Abstract
Chinese cherry [Prunus. pseudocerasus Lindl., syn. Cerasus. pseudocerasus (Lindl.) G.Don], an economically important tetraploid fruit crop native to southwestern China, is celebrated as “the earliest fruit of spring”. Understanding the inheritance and heterosis of major agronomical traits is essential for advancing its breeding. In this study, we conducted a three-year observation and inheritance analysis of 32 economic traits in the reciprocal F1 populations (NH, n = 114; HN, n = 87) derived from Chinese cherry landraces “Nanzaohong” and “Hongfei”. The results revealed a broad segregation for all traits in F1 offspring. Fruit size exhibited an inheritance tendency toward smaller dimensions, with some individuals displaying extreme values (Fruit weight, HH = 3.90~12.15%) that highlighted the potential for selecting larger fruits. The hybrids showed a tendency for sweeter fruit flavor, with total soluble solids (RHm = 7.00~19.35%) and soluble sugar (RHm = 11.09% and 17.47%) exhibiting hybrid vigor, along with a decreasing tendency in titratable acid (RHm = −16.08~−1.05%). The flowering and fruiting phenology tended to occur earlier, with extremely early and late flowering lines offering the potential to extend the ornamental and harvesting periods. Fruit bitterness (H2 = 0.98 and 0.95) and fruit skin color (H2 = 0.93 and 0.89) displayed the highest heritability. Correlation analysis revealed strong internal correlations among trait categories, confirming the reliability of the data collection and analysis. Moreover, no significant differences were observed between the maternal and the paternal effect on the inheritance for agronomic traits attributes. This study systematically clarifies the inheritance trends of agronomic traits in Chinese cherry, providing a foundation for the rational selection of parental lines in breeding strategies and laying the groundwork for future molecular genetic research.
1. Introduction
Chinese cherry [Prunus pseudocerasus Lindl., syn. Cerasus pseudocerasus (Lindl.) G.Don] is an economically important perennial fruit crop in the Rosaceae family [1]. It is one of the four main edible cherry species native to southwestern China [2]. Its economic value comes from the fruit’s delectable taste, health benefits, and the ornamental appeal of its attractive flowers [3]. In recent years, it has become a key element in the “integration of agriculture, culture, and tourism” within China’s modern rural industry. In 2022, cherries were grown on 8555 hectares, with a total production of 35,730 tons in China (www.fao.org/faostat/en/, accessed on 1 November 2024). However, the major cultivars of Chinese cherry remain as landraces with only partially desirable traits, creating an urgent need for cultivars with a broader array of desirable characteristics for modern breeding and industrial development.
In edible cherries, crossbreeding has been widely recognized as an effective approach for improving key agronomic traits. In the past decades, more than 20 bi-parental populations of sweet cherries and 6 sour cherry crosses have been established for new varieties’ breeding and genetic research, including F1 and a limited number of F2 populations [4]. The target breeding program of Chinese cherry was first reported in 2020, when several F1 segregating populations were constructed, including reciprocal crosses [5]. Reciprocal crosses, which invert the sexual roles of parental genotypes, and the resulting phenotypic differences, are valuable tools for exploring reciprocal effects, such as cytoplasmic inheritance, maternal effects, or genomic imprinting on trait inheritance [6]. Significant reciprocal effects have been well documented in field crops and vegetables, such as maize [7], olive [8], tomato [6], and pepper [9]. However, the reciprocal effect in cherry fruit remains unknown, and the phenotypic differences between reciprocal hybrids need further exploration.
In cherry consumption, the consumer generally prefers fruits with a large size, a vibrant color, firmness, and a balanced ratio of total soluble solid (SSC) to titratable acidity (TA) [10]. These desirable traits are primarily quantitatively inherited and controlled by polygenes [11], promoting significant phenotypic and genetic exploration to understand the inheritance mechanisms in cherry. Large phenotypic segregation and high heritability are commonly observed in cherry fruit-related and phenological traits, which are essential for genetic improvement [12]. Based on various estimation methods, a large broad-sense heritability (H2) has been observed in traits such as fruit firmness (0.77~0.85) [13,14,15], fruit size (0.92~0.93) [15], and flowering date (0.85~0.97) [16,17]. Furthermore, over 300 genetic loci associated with traits such as fruit size [18], flavor [15], firmness [19,20], maturity date [21], disease resistance [22], and stress tolerance [23] of sweet and sour cherry have been identified, advancing breeding processes to meet planting demands and adapt to market and climate changes. For Chinese cherry, the F1 progeny from “HF” and “PJHH” showed a rich diversity of trait distribution [24], while studies on the phenotypic and genetic exploration remain limited.
In this study, F1 true hybrids from reciprocal crosses derived from widely cultivated Chinese cherry landraces “Nanzaohong” (early-maturing, orange-red, NZH) and “Hongfei” (purple-red, large fruit, HF) were used to (i) observe and analyze genetic variation across over 32 agronomic traits; (ii) perform a correlation analysis of these traits; and (iii) conduct a significant differential analysis of traits between the reciprocal cross NZH × HF and HF × NZH. This study aims to clarify the genetic tendencies of important traits in Chinese cherry and provide a basis for rational parent selection in breeding strategies, along with laying a foundation for future molecular genetic research.
2. Materials and Methods
2.1. Plant Materials
The plant materials consisted of the F1 segregating population of 201 individuals derived from reciprocal crosses between two Chinese cherry landraces: “Nanzaohong” (NZH) and “Hongfei” (HF). Specifically, the population included 114 individuals from the cross NZH × HF (NH) and 87 from the reciprocal cross HF × NZH (HN). The parent NZH is an early-maturing variety with fruit weighing 3.30–4.45 g, characterized by orange-red skin, a kidney shape, and a sour/sweet flavor. The parent HF is a mid-maturing large-fruited variety (4.50–5.50 g) with purple-red skin, a heart shape, and a sweet/sour flavor. The population was established in 2016 and planted at the Cherry Germplasm Repository of Sichuan Province (Chongzhou, Chengdu, China) in 2017, with fruiting beginning in 2019 and stabilizing by 2021 [5]. All materials were subjected to uniform field management practices, including irrigation, fertilization, and pest control. From 2021 to 2023, 100 mature disease-free fruits displaying typical flavor characteristics of each genotype were collected within 12 h of harvest for observation and measurement. Fruit phenotypes of parents and representative F1 individuals are shown in Figure 1. The remaining fruits were stored at −80 °C for subsequent intrinsic quality assessments.

