Microbiological Biostimulants in the Improvement of Extended Storage Quality of In Vitro-Derived Plants of Popular Ornamental Perennials
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Microbiological Preparations Used in This Study
- BactoFungiStop (BactoTech, Toruń, Poland), which contains a mixture of live soil bacteria (plant growth-promoting rhizobacteria of the Bacillus genus) collected and selected from natural habitats and probiotic microorganisms that restore the microbiological balance of the soil, support the decomposition of organic substances, stimulate the biosynthesis of phytohormones, vitamins, and amino acids, colonize plant tissue, and induce plant systemic responses, as recommended by the manufacturer for preventing fungal infections; concentration: 1.0% (v/v).
- AzotoPower (BioLider, Łódź, Poland), which contains a high concentration of isolates of bacteria from the Azotobacter and Arthrobacter genera, supports the fixation of atmospheric nitrogen and makes it available to plants, thereby stimulating plant growth, enhancing the biosynthesis of phytohormones, improving the development of root systems and the efficacy of plant nutrition, and supporting plant stress resistance; concentration: 0.05% (w/v).
- Guard (Target, Kartoszyno, Poland), which contains, according to the manufacturer’s description, “useful strains of bacteria” that restore the microflora of above-ground parts of plants, protect the plants against the development of diseases, and accelerate the regeneration of plants after stress of various origins; concentration: 0.4% (w/v).
2.3. Biometrical and Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilkins, H.; Anderson, N.O. Creation of New Floral Products. In Flower Breeding and Genetics; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Kowalska, J.; Antkowiak, M.; Tymoszuk, A. Effect of Plant Seed Mixture on Overwintering and Floristic Attractiveness of the Flower Strip in Western Poland. Agriculture 2023, 13, 467. [Google Scholar] [CrossRef]
- Shim, D.; Ko, J.-H.; Kim, W.-K.; Wang, Q.; Keathley, D.E.; Han, K.-H. A molecular framework for seasonal growth-dormancy regulation in perennial plants. Hort. Res. 2014, 1, 14059. [Google Scholar] [CrossRef]
