Impact of Reduced Nitrogen Inputs on Soil Organic Carbon and Nutrient Dynamics in Arable Soil, Northern Thailand: Short-Term Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Property Analysis
2.2. Methodology for of Soil Carbon Fraction Pools
2.3. Calculations and Data Analysis
3. Results
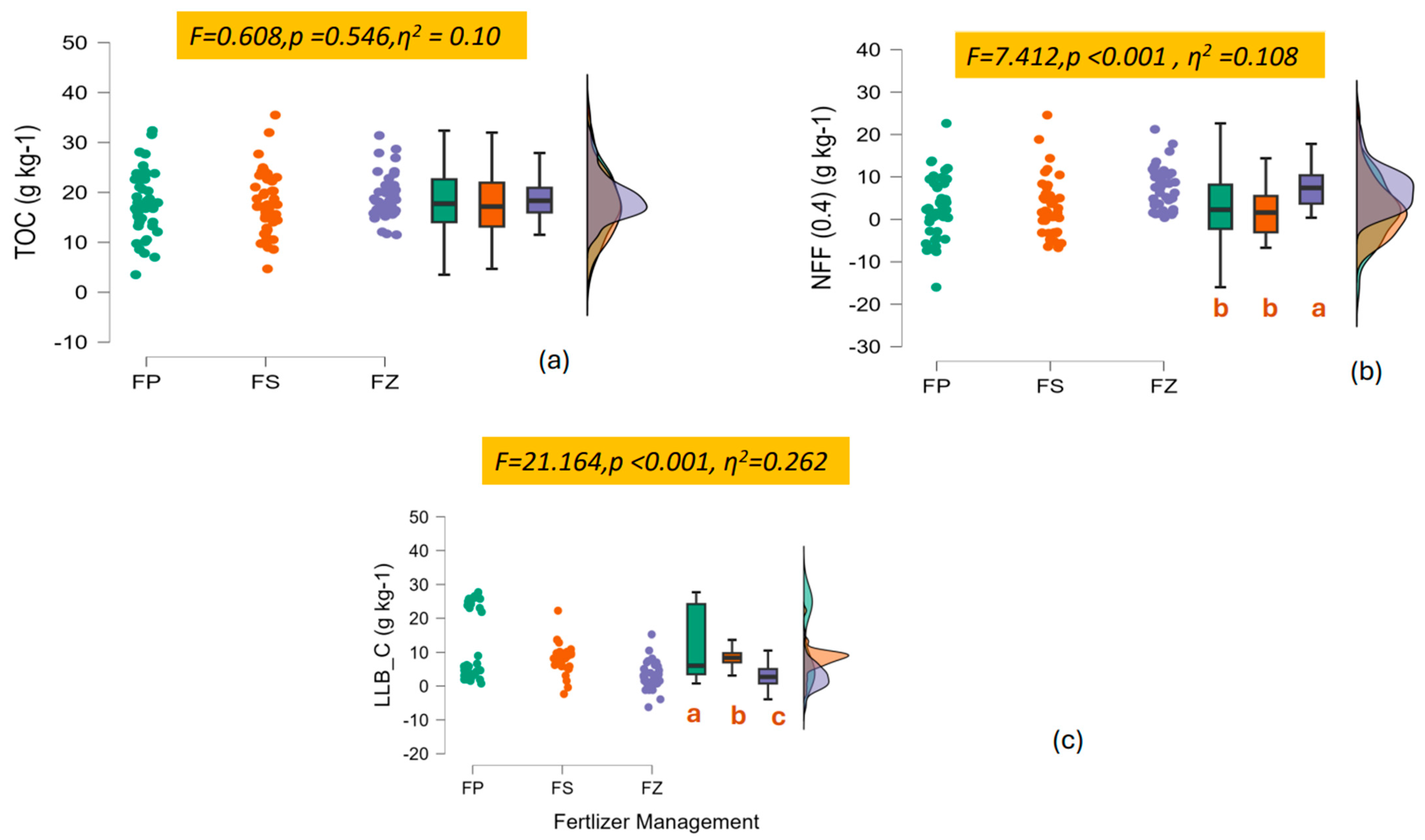
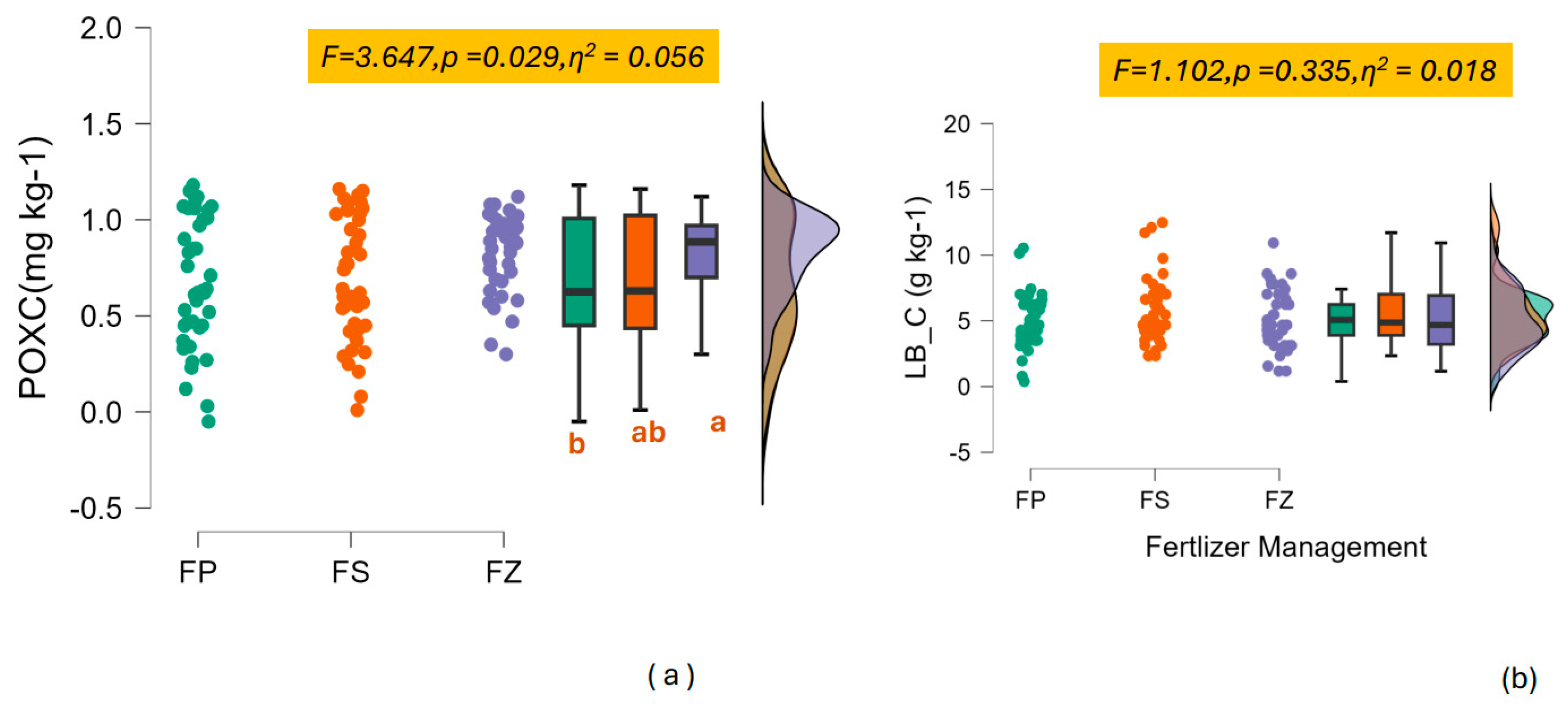
