ABA Affects Distinctive Rice Caryopses Physicochemical Properties on Different Branches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Growth Conditions
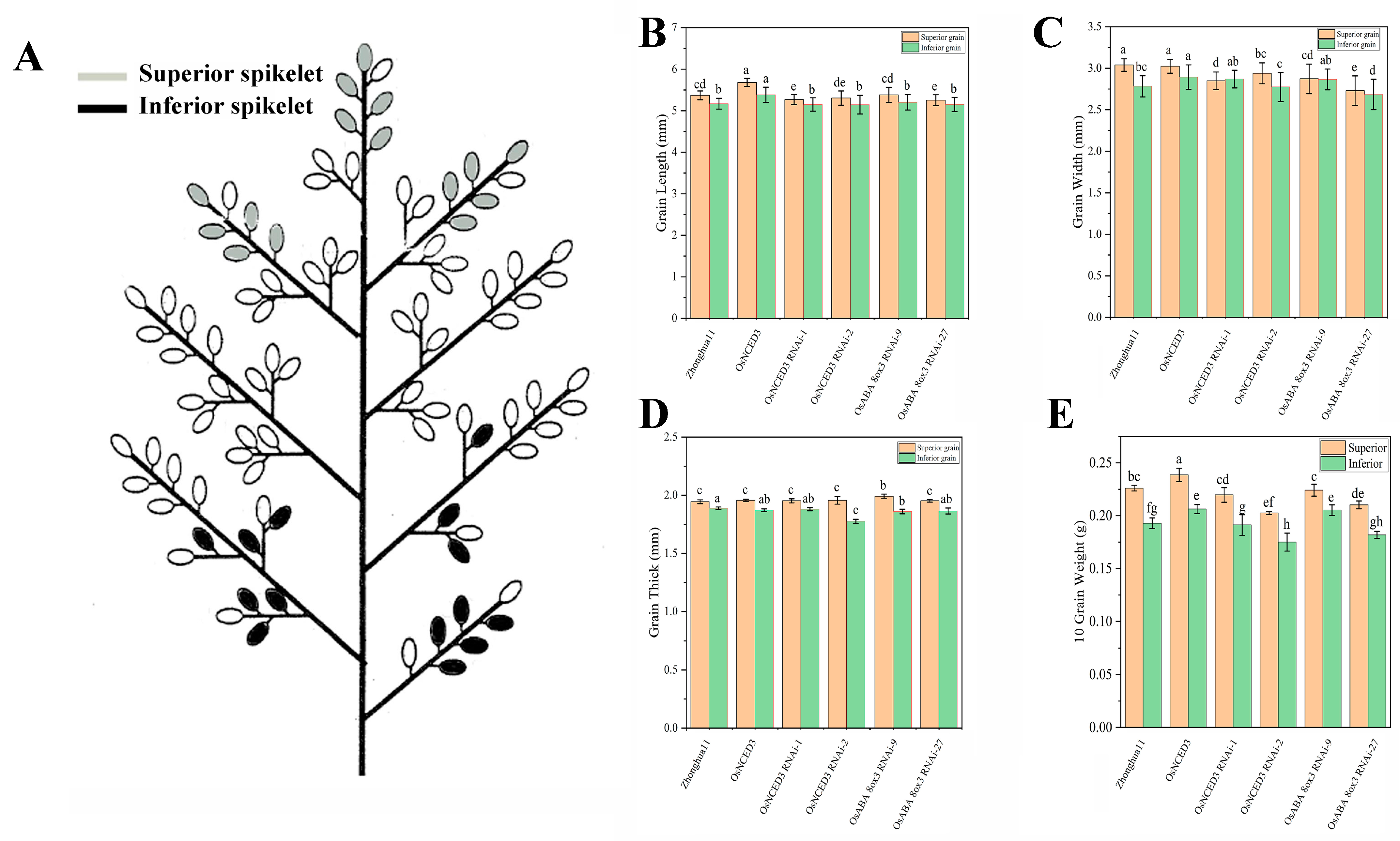
2.2. Observation and Size Measurement of Superior and Inferior Grains
2.3. Starch Isolation
2.4. Determination of Soluble Sugar and Total Starch Content
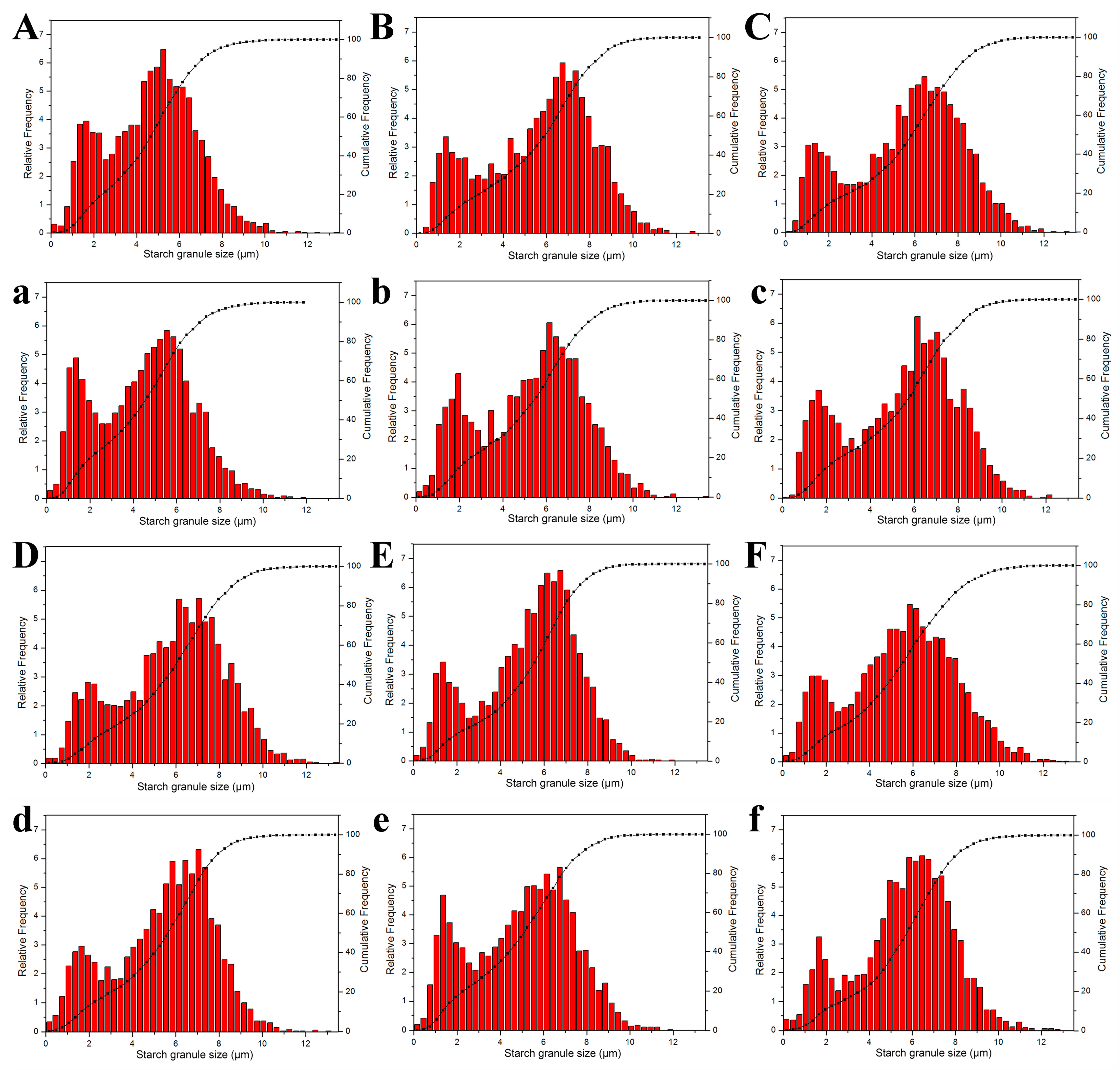
2.5. Observation of Superior and Inferior Grain Starch Morphology and Starch Particle Size Distribution
2.6. Determination of Apparent Amylose Content, Swelling Potential and Solubility
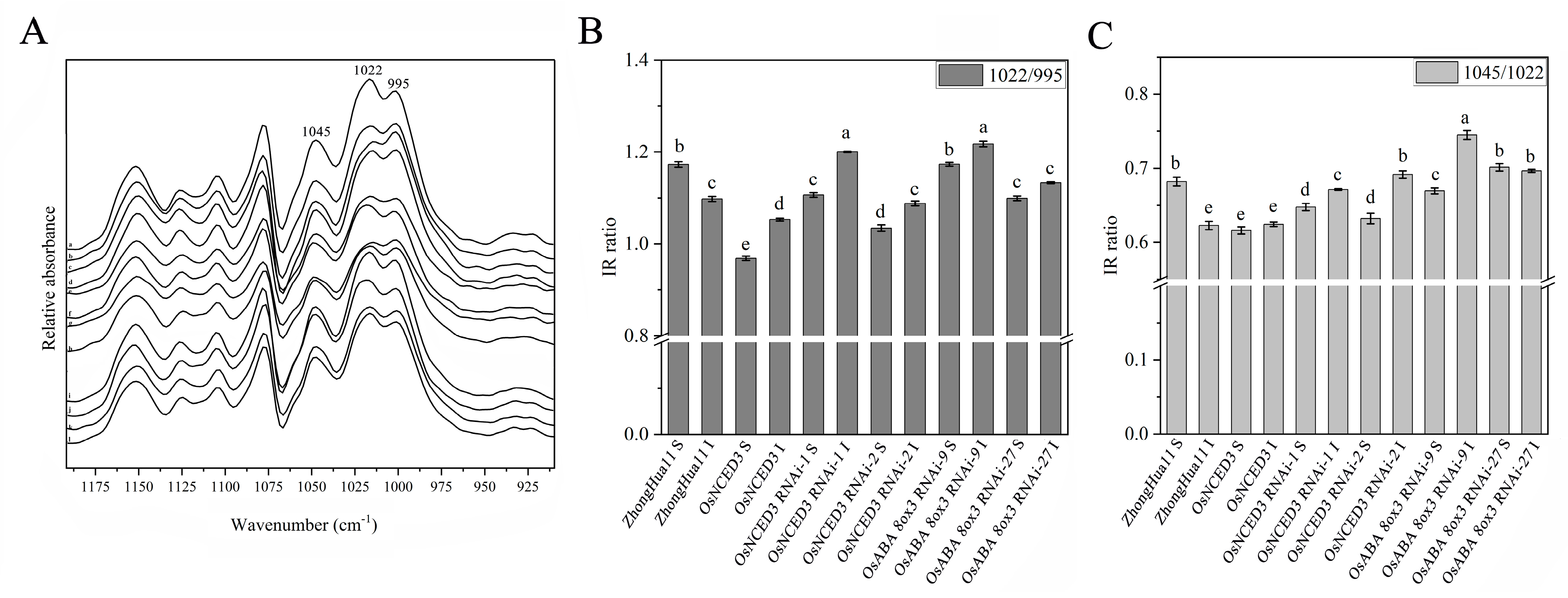
2.7. Fourier Transform Infrared (FTIR) Analysis of Starch
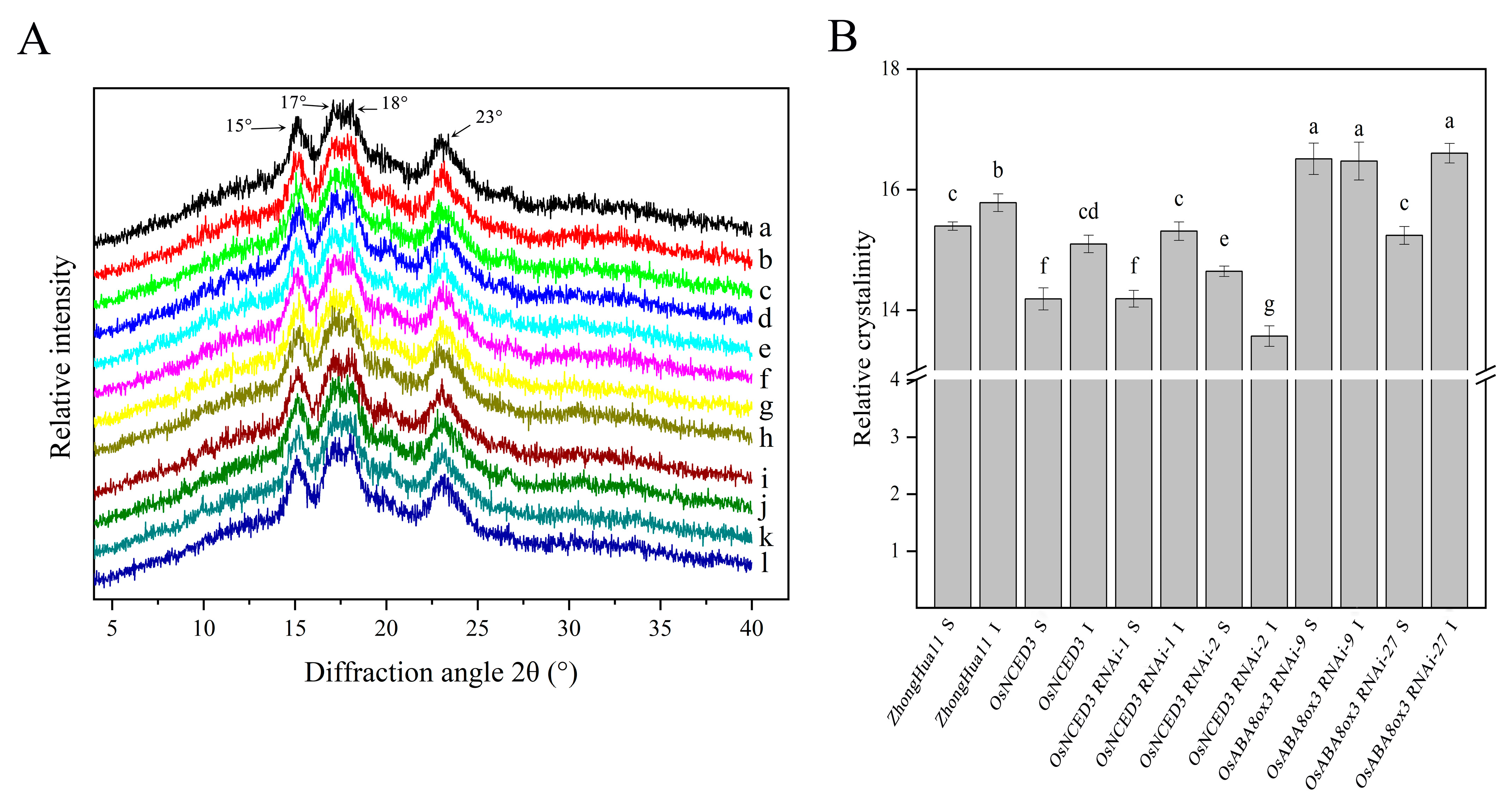
2.8. X-Ray Diffraction Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effects of ABA Transgenic Lines on the Appearance Quality in Rice
3.2. OsNCED3 and OsABA8ox3 Affect Total Starch and Soluble Sugar in Superior and Inferior Grains
3.3. OsNCED3 and OsABA8ox3 Promote the Enlargement of Starch Granules
3.4. Effect of OsNCED3 and OsABA8ox3 on Apparent Amylose Content Is Reversed in Superior and Inferior Grains
3.5. OsNCED3 and OsABA8ox3 Affect the Chemical Properties of Superior and Inferior Grain Starch in Rice
3.6. OsNCED3 and OsABA8ox3 Affect the Order of Superior and Inferior Grain Starch in Rice
3.7. OsNCED3 and OsABA8ox3 Affect the Crystal Structure of Superior and Inferior Grain Starch in Rice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Lin, G.; Yu, X.; Wu, Y.; Chen, G.; Xiong, F. Endosperm enrichment and physicochemical properties of superior and inferior grain starch in super hybrid rice. Plant Biol. 2020, 22, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Changan, S.S.; Ali, K.; Kumar, V.; Garg, N.K.; Tyagi, A. Abscisic acid biosynthesis under water stress: Anomalous behavior of the 9-cis-epoxycarotenoid dioxygenase1 (NCED1) gene in rice. Biol. Plant. 