Figure 1.
Fruit phenotypes of parents “Hongfei” (A), “Nanzaohong” (B) and representative F1 individuals (C–K).
2.2. Trait Investigation and Phenotyping
A total of 32 traits related to the phenology, flowers, and fruits of Chinese cherries were investigated and measured. Flower bud color (FlC (bud)) and petal color (FlC (petal)) were assessed based on the phenotyping protocol of Chinese cherry (Table S1) [25]. Fruit and fruit stalk traits, including fruit skin color (FC (skin)), flesh color (FC (flesh)), color around the stone (FC (stone)), fruit shape (FSh), fruit top shape (FSh (top)), and hairy stalk (HS), were also evaluated according to the phenotyping protocol of Chinese cherry with some modifications (Table S1). The sweet/sour flavor (Ff) and bitterness of the fruits (bitter) were assessed using a panel-tasting method, with flavor ratings derived from the collective descriptions of tasters [26]. Fruit weight (FW) and stone weight (FW (stone)) were measured using a precision electronic balance (Jinxuan, TP-001, Foshan, China), while fruit dimensions (AIRAJ, ARZ - 1331, Qingdao, China)—longitudinal diameter (FLoD), transverse diameter (FTrD), lateral diameters (FLaD), fruit stalk length (FSL), and flower diameter (FlD)—were recorded with a digital caliper. Fruit skin color parameters (L, a, b) were determined using a colorimeter (KONICA MINOLTA, CM-2600d, Japan). Soluble sugar (SS) and anthocyanin (An) content were assessed following Hou’s methods [27]. Total soluble solids (TSS) and titratable acidity (TA) were measured using a refractometer (ATAGO, PAL-BX/ACID 16, Tokyo, Japan), and juice pH was recorded with a pH meter (METTLER TOLEDO, FE28, Zurich, Switzerland). The fruit shape index (FSh (index)) was calculated as the FLoD/FLoD and the solid/acid ratio was computed as TSS/TA.
Phenological stages, including the beginning of flowering date (FD (beginning)), full flowering date (FD (full)), end of flowering date (FD (end)), and fruit maturity date (MD), were categorized according to the BBCH (Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie) scale for sweet cherries [28]. The fruit development period (FDP) was calculated as the time from FD (full) to MD.
2.3. Statistical Analysis
Phenotypic data were statistically analyzed using Excel 2019 and IBM SPSS Statistics 25.0. The mean, maximum, minimum, standard deviation, and coefficient of variation (CV) were calculated for each trait per year. Skewness and kurtosis of normal distribution were tested using the Kolmogorov–Smirnov test (p < 0.05). Broad-sense heritability (H2 = Vg/(Vg + Ve/n) was estimated using a linear mixed-effects model fitted with the ‘lme4’ package in R (v4.2.0) [29], where Vg is the genetic variance, Ve is the residual variance, and n is the number of individuals.
Based on the collected phenotypic data, heterosis was analyzed for each trait in the reciprocal cross. The following calculation formulas were used: mid-parent heterosis (Hm = MF1 − MP) and mid-parent heterosis rates (RHm = Hm/MP), best parent heterosis (Hb = MF1 − DP) and best parent heterosis rates (RHb = Hb/DP), where MP is the mid-parent value, MF1 is the mean value of F1 population, and DP refers to the value of dominant parent. The ratio of higher than high parent (HH), ratio of higher than mid-parent (HM), and ratio of lower than low parent (LL) were also summarized.
For significant analysis, a Student’s t-test and a Welch’s t-test were employed for normally distributed traits, while traits with non-normal distributions were analyzed using the Mann–Whitney U test. The Benjamini–Hochberg correlation for multiple comparisons was applied to reduce the risk of false positives. Correlation analyses and graphical representations were performed using R (v4.2.0) with ‘corrplot’ and ‘ggplot2’ packages.
3. Results
3.1. Phenotypic Variation of Agronomic Traits
3.1.1. Fruit-Related Traits
Fruit Size
In stone fruit, fruit size is influenced by longitudinal, transverse, and lateral diameters, along with fruit weight, with stone weight also contributing to fruit size to some extent. As shown in Table 1 and Figure 2, the greatest variation was observed for stone weight, with a CV of 23% for NH cross and 21.87% for HN cross. The fruit weight also exhibited a high level of variation, with CVs ranging from 18.90% to 21.68% in the NH cross and 17.17% to 19.42% in the HN cross. Over three years, fruit weight ranged from 1.80 to 7.46 g in the NH cross and from 1.85 to 6.48 g in the HN cross. The CVs for fruit diameter traits (FloD, FTrD, and FlaD) were consistently below 10%, indicating relatively small variations in these traits.

Table 1.
The phenotypic variation of traits in reciprocal F1 populations derived from “Nanzaohong” and “Hongfei”.
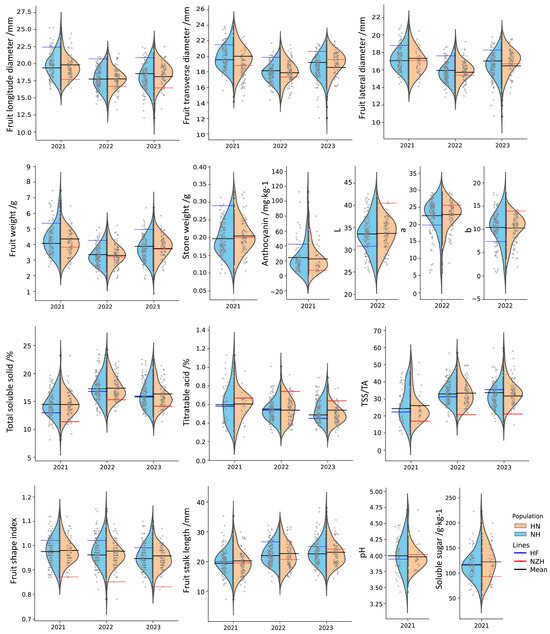
Figure 2.