3.1. The Decrease in N Addition Impacts the Carbon Fractions
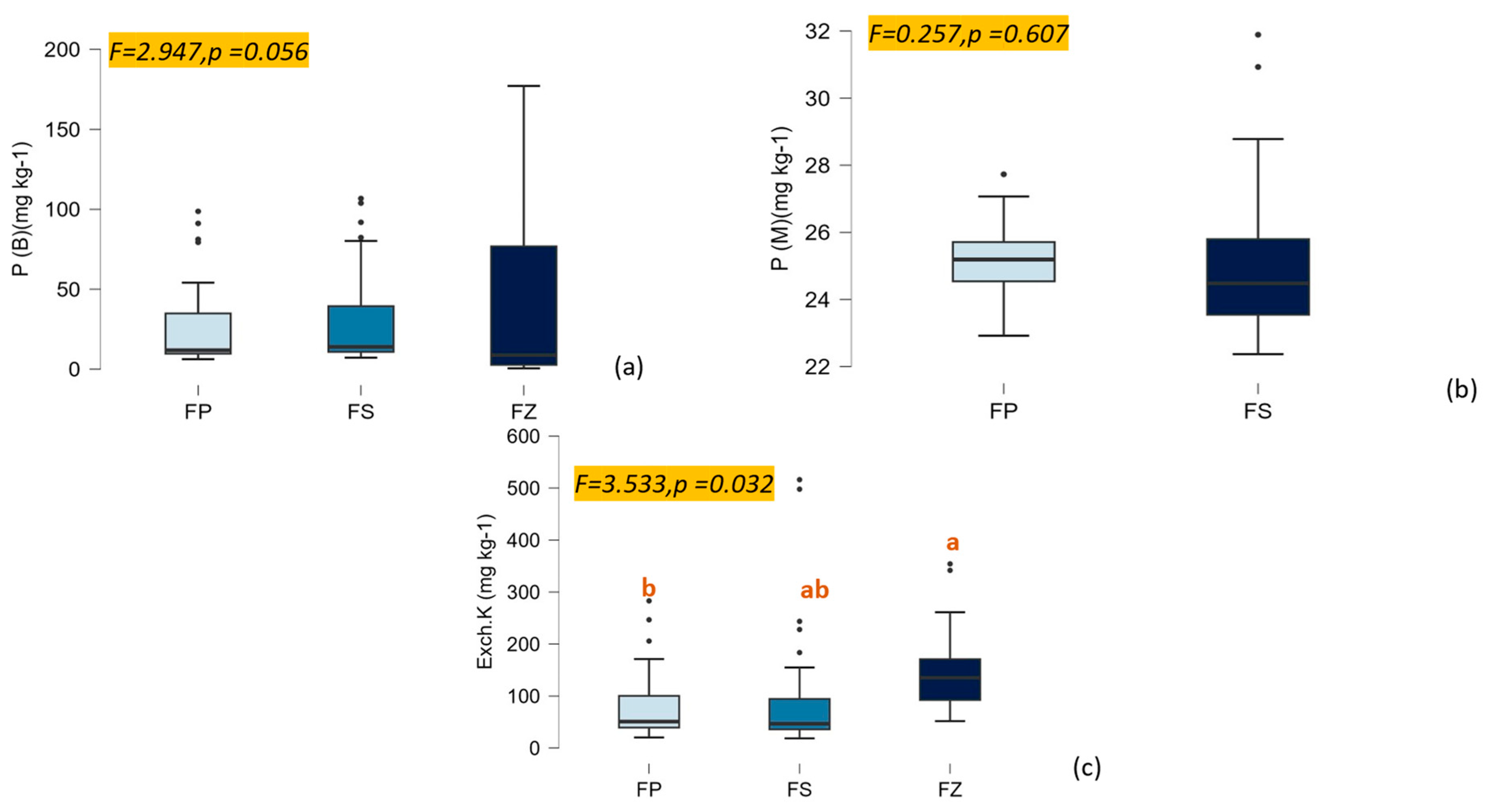
3.2. The Decrease in N Addition Impacts the Availability of NPK in Soil
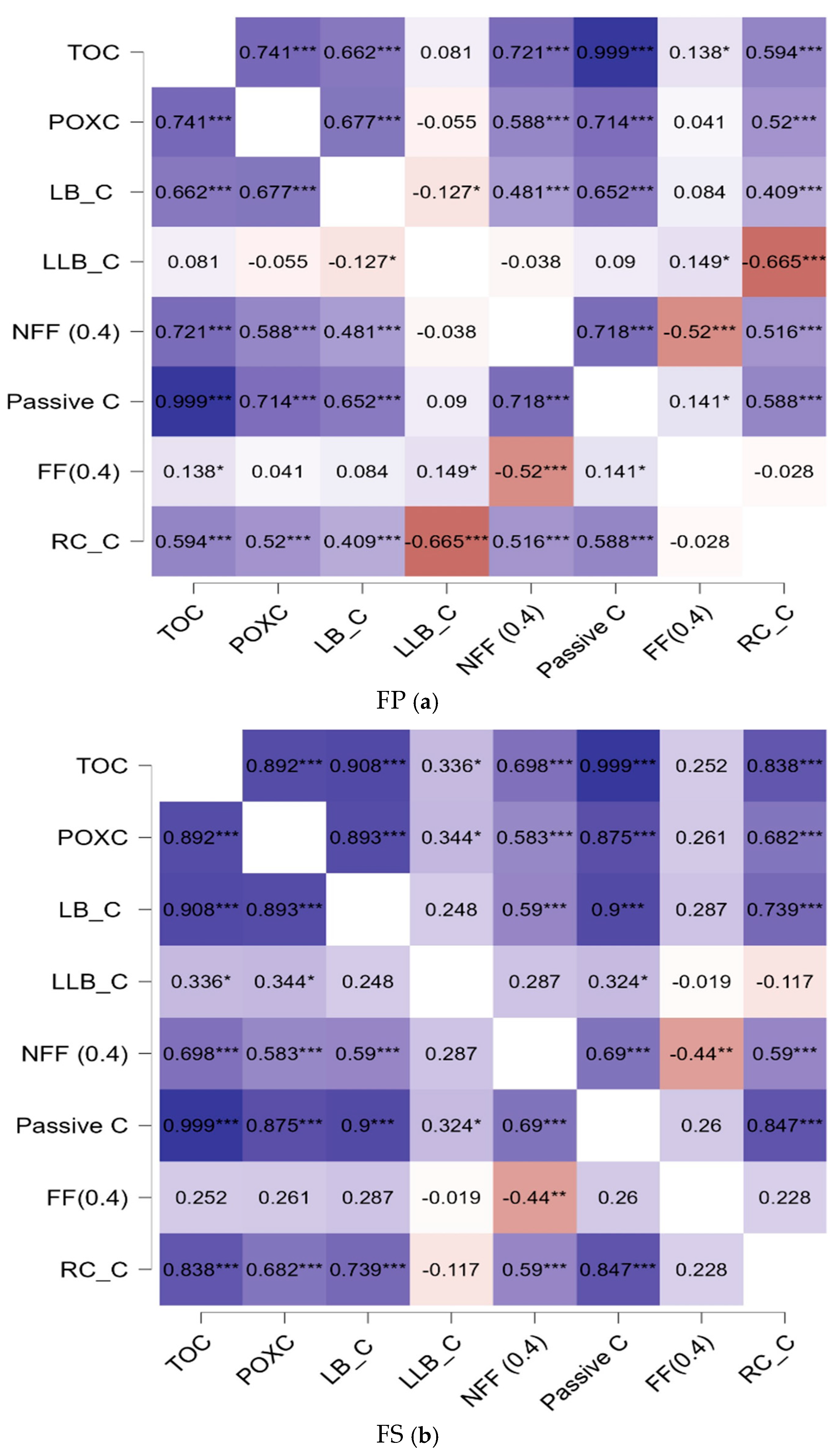
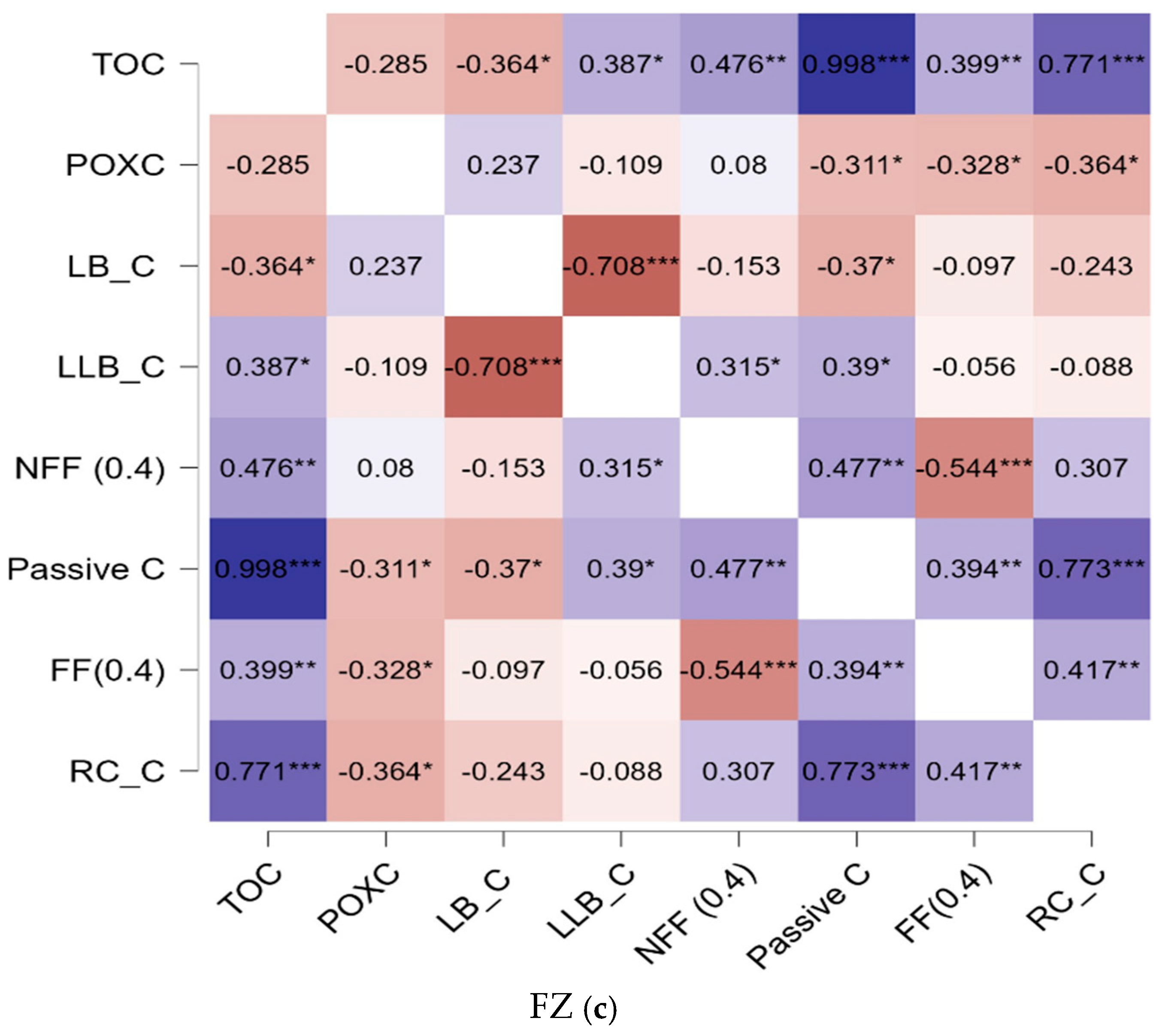
3.3. Relationships Exist Between Carbon Fractions and the Effects of Reducing N in Synthetic Fertilizer on the Dynamics of Soil Carbon