- Clapa, D.; Hârța, M.; Szabo, K.; Teleky, B.-E.; Pamfil, D. The Use of Wheat Starch as Gelling Agent for In Vitro Proliferation of Blackberry (Rubus fruticosus L.) Cultivars and the Evaluation of Genetic Fidelity after Repeated Subcultures. Horticulturae 2023, 9, 902. [Google Scholar] [CrossRef]
- Papafotiou, M.; Vlachou, G.; Martini, A.N. Investigation of the Effects of the Explant Type and Different Plant Growth Regulators on Micropropagation of Five Mediterranean Salvia spp. Native to Greece. Horticulturae 2023, 9, 96. [Google Scholar] [CrossRef]
- Haida, Z.; Sinniah, U.R.; Nakasha, J.J.; Hakiman, M. Shoot Induction, Multiplication, Rooting and Acclimatization of Black Turmeric (Curcuma caesia Roxb.): An Important and Endangered Curcuma Species. Horticulturae 2022, 8, 740. [Google Scholar] [CrossRef]
- Koufan, M.; Belkoura, I.; Mazri, M.A. In Vitro Propagation of Caper (Capparis spinosa L.): A Review. Horticulturae 2022, 8, 737. [Google Scholar] [CrossRef]
- Pourhassan, A.; Kaviani, B.; Kulus, D.; Miler, N.; Negahdar, N. A Complete Micropropagation Protocol for Black-Leaved Zamioculcas zamiifolia (Lodd.) Engl. ‘Dowon’. Horticulturae 2023, 9, 422. [Google Scholar] [CrossRef]
- Erol, M.H.; Dönmez, D.; Biçen, B.; Şimşek, Ö.; Kaçar, Y.A. Modern Approaches to In Vitro Clonal Banana Production: Next-Generation Tissue Culture Systems. Horticulturae 2023, 9, 1154. [Google Scholar] [CrossRef]
- Ling, W.T.; Tan, L.V.; Khor, S.P.; Sriskanda, D.; Subramaniam, S.; Chew, B.L. Rapid In Vitro Propagation of Fig (Ficus carica L.) ‘Violette de Solliès’ Supported by Molecular and Microscopy Analyses. Horticulturae 2022, 8, 1025. [Google Scholar] [CrossRef]
- Ribeiro, H.; Ribeiro, A.; Pires, R.; Cruz, J.; Cardoso, H.; Barroso, J.M.; Peixe, A. Ex Vitro Rooting and Simultaneous Micrografting of the Walnut Hybrid Rootstock ‘Paradox’ (Juglans hindsi × Juglans regia) cl. ‘Vlach’. Agronomy 2022, 12, 595. [Google Scholar] [CrossRef]
- Salgado Pirata, M.; Correia, S.; Canhoto, J. Ex Vitro Simultaneous Acclimatization and Rooting of In Vitro Propagated Tamarillo Plants (Solanum betaceum Cav.): Effect of the Substrate and Mineral Nutrition. Agronomy 2022, 12, 1082. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Kulus, D.; Błażejewska, A.; Nadolan, K.; Kulpińska, A.; Pietrzykowski, K. Application of Wide-spectrum Light-emitting Diodes in the Indoor Production of Cucumber and Tomato Seedlings. Acta Agrobot. 