Cucumber (Cucumis sativus L.) Growth and Productivity under Solar Radiation-Based Quantitative Nutrient Management in Hydroponic System
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Location and Experimental Facility
2.2. Plant Materials and Cultivation Method
2.3. Treatments
2.4. Measurements
2.4.1. Water and Nutrient Consumptions
2.4.2. Vegetative Growth Characteristics
2.4.3. Plant Physiology
2.4.4. Plant Productivity
2.4.5. Fruit Growth and Fruit Quality
2.5. Data Analysis
3. Results
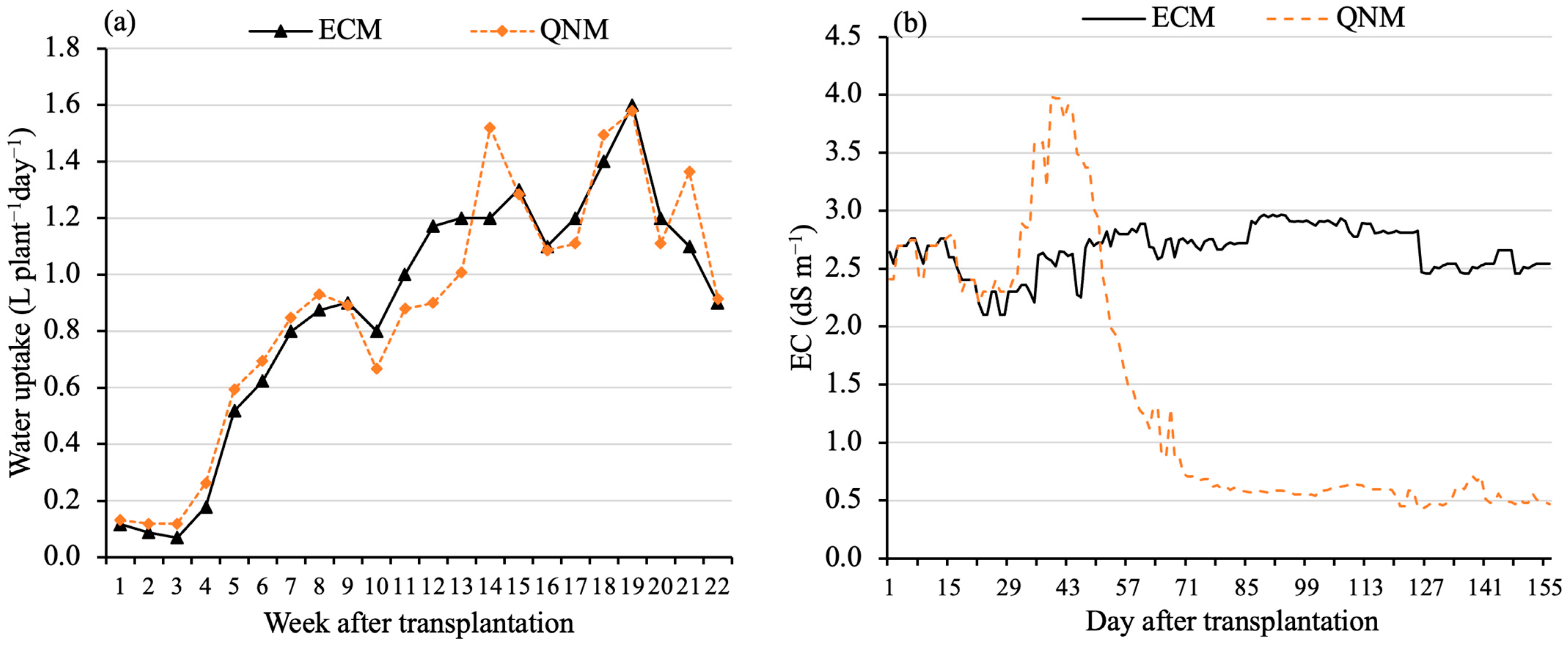
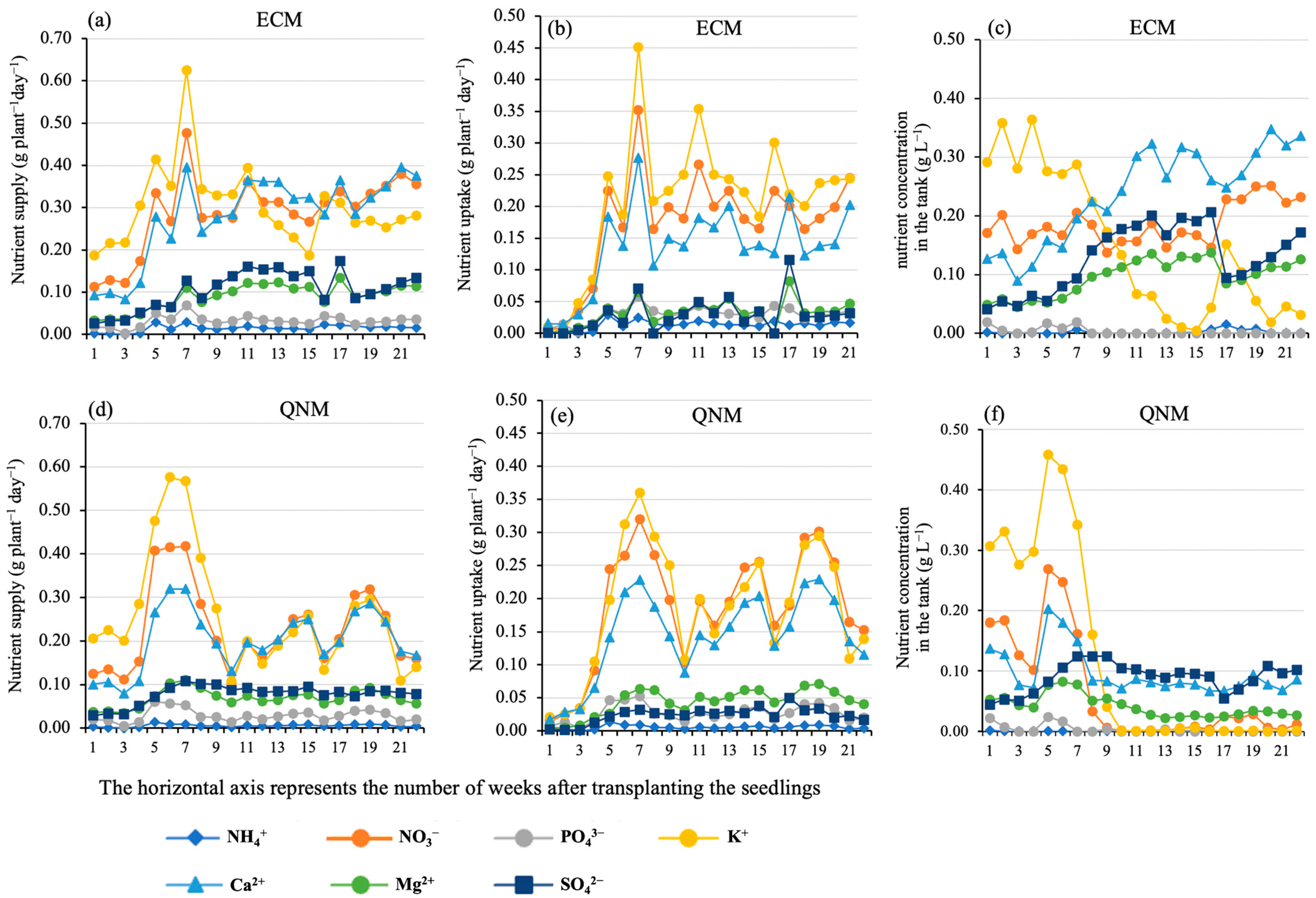
3.1. Water and Nutrient Consumptions
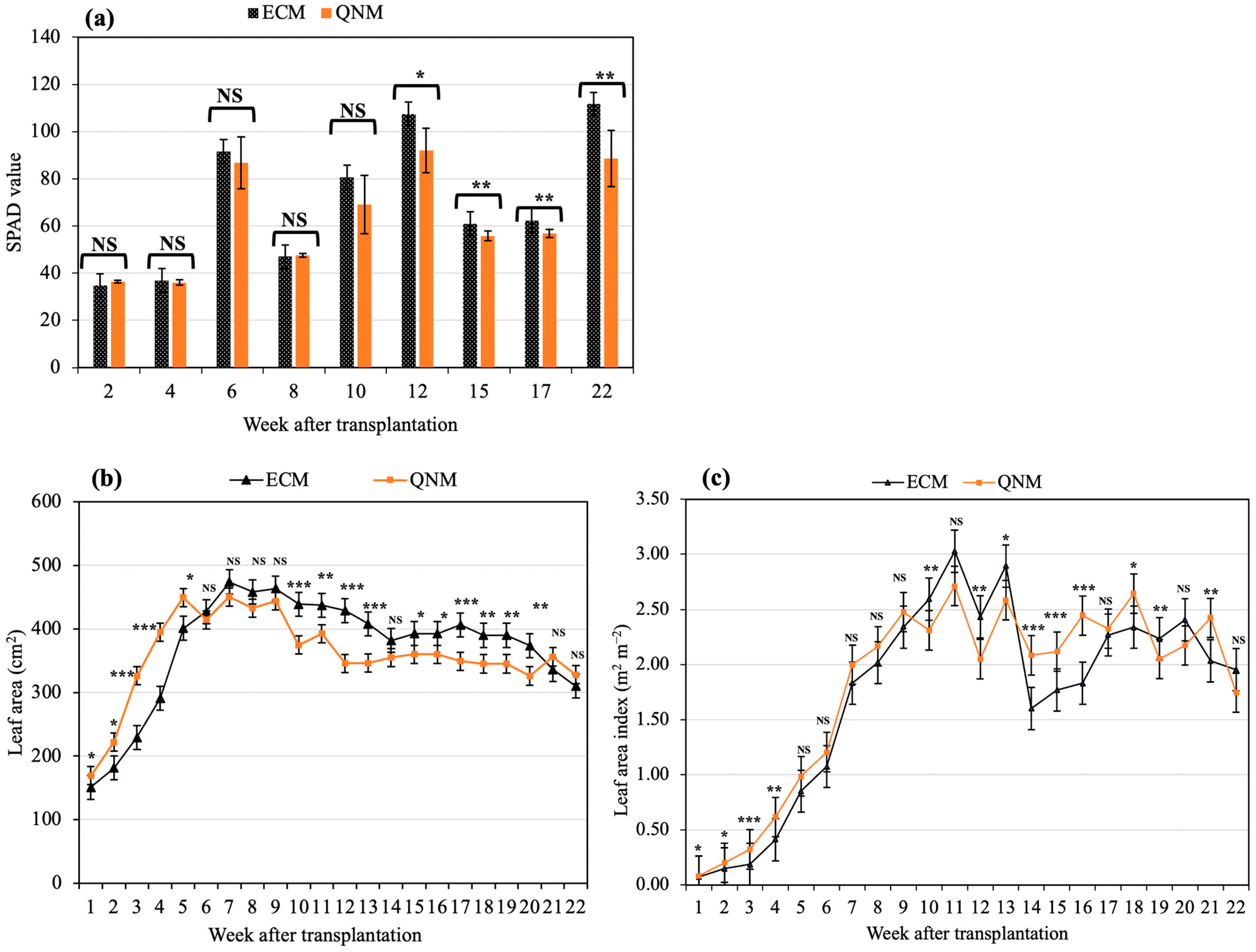
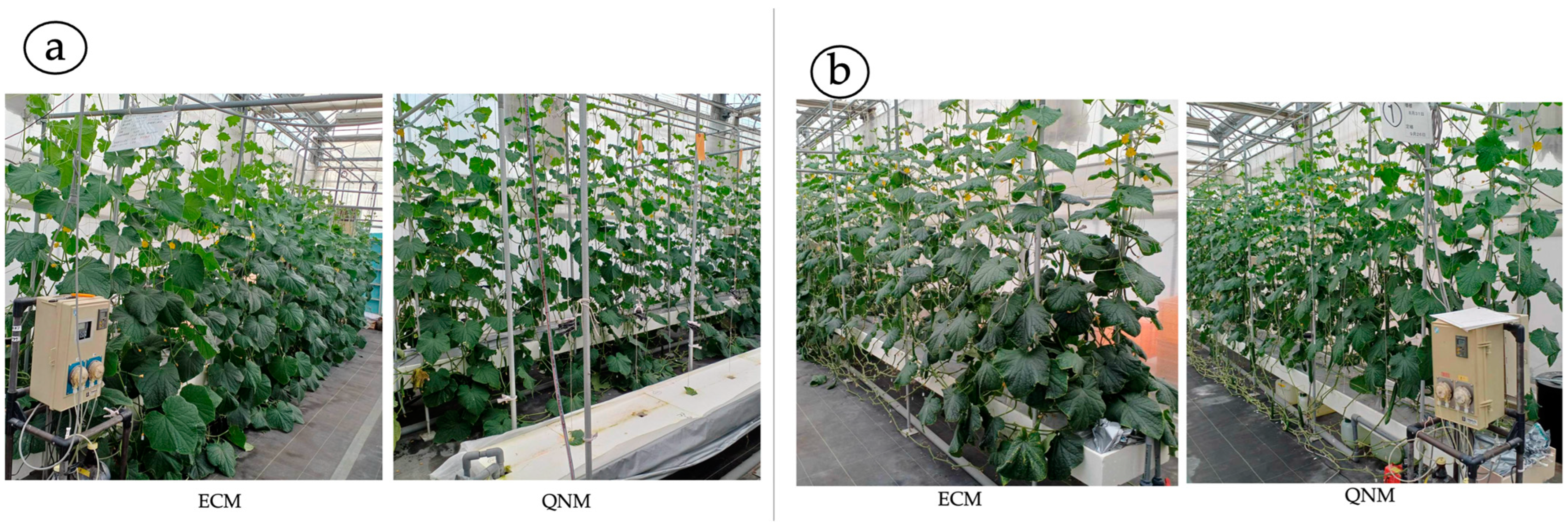
3.2. Vegetative Growth Characteristics
3.3. Plant Physiology
3.4. Plant Productivity and Dry Matter Partitioning
3.5. Fruit Growth and Fruit Quality
4. Discussion
4.1. Nutrient Wastes and Nutrient Use Efficiency
4.2. Vegetative Growth Characteristics
4.3. Plant Physiology
4.4. Plant Productivity
4.5. Fruit Growth and Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, D.; Andreev, K.; Dupre, M.E. Major trends in population growth around the world. China CDC Wkly. 2021, 3, 604–613. [Google Scholar] [CrossRef]
- Mok, W.K.; Tan, Y.X.; Chen, W.N. Technology innovations for food security in Singapore: A case study of future food systems for an increasingly natural resource-scarce world. Trends Food Sci. Technol. 2020, 102, 155–168. [Google Scholar] [CrossRef]
- Profiroiu, M.C.; Radulescu, C.V.; Burlacu, S.; Guţu, C. Changes and trends in the development of the world economy. In Competitivitatea şi Inovarea în Economia Cunoaşterii, 22nd ed.; Grigore Belostecinic, G., Guţu, C., Condraţchi, L., Bragoi, D., Feuraş, E., Copăceanu, C., Toacă, Z., Cobzari, L., Livandovschi, R., Zaporojan, V., et al., Eds.; Academia de Studii Economice a Moldovei (ASEM): Chişinău, Moldova, 2020; pp. 324–330. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2018. Building Climate Resilience for Food Security and Nutrition; FAO: Rome, Italy, 2018; pp. 1–162. [Google Scholar]
