Faunistic Study of Auchenorrhyncha in Olive Orchards in Greece, Including First Records of Species
Abstract
1. Introduction
2. Materials and Methods
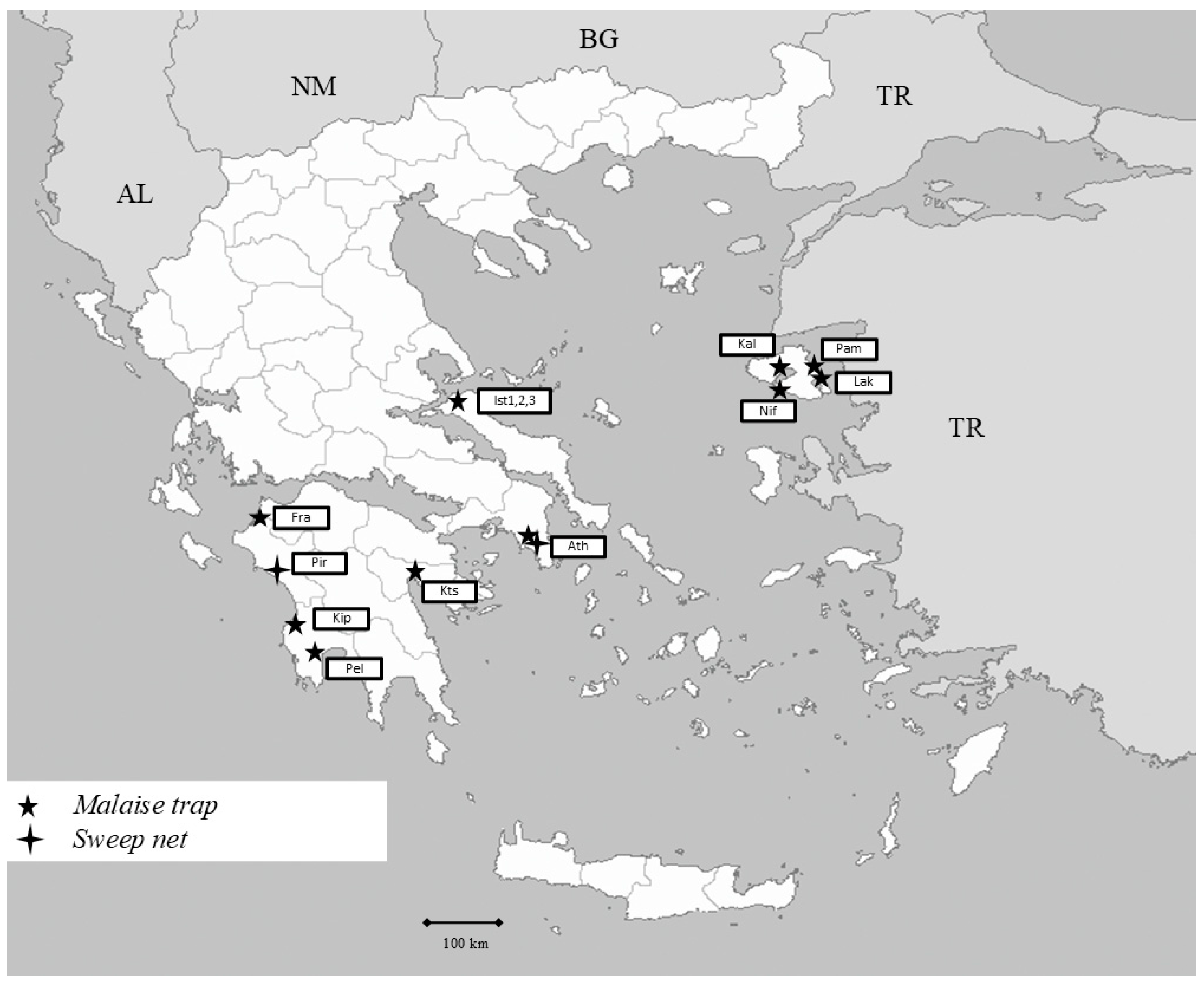
2.1. Sampling Areas
2.1.1. Sterea Ellada
2.1.2. Northeast Aegean
2.1.3. Peloponnese
2.2. Classification
2.3. Data Analysis
3. Results
3.1. Species Composition
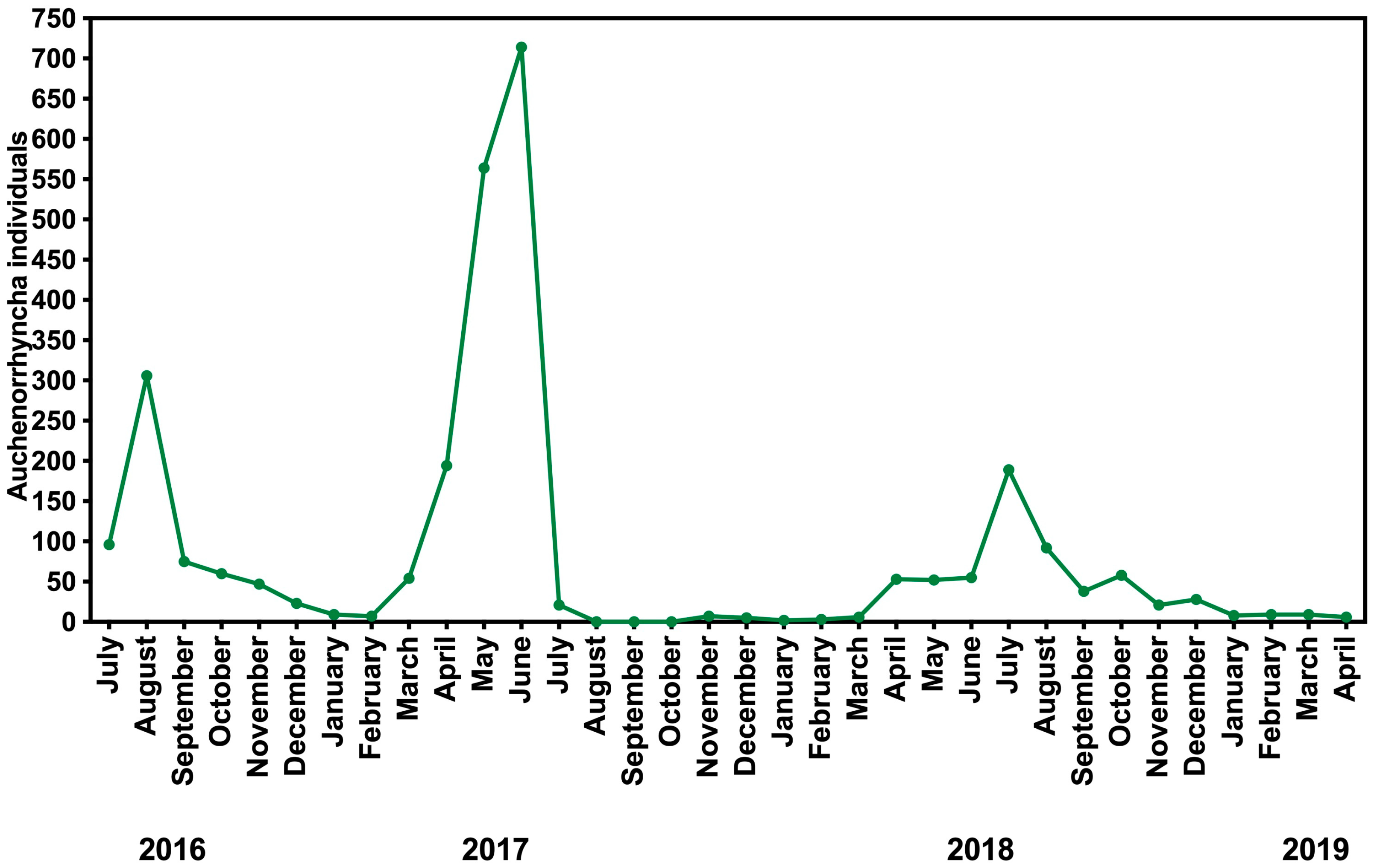
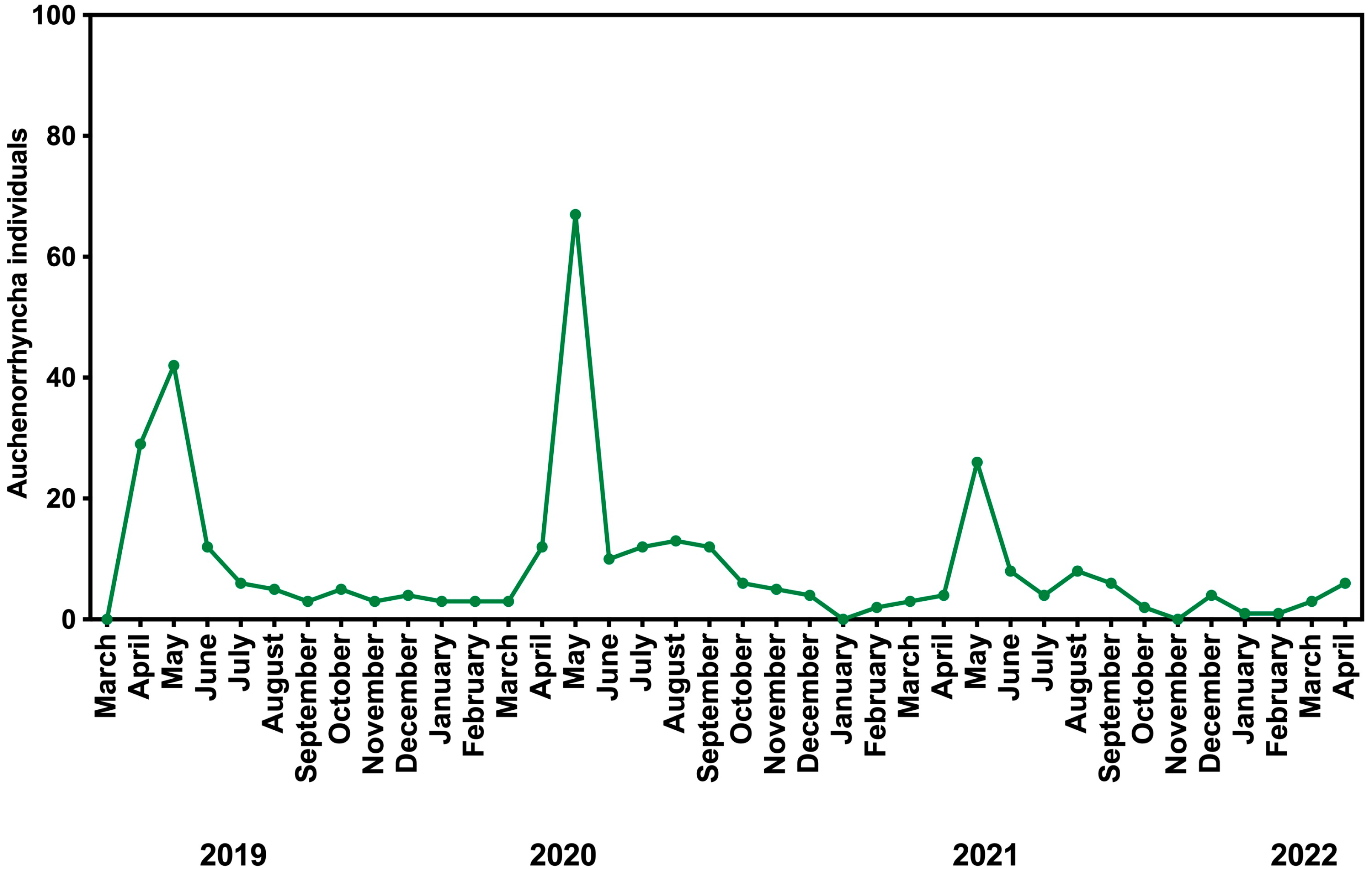
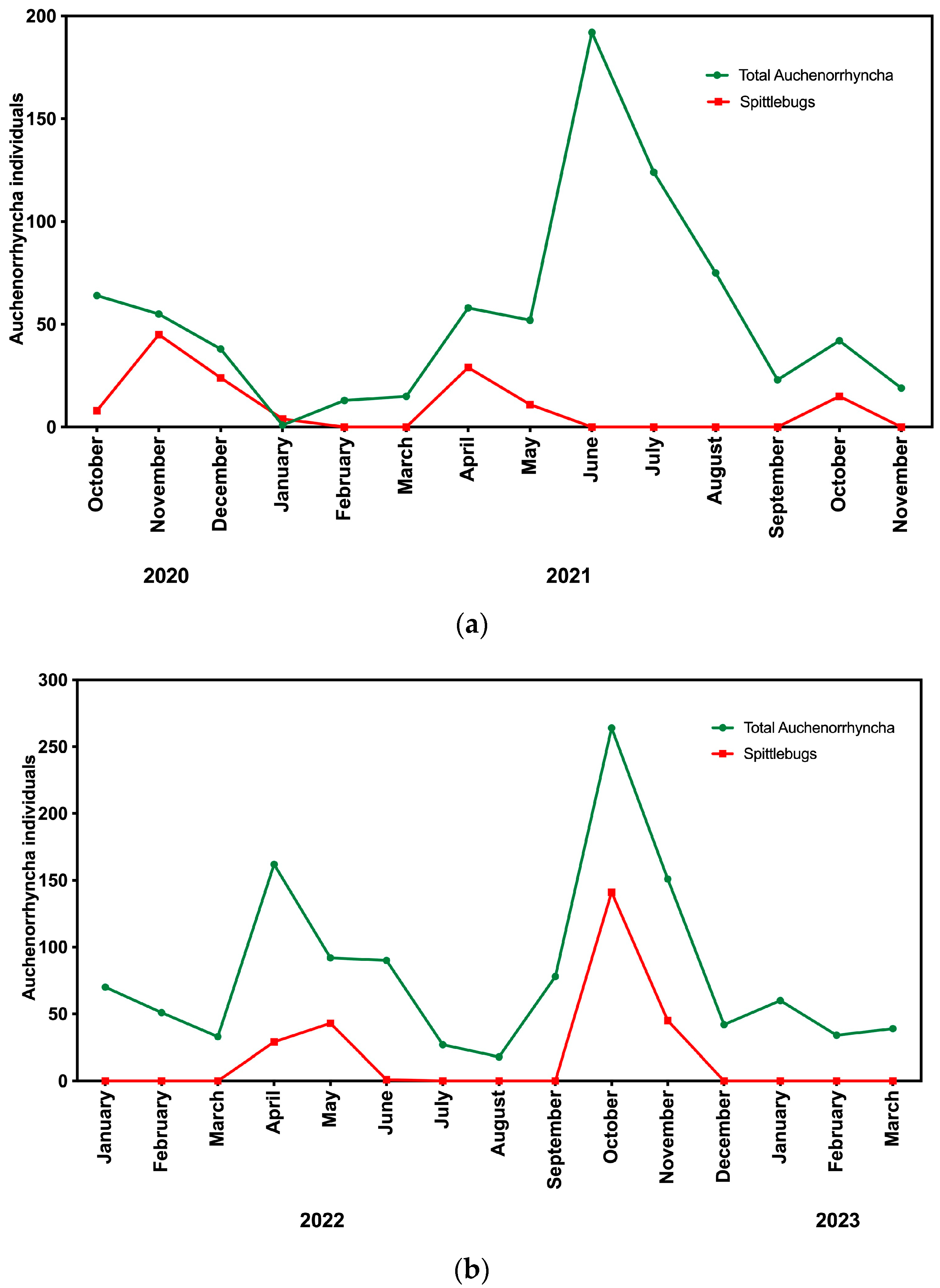
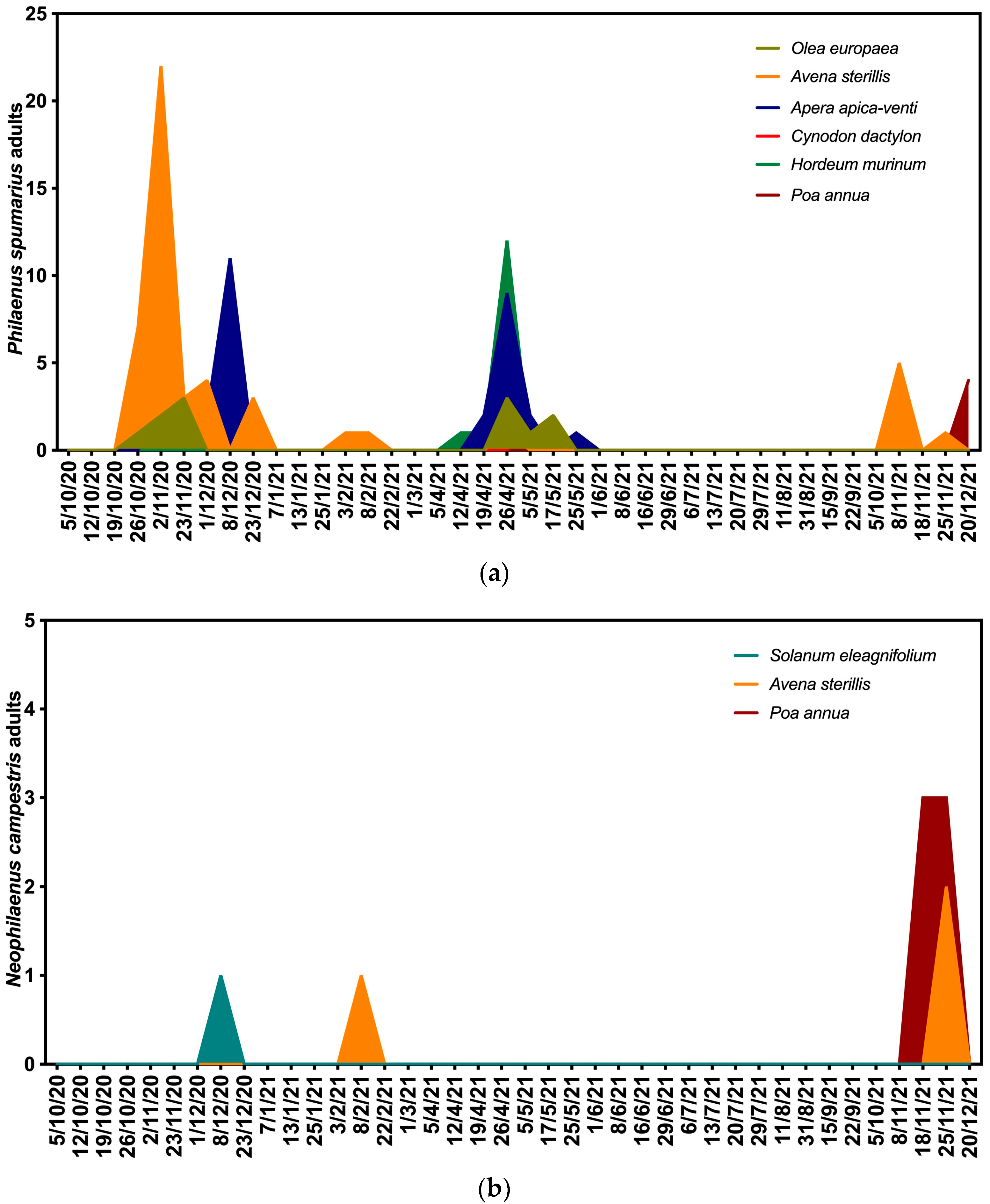
3.2. Seasonal Fluctuations
3.3. Sweep Net Results
4. Discussion
4.1. Potential Vectors
4.2. Biodiversity Indices
4.3. Seasonal Fluctuations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coletta-Filho, H.D.; Francisco, C.S.; Lopes, J.R.S.; De Oliveira, A.F.; Da Silva, L.F.D.O. First Report of Olive Leaf Scorch in Brazil, Associated with Xylella fastidiosa Subsp. pauca. Phytopathol. Mediterr. 2016, 55, 130–135. [Google Scholar] [CrossRef]
- Li, W.-B.; Pria, W.D., Jr.; Teixeira, D.C.; Miranda, V.S.; Ayres, A.J.; Franco, C.F.; Costa, M.G.; He, C.-X.; Costa, P.I.; Hartung, J.S. Coffee Leaf Scorch Caused by a Strain of Xylella fastidiosa from Citrus. Plant Dis. 2001, 85, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Rapicavoli, J.; Ingel, B.; Blanco-Ulate, B.; Cantu, D.; Roper, C. Xylella fastidiosa: An Examination of a Re-Emerging Plant Pathogen: Xylella fastidiosa. Mol. Plant Pathol. 2018, 19, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, G.; Potere, O.; Boscia, D.; Altamura, G.; Djelouah, K.; Elbeaino, T.; Frasheri, D.; Lorusso, D.; Palmisano, F.; Pollastro, P.; et al. Detection of Xylella fastidiosa in Olive Trees by Molecular and Serological Methods. J. Plant Pathol. 2014, 96, 7–14. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of Dna Sequences Related to Xylella fastidiosa in Oleander, Almond and Olive Trees Exhibiting Leaf Scorch Symptoms in Apulia (Southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar] [CrossRef]
- EPPO. Situation of Xylella fastidiosa in France; EPPO Reporting Service no. 10—2016 Num. article: 2016/193 2016; Bayer Corporation: Leverkusen, Germany, 2016. [Google Scholar]