2018, 62, 663–670. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Zhu, G.; Ye, N.; Zhang, J. Glucose-induced delay of seed germination in rice is mediated by the suppression of ABA catabolism rather than an enhancement of ABA biosynthesis. Plant Cell Physiol. 2009, 50, 644–665. [Google Scholar] [CrossRef]
- Saika, H.; Okamoto, M.; Miyoshi, K.; Kushiro, T.; Shinoda, S.; Jikumaru, Y.; Fujimoto, M.; Arikawa, T.; Takahashi, H.; Ando, M.; et al. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8′-hydroxylase in rice. Plant Cell Physiol. 2007, 48, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Dai, X.; Zhang, W.H. A rice F-box gene, OsFbx352, is involved in glucose-delayed seed germination in rice. J. Exp. Bot. 2012, 63, 5559–5568. [Google Scholar] [CrossRef]
- Xu, X.Z.; Wang, T.; Wan, W.; Li, S.H.; Zhu, G.H. Aba biosynthesis gene osnced3 confers drought stress tolerance in rice. Acta Agron. Sin. 2018, 44, 24. [Google Scholar] [CrossRef]
- Hwang, S.G.; Lee, C.Y.; Tseng, C.S. Heterologous expression of rice 9-cis-epoxycarotenoid dioxygenase 4 (OsNCED4) in Arabidopsis confers sugar oversensitivity and drought tolerance. Bot. Stud. 2018, 59, 2. [Google Scholar] [CrossRef]
- Ye, N.H.; Zhu, G.H.; Liu, Y.G.; Li, Y.X.; Zhang, J.H. ABA Controls H2O2 accumulation through the induction of OsCATB in rice leaves under water stress. Plant Cell Physiol. 2011, 52, 689–698. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Y.; Xie, N.; Guo, Y.; Zhang, F.; Xiang, Z.; Wang, R.; Wang, F.; Gao, Q.; Tian, L.; et al. OsNCED5, a 9-cis-epoxycarotenoid dioxygenase gene, regulates salt and water stress tolerance and leaf senescence in rice. Plant Sci. 2019, 287, 110188. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Yu, X.R.; Yang, Y.; Chen, X.Y.; Wang, Q.J.; Zhang, X.H.; Ran, L.P.; Xiong, F. Morphology and Physicochemical Properties of Starch in Wheat Superior and Inferior Grains. Starch/Stärke 2018, 70, 1700177. [Google Scholar] [CrossRef]
- Okamoto, M.; Tanaka, Y.; Abrams, S.R.; Kamiya, Y.; Seki, M.; Nambara, E. High humidity induces abscisic acid 8′-hydroxylase in stomata and vasculature to regulate local and systemic abscisic acid responses in Arabidopsis. Plant Physiol. 2009, 149, 825–834. [Google Scholar] [CrossRef]
- Todoroki, Y.; Kobayashi, K.; Shirakura, M.; Aoyama, H.; Takatori, K.; Nimitkeatkai, H.; Jin, M.H.; Hiramatsu, S.; Ueno, K.; Kondo, S.; et al. Abscinazole-F1, a conformationally restricted analogue of the plant growth retardant uniconazole and an inhibitor of ABA 8′-hydroxylase CYP707A with no growth-retardant effect. Bioorganic Med. Chem. 2009, 17, 6620–6630. [Google Scholar] [CrossRef]
- Mega, R.; Meguro-Maoka, A.; Endo, A.; Shimosaka, E.; Murayama, S.; Nambara, E.; Seo, M.; Kanno, Y.; Abrams, S.R.; Sato, Y. Sustained low abscisic acid levels increase seedling vigor under cold stress in rice (Oryza sativa L.). Sci. Rep. 2015, 5, 13819. [Google Scholar] [CrossRef]
- Fu, K.; Song, W.H.; Chen, C.; Mou, C.L.; Huang, Y.S.; Zhang, F.L.; Hao, Q.X.; Wang, P.; Ma, T.F.; Chen, Y.P.; et al. Improving pre-harvest sprouting resistance in rice by editing OsABA8ox using CRISPR/Cas9. Plant Cell Rep. 2022, 41, 2107–2110. [Google Scholar] [CrossRef]
- Cai, S.L.; Jiang, G.B.; Ye, N.H.; Chu, Z.Z.; Xu, X.Z.; Zhang, J.H.; Zhu, G.H. A key ABA catabolic gene, OsABA8ox3, is involved in drought stress resistance in rice. PLoS ONE 2015, 10, e0116646. [Google Scholar] [CrossRef]
