The Synergistic Effects of Different Phosphorus Sources: Ferralsols Promoted Soil Phosphorus Transformation and Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
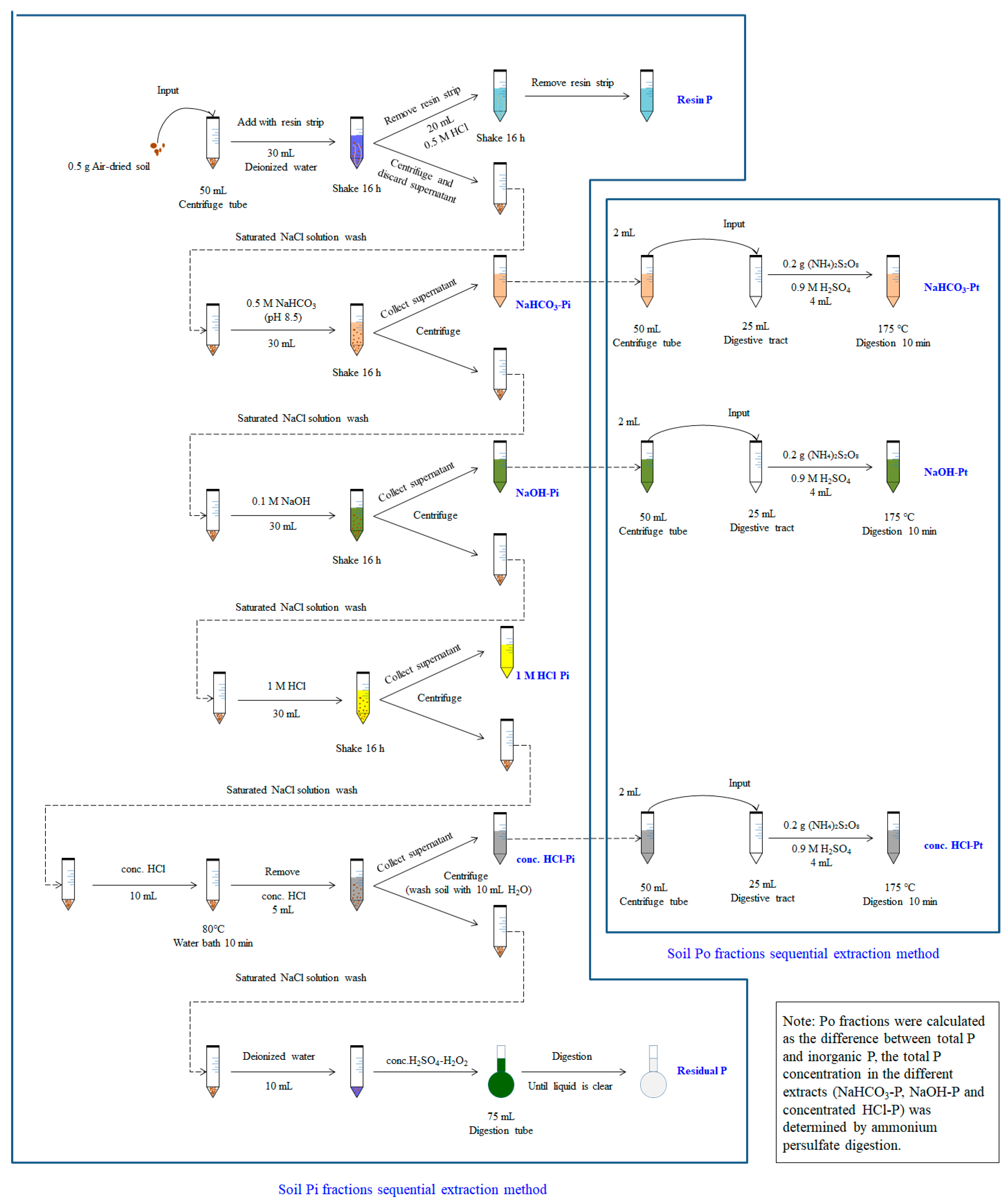
2.2. Sample Collection and Analysis
2.3. Data Processing and Statistical Analysis
3. Results
3.1. P Uptake of Maize
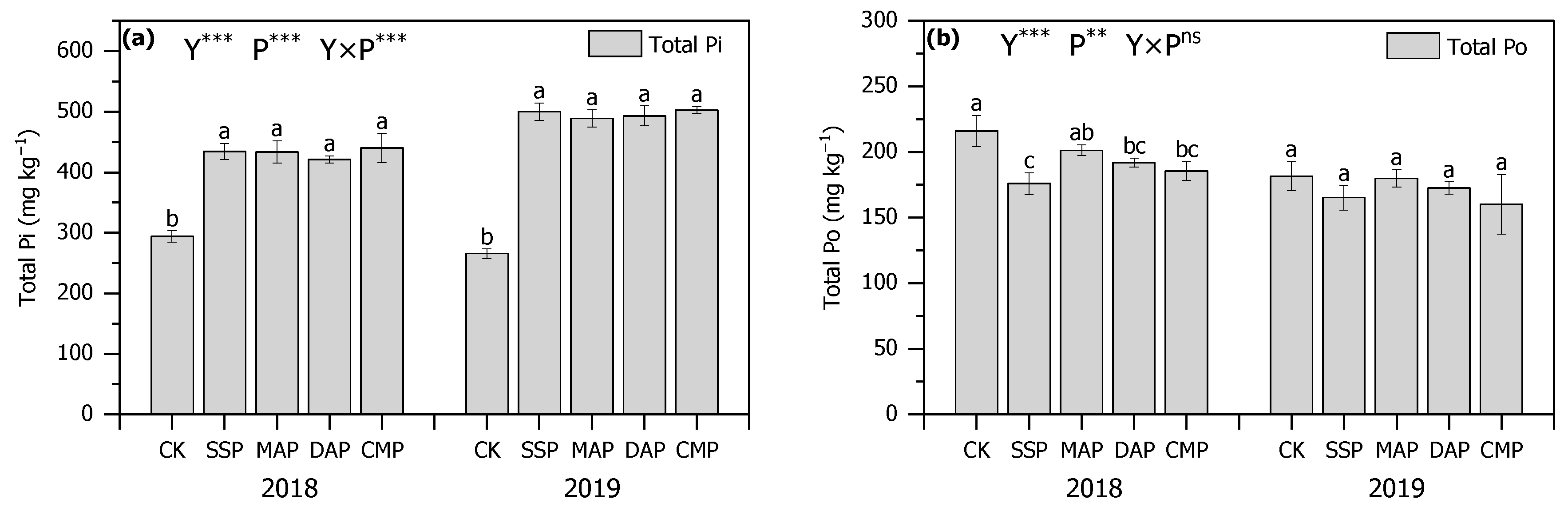
3.2. Olsen-P, Total P, and P Activation Coefficient of Soil
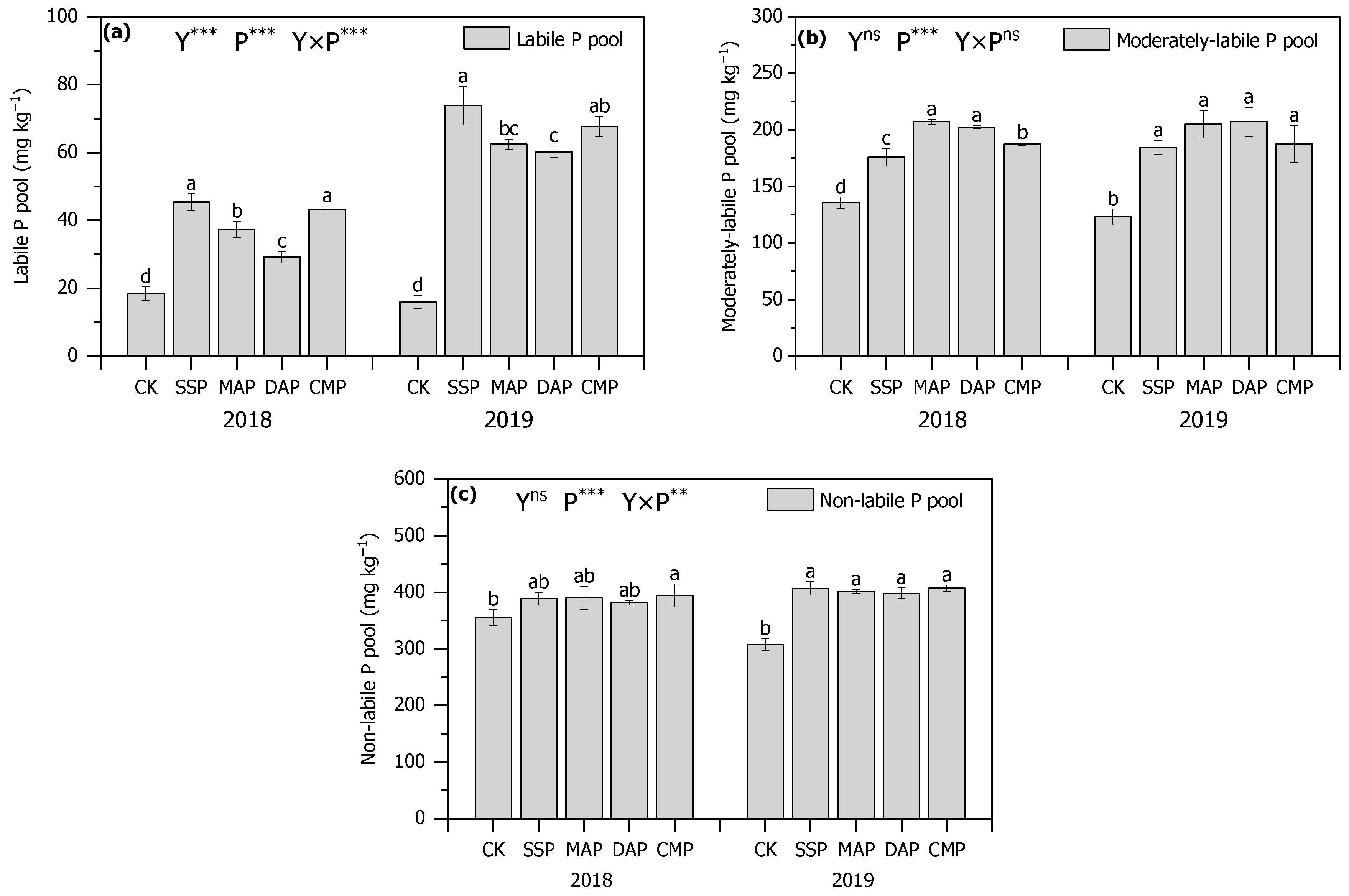
3.3. P Fractions of Soil
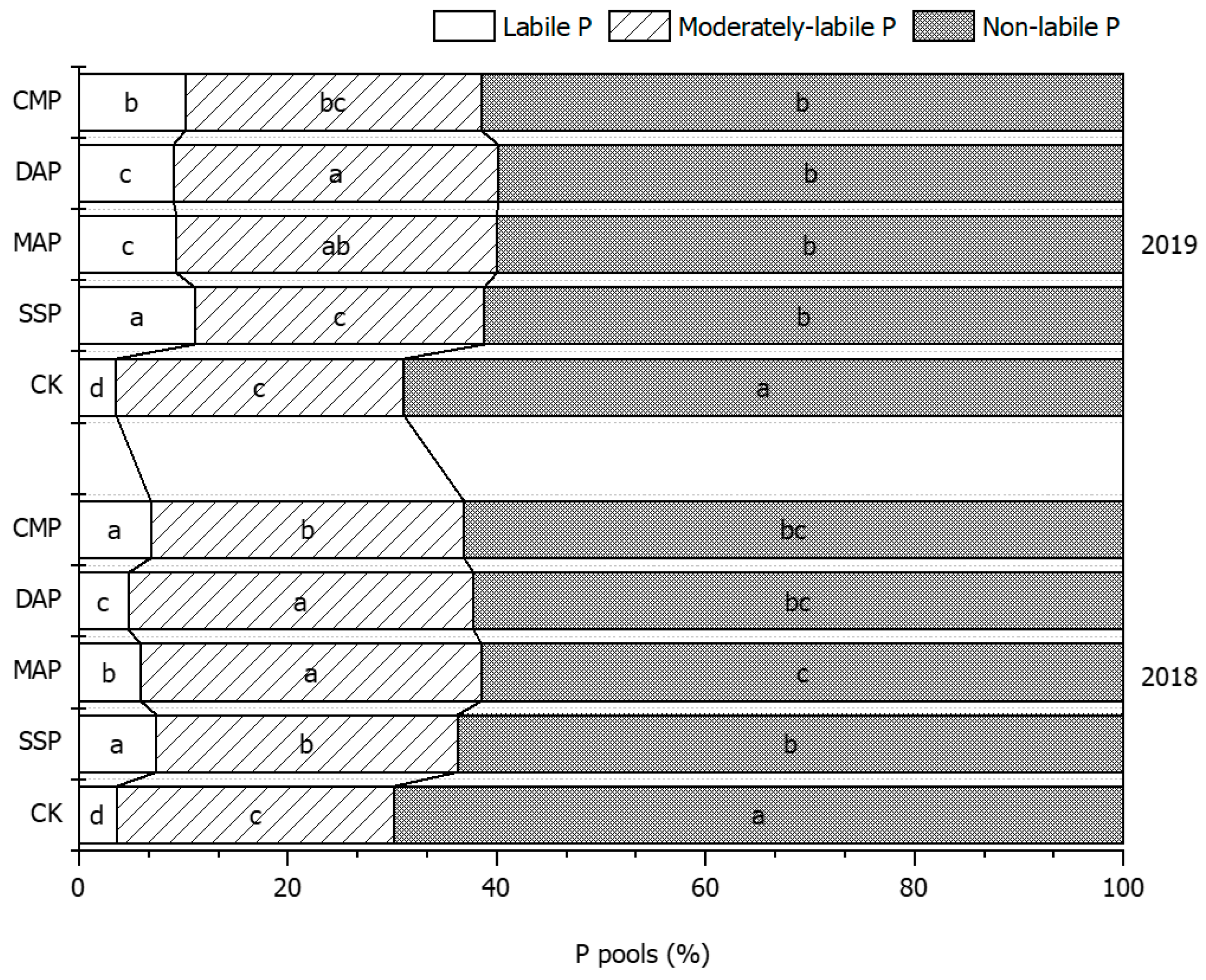
3.4. P Pool Composition of Soil
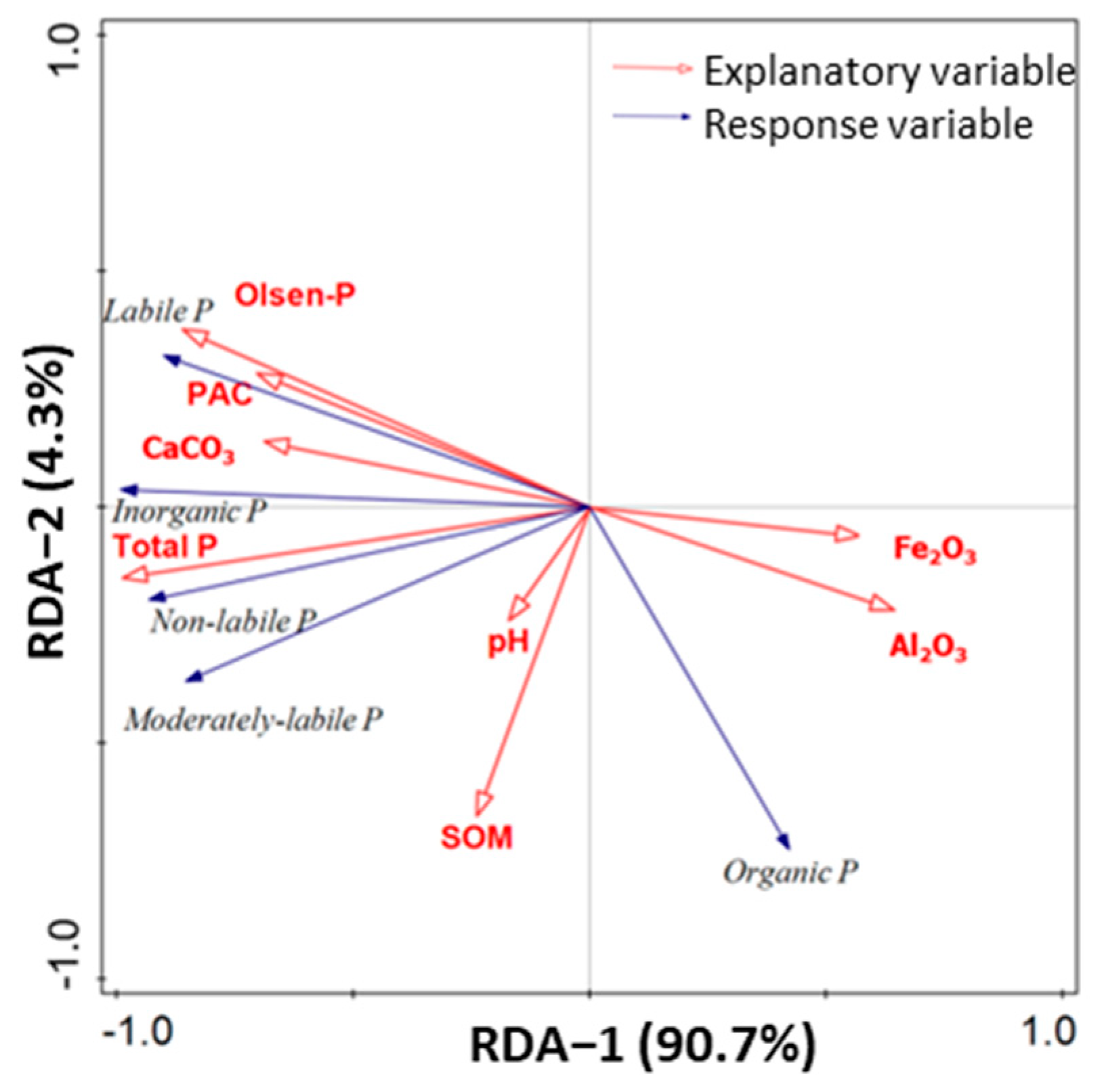
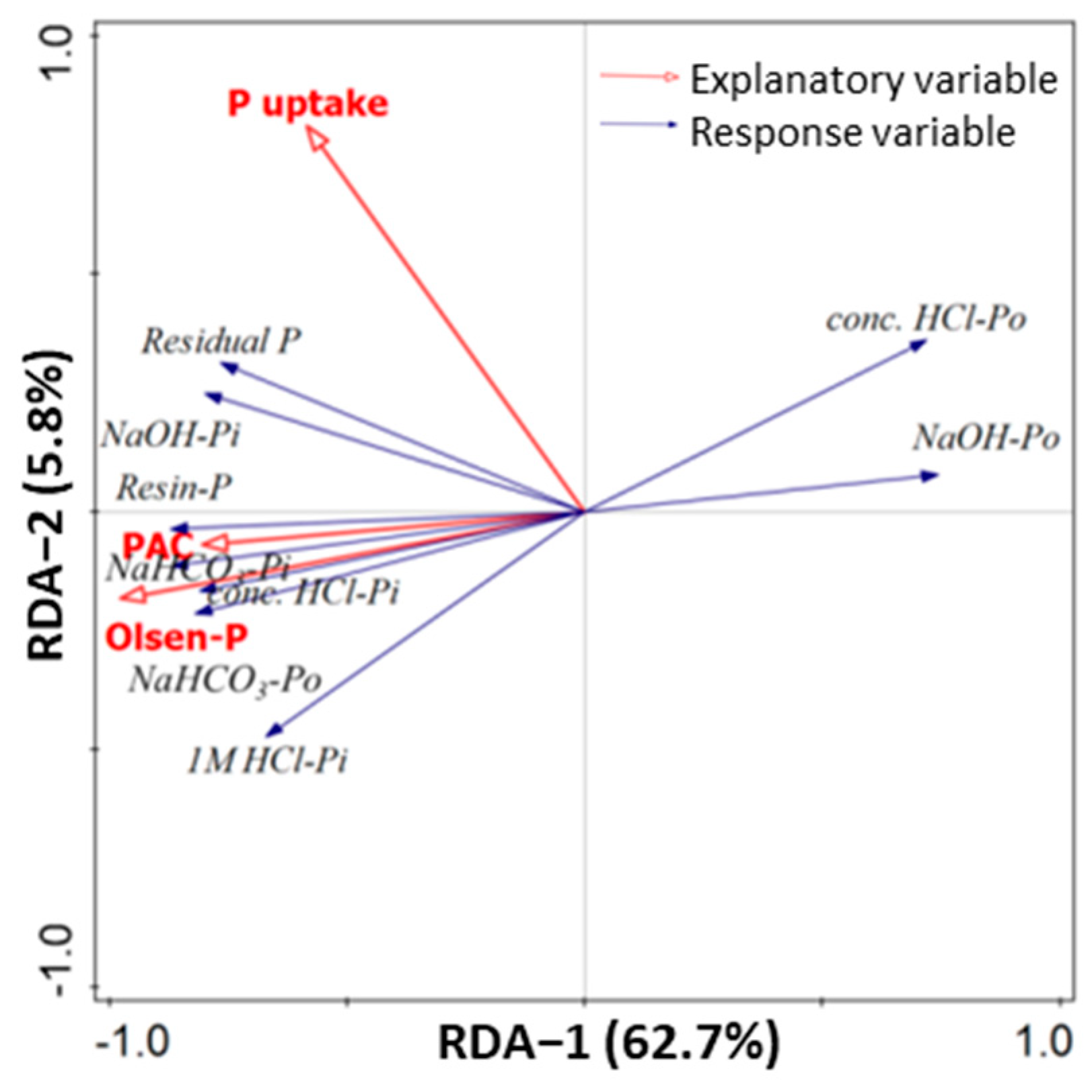
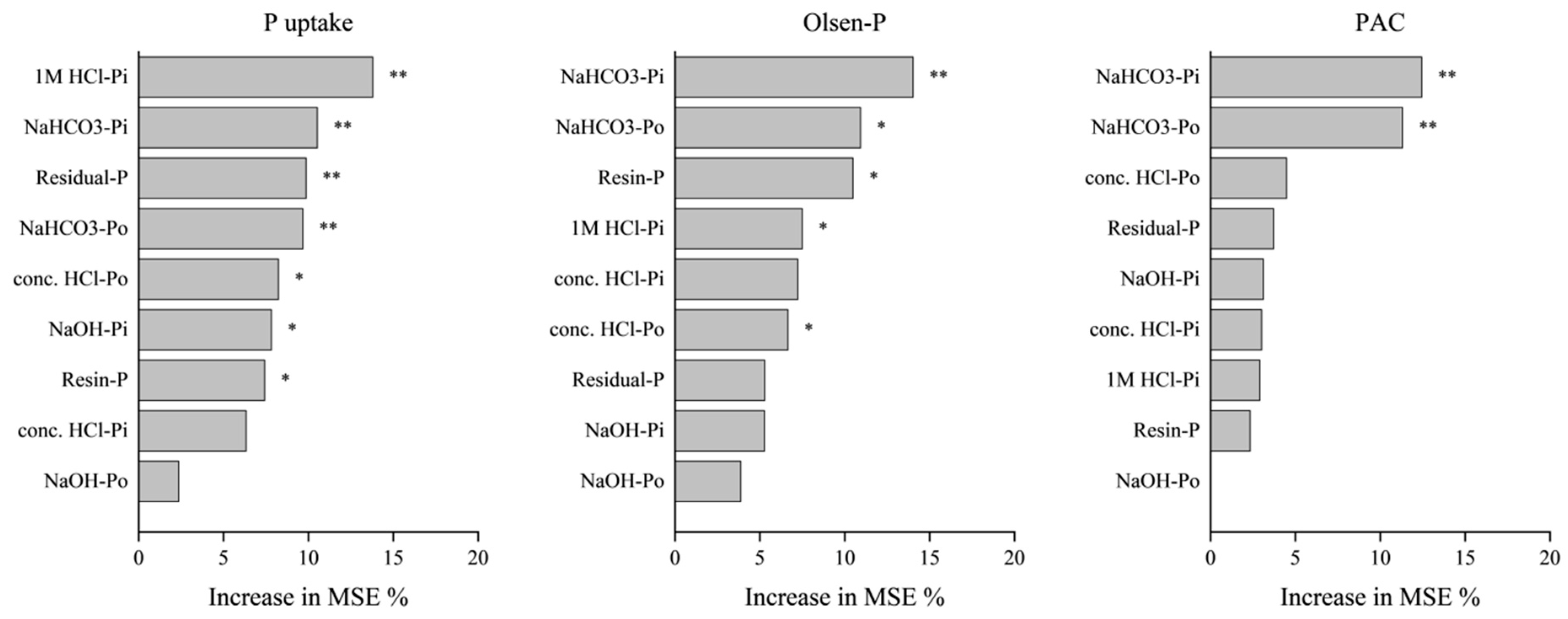
3.5. Relationships of Soil P Fractions with Crop P Uptake and Soil Chemical Factors
4. Discussion
4.1. Soil Available P and Crop P Uptake
4.2. Soil P Fractions and P Bioavailability
4.3. Relationships between Soil Available P and Soil P Fractions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferrol, N.; Azcón–Aguilar, C.; Pérez–Tienda, J. Arbuscular mycorrhizas as key players in sustainable plant phosphorus acquisition: An overview on the mechanisms involved. Plant Sci. 2019, 280, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Zhang, C.; Li, H.; Lambers, H.; Zhang, F. Changes in soil phosphorus fractions following sole cropped and intercropped maize and faba bean grown on calcareous soil. Plant Soil 2020, 448, 587–601. [Google Scholar] [CrossRef]
- Quesada, C.A.; Liovd, J.; Andeson, L.O.; Fyllas, N.M.; Schwarz, M.; Czimczik, C.I. Soils of Amazonia with particular reference to the rain for sites. Biogeosciences 2011, 6, 1415–1440. [Google Scholar] [CrossRef]