Violin plot distributions and phenotype values of fruit-related traits of F1 reciprocal crosses (NH and HN) over three consecutive years.
Fruit Color
Through a three-year investigation, the F1 generation exhibited a wide range of segregation in fruit skin color (FC (skin)), including yellow with red blush, orange-red, red, purple-red, and black-purple (Table 2, Figure 1). Among these, red was the predominate color, comprising 56.48% to 72.41% of the population, while yellow with red blush (0.94% to 2.78%) and black-purple fruits (1.15% to 5.66%) were less common. In terms of flesh color (FC (flesh)), parent line NZH had creamy white flesh and HF had pink flesh. The F1 segregation in both reciprocal crosses was stable, primarily concentrated in yellow (49.12~59.26% for NH and 48.84~66.67% for HN) and creamy white (19.44~32.46% for NH and 22.06~36.05% for HN). The anthocyanin content (An) varied widely in both NH cross (3.12~112.51 mg·kg−1) and HN cross (1.80~79.94 mg·kg−1), with the largest CV of 86.63% and 75.24%, respectively. The CVs for color parameters were highest for b*, followed by a* and L* (Table 2).

Table 2.
The phenotypic segregation of descriptive traits in reciprocal F1 populations.
Fruit Flavor
The flavor profiles of fruit, including sweetness and acidity, are determined by the balance of sugars and acids. The parent lines NZH and HF differ in taste, with NZH being sour/sweet with light bitterness, and HF having a sweet/sour flavor. In the F1 generation, 43.93% to 65.18% of individuals had sweet/sour flavors, followed by sweet and sour/sweet flavors, with sour flavors appearing less frequently (1.16% to 6.54%) (Table 2). Titratable acidity (TA) displayed considerable variability in both crosses (CV: 21.27~33.46% for NH; 19.03~25.46% for HN). Bitterness, an undesirable trait in cherries, was observed in approximately 30% of individuals in the reciprocal F1 population (Table 2).
Fruit Shape
A diversity of fruit shapes was observed, with reniform, near round, and heart being the dominant shapes, while oblate and ellipse shapes were less common. Regarding the fruit top shape, NZH and HF exhibited concave and convex shapes. The proportion of convex, flat, and concave shapes were nearly equal, while only a small percentage (1.15% to 6.54%) of individuals exhibited spiculate shapes (Table 2). For the fruit shape index, a relatively low variability (CV: 4.53~5.77%) was observed (Table 1).
Fruit Stalk
The length of fruit stalk (FSL) varied widely, ranging from 7.24 to 35.30 mm in the NH cross and from 9.93 to 30.01 mm in the HN cross, with a CV between 15.6% and 21.26% (Table 1). The fruit stalk of NZH was hairy, while HF was glabrous. In both NH and HN crosses, more than 50% of F1 offspring had hairy stalks. Surprisingly, between 3.51% and 20.93% of F1 offspring had glabrous stalks, offering potential for selecting cherries with stalks that are easier to harvest.
3.1.2. Phenological Traits and Flower-Related Traits
This study focuses on the phenological characteristics of flowers and fruits, which influence the economic value of Chinese cherry. As shown in Table 2 and Figure 3, there was minimal variation in the dates of the beginning of flowering (FD (beginning)), full flowering (FD (full)), end of flowering (FD (end)), and fruit maturity (MD) among F1 crosses derived from HF and NZH across three years, with CV ranging from 2.18% to 9.70%. Specifically, the MF1 of FD (beginning) and FD (full) occurred 1 to 3 days earlier than the mid-parent values, while the MF1 of FD (end) was similar to the mid-parent values, indicating an extension of the flowering period in the hybrids. FD (beginning) of NH cross ranged from 8th to 14th Feb in 2021 and from 14th to 27th Feb in 2022. The FD (full) period of the NH cross lasted 6 days in 2021, while in 2022 and 2023, it extended to more than twice that length, indicating the phenological traits were significantly affected by climate temperature. The degree of variation in the reciprocal crosses was similar. The parents NZH and HF were classified as early- and mid-maturing varieties, respectively. Compared with FD, the F1 hybrids exhibited a broader distribution in fruit maturity date (MD).
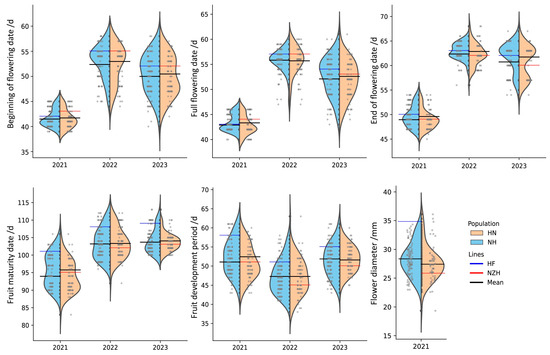
Figure 3.
Violin plot distributions and phenotype values of phenological and flower traits of F1 reciprocal crosses (NH and HN) over three consecutive years.
As shown in Table 2, the petal colors of HF and NZH are pinkish and white, respectively. In the reciprocal F1 generation, petal colors were distributed as white (34.26% and 34.67%), pinkish (45.37% and 49.33%), and pink (20.37% and 16.00%). Both parental lines had pink flower buds, while the F1 generation displayed a segregation of red, pink, and white flower buds. The proportions of red and pink accounted for 62.96% and 36.11% in the NH cross, 48.00% and 49.33% in the HN cross, while white flower buds comprised only 0.93% and 2.67%. This diversity in flower color enhances the ornamental value of the Chinese cherry. In terms of flower diameter, a wide range has been observed in HN cross (19.33~30.11 mm).
