Multiple Herbicide Resistance in Annual Ryegrass (Lolium rigidum Gaudin) in the Southeastern Cropping Region of Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population and Seed Collection
2.2. Experimental Setup and Observations
2.3. Statistical Analyses
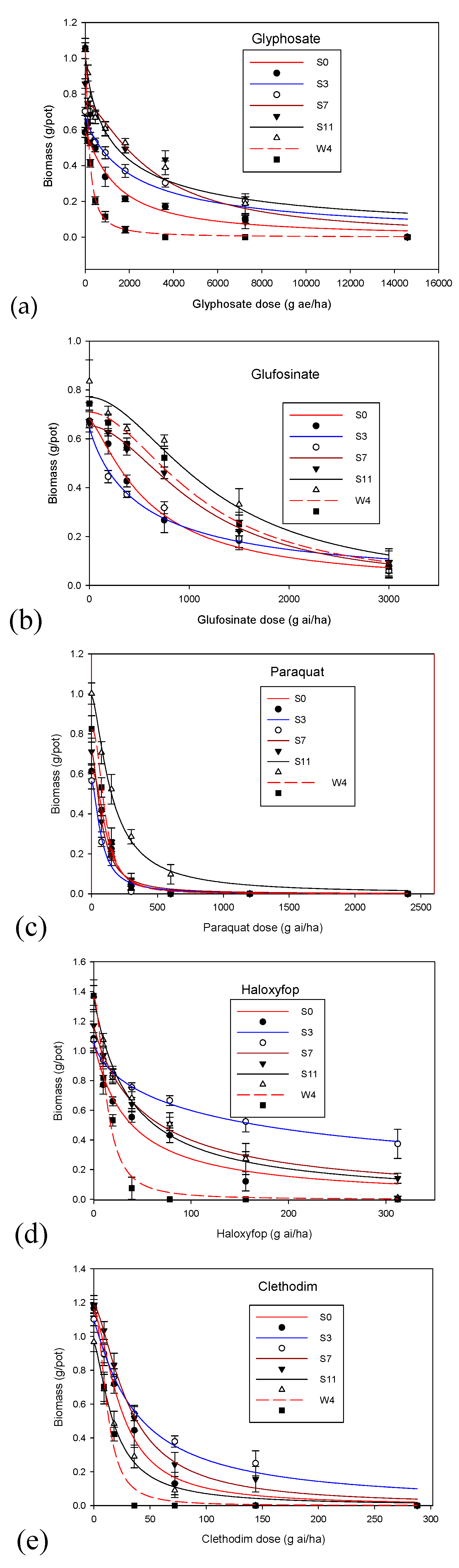
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Llewellyn, R.; Ronning, D.; Clarke, M.; Mayfield, A.; Walker, S.; Ouzman, J. Impact of Weeds in Australian Grain Production; Grains Research and Development Corporation: Canberra, Australia, 2016. [Google Scholar]
- Heap, I. The International Herbicide-Resistant Weed Database. 2024. Available online: http://www.weedscience.org (accessed on 12 July 2024).
- Pratley, J. Glyphosate resistance in annual ryegrass. In Proceedings of the 11th Annual Conference of the Grassland Society, Wagga Wagga, Australia, 10–11 July 1996. [Google Scholar]
- Powles, S.B.; Lorraine-Colwill, D.F.; Dellow, J.J.; Preston, C. Evolved resistance to glyphosate in rigid ryegrass (Lolium rigidum) in Australia. Weed Sci. 1998, 46, 604–607. [Google Scholar] [CrossRef]
- Thompson, M.; Mahajan, G.; Chauhan, B.S. Seed germination ecology of southeastern Australian rigid ryegrass (Lolium rigidum) populations. Weed Sci. 2021, 69, 454–460. [Google Scholar] [CrossRef]
- Thompson, M.; Chauhan, B.S. Changing seasonality of Lolium rigidum (annual ryegrass) in southeastern Australia. Front. Agron. 2022, 4, 897361. Available online: https://www.frontiersin.org/journals/agronomy/articles/10.3389/fagro.2022.897361/full (accessed on 12 July 2024). [CrossRef]
- GRDC. 2021. Available online: https://groundcover.grdc.com.au/weeds-pests-diseases/weeds/research-highlights-summer-adaption-of-annual-ryegrass (accessed on 13 July 2024).