- Nedelciu, C.E.; Ragnarsdottir, K.V.; Schlyter, P.; Stjernquist, I. Global phosphorus supply chain dynamics: Assessing regional impact to 2050. Glob. Food Secur. 2020, 26, 100426. [Google Scholar] [CrossRef]
- Tian, X.; Engel, B.A.; Qian, H.; Hua, E.; Sun, S.; Wang, Y. Will reaching the maximum achievable yield potential meet future global food demand? J. Clean. Prod. 2021, 294, 126285. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.; Gao, R.; Wei, H.; Chen, A.; Li, Y. Effects of long-term phosphorus fertilization and straw incorporation on phosphorus fractions in subtropical paddy soil. J. Integr. Agric. 2015, 14, 365–373. [Google Scholar] [CrossRef]
- Chen, M.; Graedel, T.E. A half-century of global phosphorus flows, stocks, production, consumption, recycling, and environmental impacts. Glob. Environ. Chang. 2016, 36, 139–152. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Zhang, H.; Yin, D.; Dari, B.; Xu, J. Soil phosphorus storage capacity as affected by repeated phosphorus addition in an Ultisol. Commun. Soil Sci. Plant Anal. 2020, 51, 1960–1968. [Google Scholar] [CrossRef]
- Su, L.; Zhao, H.; Hou, X.; Chen, Y.; Xiao, J.; Zheng, Y.; Tang, L. Activation of phosphorus pools in red soil by maize and soybean intercropping and its response to phosphorus fertilizer. Chin. J. Eco-Agric. 2023, 31, 558–566. [Google Scholar] [CrossRef]
- Yan, X.; Yang, W.; Chen, X.; Wang, M.; Wang, W.; Ye, D.; Wu, L. Soil phosphorus pools, bioavailability and environmental risk in response to the phosphorus supply in the red soil of southern China. Int. J. Environ. Res. 2020, 17, 7384. [Google Scholar] [CrossRef]