- FAO. Coping wsith Water Scarcity in Agriculture: A Global Framework for Action in A Changing Climate; FAO: Rome, Italy, 2016; pp. 1–12. [Google Scholar]
- Dolan, F.; Lamontagne, J.; Calvin, K.; Snyder, A.; Narayan, K.B.; Di Vittorio, A.V.; Vernon, C.R. Modeling the economic and environmental impacts of land scarcity under deep uncertainty. Earth’s Future 2022, 10, e2021EF002466. [Google Scholar] [CrossRef]
- Srivastava, P.; Giri, N.; Mandal, D. 137 Cs technology for soil erosion and soil carbon distribution. Curr. Sci. 2019, 116, 888–889. [Google Scholar]
- Sutton, M.A.; Bleeker, A.; Howard, C.; Erisman, J.; Abrol, Y.; Bekunda, M.; Datta, A.; Davidson, E.; De Vries, W.; Oenema, O. Our Nutrient World. The Challenge to Produce More Food & Energy with Less Pollution; Centre for Ecology & Hydrology: Edinburgh, UK, 2013. [Google Scholar]
- Roberts, T.L.; Johnston, A.E. Phosphorus use efficiency and management in agriculture. Resour. Conserv. Recycl. 2015, 105, 275–281. [Google Scholar] [CrossRef]
- United States Geological Survey. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/phosphate_rock/myb1-2015-phosp.pdf (accessed on 13 March 2023).
- Bekunda, M.; Cordell, D.; Corman, J.; Rosemarin, A.; Johnston, J.; Salcedo, I.; Syers, K. Phosphorus and food production. In UNEP Year Book 2011: Emerging Issues in Our Global Environment; Goverse, T., Bech, S., Eds.; United Nations Environment Programme: Nairobi, Kenya, 2011; pp. 35–46. [Google Scholar]
- Al Rawashdeh, R. World peak potash: An analytical study. Resour. Policy 2020, 69, 101834. [Google Scholar] [CrossRef]
- Smith, P.; Gregory, P.J. Climate change and sustainable food production. Proc. Nutr. Soc. 2013, 72, 21–28. [Google Scholar] [CrossRef]
- Garnett, T.; Appleby, M.C.; Balmford, A.; Bateman, I.J.; Benton, T.G.; Bloomer, P.; Burlingame, B.; Dawkins, M.; Dolan, L.; Fraser, D.; et al. Sustainable intensification in agriculture: Premises and policies. Science 2013, 341, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Feeding 11 billion on 0.5 billion hectare of area under cereal crops. Food Energy Secur. 2016, 5, 239–251. [Google Scholar] [CrossRef]