- Broster, J.; Koetz, E.; Wu, H. Herbicide resistance levels in annual ryegrass (Lolium rigidum Gaud.) and wild oat (Avena spp.) in southwestern New South Wales. Plant Prot. Q. 2013, 28, 126–132. [Google Scholar]
- Owen, M.J.; Martinez, N.J.; Powles, S.B. Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt. Weed Res. 2014, 54, 314–324. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Walsh, M. Differential response of winter- and summer-emerging accessions of rigid ryegrass (Lolium rigidum Gaudin) to postemergence herbicides in Australia. Weed Technol. 2022, 36, 663–670. [Google Scholar] [CrossRef]
- Goggin, D.E.; Cawthray, G.R.; Busi, R. Pyroxasulfone metabolism in resistant Lolium rigidum: Is it all down to GST activity? J. Agric. Food Chem. 2024, 72, 3937–3948. [Google Scholar] [CrossRef] [PubMed]
- Rerkasem, K.; Stern, W.R.; Goodchild, N.A. Associated growth of wheat and annual ryegrass. 1. Effect of varying total density and proportion in mixtures of wheat and annual ryegrass. Aust. J. Agric. Res. 1980, 31, 649–658. [Google Scholar] [CrossRef]
- Borger, C.P.; Hashem, A. Evaluating the double knockdown technique: Sequence, application interval, and annual ryegrass growth stage. Aust. J. Agric. Res. 2007, 58, 265–271. [Google Scholar] [CrossRef]
- Kleemann, S.; Boutsalis, P.; Preston, C. A Strategic Approach to Managing Paraquat and Glyphosate Resistance. 2022. Available online: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2022/07/a-strategic-approach-to-managing-paraquat-and-glyphosate-resistance (accessed on 13 July 2024).
- Malmo, A.; Broster, J.C.; Walsh, M.J. Looking beyond glyphosate for site-specific fallow weed control in australian grain production. Agronomy 2023, 13, 1878. [Google Scholar] [CrossRef]
- Gill, G.S. Why annual ryegrass is a problem in Australian agriculture. Plant Prot. Q. 1996, 11, 193–194. [Google Scholar]
- Lemerle, D.; Verbeek, B.; Coombes, N. Losses in grain yield of winter crops from Lolium rigidum competition depend on crop species, cultivar and season. Weed Res. 1995, 35, 503–509. [Google Scholar] [CrossRef]
