; del Río, L. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001, 52, 2115–2126. [Google Scholar] [CrossRef]
- Liu, H.; Hussain, S.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Potentially toxic elements concentration in milled rice differ among various planting patterns. Field Crops Res. 2014, 168, 19–26. [Google Scholar] [CrossRef]
- Wang, X.K.; Gong, X.; Cao, F.; Wang, Y.; Zhang, G.; Wu, F. HvPAA1 encodes a P-Type ATPase, a novel gene for cadmium accumulation and tolerance in barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2019, 20, 1732. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.M.; Peng, G.; Huang, L. Trophic transfer, bioaccumulation, and potential health risk of trace elements in water and aquatic organisms of Yundang Lagoon at Xiamen in China. Toxin Rev. 2023, 42, 242–256. [Google Scholar] [CrossRef]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. Biometals 2010, 23, 783–792. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef]
- Mezynska, M.; Brzóska, M.M. Environmental exposure to cadmium—A risk for health of the general population in industrialized countries and preventive strategies. Environ. Sci. Pollut. Res. 2018, 25, 3211–3232. [Google Scholar] [CrossRef]
- Sarma, S.N.; Saleem, A.; Lee, J.-Y.; Tokumoto, M.; Hwang, G.-W.; Chan, H.M.; Satoh, M. Effects of long-term cadmium exposure on urinary metabolite profiles in mice. J. Toxicol. Sci. 2018, 43, 89–100. [Google Scholar] [CrossRef]
- Shah, K.; Kumar, R.G.; Verma, S.; Dubey, R. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 2001, 161, 1135–1144. [Google Scholar] [CrossRef]
- Tai, Z.; Yin, X.; Fang, Z.; Shi, G.; Lou, L.; Cai, Q. Exogenous GR24 alleviates cadmium toxicity by reducing cadmium uptake in switchgrass (Panicum virgatum) seedlings. Int. J. Environ. Res. Public Health 2017, 14, 852. [Google Scholar] [CrossRef] [PubMed]
- Zoufan, P.; Jalali, R.; Hassibi, P.; Neisi, E.; Rastegarzadeh, S. Evaluation of antioxidant bioindicators and growth responses in Malva parviflora L. exposed to cadmium. Physiol. Mol. Biol. Plants 2018, 24, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Amri, B.; Khamassi, K.; Ali, M.B.; da Silva, J.A.T.; Ben Kaab, L.B. Effects of gibberellic acid on the process of organic reserve mobilization in barley grains germinated in the presence of cadmium and molybdenum. S. Afr. J. Bot. 2016, 106, 35–40. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotox. Environ. Safe 2017, 137, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.L.; Shang, S.H.; Zhang, H.T.; Jabeen, Z.; Zhang, G.P. Sodium chloride enhances cadmium tolerance through reducing cadmium accumulation and increasing anti-oxidative enzyme activity in tobacco. Environ. Toxicol. Chem. 2013, 32, 1420–1425. [Google Scholar] [CrossRef]
- Wei, T.; Liu, X.; Dong, M.; Lv, X.; Hua, L.; Jia, H.; Ren, X.; Yu, S.; Guo, J.; Li, Y. Rhizosphere iron and manganese-oxidizing bacteria stimulate root iron plaque formation and regulate Cd uptake of rice plants (Oryza sativa L.). J. Environ. Manag. 2021, 278, 111533. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Arora, P.; Kumar, S.; Chaudhury, A. Perspectives for genetic engineering of poplars for enhanced phytoremediation abilities. Ecotoxicology 2010, 19, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, C.; Liu, L.; Wang, C.; Qiao, W.; Gu, Z.; Wang, X. Lanthanum element induced imbalance of mineral nutrients, HSP 70 production and DNA-protein crosslink, Leading to hormetic response of cell cycle progression in root tips of Vicia faba L. seedlings. Dose-Response 2012, 10, 11–041. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, X.; Sun, X.; Tan, Q.; Tang, Y.; Nie, Z.; Qu, C.; Chen, Z.; Hu, C. Antioxidant enzyme systems and the ascorbate–glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere 2015, 138, 526–536. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, X.; Hou, L.; Ji, S.; Zhang, Y.; Fan, W.; Li, T.; Zhang, L.; Liu, P.; Yang, L. Effects of cadmium on transcription, physiology, and ultrastructure of two tobacco cultivars. Sci. Total Environ. 2023, 869, 161751. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, R.H.; Nowroz, F.; Nahar, K. Insight into the physiological and biochemical mechanisms of biostimulating effect of Ascophyllum nodosum and Moringa oleifera extracts to minimize cadmium-induced oxidative stress in rice. Environ. Sci. Pollut. Res. 2023, 30, 55298–55313. [Google Scholar] [CrossRef]
- Wang, M.; Zou, J.; Duan, X.; Jiang, W.; Liu, D. Cadmium accumulation and its effects on metal uptake in maize (Zea mays L.). Bioresour. Technol. 2007, 98, 82–88. [Google Scholar] [CrossRef]
- Zhang, X.Z. The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. Res. Methodol. Crop Physiol. 1992, 208–211. [Google Scholar]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidases. Methods Enzymol. 1955, 11, 764–775. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Jin, C.W.; Liu, Y.; Mao, Q.Q.; Wang, Q.; Du, S.T. Mild Fe-deficiency improves biomass production and quality of hydroponic-cultivated spinach plants (Spinacia oleracea L.). Food Chem. 2013, 138, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Groppa; Rosales, E.; Iannone, M.; Benavides, M. Nitric oxide, polyamines and Cd-induced phytotoxicity in wheat roots. Phytochemistry 2008, 69, 2609–2615. [Google Scholar] [CrossRef] [PubMed]