2023, 76, 762. [Google Scholar] [CrossRef]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. The Role of Blue and Red Light in the Orchestration of Secondary Metabolites, Nutrient Transport and Plant Quality. Plants 2023, 12, 2026. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review Report on the Role of Bioproducts, Biopreparations, Biostimulants and Microbial Inoculants in Organic Production of Fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil Microbiome: Diversity, Benefits and Interactions with Plants. Sustainability 2023, 15, 14643. [Google Scholar] [CrossRef]
- Wijesekara, T.; Xu, B. Health-Promoting Effects of Bioactive Compounds from Plant Endophytic Fungi. J. Fungi 2023, 9, 997. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B.; Skiba, D.; Barbaś, P.; Noaema, A.H. The Use of Chlorophyll Fluorescence as an Indicator of Predicting Potato Yield, Its Dry Matter and Starch in the Conditions of Using Microbiological Preparations. Appl. Sci. 2023, 13, 10764. [Google Scholar] [CrossRef]
- Szczech, M.; Maciorowski, R. Microencapsulation Technique with Organic Additives for Biocontrol Agents. J. Hort. Res. 2016, 24, 111–122. [Google Scholar] [CrossRef]
- Patel, C.; Singh, J.; Karunakaran, A.; Ramakrishna, W. Evolution of Nano-Biofertilizer as a Green Technology for Agriculture. Agriculture 2023, 13, 1865. [Google Scholar] [CrossRef]
- Lakhdar, A.; Trigui, M.; Montemurro, F. An Overview of Biostimulants’ Effects in Saline Soils. Agronomy 2023, 13, 2092. [Google Scholar] [CrossRef]
- Bauza-Kaszewska, J.; Breza-Boruta, B.; Lemańczyk, G.; Lamparski, R. Effects of Eco-Friendly Product Application and Sustainable Agricultural Management Practices on Soil Properties and Phytosanitary Condition of Winter Wheat Crops. Sustainability 2022, 14, 15754. [Google Scholar] [CrossRef]
- Castronovo, L.M.; Vassallo, A.; Mengoni, A.; Miceli, E.; Bogani, P.; Firenzuoli, F.; Fani, R.; Maggini, V. Medicinal Plants and Their Bacterial Microbiota: A Review on Antimicrobial Compounds Production for Plant and Human Health. Pathogens 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting Plant-Microbe Interactions and Microbial Consortia Application for Enhancing Sustainable Agriculture: A Review. Front. Microbiol. 2020, 11, 560406. [Google Scholar] [CrossRef] [PubMed]