3.2. Inheritance Trend of Agronomic Traits
3.2.1. Fruit-Related Traits
The MF1 for all fruit size-related traits, including FloD, FTrD, FLaD, FW, and FW (stone), were consistently smaller than the mid-parent value (MP) in both reciprocal crosses over the three years, indicating negative mid-parent heterosis rates (RHm) ranging from −0.91% to −19.37% (Table 3). This suggests that the inheritance of fruit size in Chinese cherries tends toward smaller fruit sizes. However, despite the majority of F1 individuals being smaller than the mid-parent, both reciprocal crosses still included super high parent lines, with HH between 3.90% and 12.15%. This highlights the potential for breeding excellent varieties with large fruit size. High heritability was observed for FloD (0.84 and 0.82), FTrD (0.76 and 0.78), FlaD (0.74 and 0.76), and FW (0.80 and 0.76), suggesting that fruit size is primarily under genetic control. Heritability for fruit skin color was high, with values of 0.93 for the NH cross and 0.89 for the HN cross, indicating a strong genetic control (Table 3).

Table 3.
The inheritance analysis of traits in reciprocal F1 populations derived from “Nanzaohong” and “Hongfei”.
Over three years, TSS showed high mid-parent heterosis, especially in 2021 and 2022, indicating a trend toward increased sweetness (Table 3), with relatively low H2 values of NH (0.62) and HN (0.55), suggesting significant environmental influence. The MF1 values for TA were lower than the mid-parent values, with the proportion of individuals lower than the low parent (LL) reaching 53.10% and 44.81% in 2022 (Table 3), indicating a genetic tendency toward decreased acidity. In contrast, the inheritance pattern of pH differed from that of TA, while the TSS/TA ratio was primarily influenced by TSS. This suggests that the sweeter flavor observed in the hybrid progeny of NZH and HF may result from positive heterosis in TSS and negative heterosis in acidity. The high heritability of bitterness (H2 = 0.98 and 0.95) indicates that it is primarily governed by genetic factors, highlighting the potential for further exploration of the genetic loci associated with bitterness.
For the fruit shape index, the MF1 values were similar to the mid-parent values across three consecutive years (Table 3). And the MF1 values of FSL for both NH and HN cross were consistently lower than mid-parent values, accompanied by negative heterosis, indicating an inheritance trend towards shorter stalks, with a high heritability (NH: 0.88 and HN: 0.87).
3.2.2. Phenological Traits and Flower-Related Traits
In 2022, 43.86% (NH) and 40.23% (HN) of the F1 hybrids matured earlier than the early-maturing parent NZH, while 13.16% (NH) and 13.79% (HN) matured later than the mid-maturing parent HF (Table 3). The maturity period of F1 progeny fruits was extended by up to 20 days in 2022, indicating that the harvest window for Chinese cherries could exceed 20 days. Moreover, the MF1 of MD was consistently 2 to 4 days earlier than the mid-parent values, suggesting a genetic trend toward earlier maturity in the hybrids (Table 3). The earlier and longer harvest window could prolong the market supply period, thereby increasing both the demand and the value of the Chinese cherry industry. The MF1 values (28.31 mm and 27.34 mm) of flower diameter were smaller than the mid-parent value of 30.11 mm (Table 3).
3.3. Phenotypic Difference Between NH and HN Populations
Following normality tests and variance homogeneity assessments, differential significance analysis was conducted to examine phenotypic differences between the NH and HN progenies. As shown in Table S2, none of the investigated traits exhibited p-values smaller than the significance threshold of 0.05. For instance, the adjusted p-value for fruit maturity date in 2021 was 0.09, indicating no significant difference between the NH and HN populations. Similar trends were observed for all traits across all investigated years.
In Chinese cherry, the absence of significant differences suggests that the parental role—whether as the maternal or paternal source—does not affect trait segregation or phenotypic outcomes in the F1 offspring, implying that there are no notable cytoplasmic genetic effects or interactions between nuclear and cytoplasmic genes [30]. These results provide a solid basis for future research, particularly in the merging of NH and HN populations for genetic linkage map construction and QTL analysis.
3.4. Trait Correlations
The trait correlations were similar across the years 2021, 2022, and 2023 (Figure 4 and Figure S1). Five fruit size-related traits (FloD, FTrD, FLaD, FW, and FW (stone)) were clustered together, showing highly significant positive correlations among them (r = 0.50**~0.94** in 2021) (Figure 4). The FTrD exhibited the strongest correlation with FW (r = 0.94**), indicating its dominant influence on fruit weight. FW (stone) also contributed to fruit weight (r = 0.54**). A high significant positive correlation was observed among FD (beginning), FD (full), and FD (end) in 2021 (r = 0.49**~0.88** in 2021). MD showed a high significant positive correlation with FDP (r = 0.93**) and a significant positive correlation with fruit size traits—FloD, FTrD, FLaD, FW—and fruit color traits—FC (skin) and An (0.26**, 0.22*). This suggests that late-maturing Chinese cherries are often characterized by a longer development period, larger fruit size, and deeper coloration. Notably, a significant negative correlation was observed between fruit size (FloD, FTrD, FLaD, FW) and TSS and TA in 2022 and 2023 (−0.23**~−0.39** in 2022). This may be due to the fact that fruit enlargement is often associated with an increase in water content, leading to a relative dilution of both TSS and TA, reflecting the concentration effect.
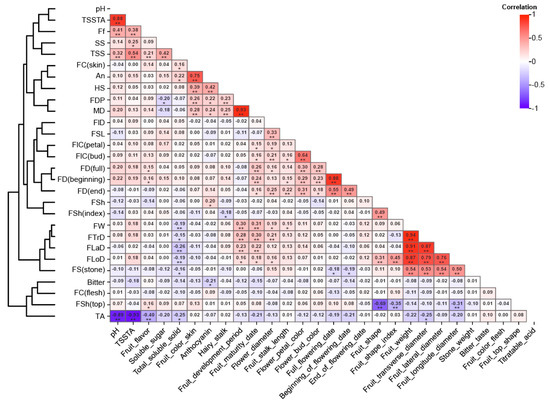
Figure 4.
Spearman’s correlation matrix and heat plot among Chinese cherry traits in 2021. Note: trait abbreviations are provided on the left and full trait names are on the bottom. * presents a significant correlation at 0.05; ** presents a highly significant correlation at 0.01.
