In Vitro Plant Regeneration of Sulla coronaria from Floral Explants as a Biotechnological Tool for Plant Breeding
Abstract
1. Introduction
2. Materials and Methods
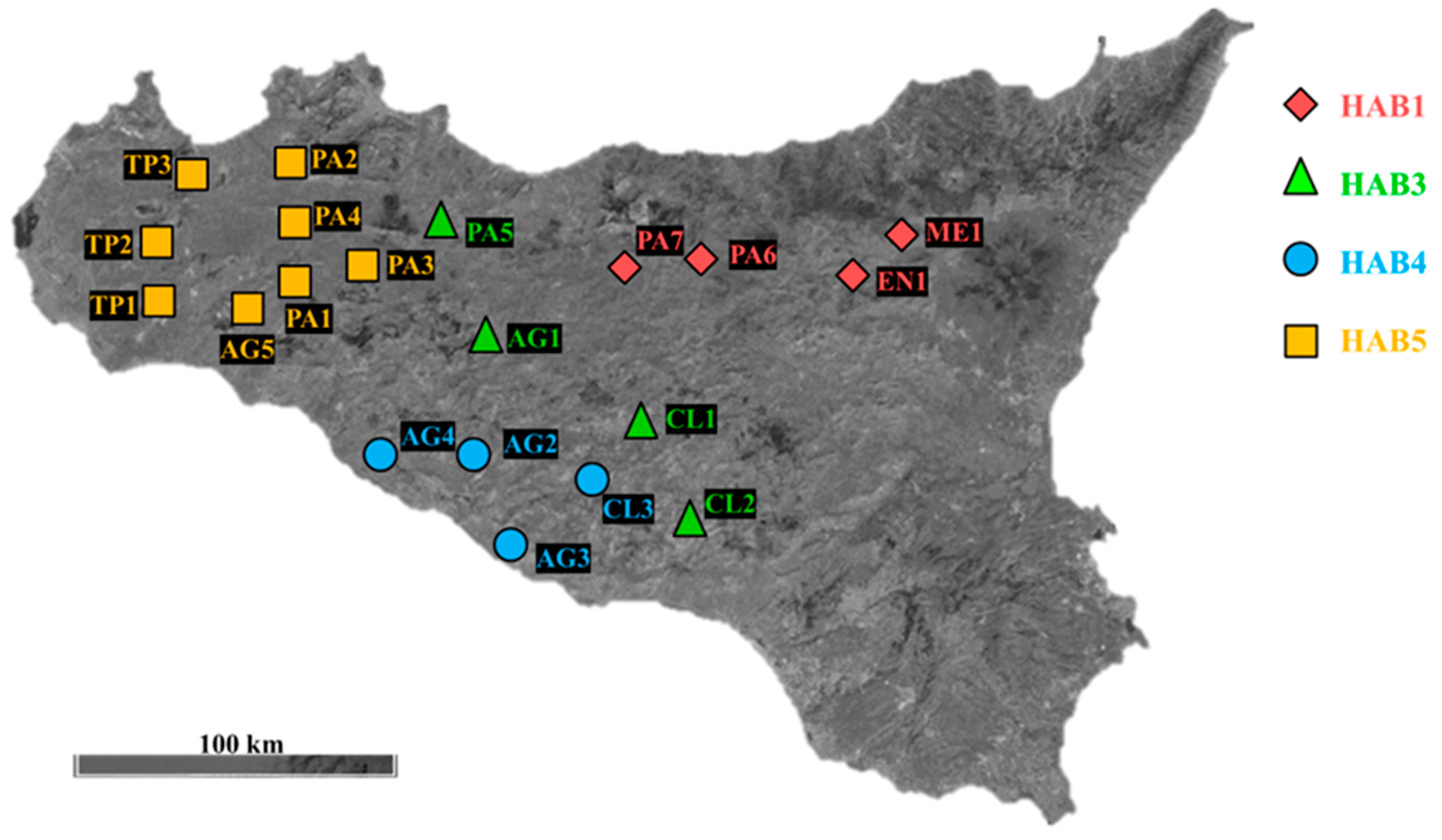
2.1. Sample Collection
2.2. Plant Materials
2.3. Culture Conditions
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Results of the Models
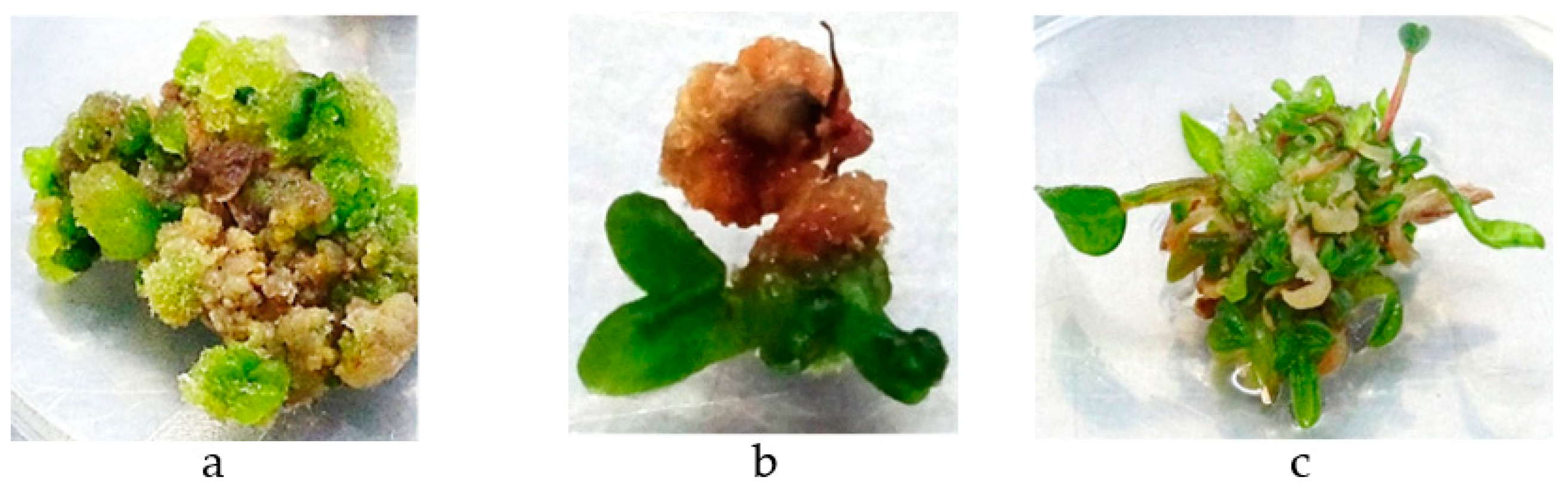
3.1.1. Callus Production
3.1.2. Meristematic Shoots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, B.H.; Ohashi, H. Generic criteria and an infrageneric system for Hedysarum and related genera (Papilionoideae–Leguminosae). Taxon 2003, 52, 567–576. [Google Scholar] [CrossRef]
- Amato, G.; Giambalvo, D.; Frenda, A.S.; Mazza, F.; Ruisi, P.; Saia, S.; Di Miceli, G. Sulla (Hedysarum coronarium L.) as Potential Feedstock for Biofuel and Protein. BioEnergy Res. 2016, 9, 711–719. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Ruisi, P.; Di Miceli, G.; Pecetti, L. Morpho-Physiological and Adaptive Variation of Italian Germplasm of Sulla (Hedysarum coronarium L.). Crop Pasture Sci. 2014, 65, 206–213. [Google Scholar] [CrossRef]
- Zullo, B.A.; Ciafardini, G. Potential of Rhizobium sullae–Sulla coronaria Symbiotic Biological Nitrogen Fixation to Supplement Synthetic Mineral Nitrogen in Olive Tree Fertilization. Agronomy 2020, 10, 270. [Google Scholar] [CrossRef]
- Rossi, R.; Amato, M.; Claps, S. Sulla (Hedysarum coronarium L.) Response to Drought Stress during Early Vegetative Stage. Plants 2023, 12, 3396. [Google Scholar] [CrossRef]
- Douglas, G.B.; Keogh, R.G.; Foote, A.G. Development of Sulla (Hedysarum coronarium) for Better Adaptation to Grazing. Proc. N. Z. Grassl. Assoc. 1998, 60, 173–179. [Google Scholar] [CrossRef]
- Yates, R.; Howieson, J.; De Meyer, S.E.; Tian, R.; Seshadri, R.; Pati, A.; Woyke, T.; Markowitz, V.; Ivanova, N.; Kyrpides, N.; et al. High-Quality Permanent Draft Genome Sequence of Rhizobium sullae Strain WSM1592; a Hedysarum coronarium Microsymbiont from Sassari, Italy. Stand. Genom. Sci. 2015, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Howieson, J.G.; O’hara, G.W.; Carr, S.J. Changing Roles for Legumes in Mediterranean Agriculture: Developments from an Australian Perspective. Field Crops Res. 2000, 65, 107–122. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Thomsen, C.D.; Graves, W.L.; Shennan, C. Cover Crops for Saline Soils. J. Agron. Crop Sci. 1999, 183, 167–178. [Google Scholar] [CrossRef]
- Ruisi, P.; Siragusa, M.; Di Giorgio, G.; Graziano, D.; Amato, G.; Carimi, F.; Giambalvo, D. Pheno-Morphological, Agronomic and Genetic Diversity among Natural Populations of Sulla (Hedysarum coronarium L.) Collected in Sicily, Italy. Genet. Resour. Crop Evol. 2011, 58, 245–257. [Google Scholar] [CrossRef]