- Anthimidou, E.; Ntoanidou, S.; Madesis, P.; Eleftherohorinos, I. Mechanisms of Lolium rigidum multiple resistance to ALS-and ACCase-inhibiting herbicides and their impact on plant fitness. Pestic. Biochem. Phys. 2020, 164, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.; Schneider, T.; Agostinetto, D.; Bianchi, M.A. Geographic distribution of ryegrass resistent to the clethodim herbicide in Rio Grande do Sul1. Planta Daninha 2016, 34, 365–376. [Google Scholar] [CrossRef]
- Neve, P.; Powles, S.B. Recurrent selection with reduced herbicide rates results in the rapid evolution of herbicide resistance in Lolium rigidum. Theor. Appl. Genet. 2005, 110, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yu, Q.; Beffa, R.; González, S.; Maiwald, F.; Wang, J.; Powles, S.B. Cytochrome P450 CYP81A10v7 in Lolium rigidum confers metabolic resistance to herbicides across at least five modes of action. Plant J. 2021, 105, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Norsworthy, J.K.; Ward, S.M.; Shaw, D.R.; Llewellyn, R.S.; Nichols, R.L.; Webster, T.M.; Bradley, K.W.; Frisvold, G.; Powles, S.B.; Burgos, E.R.; et al. Reducing the risks of herbicide resistance: Best management practices and recommendations. Weed Sci. 2012, 60, 31–62. [Google Scholar] [CrossRef]
- Aulakh, J.S.; Jhala, A.J. Comparison of glufosinate-based herbicide programs for broad-spectrum weed control in glufosinate-resistant soybean. Weed Technol. 2015, 29, 419–430. [Google Scholar] [CrossRef]
- Ganie, Z.A.; Stratman, G.; Jhala, A.J. Response of selected glyphosate-resistant broadleaved weeds to premix of fluthiacet-methyl and mesotrione (Solstice™) applied at two growth stages. Can. J. Plant Sci. 2015, 95, 861–869. [Google Scholar] [CrossRef]
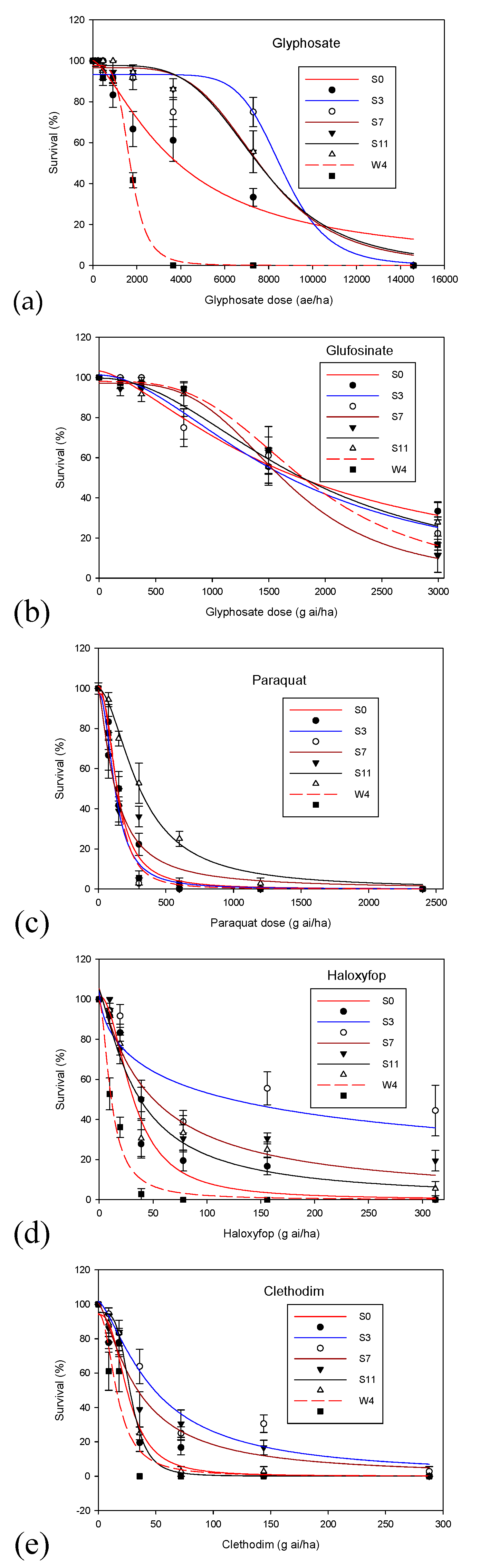
| Herbicide | Population | a ± Standard Error | b ± Standard Error | LD50 ± Standard Error | R2 | Resistance Factor |
|---|---|---|---|---|---|---|
| Glyphosate | S0 | 100 ± 5.0 | 1.4 ± 0.3 | 3846 ± 641 | 0.96 | 2.25 |