- Yurgel, S.N.; Ajeethan, N.; Smertenko, A. Response of Plant-Associated Microbiome to Plant Root Colonization by Exogenous Bacterial Endophyte in Perennial Crops. Front. Microbiol. 2022, 13, 863946. [Google Scholar] [CrossRef] [PubMed]
- Prisa, D.; Fresco, R.; Spagnuolo, D. Microbial Biofertilisers in Plant Production and Resistance: A Review. Agriculture 2023, 13, 1666. [Google Scholar] [CrossRef]
- Song, Q.; Song, X.S.; Deng, X.; Luo, J.Y.; Song, R.Q. Effects of Plant Growth Promoting Rhizobacteria Microbial on the Growth, Rhizosphere Soil Properties, and Bacterial Community of Pinus sylvestris var. mongolica Seedlings. Scand. J. Res. 2021, 36, 249–262. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Mourouzidou, S.; Ntinas, G.K.; Tsaballa, A.; Monokrousos, N. Introducing the Power of Plant Growth Promoting Microorganisms in Soilless Systems: A Promising Alternative for Sustainable Agriculture. Sustainability 2023, 15, 5959. [Google Scholar] [CrossRef]
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in Plant Growth and Development: Roles in Abiotic Stress Tolerance and Secondary Metabolites Secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef]
- Safari Motlagh, M.R.; Farokhzad, M.; Kaviani, B.; Kulus, D. Endophytic Fungi as Potential Biocontrol Agents against Sclerotium rolfsii Sacc.—The Causal Agent of Peanut White Stem Rot Disease. Cells 2022, 11, 2643. [Google Scholar] [CrossRef] [PubMed]
- Gianoli, E.; González-Teuber, M.; Vilo, C.; Guevara-Araya, M.J.; Escobedo, V.M. Endophytic Bacterial Communities are Associated with Leaf Mimicry in the Vine Boquila trifoliolata. Sci. Rep. 2021, 11, 22673. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, J.; Truba, M.; Vasileva, V. The Impact of Auxin and Cytokinin on the Growth and Development of Selected Crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- Roca, M.; Pérez-Gálvez, A. Metabolomics of Chlorophylls and Carotenoids: Analytical Methods and Metabolome-Based Studies. Antioxidants 2021, 10, 1622. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Q.; Ding, C.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Z.; Chen, Y.; et al. Effect of Low Temperature on Chlorophyll Biosynthesis and Chloroplast Biogenesis of Rice Seedlings during Greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef]