- Wang, S.; Xia, P. The use efficiency and slow-release rate of various P fertilizers with different application rates on calcareous soil. Chin. J. Soil Sci. 2008, 39, 1363–1368. [Google Scholar] [CrossRef]
- Li, H. Effects of Different Phosphorus Fertilizers on Crop Yield and Nutrient Utilization in Rice Rape Rotation System; Huazhong Agricultural University: Wuhan, China, 2020. [Google Scholar]
- Wang, Y.; Cai, Z.; Feng, G. Effects of different phosphorus application techniques on phosphorus availability in a rape system in a red soil. Acta Pedol. Sin. 2023, 60, 235–246. [Google Scholar] [CrossRef]
- Zhou, L.; Su, L.; Zhang, L.; Zhang, L.; Zheng, Y.; Tang, L. Effect of different types of phosphate fertilizer on phosphorus absorption and desorption in acidic red soil of southwest China. Sustainability 2022, 14, 9973. [Google Scholar] [CrossRef]
- Khan, A.; Lu, G.; Zhang, H.; Wang, R.; Lv, F.; Xu, J.; Yang, X.; Zhang, S. Land use changes impact distribution of phosphorus in deep soil profile. J. Soil Sci. Plant Nutr. 2019, 19, 565–573. [Google Scholar] [CrossRef]
- Li, C.; Zhang, P.; Zhang, J.; Zhu, P.; Wang, L. Forms, transformations, and availability of phosphorus after 32 years of manure and mineral fertilization in a Mollisol under continuous maize cropping. Arch. Agron. Soil Sci. 2020, 67, 1256–1271. [Google Scholar] [CrossRef]
- Beck, M.A.; Sanchez, P.A. Soil phosphorus fraction dynamics during 18 years of cultivation on a typic paleudult. Soil Sci. 1994, 34, 1424–1431. [Google Scholar] [CrossRef]