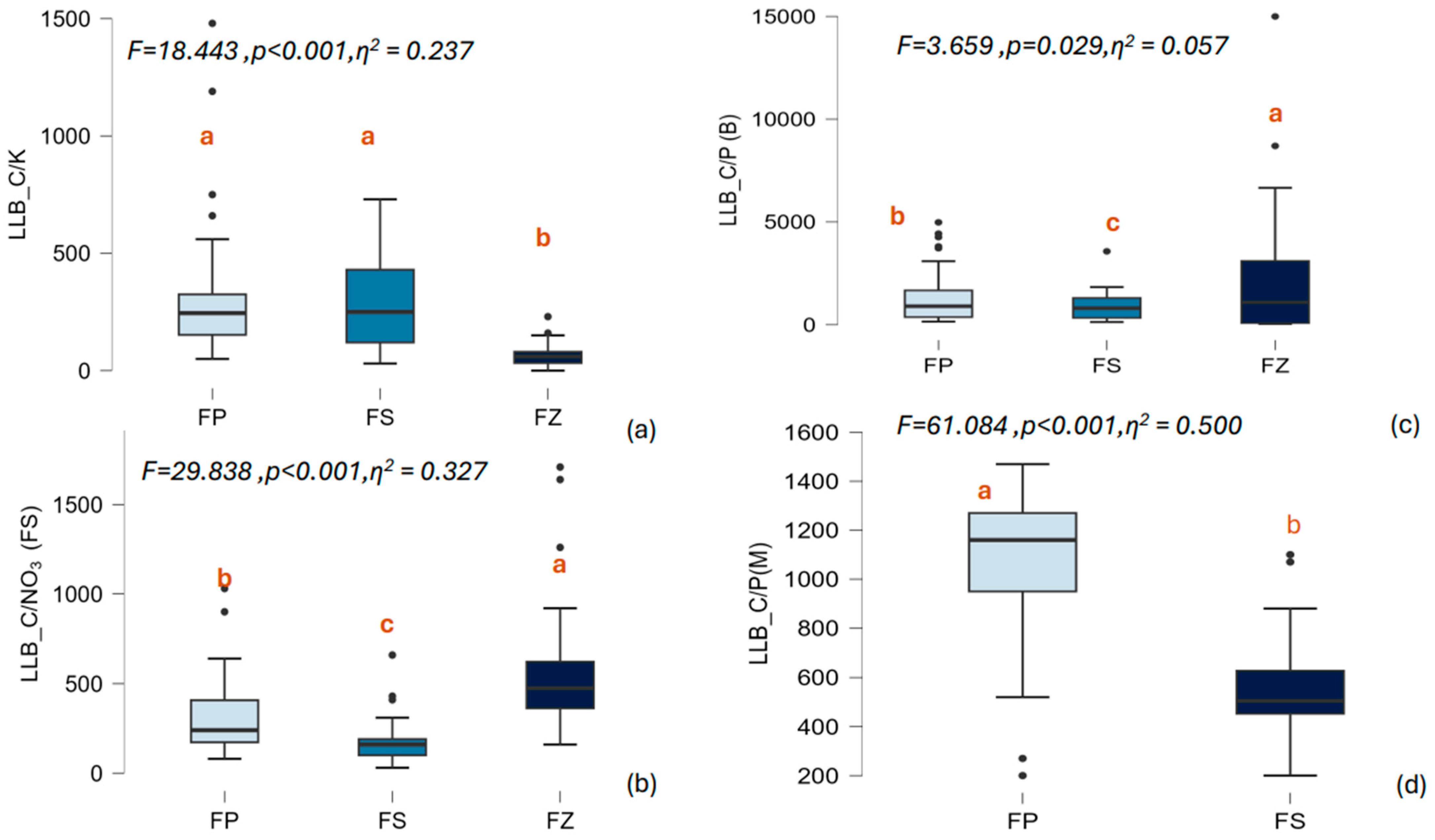
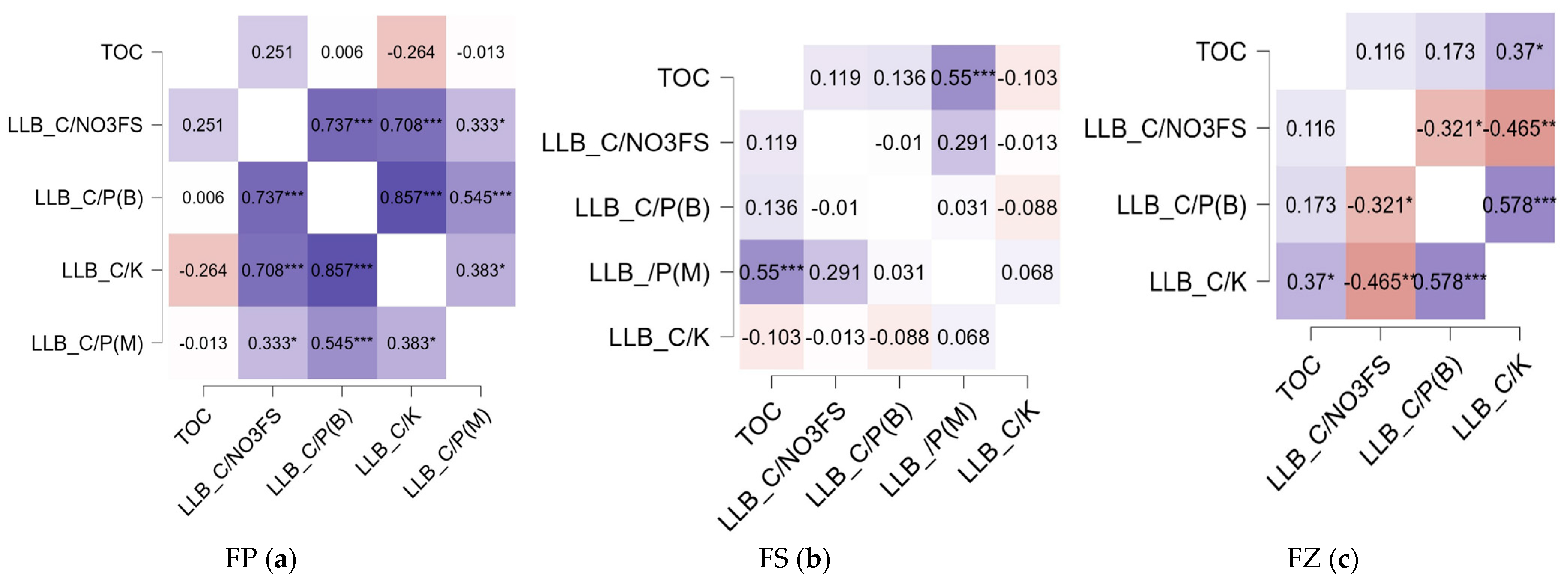
3.4. Ratio of Less Labile Carbon (LLB_C) to the NPK Content in Soil
4. Discussion
4.1. The Labile and Recalcitrant Carbon Content Decreased When Switched to SSNM
Labile Carbon Under Lower N Addition
4.2. The Impact of Availability of NPK Balanced with Carbon on SOC
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Fukase, E.; Martin, W. Economic Growth, Convergence, and World Food Demand and Supply. World Dev. 2017, 132, 104954. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Global Perspectives—Studies: Food and Agriculture Projections to 2050. Available online: https://www.fao.org/global-soil-partnership/resources/highlights/detail/en/c/1452041 (accessed on 9 March 2024).
