Abstract
The Netherlands is one of the most important countries for the production of strawberry transplants in Europe. Regulations for pesticide use and water quality become more strict each year, which is a challenge for this sector. Strawberry plants are grown from tips and raised in trays on a trayfield. One of the main plant diseases in strawberry is caused by Phytophthora cactorum. The dispersal of the disease is facilitated by sporangia and zoospores splashing from the surface of the trayfield onto the transplants in the trays. In this research, we compared, in three consecutive years, the traditional growing system with a new system in which the trays are elevated and splashes from the trayfield reaching the transplants are minimized. In two of the three years, we show that the new growing system without the use of any fungicide against P. cactorum performs as well as or even better than the traditional system with the use of the permitted pesticides. Data about Phytophthora occurring in air samples and in splash water collected at different heights support the hypothesis that the decrease in splash dispersal underlies the success of the elevated trayfield. This shows the potential of re-designing growing systems to become less dependent on pesticide use.
1. Introduction
The oomycete pathogen Phytophthora cactorum (Lebert & Cohn) J. Schröt is the causal agent of crown rot in cultivated strawberry (Fragaria × ananassa) [1]. Crown rot shows above ground symptoms ranging from stunting to wilting and necrotic lesions of leaves. Below ground symptoms comprise necrosis of the rhizome and gradually blackening roots, leading to whole plant collapse and the death of the plant. Similar symptoms in strawberry have been observed in recent years to be caused by Neopestalotiopsis spp. [2,3,4]. The two diseases often occur concurrently and can only be distinguished by molecular identification. Crown rot is now considered as a major disease of strawberry in temperate regions, causing crop losses of up to 40% [5]. The main inoculum of P. cactorum are zoospores, emerging from sporangia. Zoospores swim in free water towards the plant roots to attach to them [6]. Subsequently, they germinate, penetrate into the plant tissue, and invade the intercellular spaces between the plant cells to form new sporangia. This asexual cycle repeats several times, providing the means for dispersal and new infection cycles. Splashing water greatly facilitates in the dispersal of the motile zoospores [7]. Sexually formed oogonia from infected plant parts can lead to the persistence of the disease in the soil for many years, forming an important infection source in field-based systems.
P. cactorum is considered a major problem in strawberry nurseries, risking the rapid spread of the disease through the production of transplants and their international trade [8]. Commercial strawberry production in Northwest Europe, including the Netherlands, is increasingly moving indoors onto tabletops with a rain shelter, plastic tunnels, or greenhouses. This change in production allows for year-round, high-quality fruit production [9]. Strawberry nursery mainly takes place outdoors in the open field. In Europe, the majority of strawberry nursery plants deriving from propagation are grown in the field. In the Netherlands, however, the use of a tray plant system with soilless substrates, such as peat and coco coir, is practiced instead [10]. In such a soilless system, P. cactorum is a particular problem due to the ease of spread through the irrigation system via the motile zoospores [1]. However, nursery cultivation is under pressure due to the stricter European regulations, which are increasingly restraining the use of soil fumigation and fungicides, demanding new ways of P. cactorum disease control.
Strawberry host plant resistance has, therefore, become increasingly important. Strawberry resistance to P. cactorum is a quantitative and specific expression of many genes likely involved in the penetration and colonization of the pathogen as well as the elicitation of plant defenses is required [11] and references therein. A draft genome of a P. cactorum strawberry isolate indicated RxLR effectors as priority gene targets for the functional study of strawberry resistance to P. cactorum [12]. Although genome sequencing and comparative analysis in different investigations highlight the role of RxLR, CRN, and apoplastic effectors, not much work has been conducted in strawberry to characterize their individual role in resistance to P. cactorum [13].
The use of Pseudomonas fluorescens and various arbuscular mycorrhizal fungi as biocontrol agents against P. cactorum in strawberry in vitro and in the field have been explored [14]. However, in these trials, the biological control of the disease could not be achieved. Additional factors that have been investigated are the use of different ground covers to inhibit splash dispersal [15] and sand filtration to remove the inoculum [10]. Compost amendments with antagonistic bacteria in soilless substrates did not elevate the disease suppressiveness against P. cactorum [10]. Thermotherapy seems to have potential for reducing the number of zoospores, in contrast to the number of oospores, but the recommended 6 h long treatment still needs to be tested and implemented [16]. Thus, to date, there are no methods available that can eradicate P. cactorum from infested areas [11]. The existing cultural control strategies seem to be insufficient in controlling P. cactorum in favorable conditions for the pathogen.
Therefore, we tested a re-designed strawberry nursery system using elevated trayfields to avoid the splash dispersal of P. cactorum. However, this approach will only be successful if P. cactorum is not dispersed through the air, which is usually assumed but has not been indicated to date. We tested the following hypotheses in particular:
- (1)
- An elevated trayfield lowers the incidence of crown rot through the inhibition of splash water.