- Bonanno, A.; Di Grigoli, A.; Stringi, L.; Di Miceli, G.; Giambalvo, D.; Tornambè, G.; Vargetto, D.; Alicata, M.L. Intake and Milk Production of Goats Grazing Sulla Forage under Different Stocking Rates. Ital. J. Anim. Sci. 2007, 6, 605–607. [Google Scholar] [CrossRef]
- Tibe, O.; Meagher, L.P.; Fraser, K.; Harding, D.R.K. Condensed Tannins and Flavonoids from the Forage Legume Sulla (Hedysarum coronarium). J. Agric. Food Chem. 2011, 59, 9402–9409. [Google Scholar] [CrossRef] [PubMed]
- Terrill, T.H.; Douglas, G.B.; Foote, A.G.; Purchas, R.W.; Wilson, G.F.; Barry, T.N. Effect of Condensed Tannins upon Body Growth, Wool Growth and Rumen Metabolism in Sheep Grazing Sulla (Hedysarum coronarium) and Perennial Pasture. J. Agric. Sci. 1992, 119, 265–273. [Google Scholar] [CrossRef]
- Douglas, G.B.; Stienezen, M.; Waghorn, G.C.; Foote, A.G.; Purchas, R.W. Effect of Condensed Tannins in Birdsfoot Trefoil (Lotus corniculatus) and Sulla (Hedysarum coronarium) on Body Weight, Carcass Fat Depth, and Wool Growth of Lambs in New Zealand. N. Z. J. Agric. Res. 1999, 42, 55–64. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S.; Deroma, M.; Odoardi, M. The Accumulation of Condensed Tannins in Local Populations of Sulla. Cah. Options Méditerranéennes 2000, 45, 199–202. [Google Scholar]
- Bonanno, A.; Di Miceli, G.; Di Grigoli, A.; Frenda, A.S.; Tornambè, G.; Giambalvo, D.; Amato, G. Effects of Feeding Green Forage of Sulla (Hedysarum coronarium L.) on Lamb Growth and Carcass and Meat Quality. Animal 2011, 5, 148–154. [Google Scholar] [CrossRef]
- Yagoubi, N.; Chriki, A. Estimation of Mating System Parameters in Hedysarum coronarium L. (Leguminoseae, Fabaceae). Agronomie 2000, 20, 933–942. [Google Scholar] [CrossRef]
- Dresselhaus, T.; Hückelhoven, R. Biotic and Abiotic Stress Responses in Crop Plants. Agronomy 2018, 8, 267. [Google Scholar] [CrossRef]
- Araújo, S.S.; Beebe, S.; Crespi, M.; Delbreil, B.; González, E.M.; Gruber, V.; Lejeune-Henaut, I.; Link, W.; Monteros, M.J.; Prats, E.; et al. Abiotic Stress Responses in Legumes: Strategies Used to Cope with Environmental Challenges. Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- Ochatt, S.J. Agroecological Impact of an In Vitro Biotechnology Approach of Embryo Development and Seed Filling in Legumes. Agron. Sustain. Dev. 2015, 35, 535–552. [Google Scholar] [CrossRef]
- Altman, A. From Plant Tissue Culture to Biotechnology: Scientific Revolutions, Abiotic Stress Tolerance, and Forestry. In Vitro Cell. Dev. Biol. Plant 2003, 39, 75–84. [Google Scholar] [CrossRef]