- EPPO. First Report of Xylella fastidiosa in Spain; EPPO Reporting Service no. 11—2016 Num. article: 2016/213 2016; Bayer Corporation: Leverkusen, Germany, 2016. [Google Scholar]
- Olmo, D.; Nieto, A.; Adrover, F.; Urbano, A.; Beidas, O.; Juan, A.; Marco-Noales, E.; López, M.M.; Navarro, I.; Monterde, A.; et al. First Detection of Xylella fastidiosa Infecting Cherry (Prunus avium) and Polygala myrtifolia Plants, in Mallorca Island, Spain. Plant Dis. 2017, 101, 1820. [Google Scholar] [CrossRef]
- Landa, B.B.; Castillo, A.I.; Giampetruzzi, A.; Kahn, A.; Román-Écija, M.; Velasco-Amo, M.P.; Navas-Cortés, J.A.; Marco-Noales, E.; Barbé, S.; Moralejo, E.; et al. Emergence of a Plant Pathogen in Europe Associated with Multiple Intercontinental Introductions. Appl. Environ. Microbiol. 2020, 86, 3. [Google Scholar] [CrossRef]
- EPPO. First Report of Xylella fastidiosa subsp. multiplex in Portugal; EPPO Reporting Service no. 01/2019. Num. article 2019/017 2019; Bayer Corporation: Leverkusen, Germany, 2019. [Google Scholar]
- El-Kholy, M. Following Olive Footprints (Olea europaea L.). Cultivation and Culture, Folklore and History, Traditions and Uses; International Society for Horticultural Science: Leuven, Belgium, 2012. [Google Scholar]
- Frazier, N.W.; Freitag, J.H. Ten Additional Leafhopper Vectors of the Virus Causing Pierce’s Disease of Grapes. Phytopathology 1946, 36, 634–637. [Google Scholar]
- Hewitt, W.B.; Houston, B.R.; Frazier, N.W.; Freitag, J.H. Leaf-Hopper Transmission of the Virus Causing Pierce’s Disease of Grape and Dwarf of Alfalfa. Phytopathology 1946, 36, 117–128. [Google Scholar]
- Redak, R.A.; Purcell, A.H.; Lopes, J.R.S.; Blua, M.J.; Mizell, R.F., III; Andersen, P.C. The Biology of Xylem Fluid-Feeding Insect Vectors of Xylella fastidiosa and Their Relation to Disease Epidemiology. Annu. Rev. Entomol. 2004, 49, 243–270. [Google Scholar] [CrossRef]
- Severin, H.H.P. Spittle-Insect Vectors of Pierce’s Disease Virus. II. Life History and Virus Transmission. Hilgardia 1950, 19, 357–376. [Google Scholar]