- (2)
- Such an elevated trayfield system should outperform the traditional system with or without ground foil in regard to the control of P. cactorum and should at least perform equally well compared to a system applying pesticides.
- (3)
- P. cactorum is only spread by water and not by air.
2. Materials and Methods
2.1. Experimental Design
An elevated trayfield system was used consisting of a gutter system supporting closely placed trays 32 cm above the ground (Meteor Systems, Breda, The Netherlands), as shown in Figure 1a.
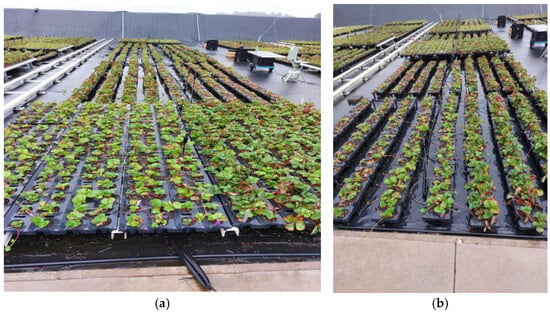
Figure 1.
(a) Elevated strawberry trayfield system (at the front) consisting of a gutter system, 32 cm above the ground, on which trays were placed containing 28 cups filled with peat and coco coir containing a transplanted strawberry tip each. At the back, the traditional system is shown where trays are placed directly on the trayfield. (b) Trays placed on foil (at the front). Behind that, trays are placed directly on the trayfield without foil.
This elevated trayfield system was compared to a traditional system, one where trays were directly placed on the trayfield, and a test system in which the trayfield was covered with a new clean polyethylen plastic foil (Figure 1b). For the inoculated fields, the foil was placed after inoculation. To be able to compare results with the practice, the traditional system was divided into two sections, one with and one without an application scheme of pesticides against P. cactorum. The spraying of pesticides was carried out with a knapsack sprayer (Azo; Wieringen; The Netherlands) when weather circumstances were suitable for spraying to guarantee precise spraying and to reduce drift. The standard application of pesticides was similar to that commonly used by propagators in the Netherlands, as described in Supplemental Table S1.
All systems were placed on an experimental trayfield, covered with an anti-root cloth, at the Delphy Innovative Soft Fruit Center at Horst, The Netherlands. The experimental field was divided into eight main plots (Supplemental Figure S1). Four of these plots were inoculated with P. cactorum, and the other four main plots were not inoculated and served as the control. Due to the slope of the experimental field to direct the water to one side, it was not possible to place the main plots randomly. The plots that were behind each other in the direction of the water flow had to be either inoculated or non-inoculated to prevent cross-contamination. This resulted in a split-plot design. Within each main plot, the four trayfield systems were randomly allocated, and within the trayfield systems, cultivars (2021) or propagation origin (2022 and 2023) were randomly assigned. The experiment consisted of two inoculation methods on four trayfield systems using two strawberry cultivars or propagation origins and with four replicates. This led to a total of sixty-four plots. The layout was the same for all three consecutive years of the trial to ensure the build-up of P. cactorum pressure.
The inoculation of the trayfield was conducted by spreading plant debris onto the trayfield from 80 P. cactorum-infested strawberry plants that were shredded into pieces of 1–5 cm. In addition, in 2021 and 2022, these plots were inoculated with P. cactorum oogonia. Oogonia were applied to the trayfield by spraying with a oogonia suspension of 7500 oogonia/mL. Oogonia were derived from a lab culture (Potato Dextrose Agar (PDA), 20 °C), originally obtained from apple and maintained for several years at Wageningen University & Research. For inoculum preparation, P. cactorum discs of 5 mm were placed on PDA plates and grown for two weeks. Plates were blended at the day of inoculation, and the material was sieved and suspended in water. A sample was taken, and oogonia were counted using a hemocytometer. The spraying of oogonia was carried out with a knapsack sprayer (Azo; Wieringen; The Netherlands) at a volume of 2.5 L.
2.2. Plant Material and Substrate
In 2021, two June bearing strawberry cultivars were used: Sonata and Malling Centenary. No differences in disease incidence nor severity between varieties were observed in 2021. Therefore, the experiment continued with Malling Centenary deriving from two different propagations origins (Van Oers, Prinsenbeek, The Netherlands, and Van den Elzen, Erp, The Netherlands) to allow more homogenous watering and cultivation. Strawberry tips were planted on 15 July 2021, 12 July 2022, and 12 July 2023. Tips were placed in either traditional trays (Beekenkamp, Maasdijk, The Netherlands) of 9 cups of 250 mL, leading to 6 trays with a total of 54 plants per plot, or in elevated trays (Meteor Systems, Breda, The Netherlands) consisting of 2 trays with each 28 cups of 200 mL, leading to 56 plants per plot. Both tray types were filled with a mixture of 45% white peat, 45% coco coir, and 10% perlite (Legro, Helmond, The Netherlands). The trayplants were fertilized with 5 kg/m3 15:9:15 N:P:K CRF Multicote 6 slow-release fertilizer (Haifa Group, Haifa, Israel) in 2021 and 2022, and 3 kg/m3 in 2023. Watering was conducted through overhead sprinkler irrigation (Meteor Systems, Breda, The Netherlands). During the vegetative phase, an EC of 1.0 was achieved (1 mmol NH4, 2.26 mmol K, 2.74 mmol Ca, 1.13 mmul Mg, 6.57 mmol NO3, 1.31 mmol SO4, 0.81 mmol P, 75 µmol Fe, 40 µmol Mn, 10 µmol Zn, 15 µmol B, 2 µmol Cu, and 2 µmol Mo). This was increased to an EC of 1.5 during the generative phase, halfway through September. Directly after placing the tips, the plants were kept wet to allow rooting.
