Effects of Organic Selenium and Nanoselenium on Drought Stress of Pak Choi (Brassica chinensis var. pekinensis. cv. ‘Suzhouqing’) and Its Transcriptomic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Experimental Design
2.2. Determination of Physiological and Biochemical Indexes
2.3. RNA Extraction and RNA-Seq Sequencing
2.4. Identification of Gene Differential Expression
2.5. GO and KEGG Enrichment Analysis of DEGs
2.6. WGCNA
2.7. Quantitative Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. Photosynthetic Pigments and Photosynthetic Indexes of Suzhouqing Leaves
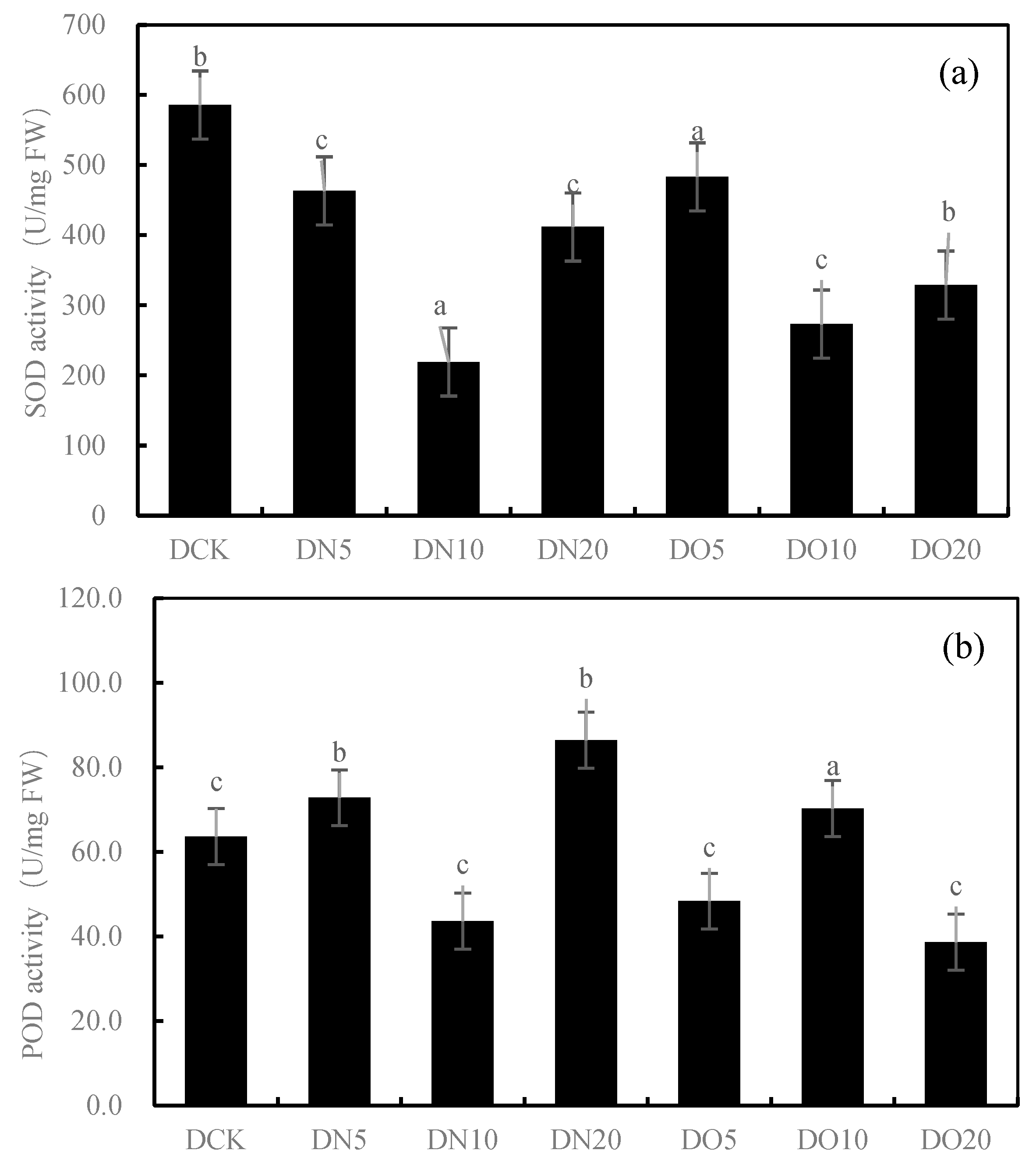
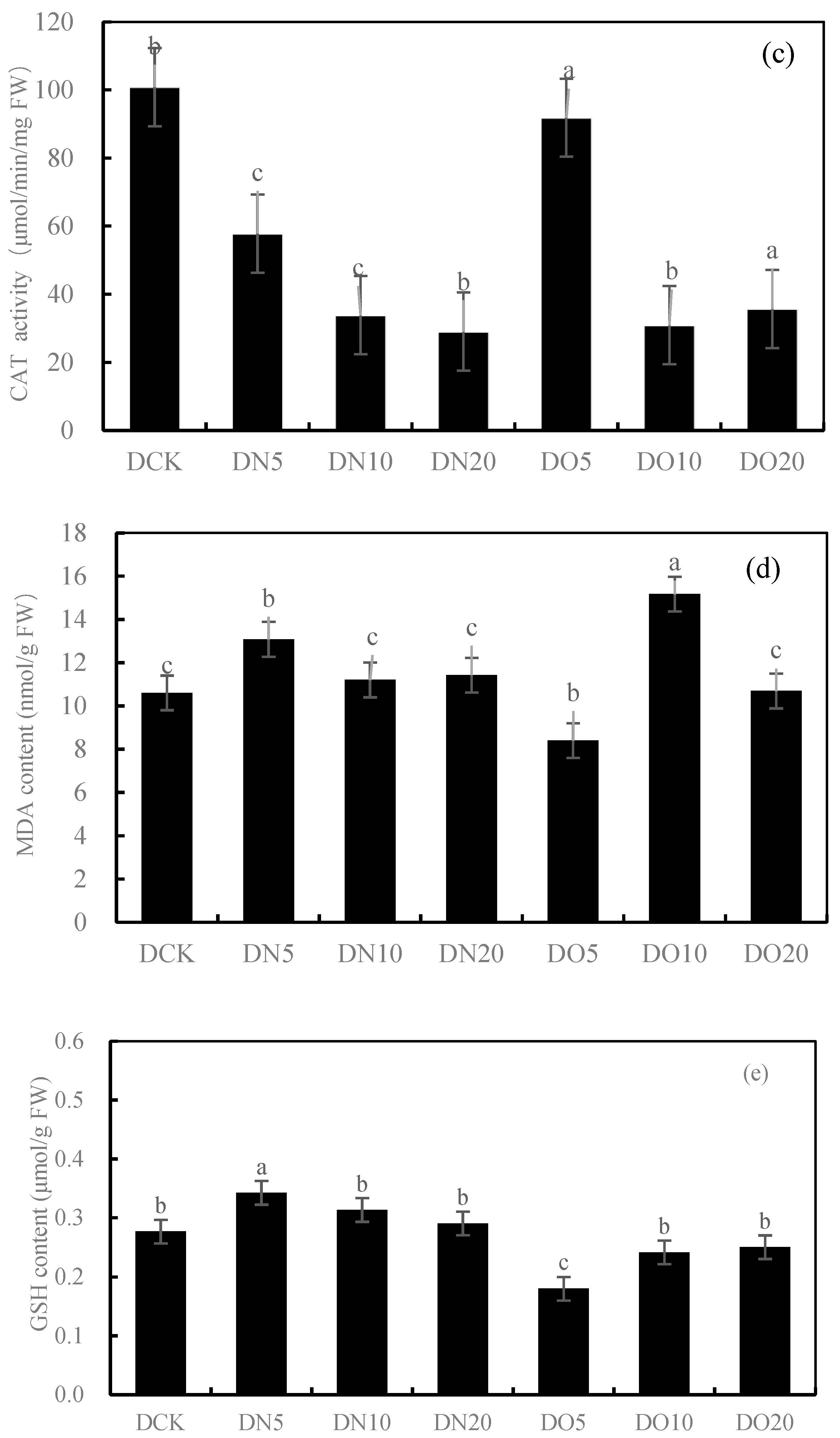
3.2. Antioxidant Properties of Leaves
3.3. Weight and Total Selenium Content of Suzhouqing Leaves
3.4. Nutritional Quality of Suzhouqing Leaves
3.5. Analysis of the Transcriptomic Changes of Nanoselenium and Organic Selenium in Response to Drought Stress
3.5.1. Sequencing Results and Quality Analysis
3.5.2. Correlation Examination between Samples
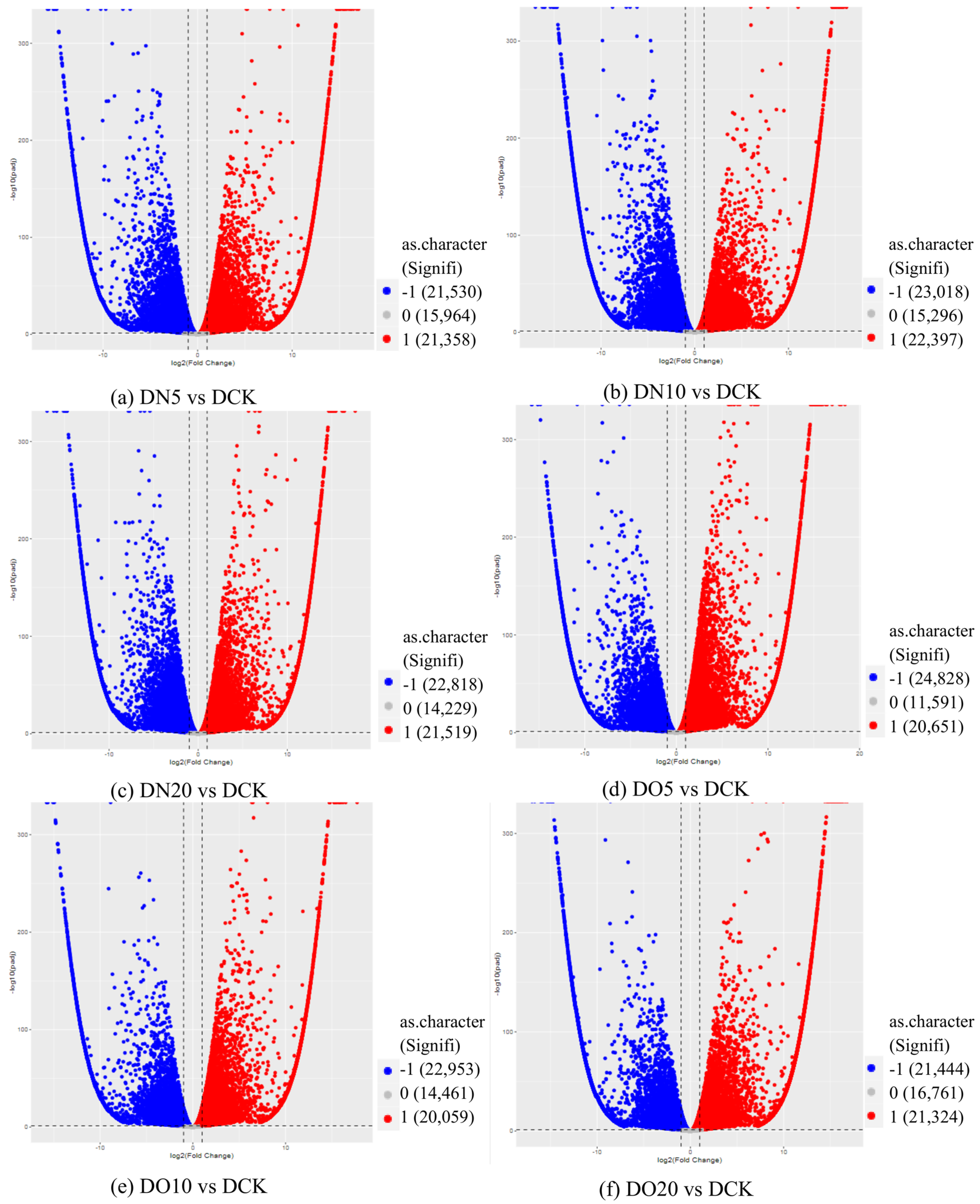
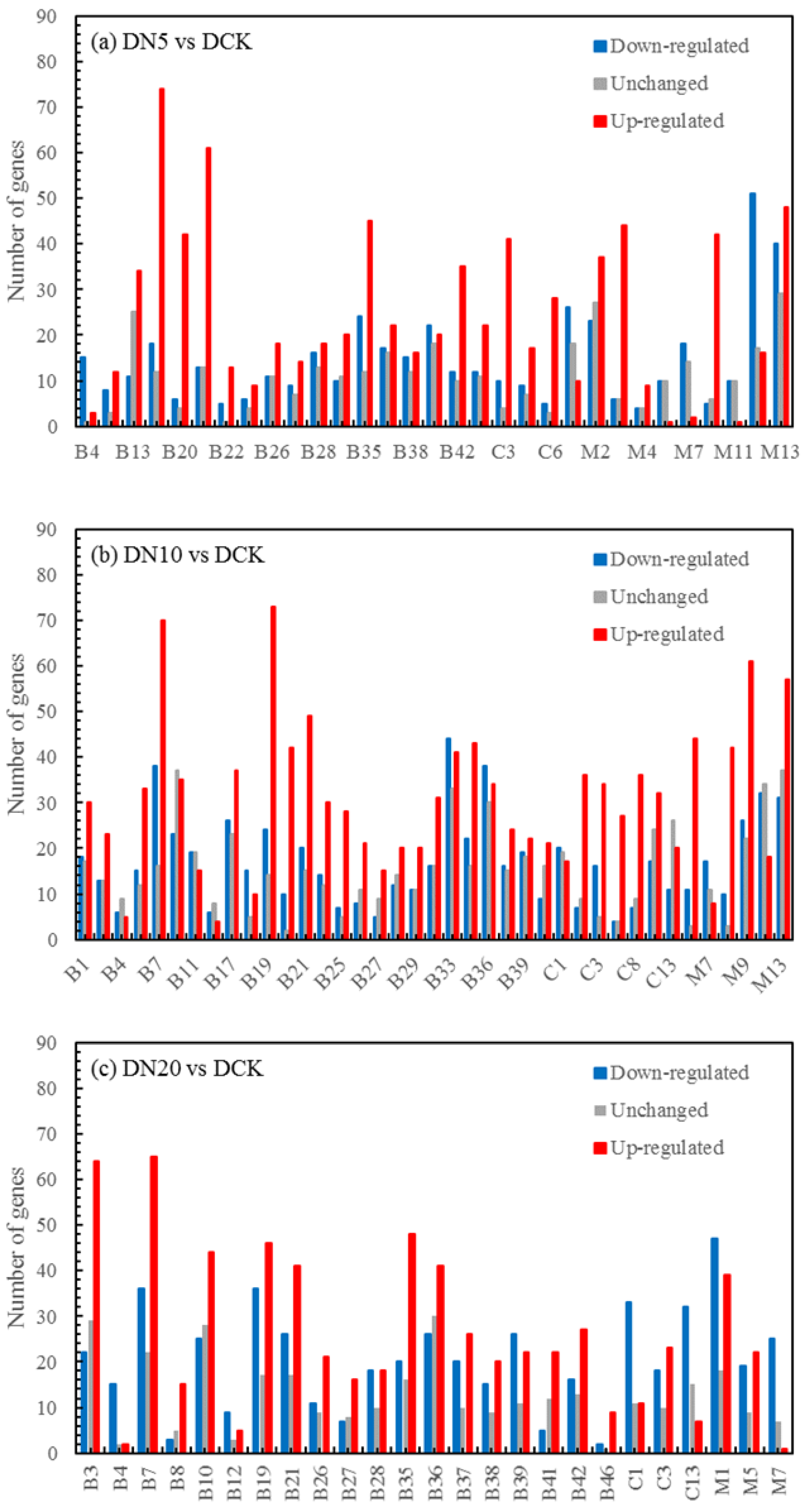
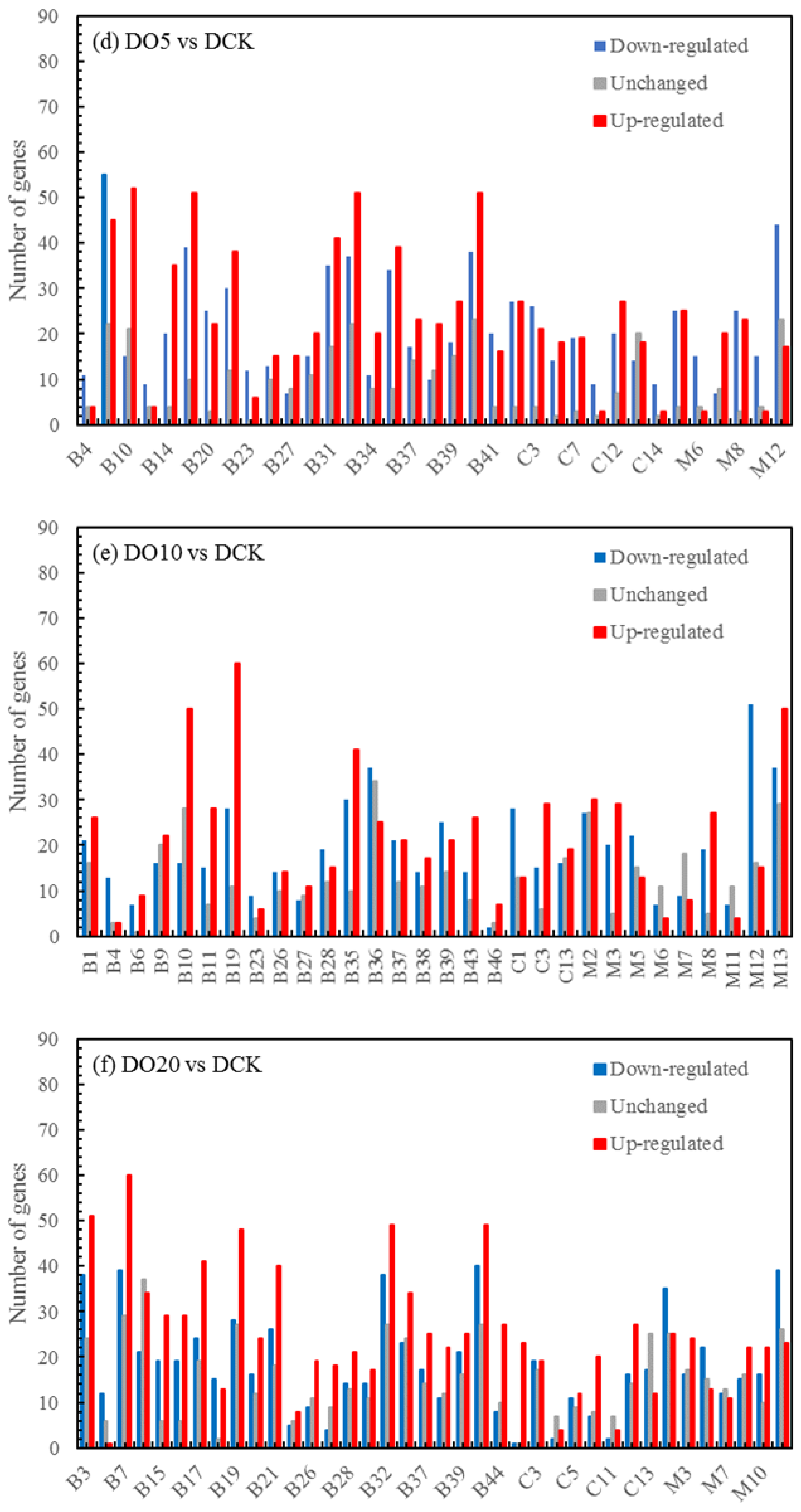
3.5.3. Differentially Expressed Genes (DEGs) Statistics
3.5.4. Volcano Map of DEGs
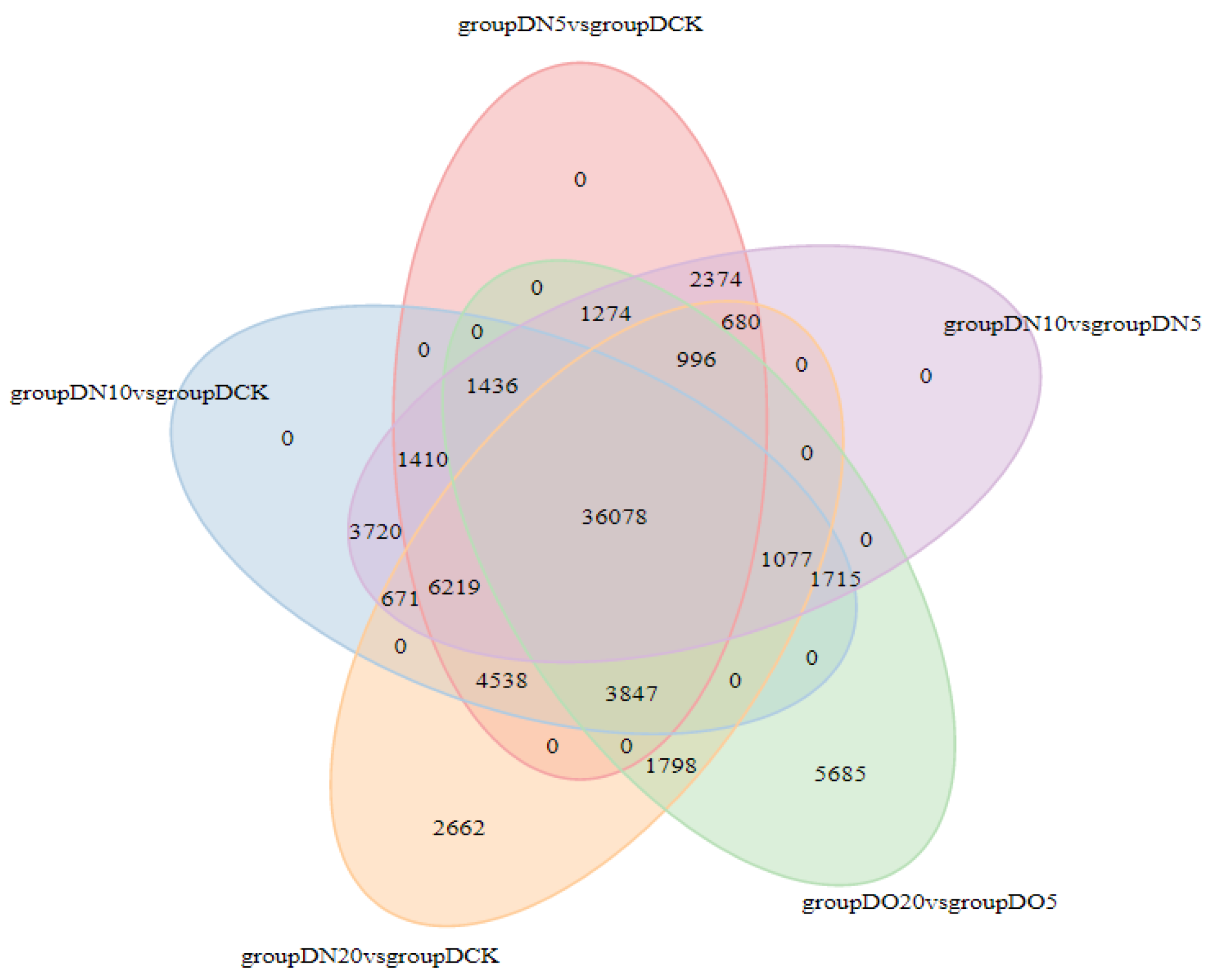
3.5.5. Venn Diagram of DEGs
3.5.6. GO Annotation and Enrichment Analysis of Differentially Expressed Genes
3.5.7. KEGG Annotation and Enrichment Analysis of Differentially Expressed Genes
3.6. WGCNA of DEGs and Identification of Hub Gene
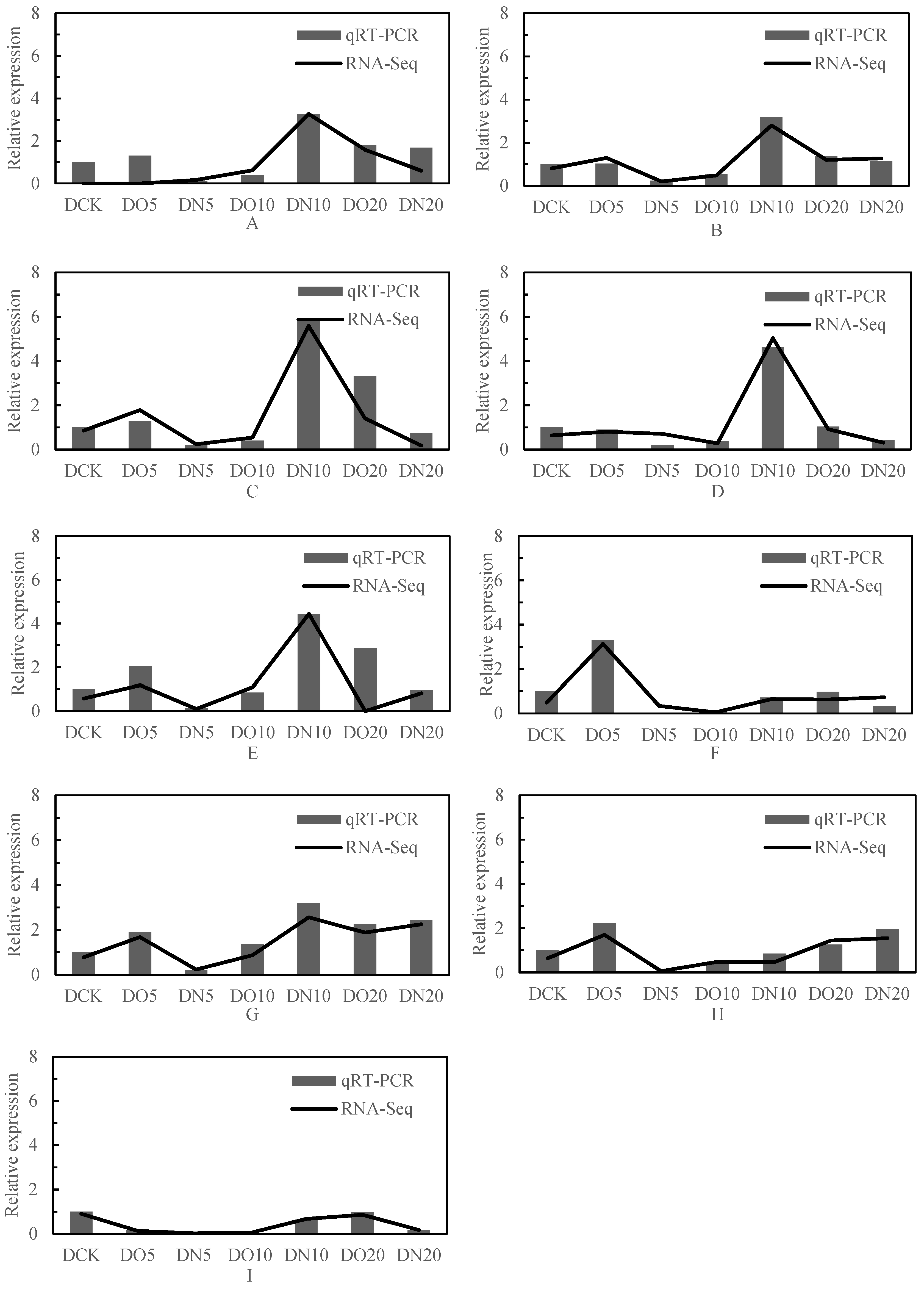
3.7. Fluorescence Quantitative PCR Analysis
4. Discussion
4.1. Effects of Organic Selenium and Nanoselenium on Leaf Photosynthesis under Drought Stress
4.2. Effects of Organic Selenium and Nanoselenium on the Regulation of the Antioxidant System of Leaves under Drought Stress
4.3. Effects of Organic Selenium and Nanoselenium on Weight, Total Selenium Content, and Leaf Nutritional Quality under Drought Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.K.; Sun, H.Y.; Han, J.J.; Yang, X.M.; Chen, H.G.; Chen, D.L.; Jiang, Y.Y. Research progress on conservation of germplasm resources of ‘Suzhouqing’. Vegetables 2015, 1, 80–82. [Google Scholar]
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Yang, X.Y. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Microbiol. Biotechnol. 2019, 103, 7385–7397. [Google Scholar] [CrossRef]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Plant Physiol. 2021, 171, 66–76. [Google Scholar] [CrossRef]
- Dubey, A.; Saiyam, D.; Kumar, A.; Hashem, A.; Abd-Allah, E.F.; Khan, M.L. Bacterial root endophytes: Characterization of their competence and plant growth promotion in Soybean (Glycine max (L.) Merr.) under drought stress. Int. J. Environ. Res. Public Health 2021, 18, 931. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Liu, Y.M.; Qin, J.; Wang, X.Z.; Zhao, H.W. Effects of microelements Se, Mn, and F on SOD activity. Acta Acad. Med. Mil. Tertiae 2014, 2, 171–173. [Google Scholar]
- Cartes, P.; Jara, A.A.; Pinilla, L.; Rosas, A.; Mora, L. Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann. Appl. Biol. 2010, 156, 297–307. [Google Scholar] [CrossRef]
- Yao, X.Q.; Chu, J.Z.; He, X.L.; Liu, B.B.; Li, J.M.; Yue, Z.W. Effects of selenium on agronomical characters of winter wheat exposed to enhanced ultraviolet-B. Ecotoxicol. Environ. Saf. 2013, 92, 320–326. [Google Scholar] [CrossRef]