- Purcell, A.H. The Ecology of Bacterial and Mycoplasma Plant Diseases Spread by Leafhoppers and Planthoppers; John Wiley & Sons: Hoboken, NJ, USA, 1985; pp. 351–380. [Google Scholar]
- Ben Moussa, I.E.B.; Mazzoni, V.; Valentini, F.; Yaseen, T.; Lorusso, D.; Speranza, S.; Digiaro, M.; Varvaro, L.; Krugner, R.; D’Onghia, A.M. Seasonal Fluctuations of Sap-Feeding Insect Species Infected by Xylella fastidiosa in Apulian Olive Groves of Southern Italy. J. Econ. Entomol. 2016, 109, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Elbeaino, T.; Yaseen, T.; Valentini, F.; Ben Moussa, I.E.; Mazzoni Valerio, V.; D’Onghia, A.M. Identification of Three Potential Insect Vectors of Xylella fastidiosa in Southern Italy. Phytopathol. Mediterr. 2014, 53, 328–332. [Google Scholar] [CrossRef]
- Chuche, J.; Sauvion, N.; Thiéry, D. Mixed Xylem and Phloem Sap Ingestion in Sheath-Feeders as Normal Dietary Behavior: Evidence from the Leafhopper Scaphoideus titanus. J. Insect Physiol. 2017, 102, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Guglielmino, A.; D’Urso, V.; Alma, A. Auchenorrhyncha (Insecta, Homoptera) from Sardinia (Italy): A Faunistic, Ecological and Zoogeographical Contribution. Mitt. Mus. Nat. Kd. Berl. Dtsch. Entomol. 2000, 47, 161–172. [Google Scholar] [CrossRef]
- Guglielmino, A.; Bückle, C.; Remane, R. Contribution to the Knowledge of the Auchenorrhyncha Fauna of Central Italy (Hemiptera, Fulgoromorpha et Cicadomorpha). Marbg. Entomol. Publ. 2005, 3, 13–98. [Google Scholar]
- Guglielmino, A.; Modola, F.; Scarici, E.; Speranza, S.; Bückle, C. The Auchenorrhyncha Fauna (Insecta, Hemiptera) of Villa Lante, Bagnaia (Italy): A Study of an Urban Ecosystem. Bull. Insectology 2015, 68, 239–253. [Google Scholar]
- Cornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Porcelli, F.; Almeida, R.P.P.; Saponari, M. Transmission of Xylella fastidiosa by Naturally Infected Philaenus spumarius (Hemiptera, Aphrophoridae) to Different Host Plants. J. Appl. Entomol. 2017, 141, 80–87. [Google Scholar] [CrossRef]