- Radomir, A.-M.; Stan, R.; Florea, A.; Ciobotea, C.-M.; Bănuță, F.M.; Negru, M.; Neblea, M.A.; Sumedrea, D.I. Overview of the Success of In Vitro Culture for Ex Situ Conservation and Sustainable Utilization of Endemic and Subendemic Native Plants of Romania. Sustainability 2023, 15, 2581. [Google Scholar] [CrossRef]
- Bidabadi, S.S.; Jain, S.M. Cellular, Molecular, and Physiological Aspects of In Vitro Plant Regeneration. Plants 2020, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sahagún, A.; Acevedo-Hernández, G.; Rodríguez-Domínguez, J.M.; Rodríguez-Garay, B.; Cervantes-Martínez, J.; Castellanos-Hernández, O.A. Effect of Light Quality and Culture Medium on Somatic Embryogenesis of Agave tequilana Weber var. Azul. Plant Cell Tissue Organ Cult. 2011, 104, 271–275. [Google Scholar] [CrossRef]
- Arcioni, S.; Mariotii, D.; Pezzotti, M. Hedysarum coronarium L. In Vitro Conditions for Plant Regeneration from Protoplasts and Callus of Various Explants. J. Plant Physiol. 1985, 121, 141–148. [Google Scholar] [CrossRef]
- Catalano, C.; Carra, A.; Carimi, F.; Motisi, A.; Sajeva, M.; Butler, A.; Lucretti, S.; Giorgi, D.; Farina, A.; Abbate, L. Somatic Embryogenesis and Flow Cytometric Assessment of Nuclear Genetic Stability for Sansevieria spp.: An Approach for In Vitro Regeneration of Ornamental Plants. Horticulturae 2023, 9, 138. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Snijders, T.A.; Bosker, R. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling, 2nd ed.; Sage Publishing: London, UK, 2012; xii+368p, ISBN 9781849202015 (pbk). [Google Scholar]
- Gelman, A. Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Raudenbush, S.W. Hierarchical Linear Models: Applications and Data Analysis Methods; Advanced Quantitative Techniques in the Social Sciences Series; SAGE: Thousand Oaks, CA, USA, 2002. [Google Scholar]
- McCulloch, C.E.; Searle, S.R. Generalized, Linear, and Mixed Models, 1st ed.; Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 2000; ISBN 978-0-471-19364-7. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. (Eds.) Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2004; ISBN 978-0-387-95364-9. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning; Springer Series in Statistics; Springer: New York, NY, USA, 2009; ISBN 978-0-387-84857-0. [Google Scholar]
- Carimi, F.; Barizza, E.; Gardiman, M.; Schiavo, F.L. Somatic Embryogenesis from Stigmas and Styles of Grapevine. In Vitro Cell. Dev. Biol. Plant 2005, 41, 249–252. [Google Scholar] [CrossRef]
- Carra, A.; De Pasquale, F.; Ricci, A.; Carimi, F. Diphenylurea Derivatives Induce Somatic Embryogenesis in Citrus. Plant Cell Tiss Organ Cult. 2006, 87, 41–48. [Google Scholar] [CrossRef]