4. Discussion
Similar to citrus [31] and grape [32], these traits in this study exhibited broad segregation, with some individuals displaying extremely high or low trait values, underscoring the significant potential of breeding superior new varieties. Reciprocal crosses can reveal maternal or paternal effects in certain crops [6,9,33]. However, in our study, no significant reciprocal differences were found in agronomic traits over three years, suggesting that the inheritance tendency of the traits studied in Chinese cherry are not strongly affected by the cross direction. Similarly, consistent inheritance tendencies have been observed in reciprocal crosses of apricot [34], olive [8], and blueberry [35]. In autotetraploid blueberry, reciprocal hybrids of “Nui” and “Hortblue Petite” have been mixed for genetic analysis, successfully identifying major QTLs for anthocyanin content [36]. Further research, including marker-assisted breeding (MAB) and refined QTL mapping, could involve expanding the hybrid populations to increase sample size, thereby improving the precision and reliability of the genetic findings.
Fruit size is a critical determinant of quality, yield, and market profitability of cherries. The F1 offspring derived from the cross NZH and HF exhibited a genetic tendency towards smaller fruit sizes, a pattern that highlights the significant influence of the small-fruited parent. This finding aligns with previous studies on Chinese cherry (HF×PJHH) [24], sweet cherry [19], peach [37], plum [38,39], and apricot [40], where similar tendencies were observed in F1 populations. This phenomenon likely results from long-term selection for larger fruit within the Rosaceae family, which has led to strong non-additive effects. In hybrid progeny, these effects tend to dissipate, causing a regression [41]. Therefore, small-fruited varieties should be avoided as parents to minimize the need for multiple generations of crossing. In our study, five fruit size-related traits (FloD, FTrD, FLaD, FW, and FW (stone)) exhibited significant positive correlations. This result is consistent with the findings of Zhang [42], who identified clustering QTLs for the highly correlated fruit size traits (FloD, FTrD, FW, FloD (stone), FTrD (stone), and mesocarp cell number). These findings suggest the presence of multiple closely linked genes or a common genetic mechanism responsible for increased fruit dimensions. Moreover, based on the segregated population for fruit size, multiple genetic loci (FW_G2a [18,43], qP-FS2.1m [15]) have been identified as contributors to the genetic architecture of cherry fruit size, providing valuable insights for future breeding programs aimed at improving fruit size in cherry.
As for fruit flavor, this is the first genetic study on cherries’ bitterness, with high heritability (H2 = 0.98 and 0.95), suggesting that the trait is primarily genetically controlled. With limocitrin-7-O-glucoside identified as a key bitter compound in Chinese cherry [3], this study lays the groundwork for further exploration of the genetic loci and mechanisms underlying bitterness, following similar efforts in the bitterness exploration of almonds [44]. In our study, hybrid vigor (RHm = 3.48~7.47%) was observed in the TSS/TA content of the F1 hybrids from HF and NZH crosses. This contrasts with the decreasing trend of TSS/TA in jujube [45] and apricot (“Chuanzhihong” and “Saimaiti”) [34], but is consistent with five other apricot crosses [46]. In the F1 population from the HF × PJHH cross of Chinese cherry, hybrid vigor for TSS was also observed. However, due to the higher TSS content in NZH compared with PJHH, the F1 population with NZH as a parent exhibited higher average TSS values (14.47~17.39%) than those with PJHH as a parent (13.05% in 2021 and 16.12% in 2022). These results suggest that the expression of hybrid vigor is influenced by the parent line differences, which aligns with findings in jujube [45]. Similarly, in line with our findings about the lowest heritability detected in TSS, Altan [46] and Calle [15] found the lowest heritability for apricot (H2 = 0.39) and sweet cherry (H2 = 0.62), supporting the notion that TSS is influenced by complex genetic interactions and environmental factors [47].
The significant variation in flowering and fruit maturity dates across years observed in our study is similar to Branchereau’s research [17], who found that the flowering date of sweet cherry is highly dependent on the environment and therefore not stable across years and locations, as detected in twenty unique location × year environments. Nevertheless, our study also demonstrated that flowering time is quantitatively controlled in Chinese cherry, exhibiting a relatively high heritability (H2 = 0.68~0.79), slightly lower than estimates reported in prior studies of sweet and sour cherry [48,49,50].
Interestingly, the F1 hybrids in this study exhibited a tendency for early flowering, in contrast to the late flowering tendency observed in F1 populations of apricot [51,52], mume [53], and sweet cherry [16]. The FD (beginning) and FD (full) occurred 1–3 days earlier than the mid-parent values, and MD occurred 2–4 days earlier than the mid-parent values, demonstrating considerable economic implications in the market. However, the late flowering allele (Lb) has been identified as dominant in almonds [54], and in sweet cherries, early flowering is more common in F2 progeny [16]. We speculate that the tetraploid nature of Chinese cherry, with its complex genetic makeup, likely drives early flowering through additive genetic effects. The early maturing parent NZH appears to contribute favorable alleles for earlier fruit maturation in the progeny. This study also suggests that the very early-maturing parent could promote early fruit maturation trends, highlighting the critical importance of parental selection in breeding programs.
5. Conclusions
This study, based on three years of phenotypic observation and inheritance analysis of 32 traits in the reciprocal F1 hybrids (NH, n = 114; HN, n = 87) of Chinese cherry landraces “NZH” and “HF”, investigates their phenotypic variation and inheritance tendency. The findings reveal genetic tendencies towards a sweeter flavor, smaller fruit size, earlier flowering and maturing, and a shorter fruit development period. No significant differences were observed between the reciprocal crosses in trait segregation or phenotypic outcomes. Moving forward, further studies are needed to better understand how phenotypic variation and inheritance tendencies are formed and regulated in Chinese cherry. These findings lay the foundation for further exploration into the genetic control of desirable traits and provide a framework for optimizing parental selection in breeding programs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14122862/s1, Figure S1: Spearman’s correlation matrix and heat plot among Chinese cherry traits in 2022 and 2023; Table S1: Phenotyping protocol of rating traits of Chinese cherry; Table S2: Significant differential test of reciprocal F1 populations of Chinese cherry NZH and HF.