- Tsagkarakis, A.E.; Afentoulis, D.G.; Matared, M.; Thanou, Z.N.; Stamatakou, G.D.; Kalaitzaki, A.P.; Tzobanoglou, D.K.; Goumas, D.; Trantas, E.; Zarboutis, I.; et al. Identification and Seasonal Abundance of Auchenorrhyncha with a Focus on Potential Insect Vectors of Xylella fastidiosa in Olive Orchards in Three Regions of Greece. J. Econ. Entomol. 2018, 111, 2536–2545. [Google Scholar] [CrossRef]
- Theodorou, D.; Koufakis, I.; Thanou, Z.N.; Kalaitzaki, A.P.; Chaldeou, E.; Afentoulis, D.G.; Tsagkarakis, A.E. Management System Affects the Occurrence, Diversity and Seasonal Fluctuation of Auchenorrhyncha, Potential Vectors of Xylella fastidiosa, in the Olive Agroecosystem. Bull. Insectology 2021, 74, 27–40. [Google Scholar]
- Ribaut, H. Homoptères Auchénorhynches I. (Typhlocybidae). In Faune de France; Lechevalier, P., Ed.; Paul Lechevalier et fils: Paris, France, 1936; Volume 31. [Google Scholar]
- Ribaut, H. Homoptères Auchénorhynches II. (Jassidae). In Faune de France; Lechevalier, P., Ed.; Paul Lechevalier et fils: Paris, France, 1952; Volume 57. [Google Scholar]
- Ossiannilsson, F. Nagra for Sverige Nya Stritar (Hom. Auchenorrh.). Med. En. Synonymisk Anmarkning. Entomol. Tidskr. 1955, 76, 131–133. [Google Scholar]
- Ossiannilsson, F. The Auchenorrhyncha (Homoptera) of Fennoscandia and Denmark. Part 2: The Families Cicadidae, Cercopidae, Membracidae, and Cicadellidae (Excl. Deltocephalinae). Entomol. Scand. 1981, 7, 223–593. [Google Scholar]
- Ossiannilsson, F. The Auchenorrhyncha (Homoptera) of Fennoscandia and Denmark. Part 3: The Family Deltocephalinae, Catalogue, Literature and Index. Fauna Entomol. Scand. 1983, 7, 594–979. [Google Scholar]
- Guglielmino, A.; Bückle, C. Revision of Errhomeninae and Aphrodinae (Hemiptera, Cicadomorpha) in Italy with Remarks on Their Variability and Distribution in Adjacent Regions and Description of Three New Taxa. Zootaxa 2015, 3906, 1–66. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, D.A.; Anufriev, G.A.; Bartlett, C.R.; Blanco-Rodríguez, E.; Borodin, O.I.; Cao, Y.-H.; Deitz, L.L.; Dietrich, C.H.; Dmitrieva, M.O.; El-Sonbati, S.A.; et al. World Auchenorrhyncha Database. TaxonPages. Available online: https://Hoppers.Speciesfile.Org (accessed on 25 September 2024).