2.3. Evaluation of Crown Rot
For evaluation, only plants in the middle of the plot were assessed. The trays of each plot that were the furthest out from the center were not assessed, and they functioned as a buffer zone for the drift of pesticides. Nursery plant loss due to crown rot was recorded. At the end of each nursery period, on 7 December 2021, 6 December 2022, and 7 December 2023, all remaining plants were assessed for root symptoms by cutting the rhizomes in half. Disease incidence as the percentage of diseased plants was assessed. Disease severity was scored according to the index of root browning: 0: No visible browning; 1: very light browning; 2: < 10% browning; 3: 10–25% browning; 4: 25–50% browning; and 5: >50% browning (Supplemental Figure S2).
2.4. Spore Detection in Water and Air Samples
Water and air samples were collected for the molecular identification of P. cactorum and N. clavispora. Splash and rainwater were sampled together in rain gauges at the center of the experimental field, mounted at 5, 32, and 52 cm height from the ground. The water samples were collected once a week and stored at −20 °C until processing. In 2021, no spores in the collected water could be detected. In 2022 and 2023, rhododendron leaves were placed into the rain gauges, having been reported to be sensitive bait for the recovery of Phytophthora zoospores [17,18]. These leaves were collected each week and stored at 4 °C until processing. A total of 1 gram of rhododendron leaves with disease symptoms was ground into powder, of which 100 mg were used for DNA isolation.
For the collection of spores from the air, a Burkhard spore sampler (Burkhard, Hertfordshire, UK) with a suction capacity of 600 L air/h was situated in the center of the experimental field. Spores were impacted on adhesive-coated tape supported on a drum. The circumference of the drum is equivalent to the tape revolving during one week, after which the tape needs replacement. Tapes were replaced weekly and stored at −20 °C until processing. For processing, the tape was cut into 7 pieces representing a day each.
DNA isolation was performed with the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). The extracted DNA was resolved in 50 μL TE buffer. qPCR was performed for the detection of P. cactorum, N. clavispora, and strawberry powdery mildew, Podosphaera aphanis. P. aphanis is a known, frequently occurring airborne pathogen, which can easily be detected by a Burkhard spore trap. Therefore, the tapes were also checked for powdery mildew spores to ensure the spore trap worked properly.
The TaqMan qPCR for the detection of P. cactorum was based on the target geneYpt1 [19], as described in Table 1. The PCR reactions were performed following the protocol and primers developed by Li et al. [19] with some modifications. The reactions took place in a total volume of 25 μL containing 2.5 μL genomic DNA solution, 12.5 μL 2× GoTaq®qPCR Probe Master Mix (Promega, Madison, WI, USA), 0.3 µM primer, and 0.2 µM P. cactorum probe. PCR amplification was programmed with one cycle of denaturation at 95° C for 10 s and 40 cycles of 95 °C for 5 s and 62 °C for 34 s and were performed using a Biorad CFX96 real-time PCR system (Biorad, Hercules, CA, USA).

Table 1.
Primers and probes used for DNA amplification in the TaqMan and Syber Green qPCR assays.
The SYBR green qPCRs for the detection of N. clavispora and P. aphanis were based on primers developed on the basis of ITS sequences from the NCBI GeneBank, using the Primer 3 software [20,21]. The primers used are described in Table 1. The reactions took place in a total volume of 25 μL containing 2.5 μL genomic DNA solution, 12.5 μL 2x GoTaq®qPCR Probe Master Mix (Promega, Madison, WI, USA) and 0.3 µM of each primer. PCR amplification was programmed with one cycle of denaturation at 95 °C for 10 s and 40 cycles of 95 °C for 10 s, 58 °C for 15 s, and 72 °C for 20 s and were performed using a Biorad CFX96 real-time PCR system (Biorad, Hercules, CA, USA).
2.5. Disease Detection in Tips, Plant Debris, and Rhizomes
On each year, randomly taken tips were analyzed for the presence of P. cactorum and N. clavispora with the above-described molecular method. In the first year, 60 tips from each cultivar were analyzed, followed by 60 tips from each origin in the second and finally third year. The analyses was carried out on 8 samples of 15 combined tips across all cultivars/origins.