- Huang, T.; Bai, Y.N.; Mi, J.; Li, J.X.; He, X.R.; Lu, L.; Zhang, B.; Duan, L.Y.; Yan, Y.M.; Qin, K. Effects of external application of copper and selenium trace elements on quality and storage resistance of Lycium barbarum. Non-Wood For. Res. 2023, 41, 252–262. [Google Scholar]
- Kumar, M.; Bijo, A.J.; Baghel, R.S.; Reddy, C.R.K.; Jha, B. Selenium and spermine alleviate cadmium induced toxicity in the red seaweed Gracilaria duraby regulating antioxidants and DNA methylation. Plant Physiol. Biochem. 2012, 51, 129–138. [Google Scholar] [CrossRef]
- Fan, S.Y. Study on Physiological Mechanisms for Exogenous Selenium-Mediated Drought and Salt Tolerance in Tomato. Master’s Thesis, Northwest A&F University, Yangling, China, 2022. [Google Scholar]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y.; Waraich, E.A.; Khan, S.Z. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef]
- Habibi, G. Effect of drought stress and selenium spraying on photosynthesis and antioxidant activity of spring barley. Acta Agric. Slov. 2013, 101, 31–39. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol. Trance Elem. Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- Van Hoewyk, D.; Takahashi, H.; Inoue, E.; Hess, A.; Tamaoki, M.; Pilson-Smits, E.A.H. Transcriptome analyses give insights into selenium stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol. Plant. 2008, 132, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Cakir, O.; Candar-Cakir, B.; Zhang, B. Small RNA and degradome sequencing reveals important microRNA function in Astragalus chrysochlorus response to selenium stimuli. Plant Biotechnol. J. 2015, 14, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.Y.; Wu, H.; Niu, J.Q. Effects of Water Stress on Physiological Index of Grapevine Leaves. SINO Overeas Grapebine Wine 2005, 2005, 11–14. [Google Scholar]
- Wang, X.K.; Huang, J.L. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2014; pp. 131–133. [Google Scholar]
- Ukeda, H.; Kawana, D.; Maeda, S.; Sawamura, M. Spectrophotometric assay for superoxide dismutase based on the reduction of highly water-soluble tetrazolium salts by xanthine-xanthine oxidase. J. Agric. Chem. Soc. Jpn. 1999, 63, 485–488. [Google Scholar] [CrossRef]
- Doerge, D.R.; Divi, R.L.; Churchwell, M.I. Identification of the colored guaiacol oxidation product produced by peroxidases. Anal. Biochem. 1997, 250, 10–17. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A.H. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Spitz, D.R.; Oberley, L.W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989, 179, 8–18. [Google Scholar] [CrossRef]
- Nishimoto, S.; Koike, S.; Inoue, N.; Suzuki, T.; Ogasawara, Y. Activation of Nrf2 attenuates carbonyl stress induced by methylglyoxal in human neuroblastoma cells: Increase in GSH levels is a critical event for the detoxification mechanism. Biochem. Biophys. Res. Commun. 2017, 483, 874. [Google Scholar] [CrossRef]
- Buysse, J.A.N.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Campion, E.M.; Loughran, S.T.; Walls, D. Protein quantitation and analysis of purity. Methods Mol. Biol. 2011, 681, 229–258. [Google Scholar] [PubMed]