- Curry, J.P. The Arthropods Associated with the Decomposition of Some Common Grass and Weed Species in the Soil. Soil Biol. Biochem. 1973, 5, 645–657. [Google Scholar] [CrossRef]
- Cusack, P.D.; Evans, G.O.; Brennan, P.A. A Survey of the Mites of Stored Grain and Grain Products in the Republic of Ireland. Sci. Proc. R. Dublin Soc. 1975, 3, 273–329. [Google Scholar]
- Emmanuel, N. Aspects of the Biology of Mites Associated with Cereals During Growth and Storage. Ph.D. Thesis, University College Dublin, Dublin, Ireland, 1977. [Google Scholar]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Malenovský, I.; Baňař, P.; Kment, P. A Contribution to the Faunistics of the Hemiptera (Cicadomorpha, Fulgoromorpha, Heteroptera, and Psylloidea) Associated with Dry Grassland Sites in Southern Moravia (Czech Republic). Acta Musei Morav. Sci. Biol. 2011, 96, 41–187. [Google Scholar]
- Nickel, H.J. The Leafhoppers and Planthoppers of Germany (Hemiptera, Auchenorrhyncha): Patterns and Strategies in a Highly Diverse Group of Phytophagous Insects. In Pensoft Series Faunistica; Pensoft Publ: Sofia, Bulgaria, 2003; ISBN 978-954-642-169-2. [Google Scholar]
- Nickel, H.J.; Remane, R. Check List of the Planthoppers and Leafhoppers of Germany, with Notes on Food Plants, Diet Width, Life Cycles, Geographic Range and Conservation Status (Hemiptera, Fulgoromorpha and Cicadomorpha). Beiträge Zur. Zikadenkunde 2002, 5, 27–64. [Google Scholar]
- Mazzoni, V. Contribution to the Knowledge of the Auchenorrhyncha (Hemiptera Fulgoromorpha and Cicadomorpha) of Tuscany (Italy). Redia 2005, 88, 85–102. [Google Scholar]
- Söderman, G. Taxonomy, Distribution, Biology and Conservation Status of Finnish Auchenorrhyncha. In The Finnish Environment; Finnish Environment Institute: Helsinki, Finland, 2007. [Google Scholar]
- Antonatos, S.; Papachristos, D.P.; Kapantaidaki, D.E.; Lytra, I.C.; Varikou, K.; Evangelou, V.I.; Milonas, P. Presence of Cicadomorpha in Olive Orchards of Greece with Special Reference to Xylella fastidiosa Vectors. J. Appl. Entomol. 2020, 144, 1–11. [Google Scholar] [CrossRef]
- Thanou, Z.N.; Kontogiannis, E.G.; Tsagkarakis, A.E. Impact of Weeds on Auchenorrhyncha Incidence and Species Richness in Citrus Orchards. Phytoparasitica 2021, 49, 333–347. [Google Scholar] [CrossRef]
- Albre, J.; García, C.; José, M.; Gibernau, M. Ecology of the Meadow Spittlebug Philaenus Spumarius in the Ajaccio Region (Corsica)—I: Spring. Bull. Entomol. Res. 2021, 111, 246–256. [Google Scholar] [CrossRef]
- Albre, J.; Gibernau, M. Diversity and Temporal Variations of the Hemiptera Auchenorrhyncha Fauna in the Ajaccio Region (France, Corsica). Ann. Soc. Entomol. Fr. 2019, 55, 497–508. [Google Scholar] [CrossRef]
- Bosco, D.; Alma, A.; Arzone, A. Studies on Population Dynamics and Spatial Distribution of Leafhoppers in Vineyards (Homoptera: Cicadellidae). Ann. Appl. Biol. 1997, 130, 1–11. [Google Scholar] [CrossRef]
- Karavin, M.; Çalişkan, E.; Dede, O. Determination of Auchenorrhyncha Species Distributed in Apple Orchards in Amasya, Turkey with a New Record for Turkish Fauna. Int. J. Sci. Lett. 2021, 3, 32–51. [Google Scholar] [CrossRef]
- Tholt, G.; Kiss, B. Host Range of Psammotettix Alienus (Dahlbom). Növényvédelem 2011, 47, 229–235. [Google Scholar]
- Holzinger, W.; Kammerlander, I.; Nickel, H. The Auchenorrhyncha of Central Europe. Die Zikaden Mitteleuropas, Volume 1: Fulgoromorpha, Cicadomorpha Excl. Cicadellidae; Brill: Leiden, The Netherlands, 2003; 674p. [Google Scholar]
- Malenovský, I.; Lauterer, P. Leafhoppers and Planthoppers (Hemiptera: Auchenorrhyncha) of the Bílé Karpaty Protected Landscape Area and Biosphere Reserve (Czech Republic). Acta Musei Morav. Sci. Biol. 2012, 96, 155–322. [Google Scholar]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable Pest Regulation in Agricultural Landscapes: A Review on Landscape Composition, Biodiversity and Natural Pest Control. Proc. R. Soc. B 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Ribaut, H. On the Insect Fauna of Cyprus. Results of the Expedition of 1939 by Harald, Hakan and P.H. Lindberg. Soc. Sci. Fenn. 1948, 1–2, 2–14. [Google Scholar]
- Bașpinar, H.; Yıldırım, E.M.; Xing JiChun, X.J. Determination and Population Fluctuations of Cicadellidae (Hemiptera: Cicadomorpha) Species in Pomegranate Orchards in Aydin Province, Turkey. Türkiye Entomoloji Derg. 2013, 37, 3–11. [Google Scholar]
- Gnezdilov, V.M.; Drosopoulos, S.; Wilson, M.R. New Data on Taxonomy and Distribution of Some Fulgoroidea (Homoptera, Cicadina). Zool. Inst. 2004, 12, 217–223. [Google Scholar]
- Thanou, Z.N.; Afentoulis, D.G.; Koufopoulou, P.; Ampatzi, A.P.; Lekkou, S.D.; Koutsogiannopoulou, A.; Bravou, A.A.; Stamatakou, G.D.; Voulgaraki, K.N.; Piperkas, A.; et al. New Records and Updated Checklist of Cicadomorpha (Hemiptera: Auchenorrhyncha) Species from Greece. Zootaxa 2018, 4413, 133. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Di Carolo, M.; Fumarola, G.; Tauro, D.; Tedone, B.; Ancona, S.; Palmisano, V.; Carrieri, M.; Cavalieri, V. Comparing Different Sticky Traps to Monitor the Occurrence of Philaenus spumarius and Neophilaenus campestris, Vectors of Xylella fastidiosa, in Different Crops. Insects 2023, 14, 777. [Google Scholar] [CrossRef]
- Thompson, V.; Harkin, C.; Stewart, A.J.A. The Most Polyphagous Insect Herbivore? Host Plant Associations of the Meadow Spittlebug, Philaenus spumarius (L.). PLoS ONE 2023, 18, e0291734. [Google Scholar] [CrossRef]