In 2023, the plant debris and rhizomes of plants with a positive visual score were analyzed for the presence of P. cactorum and N. clavispora with the above-described molecular method. One plant debris and four rhizomes samples were analyzed for all four replicates of the inoculated plots only, and only of the elevated and the traditional system without pesticide use against P. cactorum. In total, 16 samples of plant debris and 64 rhizomes samples were analyzed.
2.6. Statistics
Crown rot incidence and severity were analyzed per year by three-way ANOVA with trayfield system, inoculation/non-inoculation, and cultivar/origin as the main factors. Due to the split-plot design, only the following interactions were tested: inoculation*trayfield system and trayfield system*cultivar/origin. Significant differences were further analyzed by Bonferroni-corrected post hoc tests. Data met the assumption of normal distribution. Statistical analyses were performed with the SPSS v. 28 software.
The presence of P. cactorum and N. clavispora in the rhododendron baits from the splash water samples at different heights and the presence of P. cactorum, N. clavispora, and P. aphanis in air samples were analyzed by paired sample proportion tests (mcNemar) [22].
3. Results
3.1. Crown Rot Incidence
Overall, the disease incidence of P. cactorum increased over the years (Figure 2).
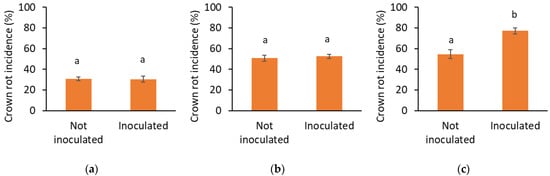
Figure 2.
Crown rot incidence (%) in (a) 2021, (b) 2022, and (c) 2023 in non-inoculated and inoculated plots. Data are presented as mean ± SEM. Letters indicate significant differences between treatments at p < 0.05 based on ANOVA.
However, only in the last year, a significantly higher crown rot incidence was observed in the inoculated plots compared to the non-inoculated ones (p ≤ 0.001). In 2021, the disease occurred less often in the elevated trayfield system compared to the traditional trayfield system without use of pesticides against P. cactorum (p = 0.007) (Figure 3a), while in 2023, the elevated trayfield showed significantly less diseased plants compared to all the other three systems, reducing the incidence by 50% (p ≤ 0.001) (Figure 3c). The year of 2023 was the only one in which crown rot symptoms were observed before the end of the nursery. At the end of the first month, less plants with crown rot symptoms in the inoculated plots were scored in the elevated system compared to the traditional systems with and without crop protection against P. cactorum (Supplemental Figure S3). No differences between the cultivation systems were observed in 2022 (Figure 3b). Variety or origin did not have any effect on crown rot incidence in any year.
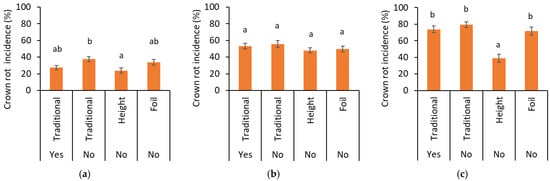
Figure 3.
Crown rot incidence in (a) 2021, (b) 2022, and (c) 2023 analyzed over non-inoculated and inoculated plots for the traditional trayfield system with (yes) and without (no) the application of pesticides against P. cactorum in the elevated trayfield system (Height) and the traditional trayfield system with foil (Foil). Data represent mean ± SEM. Letters indicate significant differences between treatments at p < 0.05 based on ANOVA followed by Bonferroni-corrected post hoc tests.
3.2. Crown Rot Severity
Similarly, disease severity was not different between inoculated and non-inoculated plots in the first 2 years, while in 2023, plants in the inoculated plots were more severely infected (p ≤ 0.001) (Figure 4).
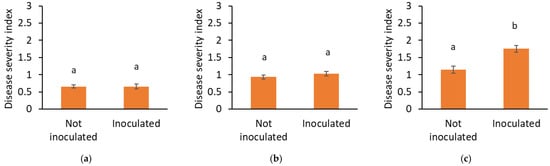
Figure 4.
Crown rot severity in (a) 2021, (b) 2022, and (c) 2023 in non-inoculated and inoculated plots. Data are presented as mean ± SEM. Letters indicate significant differences between treatments at p < 0.05 based on ANOVA.
In 2021, the crown rot severity in the elevated trayfield system was significantly lower compared to the traditional trayfield system without pesticides, but was comparable to that with pesticides (p = 0.014) (Figure 5a). In 2023, the diseased plants in the elevated trayfield system were much lower effected compared to all the other three systems (p ≤ 0.001), lowering crown rot severity substantially (Figure 5c). No differences were observed between the traditional trayfield system with or without pesticides. In 2022, no effects of the elevated tray systems were observed (Figure 5b). Variety or origin did not influence severity in any of the years.