- Chen, Y.Y.; Fu, X.M.; Mei, X.; Zhou, Y.; Cheng, S.H.; Zeng, L.T.; Dong, F.; Yang, Z.Y. Proteolysis of chloroplast proteins is responsible for accumulation of free amino acids in dark-treated tea (Camellia sinensis) leaves. J. Proteom. 2017, 157, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Feng, J.; Wu, J.F.; Wang, K.; Wu, S.; Liu, H.C.; Jiang, M.G. Transcriptome analysis of the growth-promoting effect of volatile organic compounds produced by Microbacterium aurantiacum GX14001 on tobacco (Nicotiana benthamiana). BMC Plant Biol. 2022, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.M.; Oshlack, A. Corset: Enabling differential gene expression analysis for de novo assembled transcriptomes. Genome Biol. 2014, 15, 410–424. [Google Scholar] [PubMed]
- Eom, S.H.; Ahn, M.-A.; Kim, E.; Lee, H.J.; Lee, J.H.; Wi, S.H.; Kim, S.K.; Lim, H.B.; Hyun, T.K. Plant response to cold stress: Cold stress changes antioxidant metabolism in heading type kimchi cabbage (Brassica rapa L. ssp. Pekinensis). Antioxidants 2022, 11, 700. [Google Scholar] [CrossRef]
- Hassan, Z.U. Melatonin Induced Selenium Tolerance in Oilseed Rape Cultivars through Physio-Biochemical Metabolism, Anatomical, and Molecular Profiling. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2019. [Google Scholar]
- Zhang, L.L. Study on Genes Related to Flavonoid Biosynthesis in Foxtail Millet Based on WGCNA. Master’s Thesis, Shanxi Agricultural University, Taigu, China, 2021. [Google Scholar]
- Kong, Q.H. Effects of Biological Nano Se on Yield and Physiological-Biochemical Characteristics of Foxtail Millet. Master’s Thesis, Shandong Normal University, Jinan, China, 2020. [Google Scholar]
- Yao, X.; Chu, J.; Wang, G. Effects of selenium on wheat seedlings under drought stress. Biol. Trace Elem. Res. 2009, 130, 283–290. [Google Scholar] [CrossRef]
- Chen, B.; Li, J.W.; Wang, X.D.; Xu, Z.C. Effects of exogenous selenium on growth and physiological characteristics of flue-cured tobacco under drought stress. Plant Physiol. J. 2018, 54, 165–172. [Google Scholar]
- Yue, H.F. Effects of Exogenous Selenium on Drought Tolerance of Waxy Maize Seedlings and Its Transcriptomic Analysis. Master’s Thesis, Zhongkai University of Agriculture and Engineering, Zhongkai, China, 2022. [Google Scholar]
- Sattar, A.; Cheema, M.A.; Sher, A.; Ijaz, M.; Ali, Q. Physiological and biochemical attributes of bread wheat (Triticum aestivum L.) seedlings are influenced by foliar application of silicon and selenium under water deficit. Acta Physiol. Plant 2019, 41, 146. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Ahmad, Z.; Anjum, S.; Skalicky, M.; Waraich, E.A.; Tariq, R.M.S.; Ayub, M.A.; Hossain, A.; Hassan, M.; Brestic, M.; Islam, M.S.; et al. Selenium alleviates the adverse effect of drought in oilseed crops Camelina (Camelina sativa L.) and Canola (Brassica napus L.). Molecules 2021, 26, 1699. [Google Scholar] [CrossRef]
- Sardari, M.; Rezayian, M.; Niknam, V. Comparative study for the effect of selenium and nano-selenium on wheat plants grown under drought Stress. Russ. J. Plant Physiol. 2022, 69, 127. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Daneshvar Hakimi Meybodi, N.; Peijnenburg, W. Mitigation of the effect of drought on growth and yield of pomegranates by foliar spraying of different sizes of selenium nanoparticles. J. Sci. Food Agric. 2021, 101, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Moloi, M.J.; Khoza, B.M. The Effect of selenium foliar application on the physiological responses of edamame under different water treatments. Agronomy 2022, 12, 2400. [Google Scholar] [CrossRef]