- Khan, S.; Purohit, A.; Vadsaria, N. Hydroponics: Current and future state of the art in farming. J. Plant Nutr. 2021, 44, 1515–1538. [Google Scholar] [CrossRef]
- Butler, J.D.; Oebker, N.F. Hydroponics as a Hobby—Growing Plants without Soil; Circular 844; Information Office, College of Agriculture, University of Illinois: Urbana, IL, USA, 2006. [Google Scholar]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.P.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Bradley, P.; Marulanda, C. Simplified hydroponics to reduce global hunger. Acta Hortic. 2001, 554, 289–296. [Google Scholar] [CrossRef]
- Sheikh, B.A. Hydroponics: Key to sustain agriculture in water stressed and urban environment. Pak. J. Agric. Agric. Eng. Vet. Sci. 2006, 22, 53–57. [Google Scholar]
- Jovicich, E.; Cantliffe, D.J.; Simonne, E.H.; Stoffella, P.J. Comparative water and fertilizer use efficiencies of two productionsystems for cucumbers. Acta Hortic. 2007, 731, 235–241. [Google Scholar] [CrossRef]
- Patel, C.; Panigrahi, J. Starch glucose coating-induced postharvest shelf-life extension of cucumber. Food Chem. 2019, 288, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Wehner, T.C. Cucumbers, watermelon, squash and other cucurbits. In Encyclopedia of Food and Culture; University of Michigan: Ann Arbor, MI, USA, 2007; pp. 474–479. [Google Scholar]
- Pardossi, A.; Malorgio, F.; Tognoni, F. Control of mineral nutrition in melon plants grown with nft. Acta Hortic. 1995, 396, 173–180. [Google Scholar] [CrossRef]
- Samarakoon, U.C.; Weerasinghe, P.A.; Weerakkody, A.P. Effect of electrical conductivity [EC] of the nutrient solution on nutrientuptake, growth and yield of leaf lettuce (Lactuca sativa L.) in stationary culture. Trop. Agric. Res. 2006, 18, 13–21. [Google Scholar]