- Audette, Y.; O’Halloran, I.P.; Evans, L.J.; Martin, R.C.; Voroney, R.P. Kinetics of phosphorus forms applied as inorganic and organic amendments to a calcareous soil II: Effects of plant growth on plant available and uptake phosphorus. Geoderma 2016, 279, 70–76. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B. Method to measure microbial phosphate in soils. Soil Biol. Biochem. 1982, 14, 377–385. [Google Scholar] [CrossRef]
- Nakayama, Y.; Wade, J.; Margenot, A.J. Does soil phosphomonoesterase activity reflect phosphorus pools estimated by Hedley phosphorus fractionation? Geoderma 2021, 401, 115279. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Zhou, J.; Bing, H.; Shenyan, P. Air-drying changes the distribution of Hedley phosphorus pools in forest soils. Pedosphere 2020, 30, 272–284. [Google Scholar] [CrossRef]
- Liao, D.; Zhang, C.; Lambers, H.; Zhang, F. Changes in soil phosphorus fractions in response to long-term phosphate fertilization under sole cropping and intercropping of maize and faba bean on a calcareous soil. Plant Soil 2021, 463, 589–600. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, J.; Zhang, H.; Schroder, J.; He, Y. Phosphorus availability and sorption as affected by long-term fertilization. Agron. J. 2014, 106, 1583–1592. [Google Scholar] [CrossRef]
- Johnson, C.M.; Ulrich, A. Analytical methods for use in plant analysis. Calif. Agric. Exp. Stn. Bull. 1959, 766, 25–78. [Google Scholar]
- Soil Survey Staff. Soil Survey Laboratory Methods Manual. Soil Survey Investigations Report 42. 2004. Available online: https://www.researchgate.net/publication/255947599_Soil_Survey_Laboratory_Method_Manual (accessed on 5 June 2022).