- Sajeva, M.; Carra, A.; De Pasquale, F.; Carimi, F. Somatic Embryogenesis and Plant Regeneration from Pistil Transverse Thin Cell Layers of Lemon (Citrus limon). Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2008, 142, 199–203. [Google Scholar] [CrossRef]
- Wojciechowicz, M.K. Organogenesis and Somatic Embryogenesis Induced in Petal Cultures of Sedum Species. Acta Biol. Cracoviensia Ser. Bot. 2009, 51, 83–90. [Google Scholar]
- Vidal, J.R.; Rama, J.; Taboada, L.; Martin, C.; Ibañez, M.; Segura, A.; González-Benito, M.E. Improved Somatic Embryogenesis of Grapevine (Vitis vinifera) with Focus on Induction Parameters and Efficient Plant Regeneration. Plant Cell Tiss Organ Cult. 2009, 96, 85–94. [Google Scholar] [CrossRef]
- Prado, M.J.; Grueiro, M.P.; González, M.V.; Testillano, P.S.; Domínguez, C.; López, M.; Rey, M. Efficient Plant Regeneration Through Somatic Embryogenesis from Anthers and Ovaries of Six Autochthonous Grapevine Cultivars from Galicia (Spain). Sci. Hortic. 2010, 125, 342–352. [Google Scholar] [CrossRef][Green Version]
- Lakshmanan, P.; Taji, A. Somatic Embryogenesis in Leguminous Plants. Plant Biol. 2000, 2, 136–148. [Google Scholar] [CrossRef]
- Jiménez, V.M.; Thomas, C. Participation of Plant Hormones in Determination and Progression of Somatic Embryogenesis. In Somatic Embryogenesis; Mujib, A., Šamaj, J., Eds.; Plant Cell Monographs; Springer: Berlin/Heidelberg, Germany, 2006; Volume 2, pp. 103–118. ISBN 978-3-540-28717-9. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Sun, H.J.; Boo, K.H.; Lee, D.; Lee, J.-H.; Lim, P.O.; Lee, H.Y.; Riu, K.-Z.; Lee, D.-S. Effect of Plant Growth Regulator Combination and Culture Period on In Vitro Regeneration of Spinach (Spinacia oleracea L.). Plant Biotechnol. Rep. 2013, 7, 99–108. [Google Scholar] [CrossRef]
- Jeya Mary, R.; Jayabalan, N. Influence of Growth Regulators on Somatic Embryogenesis in Sesame. Plant Cell Tissue Organ Cult. 1997, 49, 67–70. [Google Scholar] [CrossRef]
- Carra, A.; Sajeva, M.; Abbate, L.; Siragusa, M.; Sottile, F.; Carimi, F. In Vitro Plant Regeneration of Caper (Capparis spinosa L.) from Floral Explants and Genetic Stability of Regenerants. Plant Cell Tissue Organ Cult. 2012, 109, 373–381. [Google Scholar] [CrossRef]
- Guo, G.; Jeong, B.R. Explant, Medium, and Plant Growth Regulator (PGR) Affect Induction and Proliferation of Callus in Abies koreana. Forests 2021, 12, 1388. [Google Scholar] [CrossRef]
- Bedir, H.; Ari, E.; Vural, G.E.; Seguí-Simarro, J.M. Effect of the Genotype, Explant Source and Culture Medium in Somatic Embryogenesis and Organogenesis in Vaccaria hispanica (Mill.) Rauschert. Plant Cell Tissue Organ Cult. 2022, 150, 329–343. [Google Scholar] [CrossRef]