- Tsukagoshi, S.; Shinohara, Y. nutrition and nutrient uptake in soilless culture systems. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 1st ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 165–172. [Google Scholar]
- Bugbee, B. Nutrient management in recirculating hydroponic culture. Acta Hortic. 2004, 648, 99–112. [Google Scholar] [CrossRef]
- Nakano, Y.; Sasaki, H.; Nakano, A.; Suzuki, K.; Takaichi, M. Growth and yield of tomato plants as influenced by nutrient application rates with quantitative control in closed rockwool cultivation. J. Jpn. Soc. Hortic. Sci. 2010, 79, 47–55. [Google Scholar] [CrossRef]
- Matsuda, R.; Suzuki, K.; Nakano, Y.; Sasaki, H.; Takaichi, M. Nutrient supply and fruit yields in tomato rockwool hydroponics under daily quantitative nutrient management: Analysis and evaluation based on leaf area index. J. Agric. Meteorol. 2011, 67, 117–126. [Google Scholar] [CrossRef]
- Ren, X.; Lu, N.; Xu, W.; Zhuang, Y.; Tsukagoshi, S.; Takagaki, M. Growth and nutrient utilization in basil plant as affected by applied nutrient quantity in nutrient solution and light spectrum. Biology 2022, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Watanabe, S.I.; Kawashima, H.; Takaichi, M. The effect of daily nutrientapplications on yield, fruit quality, and nutrient uptake of hydroponically cultivated tomato. J. Jpn. Soc. Hortic. Sci. 2006, 75, 421–429. [Google Scholar] [CrossRef]
- Paradossi, A.; Malorgio, F.; Incrocci, L.; Campiotti, C.; Tognoni, F. A comparison between two methods to control nutrient delivery togreenhouse melons grown in recirculating nutrient solution culture. Sci. Hortic. 2002, 92, 82–95. [Google Scholar] [CrossRef]
- Maruo, T.; Hoshi, H.; Hohjo, M.; Shinohara, Y.; Ito, T. Quantitative nutrient management at low concentration condition in NFT spinach culture. Acta Hortic. 2001, 548, 133–140. [Google Scholar] [CrossRef]
- Samba, N.; Nunomura, O.; Nakano, A.; Tsukagoshi, S. Effective training methods for cucumber production in newly developed nutrient film technique hydroponic system. Horticulturae 2023, 9, 478. [Google Scholar] [CrossRef]