- Olsen, R.S.; Sommer, L.E. Phosphorus. In Methods of Soil Analysis (Part 2); Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Schollenberger, C.J. Determination of soil organic matter. Soil Sci. 1931, 31, 483–486. [Google Scholar] [CrossRef]
- Fu, Q.L.; Zhang, Y.H.; Huang, W.; Hu, H.Q.; Chen, D.Q.; Yang, C. Remaining dynamics of Cry1Ab proteins from transgenic Bt corn in soil. J. Food Agric. Environ. 2007, 10, 294–298. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Bavaresco, J.; Barrón, V.; Torrent, J.; Bayer, C. Phosphorus adsorption and desorption in undisturbed samples from subtropical soils under conventional tillage or no-tillage. J. Plant Nutr. Soil Sci. 2016, 179, 198–205. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of Available P by Sequential Extraction; CRC Press: Boca Raton, FL, USA, 1993; Volume 7, pp. 5–229. [Google Scholar]
- Sun, B.; Cui, Q.; Yun, G.; Hopkins, D.W. Soil phosphorus and relationship to phosphorus balance under long-term fertilization. Plant Soil Environ. 2018, 64, 214–220. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Wang, Y. Intercropping regulation of soil phosphorus composition and microbially-driven dynamics facilitates maize phosphorus uptake and productivity improvement. Field Crops Res. 2022, 287, 108666. [Google Scholar] [CrossRef]
- Bai, Z.; Li, H.; Yang, X.; Zhou, B.; Shi, X.; Wang, B.; Li, D.; Shen, J.; Chen, Q.; Qin, W.; et al. The critical soil P levels for crop yield, soil fertility and environmental safety in different soil types. Plant Soil 2013, 372, 39. [Google Scholar] [CrossRef]