- Duan, M.; Sun, S.S. Profiling the expression of genes controlling rice grain quality. Plant Mol. Biol. 2005, 59, 165–178. [Google Scholar] [CrossRef]
- Burrell, M.M. Starch: The need for improved quality or quantity—An overview. J. Exp. Bot. 2003, 54, 451–456. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Liu, K.; Wang, P. Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. J. Exp. Bot. 2006, 57, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.W.; Feng, N.J.; Zheng, D.F.; Zhou, H.; Liu, L.; Chen, G.J.; Mu, B. Physiological mechanism of exogenous brassinolide alleviating salt stress injury in rice seedlings. Sci. Rep. 2022, 12, 20439. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, W.; Chen, S.; Zhang, Y.; Ansah, E.; An, G.; Xiong, F.; Wu, Y. Effects of Endogenous Cytokinin on Physicochemical Properties of Superior and Inferior Grain Starch in Rice. Starch/Stärke 2023, 75. [Google Scholar] [CrossRef]
- Gao, H.M.; Cai, J.W.; Han, W.L.; Huai, H.Y.; Chen, Y.F.; Wei, C.X. Comparison of starches isolated from three different Trapa species. Food Hydrocoll. 2015, 37, 174–181. [Google Scholar] [CrossRef]
- Yu, X.R.; Yu, H.; Zhang, J.; Shao, S.S.; Zhou, L.; Xiong, F.; Wang, Z. Comparison of Endosperm Starch Granule Development and Physicochemical Properties of Starches from Waxy and Non-Waxy Wheat. Int. J. Food Prop. 2015, 18, 2409–2421. [Google Scholar] [CrossRef]
- He, J.F.; Goyal, R.; Laroche, A.; Zhao, M.L.; Lu, Z.X. Water stress during grain development affects starch synthesis, composition and physicochemical properties in triticale. J. Cereal Sci. 2012, 56, 552–560. [Google Scholar] [CrossRef]
- Wei, C.X.; Xu, B.; Qin, F.L.; Yu, H.G.; Chen, C.; Meng, X.L.; Zhu, L.J.; Wang, Y.P.; Gu, M.H.; Liu, Q.Q. C-type starch from high-amylose rice resistant starch granules modified by antisense RNA inhibition of starch branching enzyme. J. Agric. Food Chem. 2010, 58, 7383–7388. [Google Scholar] [CrossRef]