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef]
- Mustafa, A.; Hu, X.; Sun, N.; Kailou, L.; Qing-hai, H.; Mohammad Tahsin Karimi, N.; Minggang, X. Long-Term Fertilization Alters the Storage and Stability of Soil Organic Carbon in Chinese Paddy Soil. Agronomy 2023, 13, 1463. [Google Scholar] [CrossRef]
- Urmi, T.A.; Rahman, M.; Islam, M.; Islam, A.; Jahan, N.A.; Mia, A.B.; Akhter, S.; Siddiqui, M.H.; Kalaji, H.M. Integrated Nutrient Management for Rice Yield, Soil Fertility, and Carbon Sequestration. Plants 2022, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Shahrzad, S.; Khadijeh, J. Positive and Negative Impact of Nitrogen Fertilizer on Soil Properties and Nutrient Dynamic. Asian J. Res. Agric. For. 2023, 9, 233–240. [Google Scholar] [CrossRef]
- Armel, Z.; Alberto, E.; Portell, X.; Rodrigo, A.; Virto, I.; Orcaray, L. A Fertilisation Strategy Combining Mineral Fertiliser and Biosolid Improves Long-Term Yield and Carbon Storage in a Calcareous Soil. Agriculture 2023, 13, 860. [Google Scholar] [CrossRef]
- Nisar, S.; Mavi, M.S.; Singh, J.; Dey, P. Integrated nutrient management in spring-maize improves yield, nutrient use efficiency and minimizes greenhouse gas intensity. Arch. Agron. Soil Sci. 2023, 69, 2522–2536. [Google Scholar] [CrossRef]
- Bashkin, V.; Alekseev, A. Global Food Security and Fundamental Role of Fertilizer Part 2. Fundamental Role of Fertilizer in Food Production. Issues Risk Anal. 2022, 19, 10–29. [Google Scholar] [CrossRef]
- Sapkota, T.B.; Jat, M.L.; Rana, D.S.; Khatri-Chhetri, A.; Jat, H.S.; Bijarniya, D.; Sutaliya, J.M.; Kumar, M.; Singh, L.K.; Jat, R.K.; et al. Crop nutrient management using Nutrient Expert improves yield, increases farmers’ income and reduces greenhouse gas emissions. Sci. Rep. 2021, 11, 1564. [Google Scholar] [CrossRef]
- Wu, H.; MacDonald, G.K.; Galloway, J.N.; Zhang, L.; Gao, L.; Yang, L.; Yang, J.; Li, X.; Li, H.; Yang, T. The influence of crop and chemical fertilizer combinations on greenhouse gas emissions: A partial life-cycle assessment of fertilizer production and use in China. Resour. Conserv. Recycl. 2021, 168, 105303. [Google Scholar] [CrossRef]
- Corcimaru, V.; Corcimaru, S. A potentially underestimated source of CO2 and other greenhouse gases in agriculture. J. Emerg. Investig. 2022, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Liu, N.; Ren, C.; Li, H.; Wang, W.; Li, N.; Ren, G.; Feng, Y.; Yang, G. Effect of Nitrogen Fertilizer on Soil CO2 Emission Depends on Crop Rotation Strategy. Sustainability 2020, 12, 5271. [Google Scholar] [CrossRef]
- Walling, E.; Vaneeckhaute, C. Greenhouse gas emissions from inorganic and organic fertilizer production and use: A review of emission factors and their variability. J. Environ. Manag. 2020, 276, 111211. [Google Scholar] [CrossRef]
- Kim, G.W.; Lim, J.Y.; Islam Bhuiyan, M.S.; Das, S.; Khan, M.I.; Kim, P.J. Investigating the arable land that is the main contributor to global warming between paddy and upland vegetable crops under excessive nitrogen fertilization. J. Clean. Prod. 2022, 346, 131197. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Jiang, Y. Response of Nitrogen Losses to Excessive Nitrogen Fertilizer Application in Intensive Greenhouse Vegetable Production. Sustainability 2019, 11, 1513. [Google Scholar] [CrossRef]
- Atieno, M.; Herrmann, L.; Nguyen, H.T.; Phan, H.T.; Nguyen, N.K.; Srean, P.; Than, M.M.; Zhiyong, R.; Tittabutr, P.; Shutsrirung, A.; et al. Assessment of biofertilizer use for sustainable agriculture in the Great Mekong Region. J. Environ. Manag. 2020, 275, 111300. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhong, Y.; Yang, J.; Wu, Y.; Li, H.; Zheng, L. Effect of nitrogen fertilizer rates on carbon footprint and ecosystem service of carbon sequestration in rice production. Sci. Total Environ. 2019, 670, 210–217. [Google Scholar] [CrossRef]
- Govindasamy, P.; Muthusamy, S.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.; Maity, A.; Halli, H.; Sujayananad, G.K.; Vadivel, R.; Das, T.K.; et al. Nitrogen use efficiency—A key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Hu, J.; Li, X.; He, Q.; Tian, G.; Wang, H.; Wang, S.; Wang, B. Do microorganism stoichiometric alterations affect carbon sequestration in paddy soil subjected to phosphorus input? Ecol. Appl. 2015, 25, 866–879. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, B.; Lu, F.; Wang, X.; Zhuang, T.; Zhang, G.; Ouyang, Z. Roles of nitrogen, phosphorus, and potassium fertilizers in carbon sequestration in a Chinese agricultural ecosystem. Clim. Change 2017, 142, 587–596. [Google Scholar] [CrossRef]
- Jin, S. Recommended nitrogen fertilization enhances soil carbon sequestration in China’s monsoonal temperate zone. PeerJ 2018, 6, e5983. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Rocci, K.S.; Lehmann, A.; Rillig, M.C. Nitrogen increases soil organic carbon accrual and alters its functionality. Glob. Change Biol. 2023, 29, 1971–1983. [Google Scholar] [CrossRef]
- Song, Y.; Song, C.; Meng, H.; Swarzenski, C.M.; Wang, X.; Tan, W. Nitrogen additions affect litter quality and soil biochemical properties in a peatland of Northeast China. Ecol. Eng. 2017, 100, 175–185. [Google Scholar] [CrossRef]
- Jing, H.; Li, J.; Yan, B.; Wei, F.; Wang, G.; Liu, G. The effects of nitrogen addition on soil organic carbon decomposition and microbial C-degradation functional genes abundance in a Pinus tabulaeformis forest. For. Ecol. Manag. 2021, 489, 119098. [Google Scholar] [CrossRef]
- Jiang, X.; Cao, L.; Zhang, R. Changes of labile and recalcitrant carbon pools under nitrogen addition in a city lawn soil. J. Soils Sediments 2014, 14, 515–524. [Google Scholar] [CrossRef]
- Liu, H.; Xue, S.; Wang, G.; Liu, B. Effects of nitrogen addition on soil oxidisable organic carbon fractions in the rhizospheric and bulk soils of Chinese pines in north-western China. Soil Res. 2017, 56, 192–203. [Google Scholar] [CrossRef]