- Wu, X.; Khan, R.; Gao, H.; Liu, H.; Zhang, J.; Ma, X. Low Light Alters the Photosynthesis Process in Cigar Tobacco via Modulation of the Chlorophyll Content, Chlorophyll Fluorescence, and Gene Expression. Agriculture 2021, 11, 755. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhu, G.; Zhou, G.; Song, X.; Hussein Ibrahim, M.E.; Ibrahim Salih, E.G. Effect of N on Growth, Antioxidant Capacity, and Chlorophyll Content of Sorghum. Agronomy 2022, 12, 501. [Google Scholar] [CrossRef]

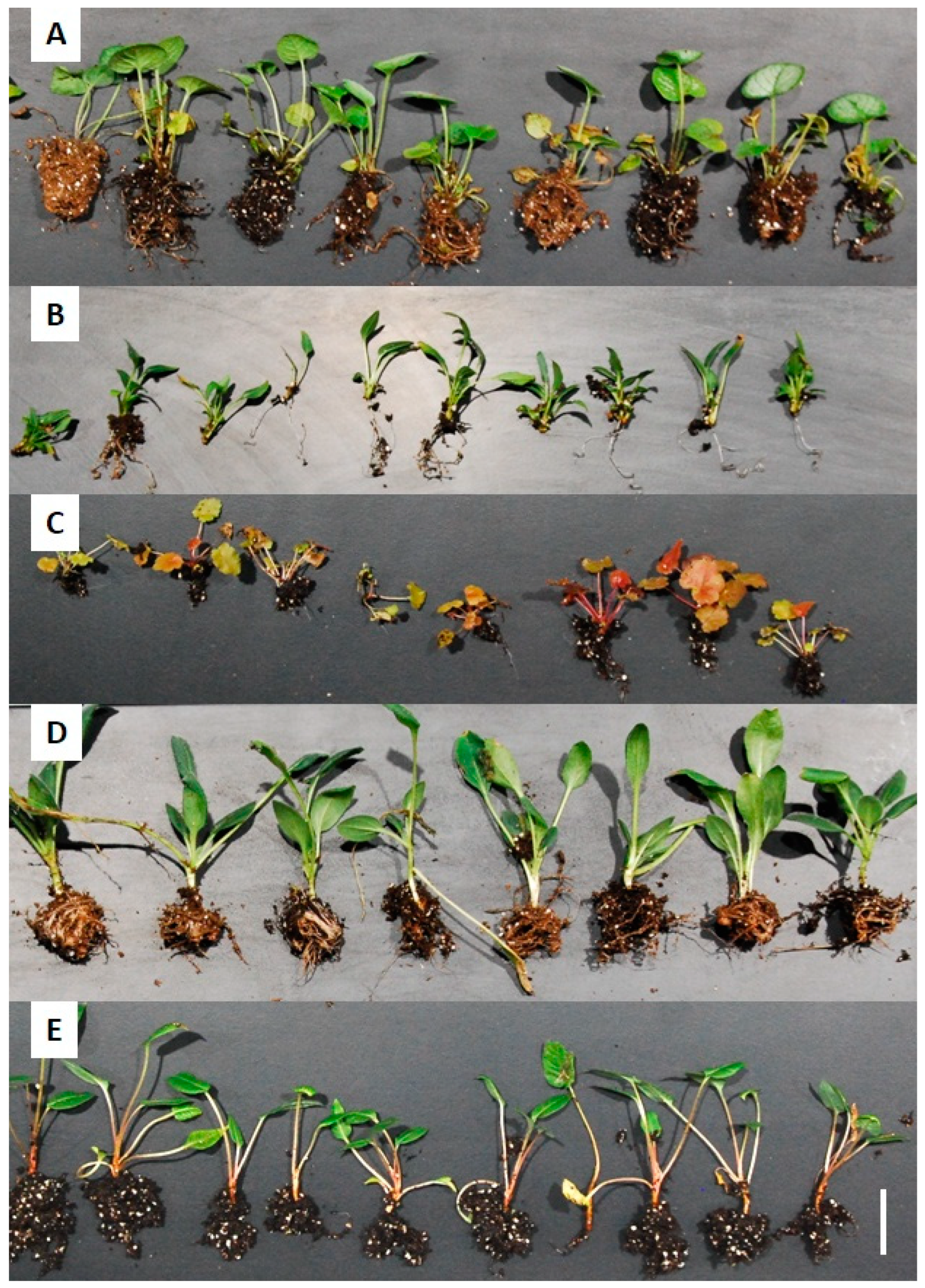

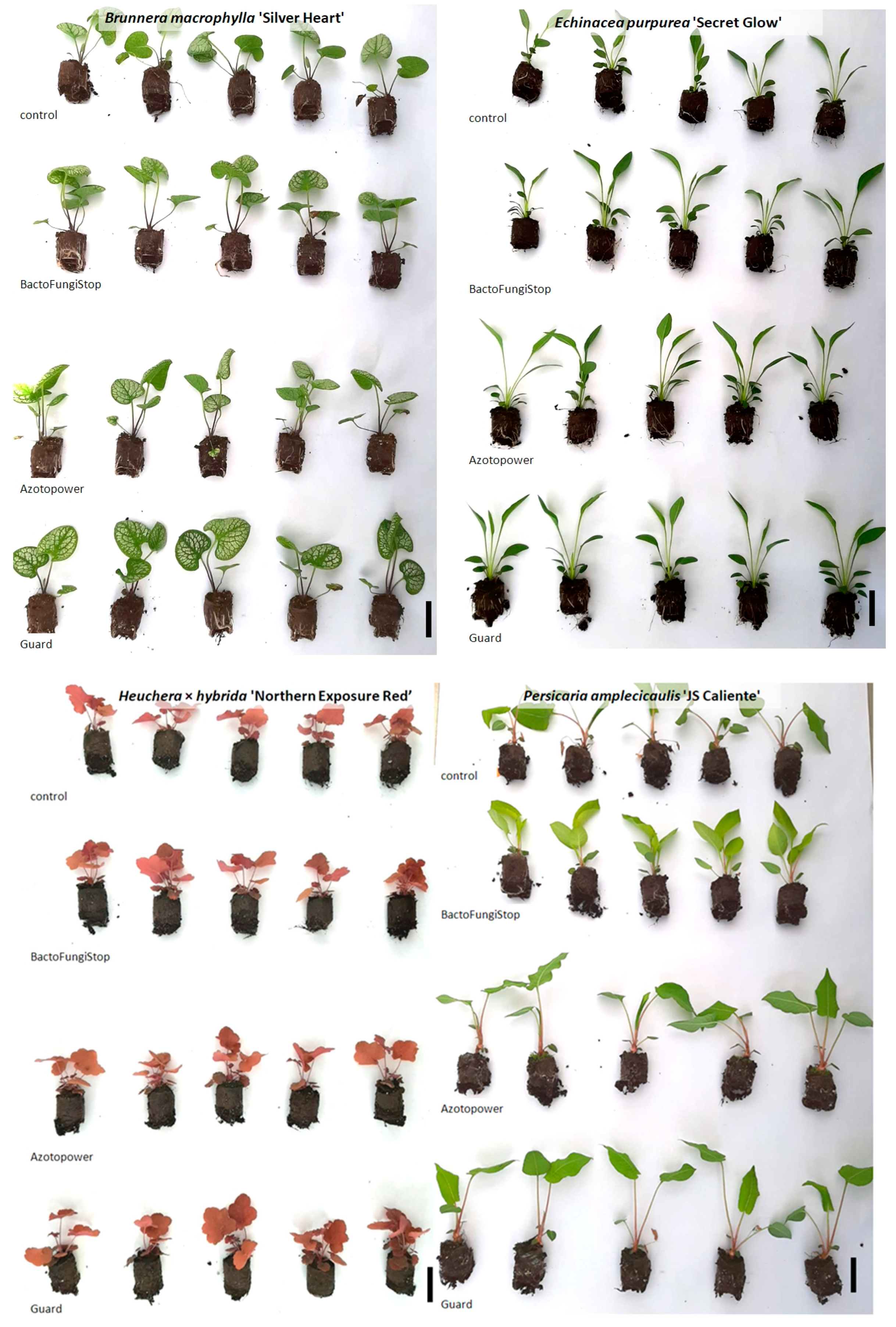

| Trait | Control | BactoFungiStop | AzotoPower | Guard |
|---|---|---|---|---|
| Shoot length (cm) | 11.1 ± 0.19 b | 12.6 ± 0.16 a | 12.6 ± 0.19 a | 12.5 ± 0.78 a |
| No. of leaves | 4.6 ± 0.60 ab | 3.6 ± 0.51 b | 5.4 ± 0.40 a | 3.8 ± 0.20 b |
| Chlorophyll content (CCI) | 14.2 ± 0.42 a | 15.0 ± 1.38 a | 15.8 ± 0.81 a | 16.6 ± 1.22 a |
| Leaf area (cm2) | 19.4 ± 0.62 a | 22.2 ± 2.31 a | 20.2 ± 2.02 a | 22.7 ± 0.62 a |
| Leaf max. width (cm) | 5.3 ± 0.06 a | 5.7 ± 0.34 a | 5.3 ± 0.31 a | 5.7 ± 0.13 a |
| Leaf avg. width (cm) | 2.0 ± 0.09 a | 2.1 ± 0.19 a | 1.9 ± 0.19 a | 2.2 ± 0.12 a |
| Leaf length (cm) | 10.2 ± 0.24 a | 11.4 ± 0.26 a | 11.3 ± 0.16 a | 11.1 ± 0.58 a |
| Leaf perimeter (cm) | 30.4 ± 0.38 a | 33.8 ± 0.84 a | 32.5 ± 0.91 a | 33.9 ± 1.28 a |
| Aspect ratio | 0.51 ± 0.01 a | 0.50 ± 0,02 a | 0.47 ± 0.02 a | 0.52 ± 0.03 a |
| Form coefficient | 20.4 ± 0.38 b | 33.8 ± 0.84 a | 32.5 ± 0.91 ab | 33.9 ± 1.28 a |
| Total root length (cm) | 233.4 ± 6.13 b | 357.0 ± 12.6 a | 341.9 ± 21.3 a | 373.2 ± 37.2 a |
| Root system area (cm2) | 45.0 ± 3.09 b | 65.8 ± 3.20 a | 71.2 ± 4.55 a | 69.5 ± 6.74 a |
| Root diameter (mm) | 0.613 ± 0.04 ab | 0.586 ± 0.01 b | 0.663 ± 0.01 a | 0.593 ± 0.01 b |
| Root system volume (cm3) | 0.70 ± 0.09 b | 0.97 ± 0.06 a | 1.18 ± 0.08 a | 1.03 ± 0.10 a |
| No. of forks in roots | 1141 ± 54 b | 1558 ± 80 a | 1512 ± 144 ab | 1846 ± 180 a |
| No. of root tips | 336 ± 42 c | 1463 ± 180 a | 938 ± 115 b | 1152 ± 197 ab |
| Trait | Control | BactoFungiStop | AzotoPower | Guard |
|---|---|---|---|---|
| Shoot length (cm) | 11.9 ± 0.51 c | 13.8 ± 0.54 b | 14.8 ± 0.45 ab | 15.9 ± 0.27 a |