- Nara, S.; Komiya, T. Studies on the relationship between water-saturated state and crystallinity by the diffraction method for moistened potato starch. Starch/Stärke 1983, 35, 407–410. [Google Scholar] [CrossRef]
- Chen, T.T.; Li, G.Y.; Islam, M.R.; Fu, W.M.; Feng, B.H.; Tao, L.X.; Fu, G.F. Abscisic acid synergizes with sucrose to enhance grain yield and quality of rice by improving the source-sink relationship. BMC Plant Biol. 2019, 19, 525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Chen, J.; Lin, S.S.; Li, Z.; Cheng, R.H.; Fang, C.X.; Chen, H.F.; Lin, W.X. Proteomic and phosphoproteomic determination of ABA’s effects on grain-filling of Oryza sativa L. inferior spikelets. Plant Sci. 2012, 185–186, 259–273. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, G.Q.; Yu, X.R.; Wu, Y.F.; Xiong, F. Rice starch accumulation at different endosperm regions and physical properties under nitrogen treatment at panicle initiation stage. Int. J. Biol. Macromol. 2020, 160, 328–339. [Google Scholar] [CrossRef]
- Man, J.M.; Yang, Y.; Zhang, C.Q.; Zhang, F.M.; Wang, Y.P.; Gu, M.H.; Liu, Q.Q.; Wei, C.X. Morphology and structural characterization of high-amylose rice starch residues hydrolyzed by porcine pancreatic α-amylase. Food Hydrocoll. 2013, 31, 195–203. [Google Scholar] [CrossRef]
- Shaik, S.S.; Carciofi, M.; Martens, H.J.; Hebelstrup, K.H.; Blennow, A. Starch bioengineering affects cereal grain germination and seedling establishment. J. Exp. Bot. 2014, 65, 2257–2270. [Google Scholar] [CrossRef]