- Li, C.H.; Wan, Y.F.; Shang, X.L.; Fang, S.Z. Integration of transcriptomic and metabolomic analysis unveils the response mechanism of sugar metabolism in Cyclocarya paliurus seedlings subjected to PEG-induced drought stress. Plant Physiol. Biochem. 2023, 1, 107856. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.Y.; Yang, R.D.; Tian, H.X.; Liang, H.Y. Effect of different ways of applying selenium on yields and related traits of broomcorn millet. Mod. Agric. Sci. Technol. 2015, 2, 29+37. [Google Scholar]
- Guo, M.M.; Guo, P.Y.; Yuan, X.Y.; Gao, H.; Gao, Z.P.; Feng, L.; Wang, B.Q.; Ning, N.; Yu, K.K.; Dong, S.Q. Effects of foliar application of Na2SeO3 on photosynthetic characteristics and yield of foxtail millet. J. Nucl. Agric. Sci. 2014, 28, 1099–1107. [Google Scholar]
- Zhang, P.F.; Zhang, A.J.; Zhang, J.H.; Wang, X.J.; Liu, J.M.; Zhou, D.M. Effects of foliar application of sodium selenite on selenium accumulation and quality in millet. Acta Agric. Boreali-Sin. 2010, 25, 231–234. [Google Scholar]
- Gao, Z.P.; Guo, P.Y.; Yuan, X.Y.; Ning, N.; Guo, M.J.; Gao, H.; Wang, B.Q.; Feng, L.; Dong, S.Q.; Wen, Y.Y. Effects of foliar spraying Na2SeO3 during the grain filling stage on quality and seeds selenium content of foxtail millet. J. Shanxi Agric. Univ. Nat. Sci. Ed. 2015, 35, 157–161. [Google Scholar]
- Song, J.Y. Effect of selenium fertilization on flag leaf physiological characteristics and grain selenium content and yield after anthesis of wheat. Acta Agric. Boreali-Sin. 2006, 21, 68–71. [Google Scholar]
- Peng, T.; Yu, J.L.; Cheng, D.F.; Gao, Y.; Zhao, W.F. Effect of spraying stage and time of selenium rich liquid on wheat yield and grain selenium content. J. Anhui Agri. Sci. 2015, 43, 104–105+108. [Google Scholar]
- Bao, Q.J. The Transcriptome Analysis of Cotton under Drought Stress. Master’s Thesis, Xinjiang University, Urumqi, China, 2018. [Google Scholar]
- Hu, Y.R. Identification and Analysis of Genes Related to Selenium Assimilation and Metabolism in Tea Plant Roots. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2016. [Google Scholar]
- Freeman, J.L.; Tamaoki, M.; Stushnoff, C.; Quinn, C.F.; Cappa, J.; Devonshire, J.; Fakra, S.C.; Marcus, M.A.; McGrath, P.; Hoewyk, D.V. Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata. Plant Physiol. 2010, 153, 1630–1652. [Google Scholar] [CrossRef] [PubMed]
- Hoewyk, D.V.; Pilon, M.; Pilon-Smits, A.H.E. The functions of NifS-like proteins in plant sulfur and selenium metabolism. Plant Sci. 2008, 174, 120–123. [Google Scholar]

| Available Potassium (g kg−1) | Available Phosphorus Content (mg kg−1) | Alkali-Hydrolyzable Nitrogen (mg kg−1) | Total Selenium Content (mg kg−1) | Organic Matter Content (g kg−1) | pH |
|---|---|---|---|---|---|
| 3.44 | 175.76 | 536.69 | 0.34 | 646.62 | 5.87 |
| Treatment | Type | Volume (mL) | Concentration (mg L−1) |
|---|---|---|---|
| DCK | Neutral water | 20 | 0 |
| DN5 | Nanoselenium | 20 | 5 |
| DN10 | Nanoselenium | 20 | 10 |
| DN20 | Nanoselenium | 20 | 20 |
| DO5 | Organic selenium | 20 | 5 |
| DO10 | Organic selenium | 20 | 10 |
| DO20 | Organic selenium | 20 | 20 |
| Treatment | Chlorophyll a Content (mg g−1 Fresh Weight) | Chlorophyll b Content (mg g−1 Fresh Weight) | Total Chlorophyll Content (mg g−1 Fresh Weight) | Chlorophyll a/b | Carotenoid Content (μg g−1) |
|---|---|---|---|---|---|
| DCK | 0.87 ± 0.02e | 0.46 ± 0.02c | 1.33 ± 0.02d | 1.89 ± 0.01a | 181.39 ± 1.01b |
| DN5 | 1.47 ± 0.02a | 0.67 ± 0.03a | 2.14 ± 0.02a | 2.19 ± 0.02d | 216.71 ± 0.63a |