- Gislerød, H.R.; Adams, P. Diurnal variations in the oxygen content and acid requirement of recirculating nutrient solutions and in the uptake of water and potassium by cucumber and tomato plants. Sci. Hortic. 1983, 21, 311–321. [Google Scholar] [CrossRef]
- Robbins, N.S.; Pharr, D.M. Leaf area prediction methods for cucumber from linear measurements. Hort. Sci. 1987, 22, 1264–1266. [Google Scholar]
- Maeda, K.; Ahn, D.-H. A review of japanese greenhouse cucumber research from the perspective of yield components. Hortic. J. 2021, 90, 263–269. [Google Scholar] [CrossRef]
- Bumgarner, N.R.; Kleinhenz, M.D. Using Brix as An Indicator of Vegetable Quality: Instructions for Measuring Brix in Cucumber, Leafy Greens, Sweet Corn, Tomato, and Watermelon; Department of Horticulture and Crop Science, The Ohio State University: Columbus, OH, USA, 2012; Fact Sheet HYG-1653-12. [Google Scholar]
- Eboibi, O.; Uguru, H. Effect of moisture content on the mechanical properties of cucumber fruit. Int. J. Scient. Eng. Res. 2018, 9, 671–678. [Google Scholar]
- Barbagallo, R.N.; Di Silvestro, I.; Patanè, C. Yield, physicochemical traits, antioxidant pattern, polyphenol oxidase activity and total visual quality of field-grown processing tomato cv. Brigade as affected by water stress in mediterranean climate. J. Sci. Food Agric. 2012, 93, 1449–1457. [Google Scholar] [CrossRef]
- Brunelle, T.; Dumas, P.; Souty, F.; Dorin, B.; Nadaud, F. Evaluating the impact of rising fertilizer prices on crop yields. Agric. Econ. 2015, 46, 653–666. [Google Scholar] [CrossRef]
- Kinoshita, T.; Masuda, M. Differential nutrient uptake and its transport in tomato plants on different fertilizer regimens. HortScience Horts 2011, 46, 1170–1175. [Google Scholar] [CrossRef]
- Savci, S. An agricultural pollutant: Chemical fertilizer. Int. J. Environ. Sci. Dev. 2012, 3, 73. [Google Scholar] [CrossRef]
- Buvaneshwari, S.; Riotte, J.; Sekhar, M.; Sharma, A.K.; Helliwell, R.; Kumar, M.S.; Ruiz, L. Potash nutrient promotes incipient salinization in groundwater irrigated semi-arid agriculture. Sci. Rep. 2020, 10, 3691. Available online: https://www.nature.com/articles/s41598-020-60365-z (accessed on 30 December 2023). [CrossRef] [PubMed]
