Isotopic Signatures and Fluxes of N2O Emitted from Soybean Plants and Soil During the Main Growth Period of Soybeans
Abstract
1. Introduction
2. Materials and Methods
2.1. Soybean Cultivation
2.2. N2O Emissions from Soybean Plants and from Soil
2.3. Gas Samples Analysis
2.4. Calculation Methods and Statistics
3. Results
3.1. N2O Emission Rates
3.2. δ15N and δ18O Signatures of N2O Emitted from Soil and Soybean
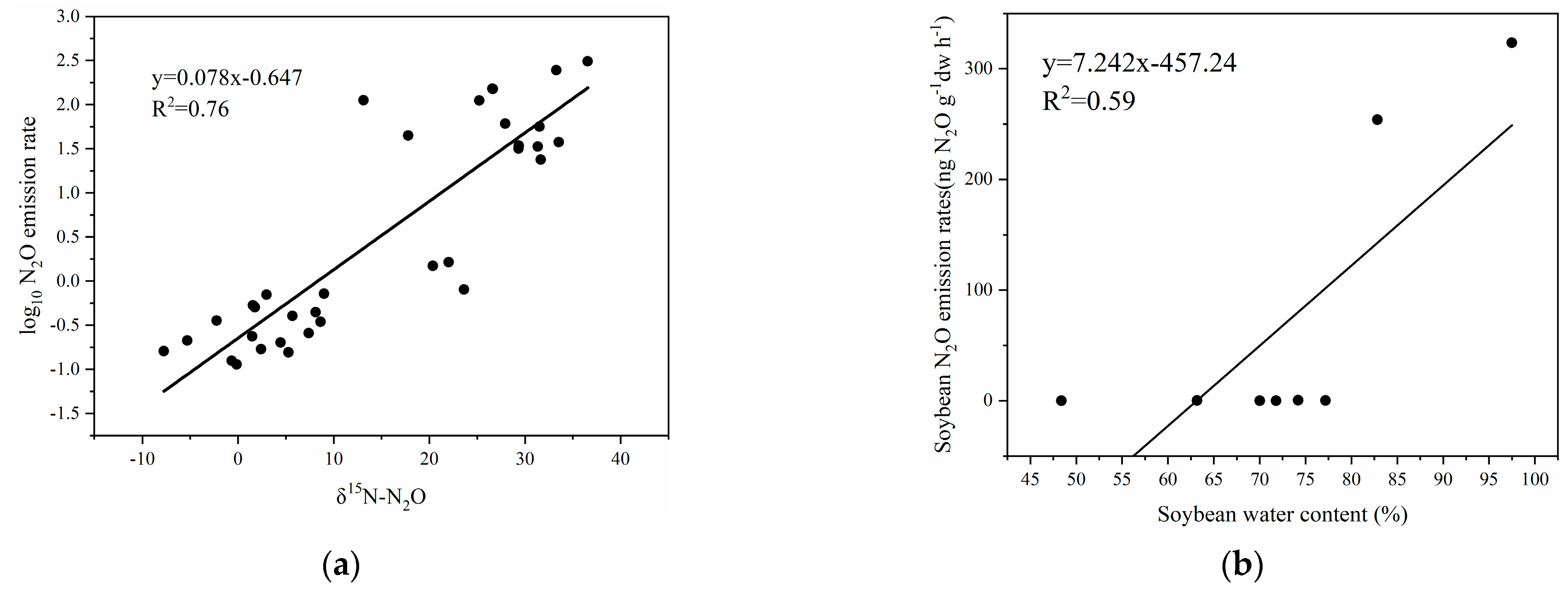
4. Discussion
4.1. Possible Processes of N2O Production by Plants
4.2. Plant N2O Fluxes in the Plant–Soil System
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chipperfield, M. Atmospheric science: Nitrous oxide delays ozone recovery. Nat. Geosci. 2009, 2, 742–743. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M. IPCC 2007: Climate Change 2007: The Physical Science Basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007; pp. 95–123. [Google Scholar]
- Davidson, E.A.; Kanter, D. Inventories and scenarios of nitrous oxide emissions. Environ. Res. Lett. 2014, 9, 105012. [Google Scholar] [CrossRef]
- Mosier, A.; Kroeze, C.; Nevison, C.; Oenema, O.; Cleemput, O.V. Closing the Global N2O Budget: Nitrous Oxide Emissions through the Agricultural Nitrogen Cycle. Nutr. Cycl. Agroecosys. 1998, 52, 225–248. [Google Scholar] [CrossRef]
- Naqvi, S.W.A.; Yoshinari, T.; Jayakumar, D.A.; Altabet, M.A.; Narvekar, P.V.; Devol, A.H.; Brandes, J.A.; Codispoti, L.A. Budgetary and biogeochemical implications of N2O isotope signatures in the Arabian Sea. Nature 1998, 394, 462–464. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Van der Hoek, K.W.; Olivier, J.G.J. Uncertainties in the global source distribution of nitrous oxide [Review]. J. Geophys. Res. Atmos. 1995, 100, 2785–2800. [Google Scholar] [CrossRef]
- Houghton, J.T.; Callander, B.A.; Varney, S.K. The Supplementary Report to the IPCC Scientific Assessment; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- IPCC. Contributions of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Harper, J.E. Evolution of Nitrogen Oxide(s) during In Vivo Nitrate Reductase Assay of Soybean Leaves. Plant Physiol. 1981, 68, 1488–1493. [Google Scholar] [CrossRef]
- Chen, G.X.; Shang, S.H.; Yu, K.W. Investigation on the emission of nitrous oxide by plant. Chin. J. Appl. Ecol. 1990, 1, 94–96. [Google Scholar]