- Gruba, P.; Socha, J.; Błońska, E.; Lasota, J. Effect of variable soil texture, metal saturation of soil organic matter (SOM) and tree species composition on spatial distribution of SOM in forest soils in Poland. Sci. Total Environ. 2015, 521–522, 90–100. [Google Scholar] [CrossRef]
- McNally, S.R.; Beare, M.H.; Curtin, D.; Meenken, E.D.; Kelliher, F.M.; Calvelo Pereira, R.; Shen, Q.; Baldock, J. Soil carbon sequestration potential of permanent pasture and continuous cropping soils in New Zealand. Glob. Change Biol. 2017, 23, 4544–4555. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Batten, G.D.; Blanchard, C.L.; McLaren, G.; Zwart, A.B.; Kirkegaard, J.A. Accurate measurement of resistant soil organic matter and its stoichiometry. Eur. J. Soil Sci. 2016, 67, 695–705. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Carbon-nutrient stoichiometry to increase soil carbon sequestration. Soil Biol. Biochem. 2013, 60, 77–86. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Passioura, J.B.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Nutrient availability limits carbon sequestration in arable soils. Soil Biol. Biochem. 2014, 68, 402–409. [Google Scholar] [CrossRef]
- Geng, J.; Cheng, S.; Fang, H.; Pei, J.; Xu, M.; Lu, M.; Yang, Y.; Cao, Z.; Li, Y. Different Molecular Characterization of Soil Particulate Fractions under N Deposition in a Subtropical Forest. Forests 2019, 10, 914. [Google Scholar] [CrossRef]
- Xu, H.; Liu, K.; Zhang, W.; Rui, Y.; Zhang, J.; Wu, L.; Colinet, G.; Huang, Q.; Chen, X.; Xu, M. Long-term fertilization and intensive cropping enhance carbon and nitrogen accumulated in soil clay-sized particles of red soil in South China. J. Soils Sediments 2020, 20, 1824–1833. [Google Scholar] [CrossRef]
- Paterson, E.; Sim, A. Soil-specific response functions of organic matter mineralization to the availability of labile carbon. Glob. Change Biol. 2013, 19, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Michelsen, A.; Rinnan, R. Vegetation and soil responses to added carbon and nutrients remain six years after discontinuation of long-term treatments. Sci. Total Environ. 2020, 722, 137885. [Google Scholar] [CrossRef]
- Song, Y.; Song, C.; Tao, B.; Wang, J.; Zhu, X.; Wang, X. Short-term responses of soil enzyme activities and carbon mineralization to added nitrogen and litter in a freshwater marsh of Northeast China. Eur. J. Soil Biol. 2014, 61, 72–79. [Google Scholar] [CrossRef]
- Shan, Z.; Yin, Z.; Yang, H.; Zuo, C.; Zhu, T. Long-Term Cultivation of Fruit Plantations Decreases Mineralization and Nitrification Rates in Calcareous Soil in the Karst Region in Southwestern China. Forests 2020, 11, 1282. [Google Scholar] [CrossRef]
- Tian, Q.; Yang, X.; Wang, X.; Liao, C.; Li, Q.; Wang, M.; Wu, Y.; Liu, F. Microbial community mediated response of organic carbon mineralization to labile carbon and nitrogen addition in topsoil and subsoil. Biogeochemistry 2016, 128, 125–139. [Google Scholar] [CrossRef]
- Tang, Z.; Sun, X.; Luo, Z.; He, N.; Sun, O.J. Effects of temperature, soil substrate, and microbial community on carbon mineralization across three climatically contrasting forest sites. Ecol. Evol. 2018, 8, 879–891. [Google Scholar] [CrossRef]
- Mehnaz, K.R.; Corneo, P.E.; Keitel, C.; Dijkstra, F.A. Carbon and phosphorus addition effects on microbial carbon use efficiency, soil organic matter priming, gross nitrogen mineralization and nitrous oxide emission from soil. Soil Biol. Biochem. 2019, 134, 175–186. [Google Scholar] [CrossRef]
- Mehnaz, K.R.; Keitel, C.; Dijkstra, F.A. Effects of carbon and phosphorus addition on microbial respiration, N2O emission, and gross nitrogen mineralization in a phosphorus-limited grassland soil. Biol. Fertil. Soils 2018, 54, 481–493. [Google Scholar] [CrossRef]
- Aumtong, S.; Chotamonsak, C.; Pongwongkam, P.; Cantiya, K. Chemical Fertilization Alters Soil Carbon in Paddy Soil through the Interaction of Labile Organic Carbon and Phosphorus Fractions. Agronomy 2023, 13, 1588. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Turner, B.L.; Stott, A.W.; Tanner, E.V.J. Nitrogen and phosphorus constrain labile and stable carbon turnover in lowland tropical forest soils. Soil Biol. Biochem. 2015, 80, 26–33. [Google Scholar] [CrossRef]
- Bar-Yosef, B.; Ben Asher, J. Simulating the effect of potassium fertilization on carbon sequestration in soil. J. Plant Nutr. Soil Sci. 2013, 176, 375–386. [Google Scholar] [CrossRef]
- DOA (Department of Agriculture). Recommendations for Applying Fertilizer Based on Soil Analysis Values. Available online: https://www.doa.go.th/th/ (accessed on 29 September 2024).
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; SSSA Book Series; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1996; pp. 961–1010. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and A Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Richardson, A.E.; Kirkby, C.A. Fate and cost effectiveness of soil carbon sequestered using supplementary nutrients applied to crop residues under field conditions. Nutr. Cycl. Agroecosystems 2023, 126, 143–161. [Google Scholar] [CrossRef]
- Ohno, T.; Zibilske, L.M. Determination of Low Concentrations of Phosphorus in Soil Extracts Using Malachite Green. Soil Sci. Soc. Am. J. 1991, 55, 892–895. [Google Scholar] [CrossRef]
- Miller, A.P.; Arai, Y. Comparative Evaluation of Phosphate Spectrophotometric Methods in Soil Test Phosphorus Extracting Solutions. Soil Sci. Soc. Am. J. 2016, 80, 1543–1550. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic Forms. In Methods of Soil Analysis; Agronomy Monographs; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1982; pp. 643–698. [Google Scholar]
- Baethgen, W.E.; Alley, M.M. A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant Kjeldahl digests. Commun. Soil Sci. Plant Anal. 1989, 20, 961–969. [Google Scholar] [CrossRef]
- Weil, R.; Stine, M.; Gruver, J.; Samson-Liebig, S. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar] [CrossRef]