| DN10 | 1.23 ± 0.02b | 0.57 ± 0.02b | 1.80 ± 0.03b | 2.16 ± 0.02c | 178.46 ± 0.42c |
| DN20 | 1.06 ± 0.02c | 0.49 ± 0.02c | 1.55 ± 0.02c | 2.16 ± 0.01a | 136.05 ± 0.27e |
| DO5 | 0.88 ± 0.02e | 0.47 ± 0.02c | 1.35 ± 0.03d | 1.87 ± 0.02b | 91.13 ± 0.44g |
| DO10 | 1.05 ± 0.02c | 0.52 ± 0.01b | 1.57 ± 0.03c | 2.02 ± 0.01d | 143.07 ± 0.48d |
| DO20 | 0.93 ± 0.03d | 0.42 ± 0.02d | 1.35 ± 0.03d | 2.21 ± 0.01e | 109.96 ± 0.70f |
| Treatment | Pn (μmol CO2 m−2 s−1) | Tr (mmol H2O m−2 s−1) | Gs (mmol H2O m−2 s−1) | Ci (μmol CO2 mol−1 Air) |
|---|---|---|---|---|
| DCK | 9.37 ± 0.02e | 5.77 ± 0.25b | 232.03 ± 1.54g | 528.62 ± 1.10f |
| DN5 | 14.09 ± 0.29b | 7.93 ± 0.19a | 370.12 ± 1.01a | 539.23 ± 0.89e |
| DN10 | 19.38 ± 0.17a | 5.65 ± 0.12b | 293.73 ± 1.93c | 547.18 ± 0.63d |
| DN20 | 19.5 ± 0.15a | 5.63 ± 0.08b | 241.49 ± 4.01e | 565.54 ± 0.68b |
| DO5 | 12.47 ± 0.02c | 7.79 ± 0.08a | 335.53 ± 1.09b | 527.15 ± 0.64f |
| DO10 | 10.67 ± 0.22d | 5.22 ± 0.25b | 215.61 ± 1.07f | 557.36 ± 0.59c |
| DO20 | 7.39 ± 0.13f | 5.17 ± 0.17b | 288.37 ± 0.85d | 573.72 ± 0.62a |
| Treatment | DCK | DN5 | DN10 | DN20 | DO5 | DO10 | DO20 |
|---|---|---|---|---|---|---|---|
| Weight (g fresh weight) | 48.71 ± 0.44f | 62.79 ± 0.25b | 67.30 ± 0.49a | 56.12 ± 0.43d | 57.25 ± 0.35c | 50.76 ± 0.24e | 56.68 ± 0.28d |
| Total selenium content (μg kg−1) | 10.21 ± 0.37g | 33.22 ± 0.27d | 42.37 ± 0.26a | 38.242 ± 0.22b | 30.19 ± 0.50f | 32.27 ± 0.36e | 36.56 ± 0.18c |
| Treatment | Soluble Sugar (mg g−1 Fresh Weight) | Soluble Protein (mg g−1 Fresh Weight) | Free Amino Acid Content (mg g−1 Fresh Weight) | Total Flavonoid Content (mg g−1 Fresh Weight) |
|---|---|---|---|---|
| DCK | 11.77 ± 0.19e | 17.36 ± 0.14c | 1.73 ± 0.02de | 0.62 ± 0.04cd |
| DN5 | 20.06 ± 0.24b | 26.84 ± 0.19a | 1.86 ± 0.03c | 1.24 ± 0.07a |
| DN10 | 12.88 ± 0.10d | 17.97 ± 0.32c | 2.09 ± 0.08b | 0.79 ± 0.04c |
| DN20 | 20.80 ± 0.17a | 28.93 ± 0.24a | 3.44 ± 0.05a | 0.63 ± 0.02d |
| DO5 | 7.85 ± 0.11g | 18.94 ± 0.24c | 1.62 ± 0.05e | 0.67 ± 0.06cd |
| DO10 | 10.56 ± 0.54f | 16.85 ± 0.66c | 1.83 ± 0.05cd | 0.69 ± 0.05d |
| DO20 | 16.87 ± 0.07c | 24.11 ± 1.98b | 2.02 ± 0.04b | 1.06 ± 0.05b |
| Treatment | Raw Base (G) | Raw Sequences | Clean Bases (G) | Clean Sequences | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|---|
| DCK | 6.67 | 44,468,500 | 6.15 | 41,582,304 | 100.0 | 99.85 | 46.00 (%) |
| DO5 | 7.22 | 48,160,344 | 6.65 | 44,924,020 | 100.0 | 99.84 | 47.00 (%) |
| DN5 | 6.66 | 44,402,076 | 6.17 | 41,736,154 | 100.0 | 99.86 | 47.50 (%) |
| DO10 | 6.11 | 40,701,126 | 5.62 | 38,076,306 | 100.0 | 99.85 | 47.00 (%) |
| DN10 | 7.03 | 46,882,738 | 6.48 | 44,169,476 | 100.0 | 99.87 | 47.00 (%) |
| DO20 | 6.99 | 46,628,854 | 6.50 | 43,870,658 | 100.0 | 99.87 | 47.00 (%) |
| DN20 | 6.79 | 45,277,238 | 6.34 | 42,817,196 | 100.0 | 99.87 | 47.00 (%) |
| Group | All | Upregulated | Downregulated |
|---|---|---|---|
| DN5vsDCK | 58,852 | 21,358 | 21,530 |
| DN10vsDCK | 60,711 | 22,397 | 23,018 |
| DN20vsDCK | 58,566 | 21,519 | 22,818 |
| DO5vsDCK | 57,070 | 20,651 | 24,828 |
| DO10vsDCK | 57,473 | 20,059 | 22,953 |
| DO20vsDCK | 59,529 | 21,324 | 21,444 |
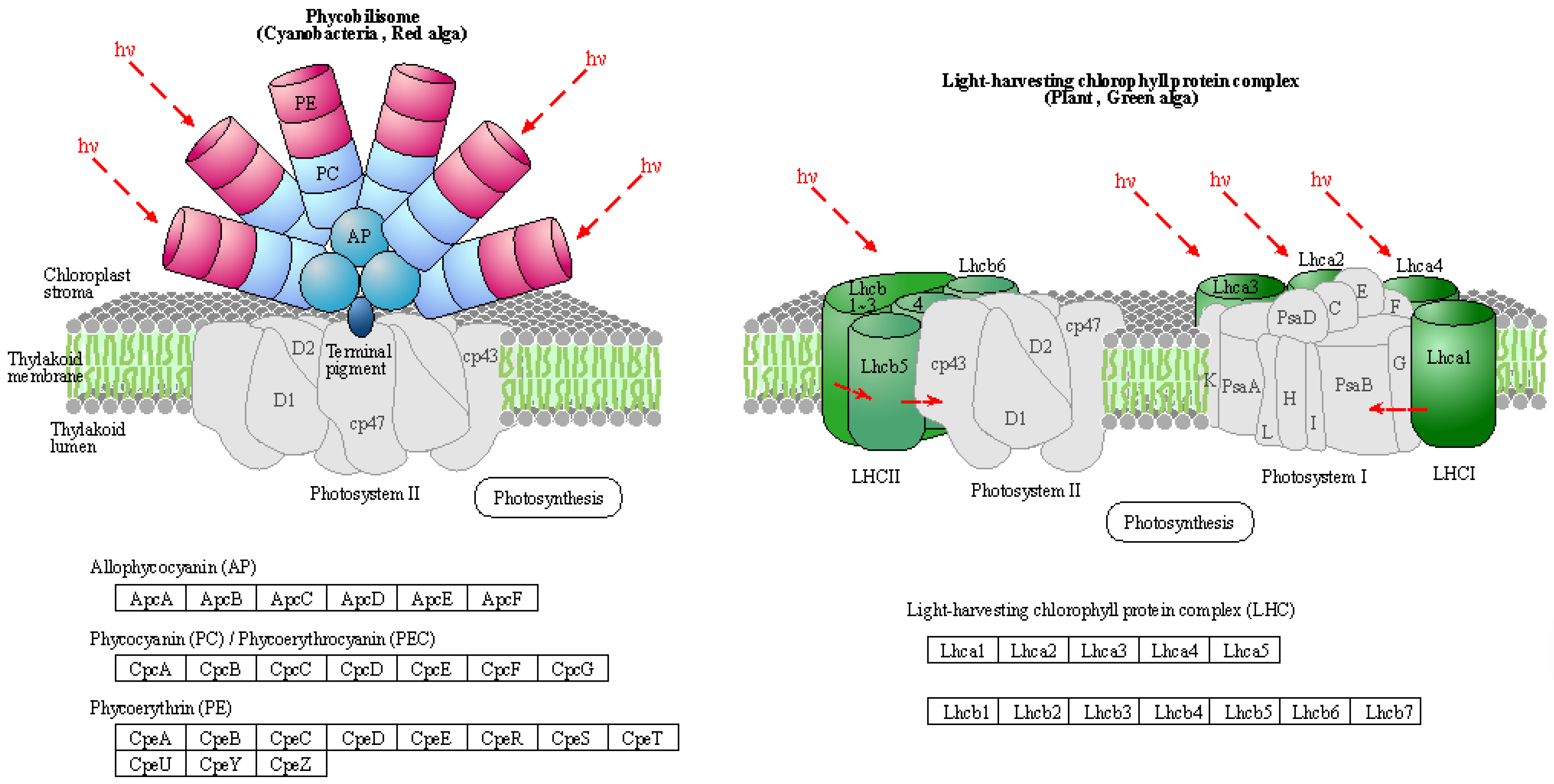
| Symbol | DN5 vs. DCK | DN10 vs. DCK | DN20 vs. DCK | DO5 vs. DCK | DO10 vs. DCK | DO20 vs. DCK |
|---|---|---|---|---|---|---|
| Lhca1 | - | Up | - | - | - | - |
| Lhca2 | - | - | - | Up | Up | - |