| No. of leaves | 9.8 ± 0.58 a | 9.8 ± 0.37 a | 10.4 ± 0.51 a | 10.6 ± 0.51 a |
| Chlorophyll content (CCI) | 16.3 ± 0.95 a | 17.2 ± 0.83 a | 16.8 ± 0.84 a | 15.6 ± 0.49 a |
| Leaf area (cm2) | 6.6 ± 0.41 a | 8.7 ± 0.98 a | 8.5 ± 0.67 a | 9.1 ± 0.79 a |
| Leaf max. width (cm) | 1.9 ± 0.06 a | 2.1 ± 0.14 a | 2.0 ± 0.13 a | 2.1 ± 0.16 a |
| Leaf avg. width (cm) | 0.6 ± 0.03 a | 0.7 ± 0.05 a | 0.7 ± 0.05 a | 0.7 ± 0.05 a |
| Leaf length (cm) | 10.6 ± 0.60 b | 12.6 ± 0.67 b | 12.9 ± 0.18 b | 13.5 ± 0.22 a |
| Leaf perimeter (cm) | 23.6 ± 1.36 c | 27.7 ± 1.42 b | 29.5 ± 0.99 ab | 32.0 ± 0.59 a |
| Aspect ratio | 0.18 ± 0.01 a | 0.17 ± 0.01 a | 0.15 ± 0.01 a | 0.15 ± 0.01 a |
| Form coefficient | 23.6 ± 1.36 c | 27.7 ± 1.42 b | 29.5 ± 0.99 ab | 32.0 ± 0.59 a |
| Total root length (cm) | 94.4 ± 9.33 c | 167.0 ± 18.9 ab | 149.0 ± 16.5 bc | 221.4 ± 31.2 a |
| Root system area (cm2) | 16.9 ± 1.44 c | 28.9 ± 3.37 b | 27.8 ± 3.09 b | 40.4 ± 5.21 a |
| Root diameter (mm) | 0.556 ± 0.01 a | 0.550 ± 0.02 a | 0.595 ± 0.01 a | 0.580 ± 0.01 a |
| Root system volume (cm3) | 0.23 ± 0.02 c | 0.40 ± 0.05 b | 0.41 ± 0.05 b | 0.58 ± 0.07 a |
| No. of forks in roots | 351 ± 51 b | 624 ± 74 ab | 561 ± 78 ab | 889 ± 178 a |
| No. of root tips | 253 ± 33 b | 778 ± 98 a | 249 ± 30 b | 740 ± 139 a |
| Trait | Control | BactoFungiStop | AzotoPower | Guard |
|---|---|---|---|---|
| Shoot length (cm) | 6.7 ± 0.32 b | 8.2 ± 0.25 a | 8.3 ± 0.30 a | 8.5 ± 0.21 a |
| No. of leaves | 10.8 ± 1.83 a | 10.8 ± 2.13 a | 14.4 ± 2.56 a | 11.4 ± 1.43 a |
| Chlorophyll content (CCI) | 4.7 ± 1.51 a | 3.4 ± 0.23 a | 4.3 ± 1.10 a | 3.4 ± 0.30 a |
| Leaf area (cm2) | 11.3 ± 0.90 b | 13.6 ± 1.04 ab | 13.1 ± 1.32 ab | 15.0 ± 0.49 a |
| Leaf max. width (cm) | 3.6 ± 0.17 b | 4.0 ± 0.14 ab | 4.0 ± 0.21 ab | 4.2 ± 0.09 a |
| Leaf avg. width (cm) | 1.80 ± 0.12 a | 1.86 ± 0.08 a | 1.80 ± 0.11 a | 2.1 ± 0.06 a |
| Leaf length (cm) | 6.3 ± 0.22 b | 7.4 ± 0.28 a | 7.3 ± 0.37 a | 7.4 ± 0.15 a |
| Leaf perimeter (cm) | 19.1 ± 0.69 b | 22.3 ± 0.84 a | 22.6 ± 0.93 a | 23.4 ± 0.64 a |
| Aspect ratio | 0.57 ± 0.02 a | 0.55 ± 0.01 a | 0.54 ± 0.02 a | 0.58 ± 0.02 a |
| Form coefficient | 19.1 ± 0.69 b | 22.3 ± 0.84 a | 22.6 ± 0.93 a | 23.4 ± 0.64 a |
| Total root length (cm) | 116.8 ± 3.84 a | 114.4 ± 13.9 a | 121.3 ± 14.1 a | 111.9 ± 9.57 a |