- Chartzoulakis, K.S. Effect of NaCl salinity on germination, growth and yield of greenhouse cucumber. J. Hort. Sci. 1992, 67, 115–119. [Google Scholar] [CrossRef]
- Helal, M.; Koch, K.; Mengel, K. Effect of salinity and potassium on the uptake of nitrogen and on nitrogen metabolism in young barley plants. Physiol. Plant 1975, 35, 310–313. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plant. Oecologia 1989, 78, 9–19. Available online: https://www.jstor.org/stable/4218825 (accessed on 30 December 2023). [CrossRef] [PubMed]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 1992, 62, 365–392. [Google Scholar] [CrossRef]
- Shipley, B. Structural interspecific determinants of specific leaf area in 34 species of herbaceous angiosperms. Funct. Ecol. 1995, 9, 312–319. [Google Scholar] [CrossRef]
- Papadopoulos, A.P.; Pararajasingham, S. The influence of plant spacing on light interception and use in greenhouse tomato (Lycopersicon esculentum Mill.): A review. Sci. Hortic. 1997, 69, 1–29. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [CrossRef]
- Qunchu, Z. Studies on genetic parameters of main characters of hot pepper and application to breeding. Acta Hortic. 1995, 402, 293–298. [Google Scholar] [CrossRef]
- Kumar, S.P.N.B.; Saravaiya, S.N. Response of parthenocarpic cucumber to fertilizers and training systems under naturally ventilated polyhouse in subtropical conditions. Int. J. Cur. Res. 2014, 6, 8051–8057. [Google Scholar]
- Xin, T.; Zhang, Z.; Li, S.; Zhang, S.; Li, Q.; Zhang, Z.H.; Yang, X. Genetic regulation of ethylene dosage for cucumber fruit elongation. Plant Cell. 2019, 31, 1063–1076. [Google Scholar] [CrossRef]
- Premalatha, M.G.S.; Wahundeniya, K.B.; Weerakkody, W.A.P.; Wicramathunga, C.K. Plant training and spatial arrangement for yield improvements in greenhouse Cucumber (Cucumis sativus L.) varieties. Trop. Agric. Res. 2006, 18, 346–357. [Google Scholar]