- Mahmood, M.; Tian, Y.; Ma, Q.; Ahmed, W.; Mehmood, S.; Hui, X.; Wang, Z. Changes in phosphorus fractions and its availability status in relation to long term P fertilization in loess plateau of China. Agronomy 2020, 10, 1818. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, S.; Zhu, P.; Huang, S.; Wang, B.; Zhao, L.; Xu, M. Characterizing differences in the phosphorus activation coefficient of three typical cropland soils and the influencing factors under long-term fertilization. PLoS ONE 2017, 12, e0176437. [Google Scholar] [CrossRef] [PubMed]
- Milić, S.; Ninkov, J.; Zeremski, T.; Latković, D.; Šeremešić, S.; Radovanović, V.; Žarković, B. Soil fertility and phosphorus fractions in a calcareous chernozem after a long-term field experiment. Geoderma 2019, 339, 9–19. [Google Scholar] [CrossRef]
- Li, D.; Wang, B.; Huang, J.; Zhang, Y.; Xu, M.; Zhang, S.; Zhang, H. Change of phosphorus in red soil and its effect to grain yield under longterm different fertilizations. Sci. Agric. Sin. 2019, 52, 3830–3841. [Google Scholar] [CrossRef]
- Murrmann, R.P.; Peech, M. Relative significance of labile and crystalline phosphates in soil. Soil Sci. 1969, 107, 249–255. [Google Scholar] [CrossRef]
- Spohn, M. Increasing the organic carbon stocks in mineral soils sequesters large amounts of phosphorus. Glob. Chang. Biol. 2020, 26, 4169–4177. [Google Scholar] [CrossRef]
- Hou, E.; Wen, D.; Kuang, Y.; Cong, J.; Chen, C.; He, X.; Heenan, M.; Lu, H.; Zhang, Y. Soil pH predominantly controls the forms of organic phosphorus in topsoils under natural broadleaved forests along a 2500 km latitudinal gradient. Geoderma 2018, 315, 65–74. [Google Scholar] [CrossRef]