| Lhca3 | - | - | - | - | - | - |
| Lhca4 | - | - | - | - | - | Up |
| Lhca5 | Up | - | - | - | - | - |
| Lhcb1 | - | Up | - | - | - | Up |
| Lhcb2 | Up | - | Up | Up | - | - |
| Lhcb3 | - | - | - | - | - | - |
| Lhcb4 | Up | - | - | - | - | - |
| Lhcb5 | - | - | - | - | - | Up |
| Lhcb6 | - | - | - | - | - | - |
| Lhcb7 | - | - | - | - | Up | Up |
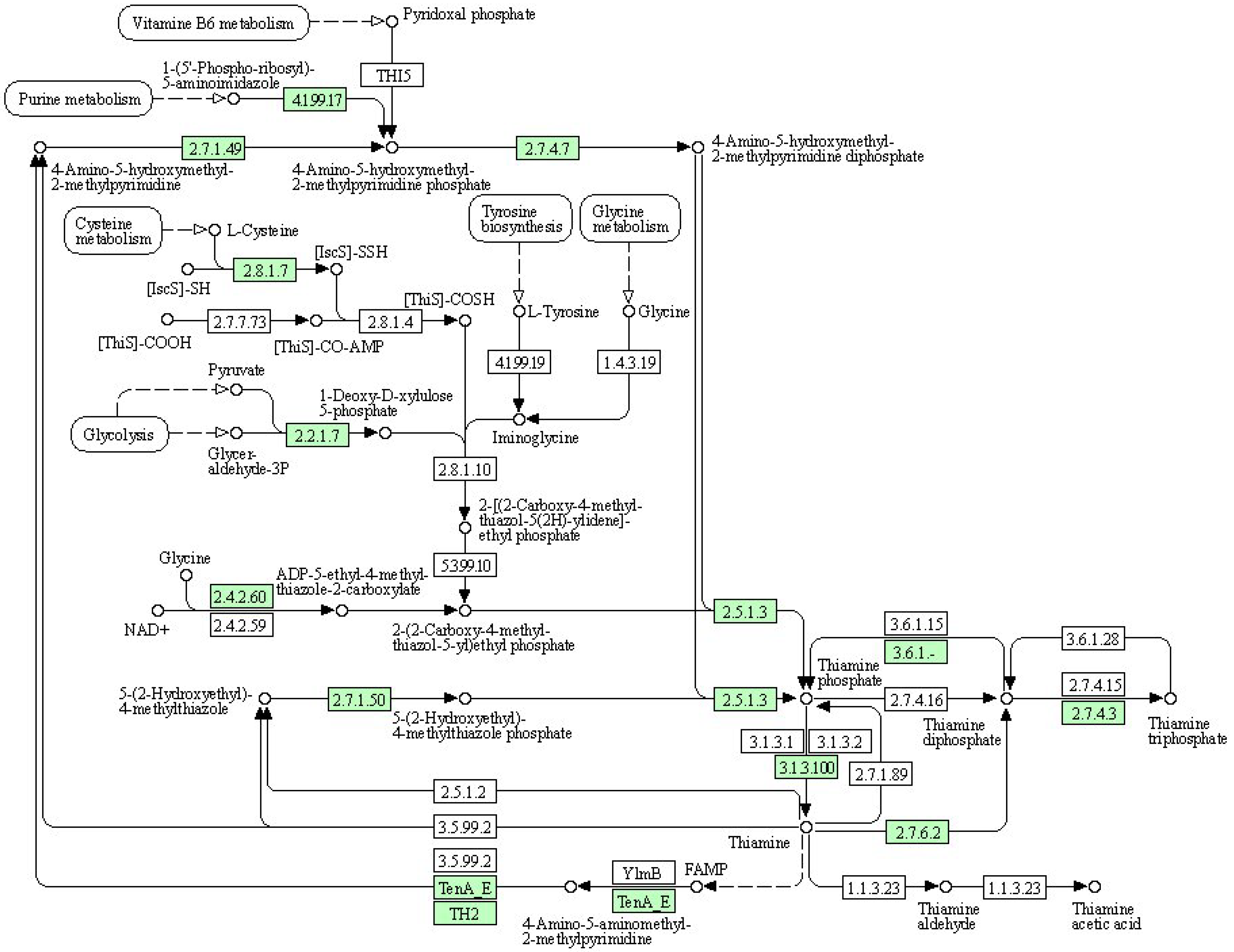
| Symbol | Description | DN5 vs. DCK | DN10 vs. DCK | DN20 vs. DCK | DO5 vs. DCK | DO10 vs. DCK | DO20 vs. DCK |
|---|---|---|---|---|---|---|---|
| THI5 | Pyrimidine precursor biosynthesis enzyme | - | - | - | - | - | - |
| 4.1.99.17 | Phosphomethylpyrimidine synthase | - | - | - | Up | - | - |
| 2.7.1.49 | Hydroxymethylpyrimidine | - | - | Up | - | - | - |
| 2.7.4.7 | Phosphomethylpyrimidine kinase | - | - | Up | - | - | - |
| 2.8.1.7 | Cysteine desulfurase | Up | Up | - | Up | Up | - |
| 2.7.7.73 | Sulfur carrier protein ThiS adenylyltransferase | - | - | - | - | - | - |
| 2.8.1.4 | tRNA uracil 4-sulfurtransferase | - | - | - | - | - | - |
| 4.1.99.19 | 2-iminoacetate synthase | - | - | - | - | - | - |
| 1.4.3.19 | Glycine oxidase | - | - | - | - | - | - |
| 2.2.1.7 | 1-deoxy-D-xylulose-5-phosphate synthase | - | - | - | - | - | - |
| 2.8.1.10 | Thiazole synthase | - | - | - | - | - | - |
| 5.3.99.10 | Thiazole tautomerase (transcriptional regulator TenI) | - | - | - | - | - | - |
| 2.4.2.60 | Cysteine-dependent adenosine diphosphate thiazole synthase | Up | - | Up | - | - | Up |
| 2.4.2.59 | Sulfide-dependent adenosine diphosphate thiazole synthase | - | - | - | - | - | - |
| 2.5.1.3 | Thiamine-phosphate pyrophosphorylase | - | - | Up | - | - | - |
| 3.6.1.15 | Nucleoside-triphosphatase | - | - | - | - | - | - |
| 3.6.1.28 | Thiamine-triphosphatase | - | - | - | - | - | - |
| 2.7.1.50 | Hydroxyethylthiazole kinase | - | - | Up | - | - | - |
| 2.5.1.3 | Thiamine-phosphate pyrophosphorylase | - | - | - | - | - | - |
| 2.7.4.16 | Thiamine-monophosphate kinase | - | - | - | - | - | - |
| 2.7.4.15 | Thiamine-diphosphate kinase | - | - | - | - | - | - |
| 2.7.4.3 | Adenylate kinase | Up | - | Up | Up | Up | Up |
| 3.1.3.1 | Alkaline phosphatase | - | - | - | - | - | - |
| 3.1.3.2 | Acid phosphatase | - | - | - | - | - | - |
| 3.1.3.100 | Thiamine phosphate phosphatase | - | - | - | - | - | - |
| 2.7.1.89 | Thiamine kinase | - | - | - | - | - | - |
| 2.5.1.2 | Thiamine pyridinylase | - | - | - | - | - | - |
| 3.5.99.2 | Thiaminase (transcriptional activator TenA) | - | - | - | - | - | - |
| 2.7.6.2 | Thiamine pyrophosphokinase | Up | Up | Up | Up | - | - |
| 1.1.3.23 | Thiamine oxidase | - | - | - | - | - | - |
| TenA_E | Formylaminopyrimidine deformylase | - | Up | - | - | Up | - |
| TH2 | Thiamine phosphate phosphatase | - | - | - | - | Up | - |
| Symbol | Description | DN5 vs. DCK | DN10 vs. DCK | DN20 vs. DCK | DO5 vs. DCK | DO10 vs. DCK | DO20 vs. DCK |
|---|---|---|---|---|---|---|---|
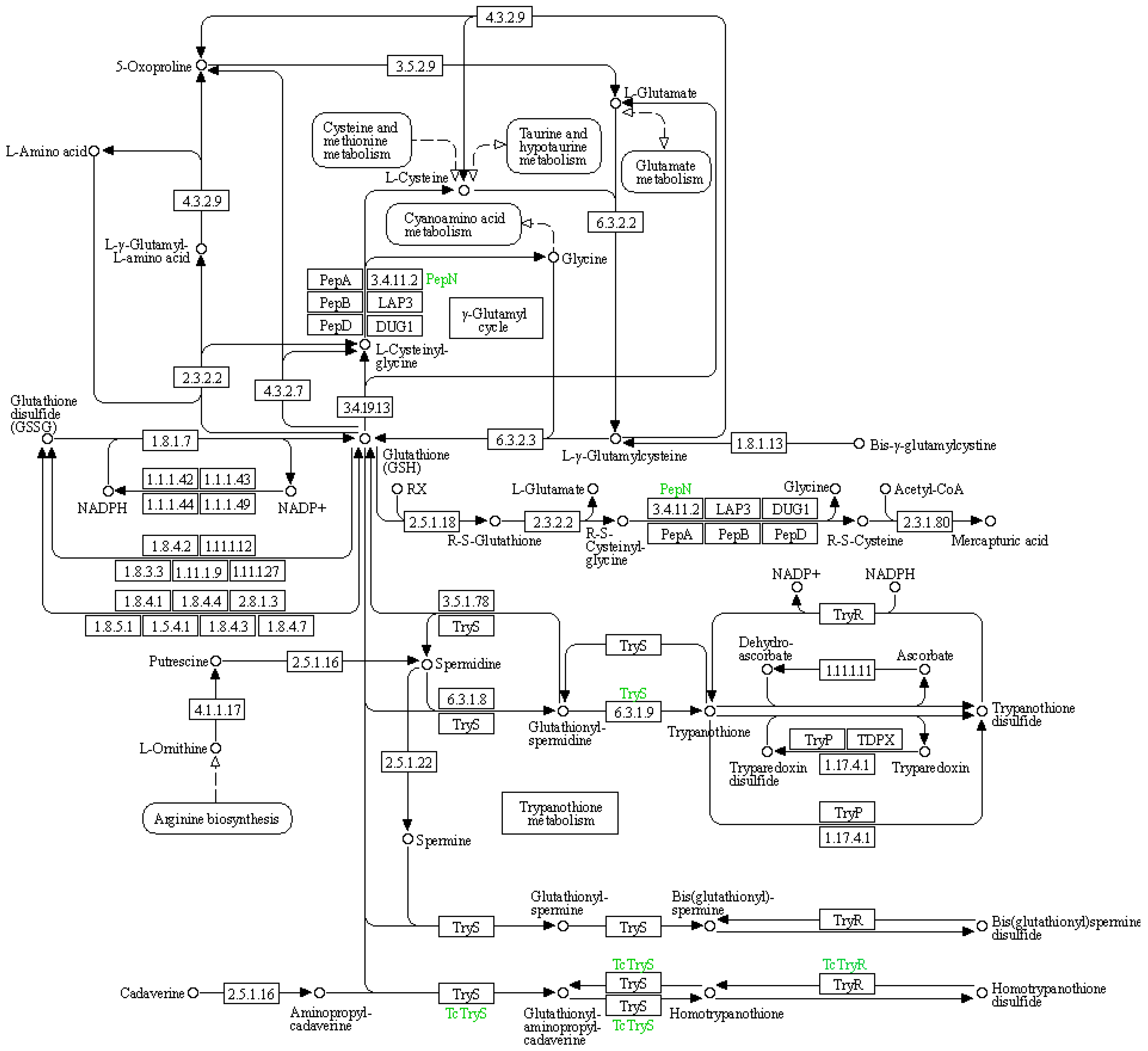
| 4.3.2.9 | Gamma-glutamylcyclotransferase | - | - | - | - | - | - |
| 3.5.2.9 | 5-oxoprolinase | - | - | - | - | - | - |
| 6.3.2.2 | Glutamate–cysteine ligase catalytic subunit | Up | - | Up | - | Up | - |
| 3.4.11.2 | Aminopeptidase N | - | - | - | - | - | - |
| 2.3.2.2 | Gamma-glutamyltranspeptidase | - | - | - | - | - | - |
| 4.3.2.7 | Glutathione-specific gamma-glutamylcyclotransferase | - | - | - | - | - | - |
| 3.4.19.13 | Glutathione hydrolase | - | - | Up | - | - | - |