| Root system area (cm2) | 16.9 ± 0.58 b | 18.5 ± 2.00 a | 20.2 ± 2.30 a | 19.5 ± 1.54 a |
| Root diameter (mm) | 0.460 ± 0.02 b | 0.518 ± 0.01 a | 0.533 ± 0.02 a | 0.558 ± 0.01 a |
| Root system volume (cm3) | 0.19 ± 0.01 a | 0.24 ± 0.02 a | 0.27 ± 0.03 a | 0.27 ± 0.02 a |
| No. of forks in roots | 619 ± 16 a | 629 ± 75 a | 647 ± 73 a | 570 ± 61 a |
| No. of root tips | 603 ± 91 a | 432 ± 86 a | 650 ± 110 a | 464 ± 49 a |
| Trait | Control | BactoFungiStop | AzotoPower | Guard |
|---|---|---|---|---|
| Shoot length (cm) | 14.3 ± 0.43 a | 12.0 ± 0.56 b | 15.4 ± 1.17 a | 16.5 ± 0.74 a |
| No. of leaves | 6.4 ± 0.40 ab | 7.6 ± 0.29 a | 5.4 ± 0.85 b | 5.6 ± 0.20 b |
| Chlorophyll content (CCI) | 6.7 ± 0.32 a | 3.5 ± 0.18 b | 6.7 ± 0.79 a | 6.6 ± 0.25 a |
| Leaf area (cm2) | 21.6 ± 2.04 a | 15.3 ± 0.44 b | 25.4 ± 1.44 a | 23.7 ± 1.22 a |
| Leaf max. width (cm) | 4.5 ± 0.17 a | 3.4 ± 0.02 b | 4.6 ± 0.12 a | 4.5 ± 0.13 a |
| Leaf avg. width (cm) | 1.8 ± 0.13 a | 1.4 ± 0.01 b | 1.9 ± 0.06 a | 1.7 ± 0.10 a |
| Leaf length (cm) | 11.9 ± 0.53 b | 10.8 ± 0.32 c | 13.4 ± 0.58 ab | 14.0 ± 0.67 a |
| Leaf perimeter (cm) | 32.6 ± 1.45 a | 25.9 ± 0.61 b | 33.6 ± 1.13 a | 35.3 ± 1.61 a |
| Aspect ratio | 0.38 ± 0.01 a | 0.31 ± 0.01 b | 0.34 ± 0.01 ab | 0.33 ± 0.02 b |
| Form coefficient | 32.6 ± 1.45 a | 25.9 ± 0.61 b | 33.65 ± 1.13 a | 35.32 ± 1.61 a |
| Total root length (cm) | 396.8 ± 43.2 b | 572.1 ± 59.9 a | 363.3 ± 40.2 b | 293.6 ± 28.4 b |
| Root system area (cm2) | 35.1 ± 3.53 b | 50.0 ± 6.02 a | 35.3 ± 4.21 b | 27.6 ± 3.10 b |
| Root diameter (mm) | 0.283 ± 0.01 b | 0.276 ± 0.01 b | 0.309 ± 0.01 a | 0.298 ± 0.01 b |
| Root system volume (cm3) | 0.25 ± 0.02 ab | 0.35 ± 0.05 a | 0.27 ± 0.04 ab | 0.21 ± 0.03 b |
| No. of forks in roots | 4172 ± 510 ab | 5143 ± 585 a | 3522 ± 361 b | 2824 ± 256 b |
| No. of root tips | 2145 ± 183 b | 5629 ± 829 a | 2967 ± 442 b | 1966 ± 235 b |
| Trait | Control | BactoFungiStop | AzotoPower | Guard |
|---|---|---|---|---|
| Shoot length (cm) | 9.7 ± 0.37 b | 12.5 ± 0.57 a | 12.7 ± 0.53 a | 12.8 ± 0.28 a |
| No. of leaves | 7.4 ± 0.40 b | 11.0 ± 0.55 a | 10.6 ± 0.75 a | 8.2 ± 0.58 b |
| Chlorophyll content (CCI) | 12.3 ± 1.27 a | 11.1 ± 0.57 a | 10.4 ± 0.56 a | 10.8 ± 0.88 a |
| Leaf area (cm2) | 14.5 ± 1.26 b | 19.1 ± 0.74 a | 18.9 ± 1.42 a | 19.2 ± 1.28 a |