- Wang, K.; Hasjim, J.; Wu, A.C.; Li, E.; Henry, R.J.; Gilbert, R.G. Roles of GBSSI and SSIIa in determining amylose fine structure. Carbohydr. Polym. 2015, 127, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.D.; Wang, J.; Huang, L.C.; Kan, L.J.; Wang, C.X.; Xiong, M.; Zhou, P.; Zhou, L.H.; Chen, C.; Zhao, D.S.; et al. ABA-mediated regulation of rice grain quality and seed dormancy via the NF-YB1-SLRL2-bHLH144 Module. Nat. Commun. 2024, 15, 4493. [Google Scholar] [CrossRef]
- Teng, Z.H.; Yu, H.H.; Wang, G.Q.; Meng, S.; Liu, B.H.; Yi, Y.K.; Chen, Y.K.; Zheng, Q.; Liu, L.; Yang, J.C.; et al. Synergistic interaction between ABA and IAA due to moderate soil drying promotes grain filling of inferior spikelets in rice. Plant J. 2022, 109, 1457–1472. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Singh, J.; Mccarthy, O.J.; Singh, H. Physico-chemical, rheological and structural properties of fractionated potato starches. J. Food Eng. 2007, 82, 383–394. [Google Scholar] [CrossRef]
- Bernazzani, P.; Peyyavula, V.K.; Agarwal, S.; Tatikomda, R.K. Evaluation of the phase composition of amylose by FTIR and isothermal immersion heats. Polymer 2008, 49, 4150–4158. [Google Scholar] [CrossRef]
- Sixou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar]
- Chen, F.Q.; Zhang, B.S.; Feng, H.F.; Zhao, Y.Q.; Zhang, X.Y. A Review of Application of X ray Diffraction in Crystal Structure Determination of Starch Granules. J. Food Sci. 2010, 31, 284–287. [Google Scholar]
- Cheetham, N.W.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Huang, J.R.; Liu, X.J. Research Progress on Determination Methods for Crystal Structures of Starch Granules. J. Food Sci. 2012, 33, 335–338. [Google Scholar]
| Sample | Soluble Sugar | Total Starch | |
|---|---|---|---|
| ZhongHua11 | Superior grain | 3.06 ± 0.12 b | 84.1 ± 2.13 a |
| Inferior grain | 2.88 ± 0.13 bc | 74.9 ± 4.04 b | |
| OsNCED3-OX | Superior grain | 2.60 ± 0.13 c | 69.5 ± 0.85 c |