- Antonatos, S.; Papachristos, D.P.; Varikou, K.; Vahamidis, P.; Kapranas, A.; Milonas, P. Seasonal Appearance, Abundance, and Host Preference of Philaenus Spumarius and Neophilaenus Campestris (Hemiptera: Aphrophoridae) in Olive Groves in Greece. Environ. Entomol. 2021, 50, 1474–1482. [Google Scholar] [CrossRef]
- Morente, M.; Cornara, D.; Plaza, M.; Durán, J.M.; Capiscol, C.; Trillo, R.; Ruiz, M.; Ruz, C.; Sanjuan, S.; Pereira, J.A.; et al. Distribution and Relative Abundance of Insect Vectors of Xylella fastidiosa in Olive Groves of the Iberian Peninsula. Insects 2018, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Drosopoulos, S.; Asche, M. Biosystematic Studies on the Spittlebug Genus Philaenus with the Description of a New Species. Zool. J. Linn. Soc. 1991, 101, 169–177. [Google Scholar] [CrossRef]
- European Food Safety Authority. Statement of EFSA on Host Plants, Entry and Spread Pathways and Risk Reduction Options for Xylella fastidiosa Wells et al. EFSA J. 2013, 11, 3468. [Google Scholar] [CrossRef]
- Delong, D.M. Some Problems Encountered in the Estimation of Insect Populations. Ann. Ent. Soc. Amer Columb. Ohio 1932, 25, 13–17. [Google Scholar]


| Species | Ath (%) | Lak (%) | Kal (%) | Nif (%) | Pam (%) | Ist1 (%) | Ist2 (%) | Ist3 (%) | Kts (%) | Pel (%) | Kip (%) | Fra (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Number | 2566 | 314 | 1442 | 311 | 320 | 2761 | 1090 | 2652 | 1231 | 366 | 1533 | 185 |
| Aphrophoridae, Aphrophorinae | ||||||||||||
| Lepyronia coleoptrata (L.) | − | − | − | − | − | + | − | + | − | − | − | − |
| Philaenus spumarius (L.) | + | + | + | + | + | + | + | + | + | + | + | + |
| Neophilaenus (Neophilaenulus) campestris (Fallén) | − | 5.79 | + | + | + | + | + | + | − | − | − | + |
| Neophilaenus (Neophilaenus) lineatus (L.) | − | − | − | − | − | + | − | − | − | − | − | − |
| Cercopidae, Cercopinae | ||||||||||||
| Cercopis sanguinolenta (Scopoli) | − | − | − | − | − | + | + | + | + | − | − | − |
| Cicadellidae, Aphrodinae | ||||||||||||
| Anoscopus albifrons (L.) | − | + | − | − | − | − | − | + | + | − | − | − |
| Aphrodes bicincta (Schrank) | − | − | − | − | − | − | + | + | − | + | − | − |
| Cicadellidae, Deltocephalinae | ||||||||||||
| Allygus modestus (Scott) | + | − | − | − | − | + | + | + | + | 8.71 | − | − |
| Anaconura acuticeps (Ribaut) | − | − | − | − | − | − | + | − | − | − | − | − |
| Anoplotettix putoni (Ribaut) | + | − | − | − | − | − | − | − | + | 8.43 | + | − |
| Anoplotettix fuscovenosus (Ferrari) | − | + | − | − | − | + | + | + | − | − | − | − |
| Arocephalus (Arocephalus) longiceps (Kirschbaum) | + | − | − | − | − | − | − | − | − | − | − | − |
| Balclutha frontalis (Ferrari) | + | + | + | + | − | 8.84 | 10.46 | + | − | − | 5.94 | + |
| Balclutha punctata (Fabricius) | 10.02 | − | − | − | − | − | − | − | − | − | 5.68 | + |
| Balclutha saltuella (Kirschbaum) | + | − | + | − | − | + | + | + | − | − | − | + |
| Cicadula (Cicadula) lineatopunctata (Matsumura) | − | − | − | − | − | + | + | + | − | − | − | − |
| Cicadulina bipunctata (Melichar) | + | + | 8.20 | − | − | 17.17 | + | + | − | − | 6.52 | − |
| Docotettix cornutus (Ribaut) | − | + | + | + | − | − | − | − | − | − | − | − |
| Eohardya fraudulenta (Horváth) | + | − | + | − | − | − | − | + | − | − | − | − |
| Epistagma (Epistagma) guttulinervis (Kirschbaum) | − | − | − | − | − | − | − | − | − | − | + | + |
| Eupelix cuspidata (Fabricius) | − | − | − | − | − | − | − | + | − | − | − | − |
| Euscelidius mundus (Haupt) | − | + | + | − | − | − | − | − | − | − | − | − |
| Euscelidius variegatus (Kirschbaum) | + | − | − | − | − | − | − | − | − | − | − | + |
| Euscelis alsia (Ribaut) | + | + | + | 5.47 | − | + | + | − | − | − | − | + |
| Euscelis lineolata (Brullé) | + | 7.40 | 9.92 | + | + | + | 14.50 | + | − | 12.64 | + | + |
| Exitianus capicola (Stål) | + | + | + | + | − | + | + | + | + | + | + | + |
| Fieberiella florii (Stål) | − | − | − | − | − | − | − | + | − | − | − | − |
| Fieberiella septentrionalis (Wagner) | − | − | − | − | − | − | − | − | + | + | − | − |
| Goniagnathus (Goniozygotes) bolivari (Melichar) | − | − | − | + | − | − | − | − | − | − | − | + |
| Goniagnathus (Goniagnathus) brevis (Herrich-Schäffer) | − | + | + | + | − | − | − | − | − | − | − | − |
| Grypotellus staurus (Ivanoff) | + | − | − | − | − | − | + | + | − | − | − | − |
| Hecalus glaucescens (Fieber) | − | − | − | − | − | + | + | − | − | − | − | − |
| Jassargus (Obtujargus) obtusivalvis (Kirschbaum) | − | − | − | − | − | − | + | − | − | − | − | − |
| Macrosteles quadripunctulatus (Kirschbaum) | − | − | + | − | + | − | − | − | − | + | − | |
| Macrosteles ramosus (Ribaut) | − | − | + | − | − | − | − | − | − | − | − | − |
| Macrosteles sexnotatus (Fallén) | − | − | + | − | − | − | − | − | − | − | − | − |
| Maiestas schmidtgeni (Wagner) | + | + | + | + | + | + | + | + | + | + | + | − |
| Melillaia desbrochersi (Lethierry) | − | + | − | − | − | − | − | − | − | − | − | − |
| Mocydia crocea (Herrich-Schäffer) | − | − | − | − | − | − | − | − | − | + | − | − |
| Neoaliturus (Circulifer) haematoceps (Mulsant & Rey) | + | + | + | − | − | + | − | − | + | − | − | − |
| Neoaliturus (Neoaliturus) fenestratus (Herrich-Schäffer) | − | − | + | − | − | + | + | + | + | − | − | − |
| Nesoclutha erythrocephala (Ferrari) | − | − | − | − | − | + | − | − | − | − | − | − |
| Opsius stactogalus (Fieber) | − | − | − | + | − | − | − | − | − | − | − | − |
| Orosius orientalis (Matsumura) | + | − | + | − | − | − | − | − | − | − | − | − |
| Paralimnus (Paralimnus) zachvatkini (Emeljanov) | − | − | − | − | − | + | + | − | − | − | − | − |