- Redel, Y.; Cartes, P.; Demanet, R.; Velásquez, G.; Poblete-Grant, P.; Bol, R.; Mora, M. Assessment of phosphorus status influenced by Al and Fe compounds in volcanic grassland soils. J. Soil Sci. Plant Nutr. 2016, 16, 490–506. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Zhang, N.; Qin, Z.; Jin, Y.; Zhu, P.; Peng, C.; Colinet, G.; Zhang, S. Phosphorus adsorption and desorption characteristics as affected by long-term phosphorus application in black soil. J. Plant Nutr. Fertil. 2022, 28, 1569–1581. [Google Scholar] [CrossRef]
- Kang Kang, J.; Hesterberg, D.; Osmond, D. Soil organic matter effects on phosphorus sorption: A path analysis. Soil Sci. Soc. Am. J. 2009, 73, 360–366. [Google Scholar] [CrossRef]
- Ye, D.; Li, T.; Liu, D.; Zhang, X.; Zheng, Z. P accumulation and physiological responses to different high P regimes in Polygonum hydropiper for understanding a P-phytoremediation strategy. Sci. Rep. 2015, 5, 17835. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jones, C.; Kim, H.J.; Jacobsen, J.S. Soil inorganic phosphorus fractions and Olsen-P in phosphorus-responsive calcareous soils: Effects of fertilizer amount and incubation time. Commun. Soil Sci. Plant Anal. 2002, 33, 855–871. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Mu, H.; Dang, T. Inorganic phosphorus fractions and phosphorus availability in a calcareous soil receiving 21-year superphosphate application. Pedosphere 2010, 20, 304–310. [Google Scholar] [CrossRef]
- Shen, Y.; Duan, Y.; McLaughlin, N.; Huang, S.; Guo, D.; Xu, M. Phosphorus desorption from calcareous soils with different initial Olsen-P levels and relation to phosphate fractions. J. Soils Sediments 2019, 19, 2997–3007. [Google Scholar] [CrossRef]