| Inferior grain | 4.23 ± 0.12 a | 64.4 ± 3.55 d | |
| OsNCED3 RNAi-1 | Superior grain | 2.78 ± 0.25 bc | 77.7 ± 2.00 b |
| Inferior grain | 4.48 ± 0.23 a | 64.4 ± 2.07 d | |
| OsNCED3 RNAi-2 | Superior grain | 2.01 ± 0.16 d | 83.5 ± 2.04 a |
| Inferior grain | 2.75 ± 0.14 bc | 78.0 ± 1.64 b | |
| OsABA8ox3 RNAi-9 | Superior grain | 3.15 ± 0.08 b | 69.7 ± 3.35 c |
| Inferior grain | 2.21 ± 0.15 cd | 69.3 ± 2.96 c | |
| OsABA8ox3 RNAi-27 | Superior grain | 2.38 ± 0.17 c | 67.5 ± 0.86 cd |
| Inferior grain | 3.23 ± 0.19 b | 67.6 ± 3.24 cd |
| Sample | AAC | 0–1.5 (μm) | 1.5–10 (μm) | Average Size | |
|---|---|---|---|---|---|
| ZhongHua11 | Superior grain | 15.16 ± 0.47 a | 7.86 ± 0.15 d | 91.49 ± 1.31 b | 4.65 ± 0.051 i |
| Inferior grain | 12.85 ± 0.14 cd | 12.52 ± 0.20 a | 86.90 ± 2.25 d | 4.47 ± 0.031 h | |
| OsNCED3-OX | Superior grain | 12.56 ± 0.38 d | 8.12 ± 0.11 cd | 89.93 ± 1.59 c | 5.56 ± 0.059 b |
| Inferior grain | 14.90 ± 0.29 ab | 7.01 ± 0.13 e | 91.60 ± 2.33 b | 5.27 ± 0.043 e | |
| OsNCED3 RNAi-1 | Superior grain | 15.50 ± 0.57 a | 8.54 ± 0.09 c | 89.72 ± 2.59 c | 5.64 ± 0.041 b |
| Inferior grain | 15.19 ± 0.59 a | 7.73 ± 0.15 d | 90.58 ± 1.79 bc | 5.45 ± 0.031 cd | |
| OsNCED3 RNAi-2 | Superior grain | 14.87 ± 0.26 ab | 4.82 ± 0.07 f | 92.67 ± 2.01 a | 5.80 ± 0.022 a |
| Inferior grain | 12.56 ± 0.46 d | 7.15 ± 0.17 e | 91.73 ± 2.51 b | 5.37 ± 0.043 d | |
| OsABA8ox3 RNAi-9 | Superior grain | 14.78 ± 0.15 ab | 8.46 ± 0.16 c | 91.15 ± 2.51 b | 5.27 ± 0.041 e |
| Inferior grain | 14.33 ± 0.54 b | 10.17 ± 0.12 b | 89.13 ± 1.35 c | 4.96 ± 0.031 g | |
| OsABA8ox3 RNAi-27 | Superior grain | 13.51 ± 0.27 c | 7.34 ± 0.11 e | 90.01 ± 1.22 c | 5.45 ± 0.054 c |
| Inferior grain | 13.29 ± 0.24 cd | 4.97 ± 0.05 f | 93.53 ± 0.99 a | 5.61 ± 0.072 b |
| Sample | Swelling Power | Solubility | |