| S3 | 93 ± 3.4 | 8.4 ± 1.4 | 8225 ± 2032 | 0.94 | 4.83 | |
| S7 | 97 ± 1.8 | 4.5 ± 1.2 | 7656 ± 329 | 0.99 | 4.50 | |
| S11 | 98 ± 1.8 | 4.2 ± 1.0 | 7593 ± 334 | 0.99 | 4.46 | |
| W4 | 98 ± 1.5 | 4.6 ± 0.9 | 1702 ± 53 | 0.99 | - | |
| Glufosinate | S0 | 103 ± 4.2 | 1.5 ± 0.3 | 1703 ± 197 | 0.98 | 1.04 |
| S3 | 101 ± 4.6 | 1.8 ± 0.4 | 1637 ± 195 | 0.97 | - | |
| S7 | 97 ± 1.7 | 3.6 ± 0.5 | 1641 ± 60 | 0.99 | 1.00 | |
| S11 | 100 ± 3.4 | 2.1 ± 0.4 | 1804 ± 159 | 0.98 | 1.10 | |
| W4 | 98 ± 0.6 | 3.3 ± 0.1 | 1828 ± 24 | 0.99 | 1.12 | |
| Paraquat | S0 | 101 ± 4.6 | 2.2 ± 0.3 | 139 ± 12 | 0.99 | 1.10 |
| S3 | 97 ± 8.1 | 2.2 ± 0.6 | 126 ± 19 | 0.97 | - | |
| S7 | 99 ± 9.0 | 1.4 ± 0.4 | 126 ± 28 | 0.96 | 1.00 | |
| S11 | 100 ± 3.2 | 1.8 ± 0.2 | 307 ± 23 | 0.99 | 2.40 | |
| W4 | 98 ± 4.5 | 2.7 ± 0.40 | 141 ± 11 | 0.99 | 1.10 | |
| Haloxyfop | S0 | 102 ± 9.4 | 2.1 ± 0.6 | 31 ± 6 | 0.96 | 2.80 |
| S3 | 104 ± 17 | 0.6 ± 0.3 | 107 ± 84 | 0.73 | 9.70 | |
| S7 | 105 ± 9.5 | 1.1 ± 0.2 | 46 ± 13 | 0.94 | 4.20 | |
| S11 | 104 ± 12 | 1.2 ± 0.4 | 35 ± 11 | 0.93 | 3.20 | |
| W4 | 99 ± 6 | 1.8 ± 0.4 | 11 ± 1.6 | 0.98 | - | |
| Clethodim | S0 | 94 ± 9 | 2.6 ± 0.9 | 26 ± 4.5 | 0.95 | 1.50 |
| S3 | 102 ± 8.1 | 1.4 ± 0.3 | 48 ± 10 | 0.96 | 2.80 | |
| S7 | 101 ± 6.6 | 1.4 ± 0.2 | 34 ± 6 | 0.97 | 2.00 | |
| S11 | 95 ± 3 | 4.1 ± 0.6 | 28 ± 1.5 | 0.99 | 1.60 | |
| W4 | 95 ± 14 | 2.1 ± 1.0 | 17 ± 4.4 | 0.92 | - |
| Herbicide | Population | a ± Standard Error | b ± Standard Error | GR50 ± Standard Error | R2 | Resistance Factor |
|---|---|---|---|---|---|---|
| Glyphosate | S0 | 0.6 ± 0.03 | 1.2 ± 0.20 | 1367 ± 205 | 0.98 | 1.04 |
| S3 | 0.7 ± 0.04 | 0.7 ± 0.05 | 2210 ± 551 | 0.96 | 1.70 | |
| S7 | 0.8 ± 0.04 | 1.4 ± 0.40 | 2923 ± 652 | 0.94 | 2.20 | |
| S11 | 1.0 ± 0.07 | 0.8 ± 0.10 | 1607 ± 5 | 0.99 | 1.20 | |
| W4 | 1.0 ± 0.01 | 1.3 ± 0.04 | 1307 ± 375 | 0.99 | - | |
| Glufosinate | S0 | 0.7 ± 0.02 | 1.3 ± 0.01 | 590 ± 57 | 0.99 | 1.10 |
| S3 | 0.6 ± 0.04 | 0.9 ± 0.20 | 535 ± 117 | 0.97 | - | |
| S7 | 0.6 ± 0.01 | 1.9 ± 0.20 | 1102 ± 64 | 0.99 | 2.00 | |