- Chan, K.; Bowman, A.; Oates, A. Oxidizible Organic Carbon Fractions and Soil Quality Changes in An Oxic Paleustalf Under Different Pasture Leys. Soil Sci. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Maia, S.M.F.; Xavier, F.A.S.; Oliveira, T.S.; Mendonça, E.S.; Araújo Filho, J.A. Organic carbon pools in a Luvisol under agroforestry and conventional farming systems in the semi-arid region of Ceará, Brazil. Agrofor. Syst. 2007, 71, 127–138. [Google Scholar] [CrossRef]
- Chertov, O.; Kuzyakov, Y.; Priputina, I.; Frolov, P.; Shanin, V.; Grabarnik, P. Modelling the Rhizosphere Priming Effect in Combination with Soil Food Webs to Quantify Interaction between Living Plant, Soil Biota and Soil Organic Matter. Plants 2022, 11, 2605. [Google Scholar] [CrossRef]
- Yang, K.; Peng, P.; Duan, F.; Tang, H.; Wu, K.; Wu, Z.; Li, F.; Chen, Y.; Zou, C.; Liu, L.; et al. Microbial Mechanisms of the Priming Effect over 12 Years of Different Amounts of Nitrogen Management. Agronomy 2023, 13, 1783. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Liu, H.; Wu, H.; Zhang, Z.; Wang, G.; Xue, S.; Liu, G. Effects of Soil Microbiological Properties on the Fractional Distribution and Stability of Soil Organic Carbon under Different N Addition Treatments. Forests 2023, 14, 1540. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, Z.; Chen, X.; Yao, X.; Ma, Z.; Wang, W. Nitrogen deficiency accelerates soil organic carbon decomposition in temperate degraded grasslands. Sci. Total Environ. 2023, 881, 163424. [Google Scholar] [CrossRef]
- Begum, R.; Jahangir, M.M.R.; Jahiruddin, M.; Islam, M.R.; Rahman, M.T.; Rahman, M.L.; Ali, M.Y.; Hossain, M.B.; Islam, K.R. Nitrogen fertilization impact on soil carbon pools and their stratification and lability in subtropical wheat-mungbean-rice agroecosystems. PLoS ONE 2021, 16, e0256397. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Change Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Du, Y.; Guo, P.; Liu, J.; Wang, C.; Yang, N.; Jiao, Z. Different types of nitrogen deposition show variable effects on the soil carbon cycle process of temperate forests. Glob. Change Biol. 2014, 20, 3222–3228. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Aluko, O.O.; Yuan, G.; Li, J.; Liu, H. The responses of soil organic carbon and total nitrogen to chemical nitrogen fertilizers reduction base on a meta-analysis. Sci. Rep. 2022, 12, 16326. [Google Scholar] [CrossRef] [PubMed]
- Elrys, A.S.; Wang, J.; Metwally, M.A.S.; Cheng, Y.; Zhang, J.B.; Cai, Z.C.; Chang, S.X.; Müller, C. Global gross nitrification rates are dominantly driven by soil carbon-to-nitrogen stoichiometry and total nitrogen. Glob. Chang Biol. 2021, 27, 6512–6524. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.A.; Li, Y.e.; Ashraf, M.N.; Ahmed, W.; Wang, B.; Sardar, M.F.; Ma, P.; Li, R.; Wan, Y.; Kuzyakov, Y. Long-term warming and elevated CO2 increase ammonia-oxidizing microbial communities and accelerate nitrification in paddy soil. Appl. Soil Ecol. 2021, 166, 104063. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Cheng, Y.; Cai, Z.; Müller, C.; Zhang, J. Composition of soil recalcitrant C regulates nitrification rates in acidic soils. Geoderma 2019, 337, 965–972. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, F. Physical disturbance accelerates carbon loss through increasing labile carbon release. Plant Soil Environ. 2020, 66, 584–589. [Google Scholar] [CrossRef]
- Kochsiek, A.; Knops, J. Effects of Nitrogen Availability on the Fate of Litter-Carbon and Soil Organic Matter Decomposition. Br. J. Environ. Clim. Change 2013, 3, 24–43. [Google Scholar] [CrossRef]
- Wang, T.; Tian, Z.; Tunlid, A.; Persson, P. Influence of Ammonium on Formation of Mineral-Associated Organic Carbon by an Ectomycorrhizal Fungus. Appl. Environ. Microbiol. 2019, 85, e03007–e03018. [Google Scholar] [CrossRef]
- Laura, R.D. Effects of neutral salts on carbon and nitrogen mineralisation of organic matter in soil. Plant Soil 1974, 41, 113–127. [Google Scholar] [CrossRef]
- Min, K.; Kang, H.; Lee, D. Effects of ammonium and nitrate additions on carbon mineralization in wetland soils. Soil Biol. Biochem. 2011, 43, 2461–2469. [Google Scholar] [CrossRef]
- Geng, J.; Fang, H.; Cheng, S.; Pei, J. Effects of N deposition on the quality and quantity of soil organic matter in a boreal forest: Contrasting roles of ammonium and nitrate. CATENA 2021, 198, 104996. [Google Scholar] [CrossRef]
- Lan, T.; Liu, R.; Suter, H.; Deng, O.; Gao, X.; Luo, L.; Yuan, S.; Wang, C.; Chen, D. Stimulation of heterotrophic nitrification and N2O production, inhibition of autotrophic nitrification in soil by adding readily degradable carbon. J. Soils Sediments 2020, 20, 81–90. [Google Scholar] [CrossRef]
- Liu, Y.; Shahbaz, M.; Fang, Y.; Li, B.; Wei, X.; Zhu, Z.; Lynn, T.M.; Lu, S.; Shibistova, O.; Wu, J.; et al. Stoichiometric theory shapes enzyme kinetics in paddy bulk soil but not in rhizosphere soil. Land Degrad. Dev. 2022, 33, 246–256. [Google Scholar] [CrossRef]
- Boddy, E.; Hill, P.W.; Farrar, J.; Jones, D.L. Fast turnover of low molecular weight components of the dissolved organic carbon pool of temperate grassland field soils. Soil Biol. Biochem. 2007, 39, 827–835. [Google Scholar] [CrossRef]
- Chen, L.; Xin, X.; Li, J.; Han, C.; Xiong, W.; Luo, Y.; Sun, R.; Zhang, J. Phosphorus Fertilization Boosts Mineral-Associated Soil Organic Carbon Formation Associated with Phagotrophic Protists. Microb. Ecol. 2023, 86, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Y.; Gao, C.; Zhang, S.; Liu, L.; Wu, L.; Li, Y.; Ding, X. Mineral-microbial interactions in nine-year organic fertilization field experiment: A mechanism for carbon storage in saline-alkaline paddy soil. Plant Soil 2023, 489, 465–481. [Google Scholar] [CrossRef]
- Mao, R.; Zhang, X.-H.; Li, S.-Y.; Song, C.-C. Long-term phosphorus addition enhances the biodegradability of dissolved organic carbon in a nitrogen-limited temperate freshwater wetland. Sci. Total Environ. 2017, 605–606, 332–336. [Google Scholar] [CrossRef]