Author Contributions
Methodology, investigation, data curation, validation, and writing—original draft preparation: Z.L., S.Y., L.H., H.W., J.Z. and W.H.; formal analysis, visualization, resources, software, supervision, and writing—review and editing: M.L., Y.L. (Yuanxiu Lin), Y.Z. (Yunting Zhang), Q.C., Y.Z. (Yong Zhang), Y.L. (Ya Luo), H.T., Y.W. and X.W. Funding acquisition: Q.C., Y.W. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Sichuan Fruit Innovation Team of National Modern Agricultural Industrial Technology System in China (grant No. SCCXTD-2024-4), the Sichuan Science and Technology Program (grant No. 2024YFHZ0302), the Natural Science Foundation of Sichuan Province (grant No. 2023NSFSC0158), the Project of Rural Revitalization Research Institute in Tianfu New Area of Sichuan Province (grant No. XZY1-04), and the Cherry Resources Sharing and Service Platform of Sichuan Province.
Data Availability Statement
All datasets supporting the conclusions of this article are included within the article. If not included in the manuscript, they are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yü, D.J.; Lu, L.T.; Gu, C.Z.; Guan, K.J.; Li, C.L.; Chen, S.H. Flora Reipublicae Popularis Sinicae, 38; Science Press: Beijing, China, 1986. [Google Scholar]
- Cao, J.; Jiang, Q.; Lin, J.; Li, X.; Sun, C.; Chen, K. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.S.; Wang, H.; Zhang, J.; Chen, Q.; He, W.; Zhang, Y.; Luo, Y.; Tang, H.R.; Wang, Y.; Wang, X.R. Comparative metabolomics profiling highlights unique color variation and bitter taste formation of Chinese cherry fruits. Food Chem. 2024, 439, 138072. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.S.; Bernard, A.; Wang, Y.; Dirlewanger, E.; Wang, X. Genomes and integrative genomic insights into the genetic architecture of main agronomic traits in the edible cherries. Hortic. Res. 2024, e269. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, J.; Liu, Z.S.; Chen, Q.; He, W.; Yang, S.F.; Lin, Y.X.; Zhang, Y.T.; Li, M.Y.; et al. Survey on intra-specific crossing and F1 seedling cultivation in seven combinations of Chinese cherry. Hortic. J. 2022, 91, 267–275. [Google Scholar] [CrossRef]
- Fortuny, A.P.; Bueno, R.A.; Pereira Da Costa, J.H.; Zanor, M.I.; Rodríguez, G.R.; Gibon, Y. Tomato fruit quality traits and metabolite content are affected by reciprocal crosses and heterosis. J. Exp. Bot. 2021, 72, 5407–5425. [Google Scholar] [CrossRef]
- Gonzalo, M.; Vyn, T.J.; Holland, J.B.; Mcintyre, L.M. Mapping reciprocal effects and interactions with plant density stress in Zea mays L. Heredity 2007, 99, 14–30. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Moreno-Alias, I.; de la Rosa-Peinazo, M.A.; Frija, A.; de la Rosa, R.; Leon, L. Floral quality characterization in Olive progenies from reciprocal crosses. Plants 2022, 11, 1285. [Google Scholar] [CrossRef]
- Naves, E.R.; Scossa, F.; Araújo, W.L.; Nunes-Nesi, A.; Fernie, A.R.; Zsögön, A. Heterosis and reciprocal effects for agronomic and fruit traits in Capsicum pepper hybrids. Sci. Hortic. 2022, 295, 110821. [Google Scholar] [CrossRef]
- Zheng, X.; Yue, C.; Gallardo, K.; Mccracken, V.; Luby, J.; Mcferson, J. What attributes are consumers looking for in sweet cherries? evidence from choice experiments. Agric. Resour. Econ. Rev. 2016, 45, 124–142. [Google Scholar] [CrossRef]
- Mackay, T.F.C.; Stone, E.A.; Ayroles, J.F. The genetics of quantitative traits: Challenges and prospects. Nat. Rev. Genet. 2009, 10, 565–577. [Google Scholar] [CrossRef]
- Paril, J.; Reif, J.; Fournier Level, A.; Pourkheirandish, M. Heterosis in crop improvement. Plant J. 2024, 117, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Campoy, J.A.; Le Dantec, L.; Barreneche, T.; Dirlewanger, E.; Quero-García, J. New insights into fruit firmness and weight control in sweet cherry. Plant Mol. Biol. Rep. 2015, 33, 783–796. [Google Scholar] [CrossRef]
- Piaskowski, J.; Hardner, C.; Cai, L.; Zhao, Y.; Iezzoni, A.; Peace, C. Genomic heritability estimates in sweet cherry reveal non-additive genetic variance is relevant for industry-prioritized traits. BMC Genet. 2018, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; Wünsch, A. Multiple-population QTL mapping of maturity and fruit-quality traits reveals LG4 region as a breeding target in sweet cherry (Prunus avium L.). Hortic. Res. 2020, 7, 127. [Google Scholar] [CrossRef]
- .Calle, A.; Cai, L.; Iezzoni, A.; Wünsch, A. Genetic dissection of bloom time in low chilling sweet cherry (Prunus avium L.) using a multi-family QTL approach. Front. Plant Sci. 2020, 10, 1647. [Google Scholar] [CrossRef]
- Branchereau, C.; Hardner, C.; Dirlewanger, E.; Wenden, B.; Le Dantec, L.; Alletru, D.; Parmentier, J.; Ivančič, A.; Giovannini, D.; Brandi, F.; et al. Genotype-by-environment and QTL-by-environment interactions in sweet cherry (Prunus avium L.) for flowering date. Front. Plant Sci. 2023, 14, 1142974. [Google Scholar] [CrossRef]