- Pathirana, R.; Carimi, F. Studies on Improving the Efficiency of Somatic Embryogenesis in Grapevine (Vitis vinifera L.) and Optimising Ethyl Methanesulfonate Treatment for Mutation Induction. Plants 2023, 12, 4126. [Google Scholar] [CrossRef]
- Jiang, W.; Hua, S.; Zhou, X.; Han, P.; Lu, Q.; Qiu, Y. Assessment of Genetic Stability and Analysis of Alkaloids Potential in Micropropagated Plants of Croomia japonica Miquel, an Endangered, Medicinal Plant in China and Japan. Plant Cell Tissue Organ Cult. 2018, 135, 1–12. [Google Scholar] [CrossRef]
- Deo, P.C.; Tyagi, A.P.; Taylor, M.; Harding, R.; Becker, D. Factors Affecting Somatic Embryogenesis and Transformation in Modern Plant Breeding. S. Pac. J. Nat. Appl. Sci. 2010, 28, 27–40. [Google Scholar] [CrossRef]
- Croser, J.S.; Lülsdorf, M.M.; Davies, P.A.; Clarke, H.J.; Bayliss, K.L.; Mallikarjuna, N.; Siddique, K.H.M. Toward Doubled Haploid Production in the Fabaceae: Progress, Constraints, and Opportunities. Crit. Rev. Plant Sci. 2006, 25, 139–157. [Google Scholar] [CrossRef]
- Fiuk, A.; Rybczyński, J.J. Genotype and Plant Growth Regulator-Dependent Response of Somatic Embryogenesis from Gentiana spp. Leaf Explants. In Vitro Cell. Dev. Biol. Plant 2008, 44, 90–99. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, A.; Qin, M.; Qin, X.; Yang, S.; Su, S.; Sun, Y.; Zhang, L. Direct and Indirect Somatic Embryogenesis Induction in Camellia oleifera Abel. Front. Plant Sci. 2021, 12, 644389. [Google Scholar] [CrossRef]
- Gutiérrez-Mora, A.; González-Gutiérrez, A.G.; Rodríguez-Garay, B.; Ascencio-Cabral, A.; Li-Wei, L. Plant Somatic Embryogenesis: Some Useful Considerations. In Embryogenesis; InTech: Rijeka, Croatia, 2012; pp. 229–248. [Google Scholar]
- Aalifar, M.; Arab, M.; Aliniaeifard, S.; Dianati, S.; Zare Mehrjerdi, M.; Limpens, E.; Serek, M. Embryogenesis Efficiency and Genetic Stability of Dianthus caryophyllus Embryos in Response to Different Light Spectra and Plant Growth Regulators. Plant Cell Tiss Organ Cult. 2019, 139, 479–492. [Google Scholar] [CrossRef]
- Colomba, E.L.; Grunberg, K.; Griffa, S.; Ribotta, A.; Mroginski, L.; Biderbost, E. The Effect of Genotype and Culture Medium on Somatic Embryogenesis and Plant Regeneration from Mature Embryos of Fourteen Apomictic Cultivars of Buffel Grass (Cenchrus ciliaris L.). Grass Forag. Sci. 2006, 61, 2–8. [Google Scholar] [CrossRef]
- Pinto, G.; Park, Y.-S.; Silva, S.; Neves, L.; Araújo, C.; Santos, C. Factors Affecting Maintenance, Proliferation, and Germination of Secondary Somatic Embryos of Eucalyptus globulus Labill. Plant Cell Tissue Organ Cult. 2008, 95, 69–78. [Google Scholar] [CrossRef]