- Meyer, N.; Welp, G.; Bornemann, L.; Amelung, W. Microbial nitrogen mining affects spatio-temporal patterns of substrate-induced respiration during seven years of bare fallow. Soil Biol. Biochem. 2017, 104, 175–184. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Fierer, N. Microbial Nitrogen Limitation Increases Decomposition. Ecology 2007, 88, 2105–2113. [Google Scholar] [CrossRef]
- Li, J.H.; Cheng, B.H.; Zhang, R.; Li, W.J.; Shi, X.M.; Han, Y.W.; Ye, L.F.; Ostle, N.J.; Bardgett, R.D. Nitrogen and phosphorus additions accelerate decomposition of slow carbon pool and lower total soil organic carbon pool in alpine meadows. Land Degrad. Dev. 2021, 32, 1761–1772. [Google Scholar] [CrossRef]
- Fang, X.-M.; Zhang, X.-L.; Chen, F.-S.; Zong, Y.-Y.; Bu, W.-S.; Wan, S.-Z.; Luo, Y.; Wang, H. Phosphorus addition alters the response of soil organic carbon decomposition to nitrogen deposition in a subtropical forest. Soil Biol. Biochem. 2019, 133, 119–128. [Google Scholar] [CrossRef]
- Zhang, F.; Niu, J.; Zhang, W.; Chen, X.; Li, C.; Yuan, L.; Xie, J. Potassium nutrition of crops under varied regimes of nitrogen supply. Plant Soil 2010, 335, 21–34. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.; Xu, M.; Li, S.; An, T.; Pei, J.; Xiao, J.; Xie, H.; Wang, J. Characteristics of differently stabilised soil organic carbon fractions in relation to long-term fertilisation in Brown Earth of Northeast China. Sci. Total Environ. 2016, 572, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Purakayastha, T.J.; Das, D.; Ahmed, N.; Kumar, R.; Biswas, S.; Walia, S.S.; Singh, R.; Shukla, V.K.; Yadava, M.S.; et al. Long-term fertilization and manuring with different organics alter stability of carbon in colloidal organo-mineral fraction in soils of varying clay mineralogy. Sci. Total Environ. 2019, 684, 682–693. [Google Scholar] [CrossRef]
- Gan, H.Y.; Schöning, I.; Schall, P.; Ammer, C.; Schrumpf, M. Soil Organic Matter Mineralization as Driven by Nutrient Stoichiometry in Soils Under Differently Managed Forest Stands. Front. For. Glob. Change 2020, 3, 99. [Google Scholar] [CrossRef]
- Tipping, E.; Somerville, C.J.; Luster, J. The C:N:P:S stoichiometry of soil organic matter. Biogeochemistry 2016, 130, 117–131. [Google Scholar] [CrossRef]
- Zhao, X.; Nafziger, E.; Pittelkow, C. Nitrogen rate strategies for reducing yield-scaled nitrous oxide emissions in maize. Environ. Res. Lett. 2017, 12, 124006. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, W. Combination of nitrogen and phosphorus fertilization enhance ecosystem carbon sequestration in a nitrogen-limited temperate plantation of Northern China. For. Ecol. Manag. 2015, 341, 59–66. [Google Scholar] [CrossRef]
- Voltr, V.; Menšík, L.; Hlisnikovský, L.; Hruška, M.; Pokorný, E.; Pospíšilová, L. The Soil Organic Matter in Connection with Soil Properties and Soil Inputs. Agronomy 2021, 11, 779. [Google Scholar] [CrossRef]
- Farahani, E.; Emami, H.; Fotovat, A.; Khorassani, R. Effect of different K:Na ratios in soil on dispersive charge, cation exchange and zeta potential. Eur. J. Soil Sci. 2019, 70, 311–320. [Google Scholar] [CrossRef]
- Xinghua, S.; Aibin, W. Organic carbon and black carbon with fertilization in paddy and upland soils. J. Zhejiang AF Univ. 2014, 31, 554. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Xia, S.; Zhang, R.; Zhang, R.; Chen, P.; Pan, J.; Liu, Y. K fertilizer alleviates N2O emissions by regulating the abundance of nitrifying and denitrifying microbial communities in the soil-plant system. J. Environ. Manag. 2021, 291, 112579. [Google Scholar] [CrossRef] [PubMed]



| Crop (No of Plot), | Age: Year | FP | FS | FZ | FP | FS | FZ | FP | FS | FZ | Fertilizer Used in FP Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (kg ha−1) | P2O5 (kg ha−1) | K2O (kg ha−1) | |||||||||
| Banana (3) | 2 3 5 | 14 14 14 | 3 2 2 | 0 0 0 | 0 0 0 | 1 1 1 | 0 0 0 | 0 0 0 | 2 2 2 | 0 0 0 | 46-0-0 46-0-0 46-0-0 |
| Cabbage (2) | 20 20 | 575.5 182.8 | 93.8 62.5 | 0 0 | 0 182.8 | 31.3 31.3 | 0 0 | 0 295.3 | 62.5 62.5 | 0 0 | 46-0-0 13-13-21 |
| Garlic (2) | 17 41 | 77.5 135 | 62.5 93.8 | 0 0 | 92.5 135 | 31.3 31.3 | 0 0 | 112.5 135.0 | 31.3 31.3 | 0 0 | 18-24-24,13-13-21 18-18-18 |
| Litchi (1) | 20 | 211 | 175 | 0 | 211 | 22 | 0 | 211 | 44.0 | 0 | 15-15-15 |
| Maize (2) | 20 20 | 168.8 115 | 31.3 31.3 | 0 0 | 75 0 | 18.8 62.5 | 0 0 | 75 0 | 39 39 | 0 0 | 46-0-0,15-15-15 46-0-0 |
| Mango (2) | 20 20 | 575 163 | 47 47 | 0 0 | 163 0 | 16 63 | 0 0 | 0 263 | 39 39 | 0 0 | 46-0-0,16-20-0 46-0-0,0-0-60 |
| Rice (2) | 36 36 | 80 230 | 37.5 37.5 | 0 0 | 100 0 | 0 0 | 0 0 | 0 0 | 0 0 | 0 0 | 16-20-0 46-0-0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aumtong, S.; Foungyen, P.; Kanchai, K.; Chuephudee, T.; Chotamonsak, C.; Lapyai, D. Impact of Reduced Nitrogen Inputs on Soil Organic Carbon and Nutrient Dynamics in Arable Soil, Northern Thailand: Short-Term Evaluation. Agronomy 2024, 14, 2587. https://doi.org/10.3390/agronomy14112587
Aumtong S, Foungyen P, Kanchai K, Chuephudee T, Chotamonsak C, Lapyai D. Impact of Reduced Nitrogen Inputs on Soil Organic Carbon and Nutrient Dynamics in Arable Soil, Northern Thailand: Short-Term Evaluation. Agronomy. 2024; 14(11):2587. https://doi.org/10.3390/agronomy14112587
Chicago/Turabian StyleAumtong, Suphathida, Phatchanuch Foungyen, Kanokorn Kanchai, Thoranin Chuephudee, Chakrit Chotamonsak, and Duangnapha Lapyai. 2024. "Impact of Reduced Nitrogen Inputs on Soil Organic Carbon and Nutrient Dynamics in Arable Soil, Northern Thailand: Short-Term Evaluation" Agronomy 14, no. 11: 2587. https://doi.org/10.3390/agronomy14112587
APA StyleAumtong, S., Foungyen, P., Kanchai, K., Chuephudee, T., Chotamonsak, C., & Lapyai, D. (2024). Impact of Reduced Nitrogen Inputs on Soil Organic Carbon and Nutrient Dynamics in Arable Soil, Northern Thailand: Short-Term Evaluation. Agronomy, 14(11), 2587. https://doi.org/10.3390/agronomy14112587