| Paramesodes lucaniae (Dlabola) | − | − | − | − | − | + | − | − | − | − | − | − |
| Phlepsius intricatus (Herrich-Schäffer) | + | − | + | + | − | + | + | + | + | + | − | + |
| Phlogotettix cyclops (Herrich-Schäffer) | − | − | − | − | − | − | − | + | − | − | − | − |
| Proceps acicularis (Mulsant & Rey) | − | + | − | − | − | − | − | − | − | − | − | − |
| Psammotettix alienus (Dahlbom) | 5.56 | + | 12.54 | + | − | + | + | + | − | + | + | + |
| Psammotettix confinis (Dahlbom) | − | − | + | − | − | − | − | − | − | − | − | − |
| Psammotettix notatus (Melichar) | + | − | − | − | + | − | − | − | + | + | − | − |
| Selenocephalus pallidus (Kirschbaum) | − | + | + | 6.11 | − | − | + | − | − | − | − | − |
| Streptanus (Streptanulus) albanicus (Horváth) | − | + | − | − | − | − | − | − | − | + | − | − |
| Synophropsis lauri (Horváth) | + | − | + | − | − | + | + | + | − | + | − | + |
| Thamnotettix zelleri (Kirschbaum) | + | 25.08 | + | 49.19 | + | + | 15.41 | − | + | + | + | − |
| Varta rubrostriata (Horváth) | − | − | − | − | − | + | + | + | − | − | − | − |
| Cicadellidae, Eurymelinae | ||||||||||||
| Acericerus vittifrons (Kirschbaum) | + | − | − | − | − | − | − | − | − | − | − | − |
| Sulamicerus stali (Fieber) | + | − | − | − | − | − | − | − | − | − | − | − |
| Cicadellidae, Iassinae | ||||||||||||
| Batracomorphus (Batracomorphus) irroratus (Lewis) | − | − | − | − | − | + | − | + | − | − | − | − |
| Cicadellidae, Megopthalminae | ||||||||||||
| Agallia consobrina (Curtis) | + | − | − | − | − | + | + | + | + | + | − | − |
| Anaceratagallia (Anaceratagallia) glabra (Dmitriev) | − | + | + | + | − | + | + | + | + | + | + | + |
| Anaceratagallia (Anaceratagallia) ribauti (Ossiannilsson) | − | − | + | + | + | − | + | + | − | − | + | − |
| Austroagallia sinuata (Mulsant & Rey) | − | + | + | + | − | + | − | − | − | − | − | − |
| Megopthalmus scabripennis (Edwards) | + | + | + | + | − | + | + | + | + | − | − | − |
| Cicadellidae, Typhlocybinae | ||||||||||||
| Anzygina honiloa (Kirkaldy) | + | + | − | − | − | − | − | − | − | − | − | − |
| Arboridia parvula (Boheman) | − | − | − | − | − | − | − | − | − | + | − | − |
| Arboridia (Arboridia) versuta (Melichar) | − | − | − | − | − | − | − | − | − | + | − | − |
| Assymetrasca decedens (Paoli) | + | + | + | + | + | + | − | − | + | − | ||
| Edwardsiana platanicola (Vidano) | − | − | − | − | − | − | − | + | − | − | − | − |
| Eupteryx (Eupteryx) collina (Flor) | − | − | − | − | − | − | − | − | − | 7.02 | − | − |
| Eupteryx (Eupteryx) curtisii (Flor) | − | − | − | − | − | − | − | + | − | − | − | − |
| Eupteryx (Eupteryx) decemnotata (Rey) | + | − | − | − | − | − | − | − | − | − | − | − |
| Eupteryx (Eupteryx) filicum (Newman) | + | − | − | − | − | − | − | − | − | − | − | − |
| Eupteryx (Eupteryx) gyaurdagica (Dlabola) | − | 14.47 | + | + | − | − | − | − | − | − | − | − |
| Eupteryx (Eupteryx) insulana (Ribaut) | − | − | + | − | − | + | + | + | − | − | − | − |
| Eupteryx (Eupteryx) melissae (Curtis) | + | − | − | − | + | + | + | + | + | − | − | − |
| Eupteryx (Eupteryx) rostrata (Ribaut) | − | − | − | − | − | − | − | − | − | + | − | − |
| Eupteryx (Eupteryx) urticae (Fabricius) | + | − | − | − | − | − | − | − | − | − | − | − |
| Eupteryx (Eupteryx) zelleri (Kirschbaum) | − | − | − | − | − | + | + | 39.52 | − | − | 7.44 | − |
| Ficocyba ficaria (Horváth) | + | − | − | − | − | + | − | + | − | − | − | − |
| Frutioidia (Frutioidia) bisignata (Mulsant & Rey) | − | − | − | − | − | − | − | + | − | − | − | − |
| Hauptidia (Hauptidia) provincialis (Ribaut) | + | + | + | − | − | + | + | + | + | 9.83 | 20.74 | − |
| Hebata (Alboneurasca) decipiens (Paoli) | + | − | 14.96 | + | 32.19 | 12.57 | 5.69 | + | 6.91 | + | 27.46 | 6.49 |
| Hebata (Signatasca) vitis (Göthe) | + | − | − | − | − | − | − | − | − | − | + | + |
| Liguropia juniperi (Lethierry) | + | − | − | − | − | − | − | − | − | − | − | − |
| Lindbergina cretica (Asche) | − | + | − | − | − | − | − | − | − | − | − | − |
| Ribautiana cruciata (Ribaut) | − | − | + | + | − | − | + | − | − | − | − | − |
| Ribautiana tenerrima (Herrich-Schäffer) | − | − | − | − | − | + | + | 18.89 | − | − | − | − |
| Zygina (Hypericiella) hyperici (Herrich-Schäffer) | − | − | − | − | − | − | − | − | + | − | − | − |
| Zygina (Zygina) angusta (Lethierry) | + | − | − | − | − | − | − | − | − | − | − | − |
| Zygina (Zygina) nivea (Mulsant & Rey) | + | − | − | − | − | − | − | − | − | − | − | − |
| Zygina (Zygina) rhamni (Ferrari) | − | − | − | − | − | + | + | + | − | − | − | − |
| Zygina (Zygina) roseipennis (Tollin) | − | − | − | + | − | − | − | − | − | − | − | − |
| Zygina (Zygina) suavis (Rey) | − | − | − | − | − | − | − | − | + | + | − | − |
| Zygina (Zygina) tiliae (Fallén) | − | − | − | − | − | − | − | − | − | + | − | − |
| Zyginella pulchra (Löw) | + | − | − | − | − | + | − | − | − | − | + | − |
| Zyginidia adamczewskii (Dworakowska) | + | − | − | + | − | − | − | − | − | − | − | − |
| Zyginidia pullula (Boheman) | 16.62 | + | 10.06 | + | 40.00 | 36.22 | 18.53 | + | 72.38 | 10.11 | + | − |
| Wagneriala sinuata (Then) | − | − | − | − | − | − | − | + | − | − | − | − |
| Delphacidae, Stenocraninae | ||||||||||||
| Stenocranus fuscovittatus (Stål) | − | − | − | − | − | + | − | + | − | − | − | − |
| Delphacidae, Delphacinae | ||||||||||||
| Laodelphax striatellus (Fallén) | − | − | + | − | − | + | − | + | − | − | − | − |
| Toya (Metadelphax) propinqua (Fieber) | − | + | + | − | − | + | + | + | + | + | − | − |
| Dictyopharidae, Dityopharinae | ||||||||||||
| Dictyophara (Dictyophara) europaea (L.) | − | − | − | − | + | − | + | + | − | − | − | − |