| S11 | 0.7 ± 0.05 | 1.9 ± 0.50 | 1237 ± 199 | 0.97 | 2.30 | |
| W4 | 0.7 ± 0.04 | 1.9 ± 0.40 | 1110 ± 137 | 0.98 | 2.10 | |
| Paraquat | S0 | 0.6 ± 0.01 | 2.1 ± 0.10 | 111 ± 4 | 0.99 | 1.50 |
| S3 | 0.6 ± 0.03 | 1.6 ± 0.40 | 74 ± 13 | 0.98 | - | |
| S7 | 0.7 ± 0.01 | 1.6 ± 0.10 | 78 ± 4 | 0.99 | 1.05 | |
| S11 | 0.9 ± 0.03 | 1.5 ± 0.10 | 152 ± 12 | 0.99 | 2.05 | |
| W4 | 0.8 ± 0.02 | 2.3 ± 0.20 | 100 ± 4 | 0.99 | 1.30 | |
| Haloxyfop | S0 | 1.0 ± 0.09 | 1.0 ± 0.20 | 36 ± 10 | 0.95 | 2.30 |
| S3 | 1.1 ± 0.02 | 0.7 ± 0.05 | 140 ± 12 | 0.99 | 10.70 | |
| S7 | 1.2 ± 0.03 | 0.98 ± 0.06 | 51 ± 4 | 0.99 | 3.90 | |
| S11 | 1.3 ± 0.08 | 1.0 ± 0.01 | 37 ± 7 | 0.98 | 2.80 | |
| W4 | 1.4 ± 0.07 | 1.9 ± 0.30 | 13 ± 1.4 | 0.99 | - | |
| Clethodim | S0 | 1.3 ± 0.05 | 1.6 ± 0.20 | 24 ± 2 | 0.99 | 2.00 |
| S3 | 1.0 ± 0.05 | 1.1 ± 0.10 | 37 ± 6 | 0.98 | 3.10 | |
| S7 | 1.2 ± 0.03 | 1.5 ± 0.10 | 31 ± 2 | 0.99 | 2.60 | |
| S11 | 0.9 ± 0.03 | 1.5 ± 0.10 | 18 ± 1.4 | 0.99 | 1.50 | |
| W4 | 1.1 ± 0.07 | 2.1 ± 0.40 | 12 ± 1.3 | 0.98 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahajan, G.; Chauhan, B.S. Multiple Herbicide Resistance in Annual Ryegrass (Lolium rigidum Gaudin) in the Southeastern Cropping Region of Australia. Agronomy 2024, 14, 2206. https://doi.org/10.3390/agronomy14102206
Mahajan G, Chauhan BS. Multiple Herbicide Resistance in Annual Ryegrass (Lolium rigidum Gaudin) in the Southeastern Cropping Region of Australia. Agronomy. 2024; 14(10):2206. https://doi.org/10.3390/agronomy14102206
Chicago/Turabian StyleMahajan, Gulshan, and Bhagirath Singh Chauhan. 2024. "Multiple Herbicide Resistance in Annual Ryegrass (Lolium rigidum Gaudin) in the Southeastern Cropping Region of Australia" Agronomy 14, no. 10: 2206. https://doi.org/10.3390/agronomy14102206
APA StyleMahajan, G., & Chauhan, B. S. (2024). Multiple Herbicide Resistance in Annual Ryegrass (Lolium rigidum Gaudin) in the Southeastern Cropping Region of Australia. Agronomy, 14(10), 2206. https://doi.org/10.3390/agronomy14102206