- Smart, D.R.; Bloom, A.J. Wheat leaves emit nitrous oxide during nitrate assimilation. Proc. Natl. Acad. Sci. USA 2001, 98, 7875–7878. [Google Scholar] [CrossRef]
- Hakata, M.; Takahashi, M.; Zumft, W.; Sakamoto, A.; Morikawa, H. Conversion of the Nitrate Nitrogen and Nitrogen Dioxide to Nitrous Oxides in Plants. Acta Biotechnol. 2003, 23, 249–257. [Google Scholar] [CrossRef]
- Lenhart, K.; Behrendt, T.; Greiner, S.; Steinkamp, J.; Well, R.; Giesemann, A.; Keppler, F. Nitrous oxide effluxes from plants as a potentially important source to the atmosphere. New Phytol. 2018, 221, 1398–1408. [Google Scholar] [CrossRef]
- Timilsina, A.; Oenema, O.; Luo, J.F.; Wang, Y.Y.; Hu, C.S. Plants are a natural source of nitrous oxide even in field conditions as explained by 15N site preference. Sci. Total Environ. 2021, 805, 150262. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.H.; Chen, G.X.; Xu, H.; Wu, J. Investigation on emission of nitrous oxide by aseptic soybean plant. J. Integr. Plant Biol. 1992, 34, 835. [Google Scholar]
- Li, N.; Chen, G.X. N2O emission by plants and influence of fertilization. Chin. J. Appl. Ecol. 1993, 4, 295–298. [Google Scholar]
- Mosier, A.R.; Mohanty, S.K.; Bhadrachalam, A.; Chakravorti, S.P. Evolution of dinitrogen and nitrous oxide from the soil to the atmosphere through rice plants. Biol. Fert. Soils 1990, 9, 61–67. [Google Scholar] [CrossRef]
- Yu, K.W.; Wang, Z.P.; Chen, G.X. Nitrous oxide and methane transport through rice plants. Biol. Fert. Soils 1997, 24, 341–343. [Google Scholar] [CrossRef]
- Rusch, H.; Rennenberg, H. Black alder (Alnus Glutinosa (L.) Gaertn.) trees mediate methane and nitrous oxide emission from the soil to the atmosphere. Plant Soil 1998, 201, 1–7. [Google Scholar] [CrossRef]
- Bowatte, S.; Newton, P.C.D.; Theobald, P.; Brock, S.; Hunt, C.; Lieffering, M.; Sevier, S.; Gebbie, S.; Luo, D.W. Emissions of nitrous oxide from the leaves of grasses. Plant Soil 2014, 374, 275–283. [Google Scholar] [CrossRef]
- Wen, Y.; Corre, M.D.; Rachow, C.; Chen, L.; Veldkamp, E. Nitrous oxide emissions from stems of alder, beech and spruce in a temperate forest. Plant Soil 2017, 20, 423–434. [Google Scholar] [CrossRef]
- Chang, C.; Janzen, H.H.; Nakonechny, E.M.; Cho, C.M. Nitrous Oxide Emission through Plants. Soil Sci. Soc. Am. J. 1998, 62, 35–38. [Google Scholar] [CrossRef]