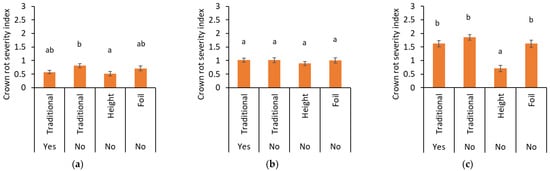
Figure 5.
Crown rot severity in (a) 2021, (b) 2022, and (c) 2023 analyzed over non-inoculated and inoculated plots for the traditional trayfield system with (yes) and without (no) the application of pesticides against P. cactorum the elevated trayfield system (Height) and the traditional trayfield system with foil (Foil). Data represent mean ± SEM. Different letters indicate significant differences between treatments at p < 0.05 based on ANOVA followed by Bonferroni-corrected post hoc tests.
3.3. Phytophthora cactorum Spread by Splash, but Not Aerially
In 2021, water samples were taken without rhododendron baits and no P. cactorum was detected. It was hypothesized that the levels were below the detection limit. Using rhododendron bait in the subsequent two years, P. cactorum could be detected in the weekly water samples (Table 2). It was determined that P. cactorum was pre-dominantly present in the rain gauge placed 5 cm above the ground, whereby the number of positive samples doubled from 22% in 2022 to 45% in 2023 (Table 2). If detected further up from the ground, the concentrations measured in the positive samples were about 370 times lower than those measured at 5 cm. While, in 2022, the concentration in the positive samples at 5 cm above the ground was double of that at 50 cm height, in 2023, there were no differences in concentrations between heights. In contrast, 90–100% of all samples tested in 2022 and 2023 contained N. clavispora. The concentrations increased strongly from year 2022 to 2023 at all heights.

Table 2.
Detection of P. cactorum and N. clavispora in water samples collected in rain gauges at different heights baited with rhododendron leaves during the cultivation periods in 2022 and 2023. Samples positive (%): percentage of weekly water samples in which the pathogen was detected in bait. Concentration (ng/g leaves): Average concentration DNA detected in rhododendron baits in positive samples. Letters indicate significant differences between the number of positive samples using the paired sample proportion test (mcNemar).
No P. cactorum was detected in the air samples in any of the three years (Table 3). N. clavispora was detected in 2022 in relatively low concentrations. In contrast, the powdery mildew P. aphanis was detected in almost all samples, at high concentrations, in all three years.

Table 3.
Detection of P. cactorum, N. clavispora, and P. aphanis in air samples collected during the cultivation periods in 2021, 2022, and 2023. Samples positive (%): percentage of daily air samples in which disease was detected. Concentration (pg/day): Average concentration DNA detected in positive samples. Letters indicate significant differences between the number of positive samples using the paired sample proportion test (mcNemar).
3.4. Phytophthora cactorum Detected in Plant Debris and Plants Without Crown Symptoms
In 2023, the presence of P. cactorum and N. clavispora was determined in fresh tips taken at start of the experiment, plant debris taken from the trayfield at the end of the experiment, and rhizomes with crown rot symptoms (Table 4). P. cactorum could not be detected in tips at the start of the experiment, whereas N. clavispora was detected in three of the eight samples. Also in 2021 and 2022, P. cactorum was not detected in tips. However, both pathogens were detected in almost all plant debris samples collected from below the elevated system as well as from below the traditional system without pesticide use against P. cactorum. The pathogens were sometimes detected together and sometimes solely. Only in one sample, no pathogen could be detected. In the rhizomes with crown rot symptoms, P. cactorum mainly occurred solely and only in six samples together with N. clavispora (Table 4). The latter did not occur solely in any of the samples. In rhizomes from plants grown on the traditional system without pesticides against P. cactorum, 25 out of the 32 samples were positive, whereas only 12 out of the 32 samples were positive for the elevated system. This coincides with the observation that the disease severity was lower in the elevated trayfield system compared to the other trayfield systems.

Table 4.
Detection of P. cactorum and N. clavispora in the tips, plant debris, and rhizomes of plants with symptoms collected in 2023. Tips: Fresh tips taken at start of the experiment. Plant Debris: Decaying plant parts taken from the trayfield at the end of the experiment. Rhizomes with symptoms: Rhizomes were inspected at the end of the experiments for crown rot symptoms, and rhizomes with a positive visual score were used for qPCR analyses.
4. Discussion
In our three-year trial, the elevated trayfield performed as well as or even better than the traditional system with the use of pesticides against P. cactorum. While, in 2021 and 2022, under relatively low or medium disease pressure, the traditional system with pesticides was comparable to the elevated system, the latter was clearly superior in suppressing P. cactorum in 2023, under a relatively high disease pressure. Covering the trayfield with foil did not result in a significant decrease in crown rot disease incidence or severity in any of the years when compared to the traditional system without the use of pesticides.