|---|---|---|---|
| ZhongHua11 | Superior grain | 31.01 ± 0.16 b | 31.48 ± 1.65 a |
| Inferior grain | 24.67 ± 0.27 c | 31.02 ± 1.73 a | |
| OsNCED3-OX | Superior grain | 19.52 ± 0.66 d | 32.43 ± 1.26 a |
| Inferior grain | 29.86 ± 1.23 b | 32.93 ± 0.71 a | |
| OsNCED3 RNAi-1 | Superior grain | 24.46 ± 0.39 c | 30.95 ± 1.22 a |
| Inferior grain | 24.30 ± 0.13 c | 31.07 ± 1.09 a | |
| OsNCED3 RNAi-2 | Superior grain | 35.27 ± 0.24 a | 32.79 ± 0.81 a |
| Inferior grain | 22.16 ± 1.37 d | 33.37 ± 1.19 a | |
| OsABA 8ox3 RNAi-9 | Superior grain | 26.70 ± 1.11 c | 32.43 ± 0.62 a |
| Inferior grain | 25.47 ± 1.96 c | 33.19 ± 1.05 a | |
| OsABA 8ox3 RNAi-27 | Superior grain | 21.65 ± 1.27 d | 29.51 ± 2.20 b |
| Inferior grain | 29.72 ± 0.55 b | 33.97 ± 3.29 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Ansah, E.O.; Zhu, L.; Fang, W.; Wang, L.; Zhang, D.; Guo, B. ABA Affects Distinctive Rice Caryopses Physicochemical Properties on Different Branches. Agronomy 2024, 14, 2632. https://doi.org/10.3390/agronomy14112632
Wu Y, Ansah EO, Zhu L, Fang W, Wang L, Zhang D, Guo B. ABA Affects Distinctive Rice Caryopses Physicochemical Properties on Different Branches. Agronomy. 2024; 14(11):2632. https://doi.org/10.3390/agronomy14112632
Chicago/Turabian StyleWu, Yunfei, Ebenezer Ottopah Ansah, Licheng Zhu, Wenchun Fang, Leilei Wang, Dongping Zhang, and Baowei Guo. 2024. "ABA Affects Distinctive Rice Caryopses Physicochemical Properties on Different Branches" Agronomy 14, no. 11: 2632. https://doi.org/10.3390/agronomy14112632
APA StyleWu, Y., Ansah, E. O., Zhu, L., Fang, W., Wang, L., Zhang, D., & Guo, B. (2024). ABA Affects Distinctive Rice Caryopses Physicochemical Properties on Different Branches. Agronomy, 14(11), 2632. https://doi.org/10.3390/agronomy14112632