| 1.8.1.7 | Glutathione reductase (NADPH) | - | - | - | - | - | - |
| 6.3.2.3 | Glutathione synthase | - | - | - | Up | - | - |
| 1.8.1.13 | Bis-gamma-glutamylcystine reductase | - | - | - | - | - | - |
| 1.1.1.42 | Isocitrate dehydrogenase | - | - | - | - | - | - |
| 1.1.1.43 | Phosphogluconate 2-dehydrogenase | - | - | - | - | - | - |
| 1.1.1.44 | 6-phosphogluconate dehydrogenase | - | - | - | - | - | - |
| 1.1.1.49 | Glucose-6-phosphate 1-dehydrogenase | - | - | - | - | - | - |
| 2.5.1.18 | Glutathione S-transferase | Up | - | Up | Up | - | Up |
| 2.3.2.2 | Gamma-glutamyltranspeptidase | - | - | - | - | - | - |
| 2.3.1.80 | N-acetyltransferase 8 | - | - | - | - | - | - |
| 1.8.4.2 | Protein-disulfide reductase | - | - | - | - | - | - |
| 1.11.1.12 | Phospholipid-hydroperoxide glutathione peroxidase | - | - | - | - | - | - |
| 1.8.3.3 | Glutathione oxidase | Up | - | Up | - | Up | - |
| 1.11.1.9 | Glutathione peroxidase | - | Up | - | - | - | Up |
| 1.11.1.27 | Peroxiredoxin 6 | - | - | - | - | - | - |
| 1.8.4.1 | Glutathione—homocystine transhydrogenase | - | - | - | Up | - | - |
| 1.8.4.4 | Glutathione—cystine transhydrogenase; | - | - | Up | - | - | - |
| 2.8.1.3 | Thiosulfate—thiol sulfurtransferase | - | - | - | - | - | - |
| 1.8.5.1 | Glutathione dehydrogenase | - | - | - | - | - | - |
| 1.5.4.1 | Pyrimidodiazepine synthase | - | - | - | - | - | - |
| 1.8.4.3 | Glutathione—CoA-glutathione transhydrogenase | Up | - | - | - | Up | - |
| 1.8.4.7 | Enzyme-thiol transhydrogenase | - | - | - | Up | - | - |
| 3.5.1.78 | Glutathionylspermidine amidase | - | - | - | - | - | - |
| 2.5.1.16 | Spermidine synthase | - | - | - | - | - | - |
| 1.11.1.11 | L-ascorbate peroxidase | - | - | - | - | - | - |
| 4.1.1.17 | Ornithine decarboxylase | - | - | - | - | - | - |
| 6.3.1.8 | Glutathionylspermidine synthase | - | Up | - | Up | - | - |
| 6.3.1.9 | Trypanothione synthetase | - | - | - | - | - | - |
| 2.5.1.22 | Spermine synthase | - | - | - | - | - | - |
| 1.17.4.1 | Ribonucleoside-diphosphate reductase subunit M1 | - | - | - | - | - | - |
| PepA | Leucyl aminopeptidase | - | - | - | - | - | - |
| PepB | PepB aminopeptidase | - | - | - | - | - | - |
| LAP3 | Cytosol aminopeptidase | - | - | - | - | - | - |
| PepD | Dipeptidase D | - | - | - | - | - | - |
| DUG1 | Cys-Gly metallodipeptidase DUG1 | - | - | - | - | - | - |
| TryS | Trypanothione synthetase | - | - | - | - | - | - |
| TryR | Trypanothione-disulfide reductase | - | - | - | - | - | - |
| TryP | Cytosolic tryparedoxin peroxidase, trypanosomatid typical 2-Cys peroxiredoxin | - | - | - | - | - | - |
| TDPX | Glutathione peroxidase-type tryparedoxin peroxidase | Up | - | - | - | - | Up |
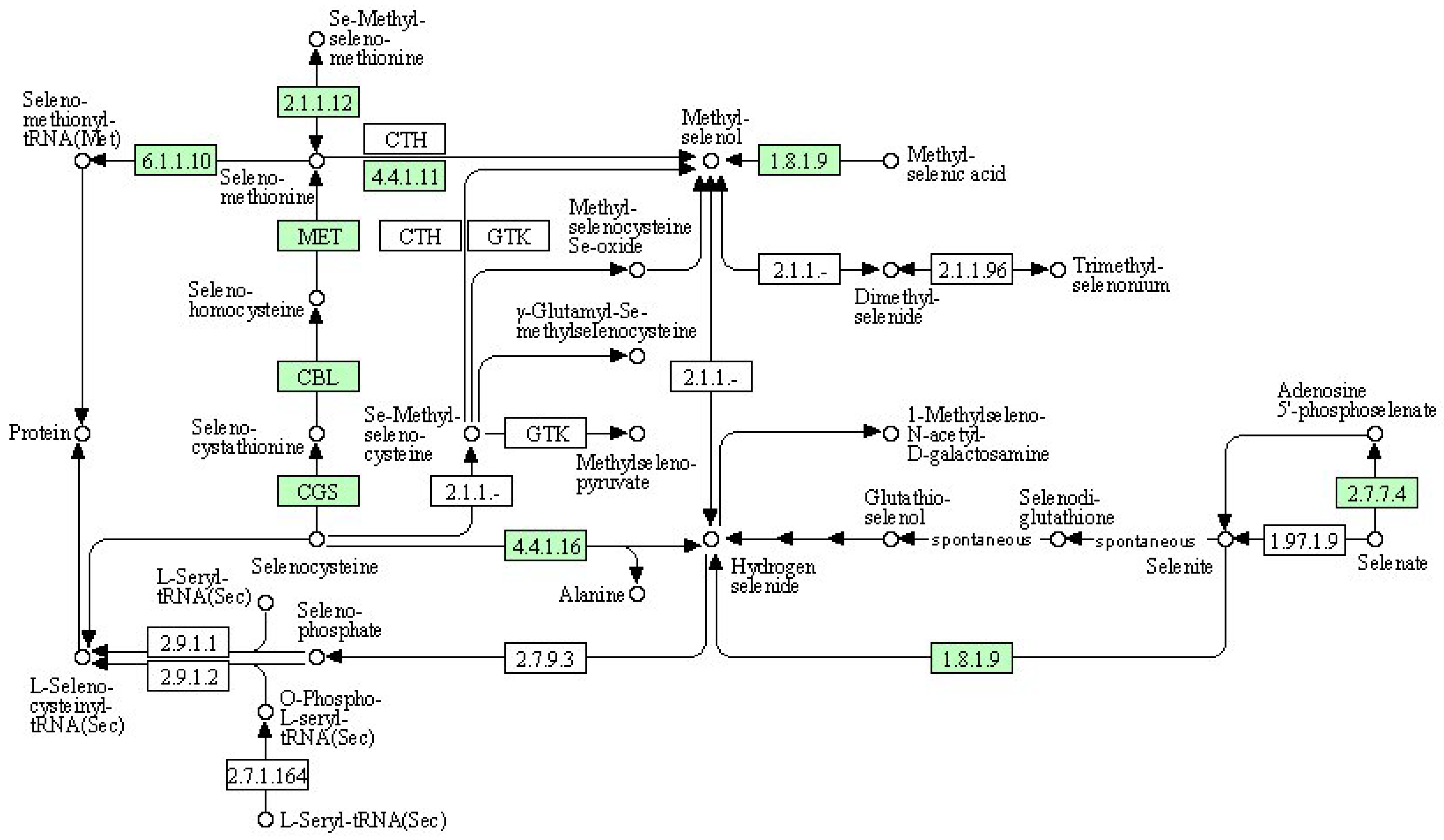
| Symbol | Description | DN5 vs. DCK | DN10 vs. DCK | DN20 vs. DCK | DO5 vs. DCK | DO10 vs. DCK | DO20 vs. DCK |
|---|---|---|---|---|---|---|---|
| 2.1.1.12 | Methionine S-methyltransferase | - | Up | - | - | - | - |
| 6.1.1.10 | Methionyl-tRNA synthetase | - | - | - | Up | Up | - |
| 4.4.1.11 | Methionine-gamma-lyase | - | - | - | - | - | Up |
| 1.8.19 | Thioredoxin reductase (NADPH) | Up | - | - | - | - | - |
| MET | 5-methyltetrahydrofolate–homocysteine methyltransferase | Up | Up | - | - | - | Up |
| CTH | Cystathionine gamma-lyase | Up | - | Up | Up | - | - |
| GTK | Cysteine-S-conjugate beta-lyase | - | - | - | - | - | - |