Our results indicate that the pesticides used for P. cactorum control are not effective under medium-to-high disease pressure. Even under low disease pressure (2021), disease control using pesticides was hardly effective. One of the reasons could be the development of resistance towards the active ingredients used. The variation in efficacy of seven different fungicide compounds used against P. cactorum has been documented by Ali [23]. Dimethomorph and promocarp, both used in our trials, were reported to be not effective to control P. cactorum, failing to inhibit zoospore release as well as mycelium growth. Besides these synthetic fungicides, our standard treatment practice included the fungus Trichoderma harzianum as a biological fungicide. Although this particular strain was not included, the effectivity of these type of microbial fungicides against P. cactorum was reported to be very variable, mainly depending on the growing medium of the plants [14]. However, none of the media tested contained any peat or coco coir as individual growing media or their mixture as used in our trials.
In 2021, 2022, and 2023, the starting material (tips) were analyzed for the presence of P. cactorum and N. clavispora. None of the tips contained P. cactorum, while a few samples in in 2023 contained N. clavispora, most likely because this pathogen can also be present in the leaves, causing symptoms in those organs [4]. This observation indicates that N. clavispora disease pressure does not only come from the trayfield, but disease can also enter the system by the starting material. In rhizomes with crown rot symptoms, P. cactorum was mainly detected solely but also in combination with N. clavispora. However, N. clavispora was never detected solely. This does not answer the question of whether N. clavispora alone can cause necrosis and the blackening of strawberry rhizomes, but indicates that P. cactorum is still the main cause of crown rot on trayfields. Therefore, prevention strategies should be targeted towards P. cactorum.
Disease pressure, determined in our trials as disease incidence and severity, increased in the consecutive three years of the trial, although in the first two years, no differences in disease severity were observed between the inoculated and non-inoculated plots. The data suggest that our inoculation of the trayfield with contaminated strawberry plant material was not strong enough to sufficiently spread P. cactorum in the first two years. Indeed, in the second year, no differences between any of the treatments were observed. In contrast, the third year showed a much higher disease pressure of the inoculated plots compared to the control. The results of the third year imply that it might have taken longer to build up a P. cactorum reservoir in the soil and/or anti-root cloth of the inoculated plots. In addition, a more virulent, naturally occurring strain of P. cactorum may have infested the plants. Differences between the pathogenicity of P. cactorum strains have evolved, suggesting discrete lineages for only strawberry-infecting isolates and isolates infecting both strawberry and apple [1]. Possibly, a P. cactorum strain with a high pathogenicity for the cultivar Malling Centenary arrived at our trial from outside our plots, resulting in much higher infection rates in the last year.
As hypothesized, the positive effect of the elevated system on the prevention of P. cactorum was based on the inhibition of splash water reaching the strawberry plants. The edges of the elevated trays fit closely, leaving little room for splash water from the bottom to reach the plants. Should, however, some splash water, mainly from the plot edges, reach the elevated plants, we demonstrated that fewer samples of splash water at the height of the elevated trays contained P. cactorum. In addition, these positive samples held a much lower concentration of the pathogen compared to splash water close to the ground. Taking into account that we could determine this effect already in our small experimental plots, with relatively strong edge effects, the inhibiting effect of splash water on the occurrence of P. cactorum may be even greater in bigger nursery fields, as occurring in practice.
The inhibition of P. cactorum though elevated trays only holds under the assumption that P. cactorum is not airborne. Indeed, no P. cactorum could be detected in the air samples in any of the three trial years. In contrast, strawberry powdery mildew, which is dispersed by air [24], was predominantly present in every trial year. In contrast, we could detect N. clavispora in the water as well as in the air samples, which may indicate its spread by water as well as by air. Pathogenic fungal species belonging to the genus Neopestalotiopsis have been reported to disperse their conidia by air or water splashing [25].
The elevated trayfield system showed a great potential in diminishing P. cactorum in strawberry nursery fields, but could not completely control the disease. We were able to prevent plant losses, but infected, asymptomatic plants were still present in all trial years, including in 2023, when, under high disease pressure, some plants were visibly diseased or even died. Therefore, a reliable, quick, non-destructive method to determine plant infection of transplants by P. cactorum is needed. The most effective methods for nondestructive internal disorder detection such as blackening are near-infrared spectroscopy (NIR), magnetic resonance imaging (MRI), and X-ray radiography [26]. Of these, MRI has been successfully applied to identify P. cactorum symptoms in the crown of hydroponically grown strawberry plantlets [27].
In order to control P. cactorum in an sustainable and environmental friendly way, we suggest to re-design the strawberry nursery system outdoors into a resilient cultivation system in which reproduction and infection is diminished by the system itself. Such a system should be effective even under climatic conditions favorable for the pathogen. We suggest considering the following measures in the re-designing process:
- (1)
- The basis ought to be resistant varieties. Since these are not available yet, less susceptible varieties should be used. Great variation in susceptibility to P. cactorum between strawberry varieties has been demonstrated [28]. However, it has not been established yet by how much the occurrence of P. cactorum could be reduced using less susceptible varieties.