- Szilágyi, S.; Horváth-Kupi, T.; Desiderio, F.; Bekefi, Z. Evaluation of sweet cherry (Prunus avium L.) cultivars for fruit size by FW_G2a QTL analysis and phenotypic characterization. Sci. Hortic. 2022, 292, 110656. [Google Scholar] [CrossRef]
- Calle, A.; Balas, F.; Cai, L.; Iezzoni, A.; López-Corrales, M.; Serradilla, M.J.; Wünsch, A. Fruit size and firmness QTL alleles of breeding interest identified in a sweet cherry ‘Ambrunés’ × ‘Sweetheart’ population. Mol. Breeding 2020, 40, 86. [Google Scholar] [CrossRef]
- Cai, L.; Quero-García, J.; Barreneche, T.; Dirlewanger, E.; Saski, C.; Iezzoni, A. A fruit firmness QTL identified on linkage group 4 in sweet cherry (Prunus avium L.) is associated with domesticated and bred germplasm. Sci. Rep. 2019, 9, 5008. [Google Scholar] [CrossRef]
- Hardner, C.M.; Hayes, B.J.; Kumar, S.; Vanderzande, S.; Cai, L.; Piaskowski, J.; Quero-Garcia, J.; Campoy, J.A.; Barreneche, T.; Giovannini, D.; et al. Prediction of genetic value for sweet cherry fruit maturity among environments using a 6K SNP array. Hortic. Res. 2019, 6, 6–15. [Google Scholar] [CrossRef]
- Mgbechi-Ezeri, J.; Porter, L.; Johnson, K.B.; Oraguzie, N. Assessment of sweet cherry (Prunus avium L.) genotypes for response to bacterial canker disease. Euphytica 2017, 213, 1. [Google Scholar] [CrossRef]
- Quero-García, J.; Letourmy, P.; Campoy, J.A.; Branchereau, C.; Malchev, S.; Barreneche, T.; Dirlewanger, E. Multi-year analyses on three populations reveal the first stable QTLs for tolerance to rain-induced fruit cracking in sweet cherry (Prunus avium L.). Hortic. Res. 2021, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.S.; Yang, X.Q.; Wang, Z.Y.; Ma, L.; Tu, H.X.; Ma, Y.; Zhou, J.T.; Zhang, J.; Wang, H.; et al. Inheritance analysis of fruit-related traits in Chinese cherry [Cerasus pseudocerasus (Lindl.) G.Don] breeding progenies. Sci. Hortic. 2023, 307, 111519. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, G.P.; Liu, Z.S.; Zhang, J.; Ma, L.; Tian, T.; Wang, H.; Chen, T.; Chen, Q.; He, W.; et al. Phenotyping in flower and main fruit traits of Chinese cherry [Cerasus pseudocerasus (Lindl.) G.Don]. Sci. Hortic. 2022, 296, 110920. [Google Scholar] [CrossRef]
- Karagiannis, E.; Sarrou, E.; Michailidis, M.; Tanou, G.; Ganopoulos, I.; Bazakos, C.; Kazantzis, K.; Martens, S.; Xanthopoulou, A.; Molassiotis, A. Fruit quality trait discovery and metabolic profiling in sweet cherry genebank collection in Greece. Food Chem. 2021, 342, 128315. [Google Scholar] [CrossRef]
- Hou, F.L. Plant Physiology Experiment; Science Press: Beijing, China, 2015. [Google Scholar]
- Fadón, E.; Herrero, M.; Rodrigo, J. Flower development in sweet cherry framed in the BBCH scale. Sci. Hortic. 2015, 192, 141–147. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv Prepr. 2014, arXiv:1406.5823. [Google Scholar]
- Joseph, B.; Corwin, J.A.; Li, B.; Atwell, S.; Kliebenstein, D.J. Cytoplasmic genetic variation and extensive cytonuclear interactions influence natural variation in the metabolome. Elife 2013, 2, e776. [Google Scholar] [CrossRef]
- Asins, M.J.; Raga, V.; Bernet, G.P.; Carbonell, E.A. Genetic analysis of reproductive, vegetative and fruit quality traits to improve Citrus varieties. Tree Genet. Genomes 2015, 11, 117. [Google Scholar] [CrossRef]
- Zinelabidine, L.H.; Torres-Pérez, R.; Grimplet, J.; Baroja, E.; Ibáñez, S.; Carbonell-Bejerano, P.; Martínez-Zapater, J.M.; Ibáñez, J.; Tello, J. Genetic variation and association analyses identify genes linked to fruit set-related traits in grapevine. Plant Sci. 2021, 306, 110875. [Google Scholar] [CrossRef]
- Fortuny, A.P.; Mengarelli, D.A.; Pereira Da Costa, J.H.; Rodríguez, G.R.; Zanor, M.I. Reciprocal effect and heterosis for tomato fruit metabolites revealed by whole transcriptomic analysis of two cultivars and their reciprocal hybrids. Sci. Hortic. 2023, 308, 111583. [Google Scholar] [CrossRef]
- Liu, J.C.; Zhang, Q.P.; Niu, T.Q.; Liu, N.; Zhang, Y.P.; Xu, M.; Ma, X.X.; Zhang, Y.J.; Liu, S.; Liu, W.S. Analysis of inherited tendency of fruit characteristics in F1 group of reciprocal crossing between "Chuanzhihong" and "Saimaiti" in apricots. J. Fruit Sci. 2020, 37, 625–634. [Google Scholar]
- Lei, L.; Xu, G.H.; Wang, L.; Du, Q.H.; Li, Y.F.; Wang, H.X. Screening and inheritance of fruit storage-related traits based on reciprocal cross of Southern×Northern high bush blueberry (Vaccinium linn). Sci. Agric. Sinica 2020, 53, 4045. [Google Scholar] [CrossRef]
- Montanari, S.; Thomson, S.; Cordiner, S.; Günther, C.S.; Miller, P.; Deng, C.H.; Mcghie, T.; Knäbel, M.; Foster, T.; Turner, J.; et al. High-density linkage map construction in an autotetraploid blueberry population and detection of quantitative trait loci for anthocyanin content. Front. Plant Sci. 2022, 13, 965397. [Google Scholar] [CrossRef] [PubMed]
- Rawandoozi, Z.J.; Hartmann, T.P.; Carpenedo, S.; Gasic, K.; Da Silva Linge, C.; Cai, L.; Van de Weg, E.; Byrne, D.H. Identification and characterization of QTLs for fruit quality traits in peach through a multi-family approach. BMC Genomics 2020, 21, 522. [Google Scholar] [CrossRef]