- Pihlatie, M.; Ambus, P.; Rinne, J.; Pilegaard, K.; Vesala, T. Plant-mediated nitrous oxide emissions from beech (Fagus sylvatica) leaves. New Phytol. 2005, 168, 93–98. [Google Scholar] [CrossRef]
- Timilsina, A.; Bizimana, F.; Pandey, B.; Yadav, R.K.P.; Dong, W.X.; Hu, C.S. Nitrous Oxide Emissions from Paddies: Understanding the Role of Rice Plants. Plants 2020, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Timilsina, A.; Zhang, C.; Pandey, B.; Bizimana, F.; Hu, C.S. Potential Pathway of Nitrous Oxide Formation in Plants. Front. Plant Sci. 2020, 11, 1177. [Google Scholar] [CrossRef] [PubMed]
- Boutton, T.; Yamasaki, S. Mass Spectrometry of Soils; Marcel Dekker: New York, NY, USA, 1996; pp. 177–204. [Google Scholar]
- Toyoda, S.; Yoshida, N.; Koba, K. Isotopocule analysis of biologically produced nitrous oxide in various environments. Mass Spectrom. Rev. 2015, 36, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Lewicka-Szczebak, D.; Dyckmans, J.; Kaiser, J.; Marca, A.; Augustin, J.; Well, R. Oxygen isotope fractionation during N2O production by soil denitrification. Biogeosciences 2016, 13, 1129–1144. [Google Scholar] [CrossRef]
- Wei, J.; Amelung, W.; Lehndorff, E.; Schloter, M.; Vereecken, H.; Brüggemann, N. N2O and NOx emissions by reactions of nitrite with soil organic matter of a Norway spruce forest. Biogeochemistry 2017, 132, 325–342. [Google Scholar] [CrossRef]
- Samuelson, M.E.; Ohlen, E.; Lind, M.; Larsson, C.M. Nitrate regulation of nitrate uptake and nitrate reductase expression in barley grown at different nitrate:ammonium ratios at constant relative nitrogen addition rate. Physiol. Plantarum 1995, 94, 254–260. [Google Scholar] [CrossRef]
- Saravitz, C.H.; Devienne-Barret, F.; Raper, C.D.; Chaillou, S.; Lamaze, T. Nitrate uptake rate by soyabean and wheat plants determined by external nitrate concentration and. Int. J. Plant Sci. 1998, 159, 305–312. [Google Scholar] [CrossRef]
- Cooper, C.E. Nitric oxide and cytochrome oxidase: Substrate, inhibitor or effector? Trends Biochem. Sci. 2002, 27, 33–39. [Google Scholar] [CrossRef]
- Wany, A.; Kumari, A.; Gupta, K.J. Nitric oxide is essential for the development of aerenchyma in wheat roots under hypoxic stress. Plant Cell Environ. 2017, 40, 3002–3017. [Google Scholar] [CrossRef]