- (2)
- Tips could probably be treated with thermotherapy to start with plant material free of P. cactorum, Botrytis cinerea, P. aphanis, and mites (Tetranychus urticae and Phytonemus pallidus). This method has only been described for use in transplants after plant nursery, but should in principle also work with strawberry tips, although the protocol probably needs some optimization for this type of plant tissue [16].
- (3)
- To prevent infection from the trayfield, elevated trayfields should be used. This will diminish the number of spores reaching plants by splash water.
- (4)
- Infection from trayfield to elevated trays can even be further diminished by placing new plastic foils on top of the trayfield each year. This is especially important in reducing the transfer of disease from one season to the next.
- (5)
- If irrigation water and rain water are collected for re-use, a sand filter should be used [10]. This filtration step will prevent the spread of mobile zoospores by the irrigation system, because these will be filtered out by the sand.
In this way, the production of tray plants free of P. cactorum without the use of pesticides should be possible. Such a system, however, needs to be designed carefully and tested in practice, at the sites of propagation companies, in order to assess feasibility, ease of use, and ultimately, the costs of the re-designed system.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14122809/s1. Table S1. Crop protection scheme against P. cactorum applied during the course of the trials. Figure S1. Experimental set-up of the different trayfield systems. Figure S2. Disease severity was scored as an index of root browning. Figure S3. Plants with crown rot symptoms (wilting or dying) one month after the start of the experiment in 2023.
Author Contributions
Conceptualization, A.E.; methodology, A.E. and K.P.; investigation, K.P.; data analysis, A.E. and J.A.B.-M.; writing—original draft preparation, J.A.B.-M. and A.E.; writing—review and editing, K.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ‘Topsector Tuinbouw en Uitgangsmaterialen’, grant number LWV 19180.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank R. Peters, J.A.M. Wilms, C.G. Topper, A. Biemans, and T. Keijsers for their technical support. We are grateful to Meteor Systems providing the elevated tray system and to Gondy Heijerman for looking after the trial at the Delphy International Soft Fruit Center and providing the crop protection scheme against P. cactorum as used in practice. We also thank J. Peller for proofreading the manuscript to improve the English language.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nellist, C.F.; Armitage, A.D.; Bates, H.J.; Sobzy, M.K.; Luberti, M.; Lewis, L.A.; Harrison, R.J. Comparative analysis of host-associated variation in Phytophthora cactorum. Front. Plant Sci. 2021, 12, 679936. [Google Scholar] [CrossRef] [PubMed]
- Chamoro, M.; Aguado, A.; de los Santos, B. First report of root and crown rot caused by Pestalotiopsis clavispora (Neopestalotiopsis clavispora) on strawberry in Spain. Plant Dis. 2016, 100, 1495. [Google Scholar] [CrossRef]
- Gilardi, G.; Bergeretti, F.; Gullino, M.L.; Garibaldi, A. First report of Neopestalotiopsis clavispora causing root and crown rot in strawberry in Italy. Plant Dis. 2019, 103, 2959. [Google Scholar] [CrossRef]
- Baggio, J.S.; Forcelini, B.B.; Wang, N.-Y.; Ruschel, R.G.; Mertely, J.C.; Peres, N.A. Outbreak of leaf spot and fruit rot in Florida strawberry caused by Neopestalotiopsis spp. Plant Dis. 2021, 105, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Stensvand, A.; Herrero, M.L.; Talgø, V. Crown rot caused by Phytophthora cactorum in Norwegian strawberry production. EPPO Bull. 1999, 29, 155–158. [Google Scholar] [CrossRef]
- Chen, X.-R.; Wen, K.; Zhou, X.; Zhu, M.-Y.; Liu, Y.; Jin, J.-H.; Nellist, C.F. The devastating oomycete phytopathogen Phytophthora cactorum: Insights into its biology and molecular features. Mol. Plant Pathol. 2023, 24, 1017–1032. [Google Scholar] [CrossRef]
- Grove, G.G.; Madden, L.V.; Ellis, M.A. Splash dispersal of Phytophthora cactorum from infected strawberry fruit. Phytopathology 1985, 75, 611–615. [Google Scholar] [CrossRef]
- Fennimore, S.A.; Duniway, J.M.; Browne, G.T.; Martin, F.N.; Ajwa, H.A.; Westerdahl, B.B.; Goodhue, R.E.; Haar, M.; Winterbottom, C. Methylbromide alternatives evaluated for Californian strawberry nurseries. Calif. Agric. 2008, 62, 62–67. [Google Scholar] [CrossRef]