- Daud, M.K.; Sun, Y.; Dawood, M.; Hayat, Y.; Variath, M.T.; Wu, Y.X.; Mishkat, U.; Najeeb, U.; Zhu, S. Cadmium-induced functional and ultrastructural alterations in roots of two transgenic cotton cultivars. J. Hazard. Mater. 2009, 161, 463–473. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, R.P. Differential toxicity of cadmium to mustard (Brassica juncia L.) genotypes under higher metal levels. J. Environ. Biol. 2011, 32, 355. [Google Scholar]
- Hernandez, L.E.; Cooke, D.T. Modification of the root plasma membrane lipid composition of cadmium-treated. Pisum. Sativum. J. Exp. Bot. 1997, 48, 1375–1381. [Google Scholar] [CrossRef]
- Borges, K.L.R.; Salvato, F.; Alcântara, B.K.; Nalin, R.S.; Piotto, F.; Azevedo, R.A. Temporal dynamic responses of roots in contrasting tomato genotypes to cadmium tolerance. Ecotoxicology 2018, 27, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xin, J.; Dai, H.; Liu, A.; Zhou, W.; Yi, Y.; Liao, K. Root morphological responses of three hot pepper cultivars to Cd exposure and their correlations with Cd accumulation. Environ. Sci. Pollut. Res. 2015, 22, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Bahn, M.; Lattanzi, F.A.; Hasibeder, R.; Wild, B.; Koranda, M.; Danese, V.; Brüggemann, N.; Schmitt, M.; Siegwolf, R.; Richter, A. Responses of belowground carbon allocation dynamics to extended shading in mountain grassland. New Phytol. 2013, 198, 116–126. [Google Scholar] [CrossRef]
- Muradoglu, F.; Gundogdu, M.; Ercisli, S.; Encu, T.; Balta, F.; Jaafar, H.Z.; Zia-Ul-Haq, M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 11. [Google Scholar] [CrossRef]
- Lagriffoul, A.; Mocquot, B.; Mench, M.; Vangronsveld, J. Cadmium toxicity effects on growth, mineral and chlorophyll contents, and activities of stress related enzymes in young maize plants (Zea mays L.). Plant Soil. 1998, 200, 241–250. [Google Scholar] [CrossRef]
- Shackira, A.M.; Puthur, J.T. Enhanced phytostabilization of cadmium by a halophyte—Acanthus ilicifolius L. Int. J. Phytorem. 2017, 19, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Bankaji, I.; Caçador, I.; Sleimi, N. Physiological and biochemical responses of Suaeda fruticosa to cadmium and copper stresses: Growth, nutrient uptake, antioxidant enzymes, phytochelatin, and glutathione levels. Environ. Sci. Pollut. Res. 2015, 22, 13058–13069. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.T.; Akram, M.S.; Tanwir, K.; Chaudhary, H.J.; Ali, Q.; Stoltz, E.; Lindberg, S. Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotox. Environ. Safe 2017, 141, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Q.; Diao, C. Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L. Bioresour. Technol. 2008, 99, 1103–1110. [Google Scholar] [CrossRef]
- Ekmekçi, Y.; Tanyolac, D.; Ayhan, B. Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J. Plant Physiol. 2008, 165, 600–611. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef]
- Grill, E.; Löffler, S.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Chen, L. Growth and physiological responses of Pennisetum sp. to cadmium stress under three different soils. Environ. Sci. Pollut. Res. 2021, 28, 14867–14881. [Google Scholar] [CrossRef]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Effect of cadmium and lead on growth, biochemical parameters and uptake in Lemna polyrrhiza L. Plant Soil Environ. 2008, 54, 262. [Google Scholar] [CrossRef]
- Nedjimi, B.; Daoud, Y. Cadmium accumulation in Atriplex halimus subsp. schweinfurthii and its influence on growth, proline, root hydraulic conductivity and nutrient uptake. Flora 2009, 204, 316–324. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Wang, P.; Deng, X.; Huang, Y.; Fang, X.; Zhang, J.; Wan, H.; Yang, C. Root morphological responses of five soybean [Glycine max (L.) Merr] cultivars to cadmium stress at young seedlings. Environ. Sci. Pollut. Res. 2016, 23, 1860–1872. [Google Scholar] [CrossRef] [PubMed]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef]
- Haque, A.M.; Tasnim, J.; El-Shehawi, A.M.; Rahman, M.A.; Parvez, M.S.; Ahmed, M.B.; Kabir, A.H. The Cd-induced morphological and photosynthetic disruption is related to the reduced Fe status and increased oxidative injuries in sugar beet. Plant Physiol. Biochem. 2021, 166, 448–458. [Google Scholar] [CrossRef]
- Biswal, B.; Joshi, P.N.; Raval, M.K.; Biswal, U.C. Photosynthesis, a global sensor of environmental stress in green plants: Stress signalling and adaptation. Curr. Sci. India 2011, 47–56. [Google Scholar]
- Matysik, J.; Alia Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 525–532. [Google Scholar]
- Gallardo, M.; Turner, N.C.; Ludwig, C. Water relations, gas exchange and abscisic acid content of Lupinus cosentinii leaves in response to drying different proportions of the root system. J. Exp. Bot. 1994, 45, 909–918. [Google Scholar] [CrossRef]
- Lin , R.; Wang, X.; Luo, Y.; Du, W.; Guo, H.; Yin, D. Effects of soil cadmium on growth, oxidative stress and antioxidant system in wheat seedlings (Triticum aestivum L.). Chemosphere 2007, 69, 89–98. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).