- Gupta, K.J.; Stoimenova, M.; Kaiser, W.M. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, In Vitro and In Situ. J. Exp. Bot. 2005, 56, 2601–2609. [Google Scholar] [CrossRef]
- de Oliveira, H.C.; Wulff, A.; Saviani, E.E.; Salgado, L. Nitric oxide degradation by potato tuber mitochondria: Evidence for the involvement of external NAD(P)H dehydrogenases. Biochim. Biophys. Acta 2008, 1777, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Wulff, A.; Oliveira, H.C.; Saviani, E.E.; Salgado, L. Nitrite reduction and superoxide-dependent nitric oxide degradation by Arabidopsis mitochondria: Influence of external NAD(P)H dehydrogenases and alternative oxidase in the control of nitric oxide levels. Nitric Oxide 2009, 21, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Kaiser, W.M. Production and Scavenging of Nitric Oxide by Barley Root Mitochondria. Plant Cell Physiol. 2010, 51, 576–584. [Google Scholar] [CrossRef]
- Machacova, K.; Borak, L.; Agyei, T.; Schindler, T.; Soosaar, K.; Mander, U.; Ah-Peng, C. Trees as net sinks for methane (CH4) and nitrous oxide (N2O) in the lowland tropical rain forest on volcanic Reunion Island. New Phytol. 2021, 229, 1983–1994. [Google Scholar] [CrossRef]
- Kadenbach, B.; Stroh, A.; Hüther, F.J.; Reimann, A.; Steverding, D. Evolutionary aspects of cytochromec oxidase. J. Bioenerg. Biomembr. 1991, 23, 321–334. [Google Scholar] [CrossRef]
- Machacova, K.; Maier, M.; Svobodova, K.; Lang, F.; Urban, O. Cryptogamic stem covers may contribute to nitrous oxide consumption by mature beech trees. Sci. Rep. 2017, 7, 13243. [Google Scholar] [CrossRef]
- Voesenek, L.A.; Sasidharan, R.; Visser, E.J.; Bailey-Serres, J. Flooding stress signaling through perturbations in oxygen, ethylene, nitric oxide and light. New Phytol. 2015, 209, 39–43. [Google Scholar] [CrossRef]
- Vanecko, S.; Varner, J.E. Studies on Nitrite Metabolism in Higher Plants. Plant Physiol. 1955, 30, 388–390. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Al-Amri, S.M.; El-Enany, A.-W.E. Enhancing rhizobium–legume symbiosis and reducing nitrogen fertilizer use are potential options for mitigating climate change. Agriculture 2023, 13, 2092. [Google Scholar] [CrossRef]
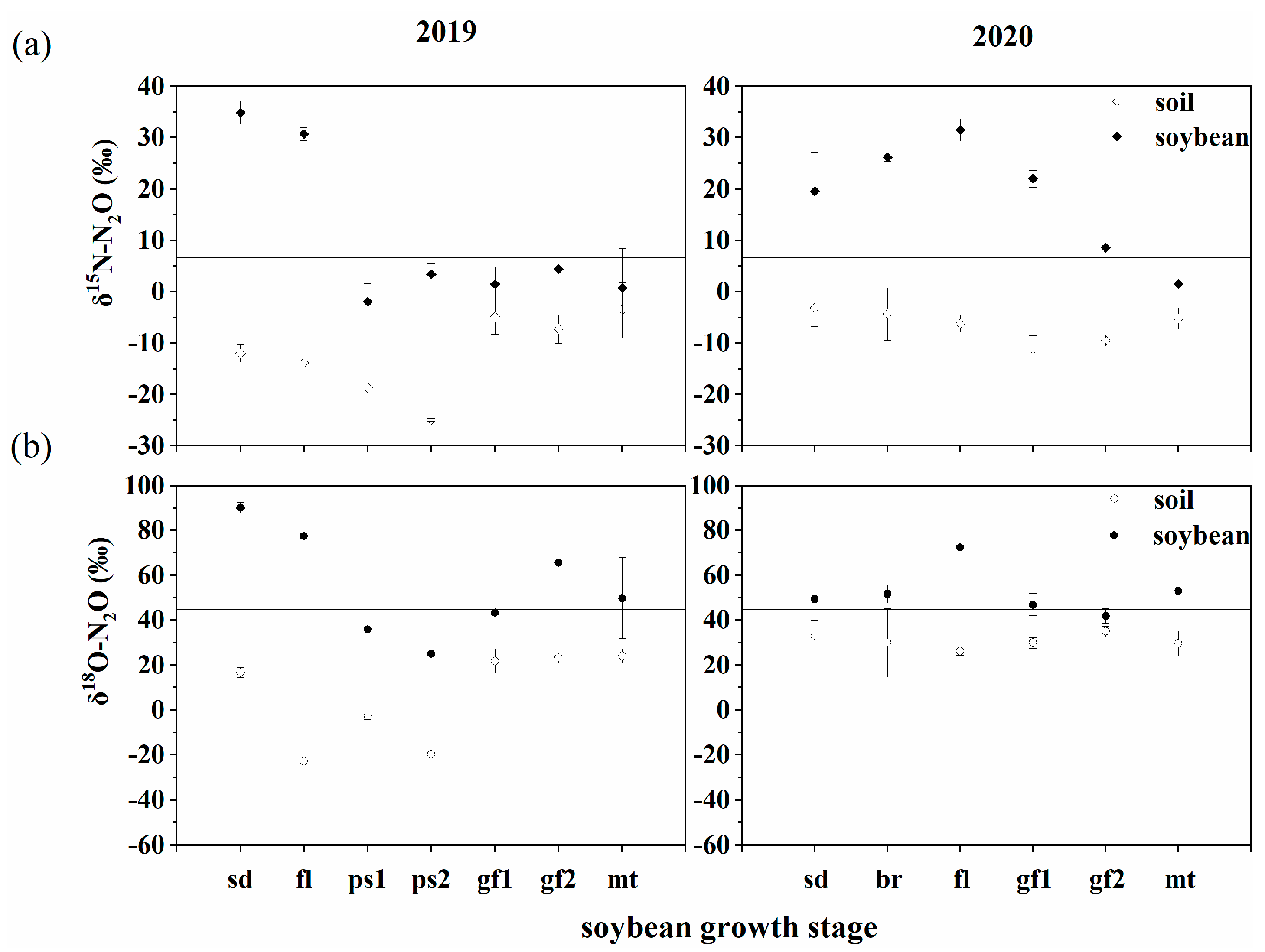