| PGR Combinations | Ref. |
|---|---|
| (S.c. 1) 5 µM N-(2-chloro-4-pyridyl)-N′-phenylurea (4-CPPU) + 5 µM 2,4-dichlorophenoxyacetic acid (2,4-D) | [26] |
| (S.c. 2) 8.8 µM 6-banzylaminopurine (BA) + 1.07 µM 1-Naphthalene acetic acid (NAA) | [27] |
| (S.c. 3) 10 µM naphthoxyacetic acid (NOA) + 4.4 µM 6-banzylaminopurine (BA) | [26] |
| Variables | Category | Significance for Callus Production | Type of Effect |
|---|---|---|---|
| Biotypes | AG1 | Reference category | |
| AG2 | *** | - | |
| AG3 | ** | - | |
| AG4 | *** | - | |
| AG5 | ** | - | |
| CL1 | *** | - | |
| CL2 | *** | - | |
| CL3 | ** | - | |
| EN1 | *** | - | |
| ME1 | *** | - | |
| PA1 | *** | - | |
| PA2 | *** | - | |
| PA3 | * | - | |
| PA4 | *** | - | |
| PA5 | *** | - | |
| PA6 | * | - | |
| PA7 | *** | - | |
| TP1 | *** | - | |
| TP2 | ** | - | |
| TP3 | *** | - | |
| PGR Combinations | S.c. 1 | Reference category | |
| S.c. 2 | n.s. | n.s. | |
| S.c. 3 | ** | - | |
| Explant Type | Ant | Reference category | |
| Ov | *** | - | |
| Pet | *** | - | |
| Wf | *** | - | |
| Light Conditions | Dark | Reference category | |
| Light | * | - | |
| Variables | Category | Significance for Meristematic Shoots | Type of Effect |
|---|---|---|---|
| PGR Combinations | S.c. 1 | Reference category | |
| S.c. 2 | * | + | |
| S.c. 3 | ** | + | |
| Explant Type | Ant | Reference category | |
| Ov | n.s. | n.s. | |
| Pet | * | - | |
| Wf | n.s. | n.s. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auteri, M.; Carra, A.; Di Miceli, G.; Iacuzzi, N.; Albano, A.; Lala, N.; Motisi, A.; Catalano, C. In Vitro Plant Regeneration of Sulla coronaria from Floral Explants as a Biotechnological Tool for Plant Breeding. Agronomy 2024, 14, 2667. https://doi.org/10.3390/agronomy14112667
Auteri M, Carra A, Di Miceli G, Iacuzzi N, Albano A, Lala N, Motisi A, Catalano C. In Vitro Plant Regeneration of Sulla coronaria from Floral Explants as a Biotechnological Tool for Plant Breeding. Agronomy. 2024; 14(11):2667. https://doi.org/10.3390/agronomy14112667
Chicago/Turabian StyleAuteri, Monica, Angela Carra, Giuseppe Di Miceli, Nicolò Iacuzzi, Alessandro Albano, Nicoletta Lala, Antonio Motisi, and Caterina Catalano. 2024. "In Vitro Plant Regeneration of Sulla coronaria from Floral Explants as a Biotechnological Tool for Plant Breeding" Agronomy 14, no. 11: 2667. https://doi.org/10.3390/agronomy14112667
APA StyleAuteri, M., Carra, A., Di Miceli, G., Iacuzzi, N., Albano, A., Lala, N., Motisi, A., & Catalano, C. (2024). In Vitro Plant Regeneration of Sulla coronaria from Floral Explants as a Biotechnological Tool for Plant Breeding. Agronomy, 14(11), 2667. https://doi.org/10.3390/agronomy14112667