- Valderrama-Soto, D.; Salazar, J.; Sepúlveda-González, A.; Silva-Andrade, C.; Gardana, C.; Morales, H.; Battistoni, B.; Jiménez-Muñoz, P.; González, M.; Peña-Neira, Á.; et al. Detection of quantitative trait loci controlling the content of phenolic compounds in an Asian plum (Prunus salicina L.) F1 population. Front. Plant Sci. 2021, 12, 679059. [Google Scholar] [CrossRef]
- Salazar, J.A.; Pacheco, I.; Shinya, P.; Zapata, P.; Silva, C.; Aradhya, M.; Velasco, D.; Ruiz, D.; Martínez-Gómez, P.; Infante, R. Genotyping by sequencing for SNP-based linkage analysis and identification of QTLs linked to fruit quality traits in Japanese plum (Prunus salicina Lindl.). Front. Plant Sci. 2017, 8, 476. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Liu, W.; Liu, N.; Zhang, Y.; Xu, M.; Liu, S.; Ma, X.; Zhang, Y. Construction of a high-density genetic map and identification of quantitative trait loci linked to fruit quality traits in apricots using specific-locus amplified fragment sequencing. Front. Plant Sci. 2022, 13, 798700. [Google Scholar] [CrossRef]
- Xuesen, C.; Nan, W.; Zongying, Z.; Zhiquan, M.; Chengmiao, Y. Understanding and thinking about some problems of fruit tree germplasm resources and genetic breeding. Sci. Agric. Sinica 2022, 55, 3395–3410. [Google Scholar] [CrossRef]
- Zhang, G.; Sebolt, A.M.; Sooriyapathirana, S.S.; Wang, D.; Bink, M.C.; Olmstead, J.W.; Iezzoni, A.F. Fruit size QTL analysis of an F1 population derived from a cross between a domesticated sweet cherry cultivar and a wild forest sweet cherry. Tree Genet. Genomes 2010, 6, 25–36. [Google Scholar] [CrossRef]
- Rosyara, U.R.; Bink, M.C.A.M.; van de Weg, E.; Zhang, G.; Wang, D.; Sebolt, A.; Dirlewanger, E.; Quero-Garcia, J.; Schuster, M.; Iezzoni, A.F. Fruit size QTL identification and the prediction of parental QTL genotypes and breeding values in multiple pedigreed populations of sweet cherry. Mol. Breeding 2013, 32, 875–887. [Google Scholar] [CrossRef]
- Sanchez-Perez, R.; Pavan, S.; Mazzeo, R.; Moldovan, C.; Aiese, C.R.; Del, C.J.; Ricciardi, F.; Lotti, C.; Ricciardi, L.; Dicenta, F.; et al. Mutation of a bHLH transcription factor allowed almond domestication. Science 2019, 364, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, W.; Pan, Y.; Ge, L.; Wu, C.; Wang, J.; Liu, M.; Yan, F. Comparison and genetic variation analysis of important fruit traits in jujube F1 hybrids by different male parents. Agronomy 2024, 14, 459. [Google Scholar] [CrossRef]
- Altan, H.; Bircan, M.; Caliskan, O. Inheritance of earliness and fruit quality characteristics in five apricot progenies. Erwerbsobstbau 2022, 64, 591–601. [Google Scholar] [CrossRef]
- Desnoues, E.; Baldazzi, V.; Génard, M.; Mauroux, J.; Lambert, P.; Confolent, C.; Quilot-Turion, B. Dynamic QTLs for sugars and enzyme activities provide an overview of genetic control of sugar metabolism during peach fruit development. J. Exp. Bot. 2016, 67, 3419–3431. [Google Scholar] [CrossRef]
- Castede, S.; Campoy, J.A.; Garcia, J.Q.; Le Dantec, L.; Lafargue, M.; Barreneche, T.; Wenden, B.; Dirlewanger, E. Genetic determinism of phenological traits highly affected by climate change in Prunus avium: Flowering date dissected into chilling and heat requirements. New Phytol. 2014, 202, 703–715. [Google Scholar] [CrossRef]
- Cai, L.; Stegmeir, T.; Sebolt, A.; Zheng, C.; Bink, M.C.A.M.; Iezzoni, A. Identification of bloom date QTLs and haplotype analysis in tetraploid sour cherry (Prunus cerasus). Tree Genet. Genomes 2018, 14, 22. [Google Scholar] [CrossRef]
- Dirlewanger, E.; Quero-Garcia, J.; Le Dantec, L.; Lambert, P.; Ruiz, D.; Dondini, L.; Illa, E.; Quilot-Turion, B.; Audergon, J.M.; Tartarini, S.; et al. Comparison of the genetic determinism of two key phenological traits, flowering and maturity dates, in three Prunus species: Peach, apricot and sweet cherry. Heredity 2012, 109, 280–292. [Google Scholar] [CrossRef]
- Salazar, J.A.; Ruiz, D.; Campoy, J.A.; Tartarini, S.; Dondini, L.; Martínez-Gómez, P. Inheritance of reproductive phenology traits and related QTL identification in apricot. Tree Genet. Genomes 2016, 12, 1. [Google Scholar] [CrossRef]
- Campoy, J.A.; Ruiz, D.; Egea, J.; Rees, D.J.G.; Celton, J.M.; Martínez-Gómez, P. Inheritance of flowering time in apricot (Prunus armeniaca L.) and analysis of linked quantitative trait loci (QTLs) using simple sequence repeat (SSR) markers. Plant Mol. Biol. Rep. 2011, 29, 404–410. [Google Scholar] [CrossRef]
- Kitamura, Y.; Habu, T.; Yamane, H.; Nishiyama, S.; Kajita, K.; Sobue, T.; Kawai, T.; Numaguchi, K.; Nakazaki, T.; Kitajima, A.; et al. Identification of QTLs controlling chilling and heat requirements for dormancy release and bud break in Japanese apricot (Prunus mume). Tree Genet. Genomes 2018, 14, 33. [Google Scholar] [CrossRef]
- Ballester, J.; Socias, I. Company, R.; Arus, P.; De Vicente, M.C. Genetic mapping of a major gene delaying blooming time in almond. Plant Breeding 2001, 120, 268–270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).