| Leaf max. width (cm) | 3.2 ± 0.22 a | 3.7 ± 0.07 a | 3.2 ± 0.28 a | 3.7 ± 0.19 a |
| Leaf avg. width (cm) | 1.6 ± 0.11 a | 1.7 ± 0.06 a | 1.5 ± 0.06 a | 1.58 ± 0.07 a |
| Leaf length (cm) | 9.3 ± 0.36 b | 11.5 ± 0.44 a | 11.0 ± 1.13 ab | 12.1 ± 0.32 a |
| Leaf perimeter (cm) | 22.1 ± 0.81 b | 27.0 ± 0.90 a | 25.5 ± 2.58 ab | 28.2 ± 0.81 a |
| Aspect ratio | 0.35 ± 0.02 a | 0.32 ± 0.02 ab | 0.28 ± 0.01 b | 0.30 ± 0.01 ab |
| Form coefficient | 22.1 ± 0.81 b | 27.0 ± 0.90 a | 25.5 ± 2.58 ab | 28.3 ± 0.81 a |
| Total root length (cm) | 731.6 ± 72.8 a | 696.0 ± 75.9 ab | 717.3 ± 54.8 a | 505.2 ± 62.2 b |
| Root system area (cm2) | 128.8 ± 10.1 a | 124.3 ± 8.79 a | 121.0 ± 10.4 a | 90.9 ± 9.16 b |
| Root diameter (mm) | 0.566 ± 0.02 a | 0.581 ± 0.04 a | 0.536 ± 0.01 a | 0.580 ± 0.02 a |
| Root system volume (cm3) | 1.81 ± 0.14 a | 1.79 ± 0.14 a | 1.63 ± 0.16 ab | 1.31 ± 0.11 b |
| No. of forks in roots | 3885 ± 419 a | 3616 ± 596 a | 3573 ± 294 a | 2560 ± 380 a |
| No. of root tips | 2481 ± 800 a | 2994 ± 500 a | 3480 ± 470 a | 1861 ± 585 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miler, N.; Tymoszuk, A.; Woźny, A.; Michalik, T.; Wiśniewska, J.; Kulus, D. Microbiological Biostimulants in the Improvement of Extended Storage Quality of In Vitro-Derived Plants of Popular Ornamental Perennials. Agronomy 2024, 14, 289. https://doi.org/10.3390/agronomy14020289
Miler N, Tymoszuk A, Woźny A, Michalik T, Wiśniewska J, Kulus D. Microbiological Biostimulants in the Improvement of Extended Storage Quality of In Vitro-Derived Plants of Popular Ornamental Perennials. Agronomy. 2024; 14(2):289. https://doi.org/10.3390/agronomy14020289
Chicago/Turabian StyleMiler, Natalia, Alicja Tymoszuk, Anita Woźny, Tomasz Michalik, Justyna Wiśniewska, and Dariusz Kulus. 2024. "Microbiological Biostimulants in the Improvement of Extended Storage Quality of In Vitro-Derived Plants of Popular Ornamental Perennials" Agronomy 14, no. 2: 289. https://doi.org/10.3390/agronomy14020289
APA StyleMiler, N., Tymoszuk, A., Woźny, A., Michalik, T., Wiśniewska, J., & Kulus, D. (2024). Microbiological Biostimulants in the Improvement of Extended Storage Quality of In Vitro-Derived Plants of Popular Ornamental Perennials. Agronomy, 14(2), 289. https://doi.org/10.3390/agronomy14020289