- Chaverria, C.J.; Hochmuth, G.J.; Hochmuth, R.C.; Sargent, S.A. Fruit yield, size, and color responses of two greenhouse cucumber types to nitrogen fertilization in perlite soilless culture. HortTechnology 2005, 15, 565–571. [Google Scholar] [CrossRef]
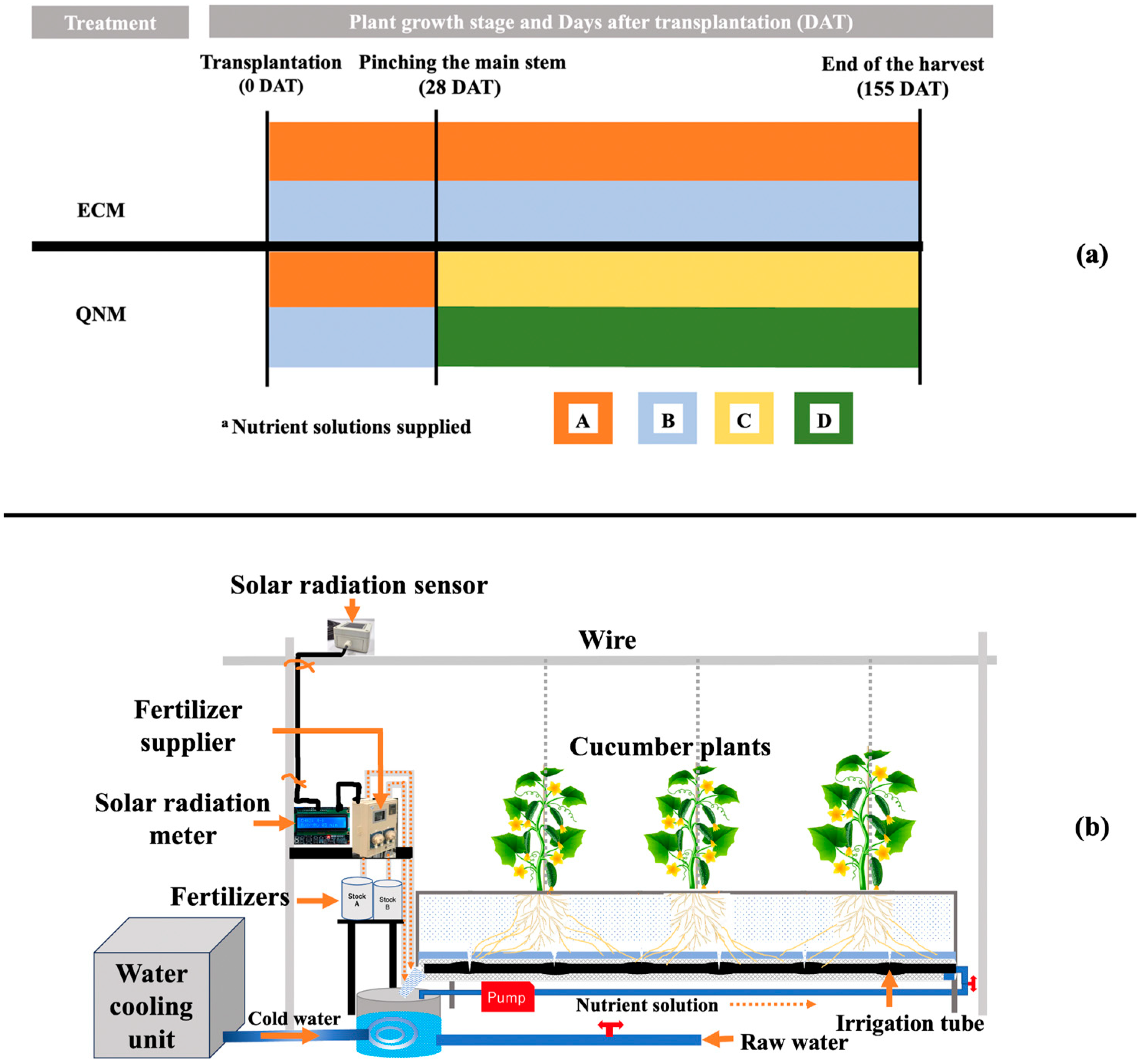


| Solution | Components and Concentrations (g L−1) | ||||||
|---|---|---|---|---|---|---|---|
| Ca (NO3)2·4H2O | KNO3 | NH4H2PO4 | MgSO4·7H2O | K2SO4 | KH2PO4 | Pre-Mixed Micronutrients | |
| A | 0 | 0.81 | 0.17 | 0.51 | 0.05 | 0 | 0.05 |
| B | 1.30 | 0 | 0 | 0 | 0 | 0 | 0 |
| C | 0 | 1.96 | 0.21 | 1.22 | 0 | 0.27 | 0.14 |
| D | 2.86 | 0 | 0 | 0 | 0 | 0 | 0 |
| Treatment | Nutrient Use Efficiency (NUE) (g Nutrient kg−1 Fruit) | Nutrient Waste (NW) (g Nutrient kg−1 Fruit) | WUE (L kg−1 Fruit) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | PO43− | K+ | Ca2+ | Mg2+ | SO42− | N | PO43− | K+ | Ca2+ | Mg2+ | SO42− | ||
| ECM | 6.0 ± 0.2 | 0.62 ± 0.02 | 5.9 ± 0.2 | 5.6 ± 0.2 | 1.8 ± 0.06 | 2.1 ± 0.07 | 2.2 ± 0.08 | 0.038 ± 0 | 1.8 ± 0.06 | 2.8 ± 0.1 | 0.23 ± 0.02 | 0.27 ± 0.02 | 17.3 ± 0.6 |
| QNM | 4.4 ± 0.2 | 0.60 ± 0.01 | 5.0 ± 0.2 | 3.9 ± 0.1 | 1.3 ± 0.05 | 1.5 ± 0.05 | 0.8 ± 0.03 | 0.040 ± 0 | 1.4 ± 0.04 | 1.1 ± 0.03 | 0.17 ± 0.01 | 0.19 ± 0.01 | 16.9 ± 0.6 |
| Significance | ** | * | ** | ** | ** | ** | ** | NS | ** | ** | ** | ** | NS |
| Reduction (%) | 26.7 | 3.2 | 15.3 | 30.4 | 27.8 | 28.6 | 66.4 | 0 | 21.2 | 60.7 | 27.9 | 30.2 | 2.3 |
| Treatment | Total Yield (kg plant−1) | Marketable Yield (kg Plant−1) | Total Fruits Number (Fruits Plant−1) | Marketable Fruits Number (Fruits Plant−1) | Non-Marketable Fruits Rate Per Plant (%) | Node Number Plant−1 | Total Plant Length (m Plant−1) | Main Stem Diameter (mm) |
|---|---|---|---|---|---|---|---|---|
| ECM | 9.0 ± 0.3 | 8.0 ± 0.3 | 91.4 ± 3.1 | 76.0 ± 2.6 | 16.8 ± 1.1 | 147 ± 2.6 | 18.1 ± 0.5 | 5.68 ± 0.05 |
| QNM | 9.1 ± 0.3 | 8.2 ± 0.2 | 86.5 ± 2.7 | 73.7 ± 2.1 | 14.6 ± 0.1 | 178 ± 6.1 | 19.8 ± 0.5 | 5.72 ± 0.06 |