| 2.1.1.- | S-adenosyl-L-methionine:methaneselenol Se-methyltransferase | Up | - | - | - | - | - |
| 2.1.1.96 | Methyltransferase | - | - | - | - | - | Up |
| CBL | Cysteine-S-conjugate beta-lyase | - | - | - | - | - | - |
| CGS | Cystathionine gamma-synthase | - | - | - | - | - | - |
| 2.7.7.4 | 3′-phosphoadenosine 5′-phosphosulfate synthase | - | - | - | - | - | - |
| 4.4.1.16 | Selenocysteine lyase | - | - | - | - | - | - |
| 1.97.1.9 | Selenate/chlorate reductase subunit alpha | - | - | - | - | - | - |
| 2.9.1.1 | L-seryl-tRNA(Ser) seleniumtransferase | - | - | - | - | - | - |
| 2.9.1.2 | O-phospho-L-seryl-tRNASec:L-selenocysteinyl-tRNA synthase | - | - | - | - | - | - |
| 2.7.9.3 | Selenide, water dikinase | - | - | - | - | - | - |
| 2.7.1.164 | O-phosphoseryl-tRNA(Sec) kinase | - | - | - | - | - | - |
| Gene id | Gene Name | Function | kTotal | kWithin | kOut | kDiff | Module |
|---|---|---|---|---|---|---|---|
| Cluster-1111.26333 | LOC103837231 | Adenylate kinase 2 | 128.913278 | 53.85615 | 75.05713 | 21.201 | Salmon |
| Cluster-1111.33663 | LOC103858064 | Glutathione peroxidase 3 | 266.4526 | 169.3169 | 97.1357 | 72.18123 | Salmon |
| Cluster-1111.62702 | LOC103842805 | Glutathione S-transferase U25 | 788.70 | 573.26 | 215.44 | 357.81 | Magenta |
| Cluster-1111.32944 | LOC103830524 | Gglutathione S-transferase U20 | 216.4186 | 126.5464 | 89.87223 | 36.67415 | Salmon |
| Cluster-1111.94080 | LOC103844552 | Thiamine pyrophosphokinase 1 | 77.49846 | 14.04587 | 63.45259 | 49.4067 | Darkorange |
| Cluster-1111.21138 | LOC103872309 | ABC transporter G family member 35 | 470.7212 | 281.3282 | 189.393 | 91.93526 | Darkred |
| Cluster-1111.51912 | LOC103867761 | ABC transporter G family member 1 | 317.63 | 200.26 | 117.38 | 82.88 | Darkorange |
| Cluster-1111.47151 | LOC103844798 | ATP-dependent zinc metalloprotease FTSH 11 | 741.37 | 564.38 | 176.99 | 387.39 | Tan |
| Cluster-1111.43447 | LOC103866104 | Cysteine synthase | 83.49206 | 27.36962 | 56.12244 | 28.7528 | Brown4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Rao, C.; Huang, L.; Wu, J.; Sun, P.; Zhan, J.; Wu, J.; Liu, S.; Zhou, C.; Hu, L.; et al. Effects of Organic Selenium and Nanoselenium on Drought Stress of Pak Choi (Brassica chinensis var. pekinensis. cv. ‘Suzhouqing’) and Its Transcriptomic Analysis. Agronomy 2024, 14, 78. https://doi.org/10.3390/agronomy14010078
Wang Y, Rao C, Huang L, Wu J, Sun P, Zhan J, Wu J, Liu S, Zhou C, Hu L, et al. Effects of Organic Selenium and Nanoselenium on Drought Stress of Pak Choi (Brassica chinensis var. pekinensis. cv. ‘Suzhouqing’) and Its Transcriptomic Analysis. Agronomy. 2024; 14(1):78. https://doi.org/10.3390/agronomy14010078
Chicago/Turabian StyleWang, Yanyan, Chulin Rao, Liu Huang, Junda Wu, Peiheng Sun, Jianyun Zhan, Jianfu Wu, Shiyu Liu, Changming Zhou, Longsong Hu, and et al. 2024. "Effects of Organic Selenium and Nanoselenium on Drought Stress of Pak Choi (Brassica chinensis var. pekinensis. cv. ‘Suzhouqing’) and Its Transcriptomic Analysis" Agronomy 14, no. 1: 78. https://doi.org/10.3390/agronomy14010078
APA StyleWang, Y., Rao, C., Huang, L., Wu, J., Sun, P., Zhan, J., Wu, J., Liu, S., Zhou, C., Hu, L., Li, N., Chen, J., & He, X. (2024). Effects of Organic Selenium and Nanoselenium on Drought Stress of Pak Choi (Brassica chinensis var. pekinensis. cv. ‘Suzhouqing’) and Its Transcriptomic Analysis. Agronomy, 14(1), 78. https://doi.org/10.3390/agronomy14010078