- Shen, J.; Li, R.; Zhang, F.; Fan, J.; Tang, C.; Rengel, Z. Crop yield, soil fertility and phosphorus fractions in response to long-term fertilization under the rice monoculture system on a calcareous soil. Field Crops Res. 2004, 86, 225–238. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Gao, H.; Zhang, R.; Yang, L.; Guo, Y.; Li, H.; Awasthi, M.; Li, G. Long-term cover crops improved soil phosphorus availability in a rain-fed apple orchard. Chemosphere 2021, 275, 130093. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Zhao, T.; Thu, N.; Zhao, H.; Zheng, Y.; Tang, L. The Synergistic Effects of Different Phosphorus Sources: Ferralsols Promoted Soil Phosphorus Transformation and Accumulation. Agronomy 2024, 14, 2372. https://doi.org/10.3390/agronomy14102372
Zhou L, Zhao T, Thu N, Zhao H, Zheng Y, Tang L. The Synergistic Effects of Different Phosphorus Sources: Ferralsols Promoted Soil Phosphorus Transformation and Accumulation. Agronomy. 2024; 14(10):2372. https://doi.org/10.3390/agronomy14102372
Chicago/Turabian StyleZhou, Long, Tilei Zhao, Nyeinnyein Thu, Hongmin Zhao, Yi Zheng, and Li Tang. 2024. "The Synergistic Effects of Different Phosphorus Sources: Ferralsols Promoted Soil Phosphorus Transformation and Accumulation" Agronomy 14, no. 10: 2372. https://doi.org/10.3390/agronomy14102372
APA StyleZhou, L., Zhao, T., Thu, N., Zhao, H., Zheng, Y., & Tang, L. (2024). The Synergistic Effects of Different Phosphorus Sources: Ferralsols Promoted Soil Phosphorus Transformation and Accumulation. Agronomy, 14(10), 2372. https://doi.org/10.3390/agronomy14102372