| Significance | NS | NS | NS | NS | NS | * | * | NS |
| Treatment | Leaf DW (g Plant−1) | Fruit DW (g Plant−1) | Stem DW (g Plant−1) | Root DW (g Plant−1) | TDM (g Plant−1) | Dry Matter Partitioning (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Leaves | Stem | Fruits | Root | ||||||
| ECM | 194.1 ± 1.2 | 316.1 ± 10.4 | 56.0 ± 0.1 | 11.2 ± 0.8 | 577.4 ± 11.8 | 33.7 ± 0.5 | 9.8 ± 0.2 | 54.5 ± 0.7 | 2.00 ± 0.04 |
| QNM | 228.5 ± 3.0 | 309.9 ± 9.9 | 65.8 ± 0.2 | 12.8 ± 0.8 | 617.0 ± 12.5 | 37.1 ± 0.4 | 10.7 ± 0.2 | 50.1 ± 0.7 | 2.09 ± 0.05 |
| Significance | *** | NS | *** | NS | * | *** | ** | *** | * |
| Treatment | Fruit Length (cm) | Fruit Shape Index | Fruit Diameter (mm) |
|---|---|---|---|
| ECM | 23.3 ± 0.06 | 0.939 ± 0.001 | 27.38 ± 0.06 |
| QNM | 22.4 ± 0.09 | 0.941 ± 0.001 | 27.29 ± 0.07 |
| Significance | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samba, N.; Nunomura, O.; Lu, N.; Johkan, M.; Nakano, A.; Tsukagoshi, S. Cucumber (Cucumis sativus L.) Growth and Productivity under Solar Radiation-Based Quantitative Nutrient Management in Hydroponic System. Agronomy 2024, 14, 296. https://doi.org/10.3390/agronomy14020296
Samba N, Nunomura O, Lu N, Johkan M, Nakano A, Tsukagoshi S. Cucumber (Cucumis sativus L.) Growth and Productivity under Solar Radiation-Based Quantitative Nutrient Management in Hydroponic System. Agronomy. 2024; 14(2):296. https://doi.org/10.3390/agronomy14020296
Chicago/Turabian StyleSamba, Nethone, Osamu Nunomura, Na Lu, Masahumi Johkan, Akimasa Nakano, and Satoru Tsukagoshi. 2024. "Cucumber (Cucumis sativus L.) Growth and Productivity under Solar Radiation-Based Quantitative Nutrient Management in Hydroponic System" Agronomy 14, no. 2: 296. https://doi.org/10.3390/agronomy14020296
APA StyleSamba, N., Nunomura, O., Lu, N., Johkan, M., Nakano, A., & Tsukagoshi, S. (2024). Cucumber (Cucumis sativus L.) Growth and Productivity under Solar Radiation-Based Quantitative Nutrient Management in Hydroponic System. Agronomy, 14(2), 296. https://doi.org/10.3390/agronomy14020296