| Year | Sampling Date (DAS) | Growth Stage | cSBF N2O Emission Rate ng N2O g−1 dw h−1 | Estimated Field N2O Flux from Plant 1 μg N2O m−2 h−1 | Soil N2O Emission Rate μg N2O m−2 h−1 | Estimated Field N2O Flux from Soil 2 μg N2O m−2 h−1 |
|---|---|---|---|---|---|---|
| 2019 | 57 III | seedling (sd) | 252.9 ± 53.5 | 81.9 ± 17.3 | 1.6 ± 0.2 | 4.0 ± 0.6 |
| 76 I | flowering (fl) | 40.6 ± 13.7 | 15.3 ± 5.2 | 10.1 ± 4.6 | 25.2 ± 11.6 | |
| 88 I | pod-setting (ps1) | 0.4 ± 0.2 | 0.2 ± 0.1 | 3.5 ± 0.8 | 8.8 ± 2.0 | |
| 102 I | pod-setting (ps2) | 0.5 ± 0.2 | 0.3 ± 0.1 | 1.8 ± 0.2 | 4.5 ± 0.6 | |
| 125 I | grain-filling (gf1) | 0.1 ± 0.0 | 0.1 ± 0.0 | 5.2 ± 3.3 | 13.1 ± 8.2 | |
| 139 I | grain-filling (gf2) | 0.3 ± 0.1 | 0.2 ± 0.0 | 3.4 ± 0.9 | 8.4 ± 2.3 | |
| 103 II | maturating (mt) | 0.2 ± 0.1 | 0.2 ± 0.1 | 10.8 ± 8.5 | 27.0 ± 21.3 | |
| 2020 | 35 IV | seedling (sd) | 72.5 ± 35.2 | 14.5 ± 7.1 | 5.1 ± 1.8 | 12.8 ± 4.5 |
| 52 IV | branching (br) | 138.0 ± 22.8 | 35.0 ± 5.8 | 8.2 ± 2.1 | 20.5 ± 5.2 | |
| 66 IV | flowering (fl) | 31.9 ± 7.3 | 9.6 ± 2.2 | 4.9 ± 0.7 | 12.2 ± 1.7 | |
| 99 IV | grain-filling (gf1) | 2.5 ± 1.7 | 1.2 ± 0.8 | 55.8 ± 12.6 | 139.5 ± 31.5 | |
| 115 IV | grain-filling (gf2) | 0.5 ± 0.2 | 0.4 ±0.2 | 36.6 ± 6.4 | 91.6 ± 15.9 | |
| 133 V | maturating (mt) | 0.2 ± 0.1 | 0.2 ± 0.1 | 13.8 ± 3.9 | 34.6 ± 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.; Chen, G.; Yu, K.; Xu, H.; Yu, X. Isotopic Signatures and Fluxes of N2O Emitted from Soybean Plants and Soil During the Main Growth Period of Soybeans. Agronomy 2024, 14, 2875. https://doi.org/10.3390/agronomy14122875
Xia Z, Chen G, Yu K, Xu H, Yu X. Isotopic Signatures and Fluxes of N2O Emitted from Soybean Plants and Soil During the Main Growth Period of Soybeans. Agronomy. 2024; 14(12):2875. https://doi.org/10.3390/agronomy14122875
Chicago/Turabian StyleXia, Zongwei, Guanxiong Chen, Kewei Yu, Hui Xu, and Xiuling Yu. 2024. "Isotopic Signatures and Fluxes of N2O Emitted from Soybean Plants and Soil During the Main Growth Period of Soybeans" Agronomy 14, no. 12: 2875. https://doi.org/10.3390/agronomy14122875
APA StyleXia, Z., Chen, G., Yu, K., Xu, H., & Yu, X. (2024). Isotopic Signatures and Fluxes of N2O Emitted from Soybean Plants and Soil During the Main Growth Period of Soybeans. Agronomy, 14(12), 2875. https://doi.org/10.3390/agronomy14122875