| Flatidae, Flatinae | ||||||||||||
| Metcalfa pruinosa (Say) | − | − | − | − | − | − | + | + | − | − | − | − |
| Phantia subquadrata (Herrich-Schäffer) | − | + | + | + | − | − | + | + | − | − | − | − |
| Issidae, Hysteropterinae | ||||||||||||
| Agalmatium bilobum (Fieber) | − | − | − | − | − | + | + | + | − | − | − | − |
| Agalmatium flavescens (Olivier) | − | + | − | − | − | − | − | − | − | + | − | − |
| Latilica antalyica (Dlabola) | + | + | − | − | − | − | − | − | − | − | − | − |
| Latilica maculipes (Melichar) | 5.73 | − | − | − | − | − | − | − | − | − | + | − |
| Management System | Locality | Richness (S) | Simpson’s Index of Diversity (1-D) | Evenness (E) |
|---|---|---|---|---|
| Organic | Athens | 62 | 0.932 | 0.240 |
| Organic | Lakerda | 52 | 0.901 | 0.194 |
| Conventional | Kalloni | 64 | 0.924 | 0.203 |
| Conventional | Nifida | 43 | 0.746 | 0.092 |
| Conventional | Pamfila | 17 | 0.735 | 0.222 |
| Conventional | Istiea | 48 | 0.824 | 0.118 |
| Organic | Istiea | 45 | 0.899 | 0.220 |
| No-treatments | Istiea | 57 | 0.815 | 0.095 |
| Organic | Koutsopodi | 55 | 0.468 | 0.034 |
| Conventional | Pelekanada | 47 | 0.928 | 0.296 |
| Conventional | Kiparissia | 35 | 0.858 | 0.201 |
| Organic | Fragka | 44 | 0.881 | 0.191 |
| Conventional | Pirgos (sweep net) | 32 | 0.897 | 0.303 |
| Organic | Athens (sweep net) | 18 | 0.637 | 0.153 |
| Species | Pir (%) | Pirgos’ Plant Species | Ath (%) | Athens’ Plant Species |
|---|---|---|---|---|
| Philaenus spumarius (L.) | 14.10 | AS | 15.85 | PL, OE, AS, HM, CD, ASV, PA, SE, MS |
| Neophilaenus (Neophilaenulus) campestris (Fallén) | 16.98 | AS | + | PA, SE, AS |
| Aphrodes bicincta (Schrank) | − | − | + | HM, SO |
| Allygidius (Dicrallygus) mayri (Kirschbaum) | + | PO | − | − |
| Allygus modestus (Scott) | − | − | + | OE, PL, MS |
| Anaconura acuticeps (Ribaut) | − | − | + | CD |
| Anoplotettix putoni (Ribaut) | + | PO | + | OE, HM, ASV |
| Balclutha frontalis (Ferrari) | 14.24 | AC | − | − |
| Balclutha punctata (Fabricius) | + | AC | + | OE, ASV |
| Balclutha saltuella (Kirschbaum) | + | AC | − | − |
| Cicadulina bipunctata (Melichar) | + | PO | − | − |
| Doratura stylata (Boheman) | + | AC, CD | − | − |
| Epistagma (Epistagma) guttulinervis (Kirschbaum) | + | CD | − | − |
| Euscelidius variegatus (Kirschbaum) | − | − | + | AS, HM, PHA |
| Euscelis lineolata (Brullé) | + | AS, CD | − | − |
| Euscelis ohausi (Wagner) | + | PO | − | − |
| Exitianus capicola (Stål) | 6.19 | CD | + | CD, AS |
| Exitianus nanus (Distant) | + | PO | − | − |
| Hecalus storai (Lindberg) | + | CD | − | − |
| Maiestas schmidtgeni (Wagner) | + | CD | − | − |
| Mocydiopsis longicauda (Remane) | + | PO | − | − |
| Mocydiopsis monticola (Remane) | + | PO | − | − |
| Nesoclutha erythrocephala (Ferrari) | + | AS, HM, CD, ASV, PA | − | − |
| Phlepsius intricatus (Herrich-Schäffer) | + | PO | − | − |
| Psammotettix alienus (Dahlbom) | 14.96 | AS, HM, CD, ASV, PA | + | CD |
| Streptanus (Streptanulus) albanicus (Horváth) | − | − | + | AS, HM |
| Synophropsis lauri (Horváth) | − | − | 8.17 | OE, ASV |
| Thamnotettix zelleri (Kirschbaum) | + | PO | + | OE, AS, MS, SA, HM, ASV |
| Anaceratagallia (Anaceratagallia) glabra (Dmitriev) | + | AS | − | − |
| Anaceratagallia (Anaceratagallia) ribauti (Ossiannilsson) | − | − | + | SO |
| Sulamicerus stali (Fieber) | − | − | 57.20 | PL |
| Hauptidia (Hauptidia) provincialis (Ribaut) | + | MA, TR | − | − |
| Hebata (Alboneurasca) decipiens (Paoli) | + | MA, TR | − | − |
| Zyginidia pullula (Boheman) | + | MA, TR | − | − |
| Asiraca clavicornis (Fabricius) | + | PO | − | − |
| Euidopsis truncata (Ribaut) | + | PO | − | − |
| Eurysella brunnea (Melichar) | + | PO | − | − |
| Toya (Metadelphax) propinqua (Fieber) | + | AC, CD | + | CD |
| Dictyophara (Dictyophara) europaea (Linnaeus) | − | − | + | AV |
| Phantia subquadrata (Herrich-Schäffer) | + | PO | − | − |
| Clybeccus declivum (Dlabola) | + | AS | − | − |
| Latilica antalyica (Dlabola) | − | − | + | OE, ASV |
| Latilica maculipes (Melichar) | + | AS | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanou, Z.; Stamouli, M.; Magklara, A.; Theodorou, D.; Stamatakou, G.; Konidis, G.; Koufopoulou, P.; Lyberopoulos, C.; Tribonia, S.; Vetsos, P.; et al. Faunistic Study of Auchenorrhyncha in Olive Orchards in Greece, Including First Records of Species. Agronomy 2024, 14, 2792. https://doi.org/10.3390/agronomy14122792
Thanou Z, Stamouli M, Magklara A, Theodorou D, Stamatakou G, Konidis G, Koufopoulou P, Lyberopoulos C, Tribonia S, Vetsos P, et al. Faunistic Study of Auchenorrhyncha in Olive Orchards in Greece, Including First Records of Species. Agronomy. 2024; 14(12):2792. https://doi.org/10.3390/agronomy14122792
Chicago/Turabian StyleThanou, Zoi, Myrto Stamouli, Anastasia Magklara, David Theodorou, Georgia Stamatakou, Georgios Konidis, Panagiota Koufopoulou, Christos Lyberopoulos, Sofia Tribonia, Petros Vetsos, and et al. 2024. "Faunistic Study of Auchenorrhyncha in Olive Orchards in Greece, Including First Records of Species" Agronomy 14, no. 12: 2792. https://doi.org/10.3390/agronomy14122792
APA StyleThanou, Z., Stamouli, M., Magklara, A., Theodorou, D., Stamatakou, G., Konidis, G., Koufopoulou, P., Lyberopoulos, C., Tribonia, S., Vetsos, P., Katribouzas, A., Kalaitzaki, A., Papadoulis, G., & Tsagkarakis, A. (2024). Faunistic Study of Auchenorrhyncha in Olive Orchards in Greece, Including First Records of Species. Agronomy, 14(12), 2792. https://doi.org/10.3390/agronomy14122792