- Mezzetti, B.; Giampieri, F.; Zhang, Y.T.; Zhong, C.F. Status of strawberry breeding programs and cultivation systems in Europe and the rest of the world. J. Berry Res. 2018, 8, 205–211. [Google Scholar] [CrossRef]
- Evenhuis, B.; Nijhuis, E.; Lamers, J.; Verhoeven, J.; Postma, J. Alternative methods to control Phytophthora cactorum in strawberry cultivated in soilless growing media. Acta Hortic. 2014, 1044, 337–342. [Google Scholar] [CrossRef]
- Chen, X.-R.; Huang, S.-X.; Zhang, Y.; Sheng, G.-L.; Zhang, B.-Y.; Li, Q.-Y.; Zhu, F.; Xu, J.-Y. Transcription profiling and identification of infection related genes in Phytophthora cactorum. Mol. Genet. Genom. 2018, 293, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Armitage, A.D.; Lysøe, E.; Nellist, C.F.; Lewis, L.A.; Cano, L.M.; Harrison, R.J.; Brurberg, M.B. Bioinformatic characterization of the effector repertoire of the strawberry pathogen Phytophthora cactorum. PLoS ONE 2018, 13, e0203205. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Marin, M.V.; Lee, M.B.; Baggio, J.S.; Peres, N.A.; Lee, S. Genomic approaches for improving resistance to Phytophthora crown rot caused by P. cactorum in strawberry (Fragraria x ananassa). Front. Agron. 2022, 4, 941111. [Google Scholar] [CrossRef]
- Hautsalo, J.; Vestberg, M.; Parikka, P.; Kukkonen, S.; Karhu, S. Biological control of strawberry crown rot is substrate dependent phenomenon. J. Berry Res. 2016, 6, 65–79. [Google Scholar] [CrossRef]
- Madden, L.V.; Ellis, M.A. Effect of ground cover on splash dispersal of Phytophthora cactorum from strawberry fruits. J. Phytopathol. 1990, 129, 170–174. [Google Scholar] [CrossRef]
- Baggio, J.; Marin, M.V.; Peres, N.A. Phytophthora crown rot of Florida strawberry: Inoculum sources and thermotherapy of transplants for disease management. Plant Dis. 2021, 105, 3496–3502. [Google Scholar] [CrossRef]
- Ghimire, S.R.; Richardson, P.A.; Moorman, G.W.; Lea-Cox, J.D.; Ross, D.S.; Hong, C.X. An in-situ baiting bioassay for detecting Phytophthora species in irrigation runoff containment basins. Plant Pathol. 2009, 58, 577–583. [Google Scholar] [CrossRef]
- Rollins, L.; Coats, K.; Elliott, M.; Chastagner, G. Comparison of five detection and quantification methods for Phytophthora ramorum in stream and irrigation water. Plant Dis. 2016, 100, 1202–1211. [Google Scholar] [CrossRef]
- Li, M.; Inada, M.; Watanabe, H.; Suga, H.; Kageyama, K. Simultaneous detection and quantification of Phytophthora nicotianae and P. cactorum, and distribution analyses in strawberry greenhouses by duplex real-time PCR. Microbes Environ. 2013, 28, 195–203. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- McNemar, Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 1947, 12, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Kumar, R.; Mazákova, J.; Maňasova, M.; Zouhar, M.; Pánek, M. Evaluation of the ability of seven active ingredients of fungicides to suppress Phytophthora cactorum at diverse life stages, and variability in resistance found among isolates. J. Fungi 2022, 8, 1039. [Google Scholar] [CrossRef]
- Blanco, C.; de los Santos, B.; Arroyo, B.F.T.; Porras, M. Relationship among concentrations of Spaerotheca macularis conidia in the air, environmental conditions and the incidence of powdery mildew in strawberry. Plant Dis. 2008, 88, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Prasannath, K.; Galea, V.L.; Akinsanmi, O.A. Molecular methods for detection and quantification of Pestalotiopsis and Neopestalotiopsis inoculum associated with macademia. Plant Pathol. 2020, 70, 1209–1218. [Google Scholar] [CrossRef]
- Nicolaï, B.; de Ketelaere, B.; Dizon, A.; Wouters, N.; Postelmans, A.; Saeys, W.; van de Looverbosch, T.; Verboven, P.; Hertog, L.M.A.T.M. Nondestructive evaluation: Detection of external and internal attributes frequently associated with quality and damage. In Postharvest Handling, 4th ed.; Florkowski, W.J., Banks, N.H., Shewfelt, R.L., Prussia, S.E., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 399–433. [Google Scholar]
- Toumainen, T.V.; Toljamo, A.; Kokko, H.; Nissi, M.J. Non-invasive assessment and visualization of Phytophthora cactorum infection in strawberry crowns using quantitative magnetic resonance imaging. Sci. Rep. 2024, 14, 2129. [Google Scholar] [CrossRef]
- Marin, M.V.; Seijo, T.E.; Baggio, J.S.; Whitaker, V.M.; Peres, N.A. Resistance of strawberry cultivars and the effects of plant ontogenesis on Phytophthora cactorum and P. nicotianae causing crown rot. Plant Dis. 2023, 107, 651–657. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).