Influence of Different Agro-Food Waste on Ammonia and Greenhouse Gas Emissions during Composting
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Agro-Food Waste
2.3. Data Analysis
3. Results and Discussion
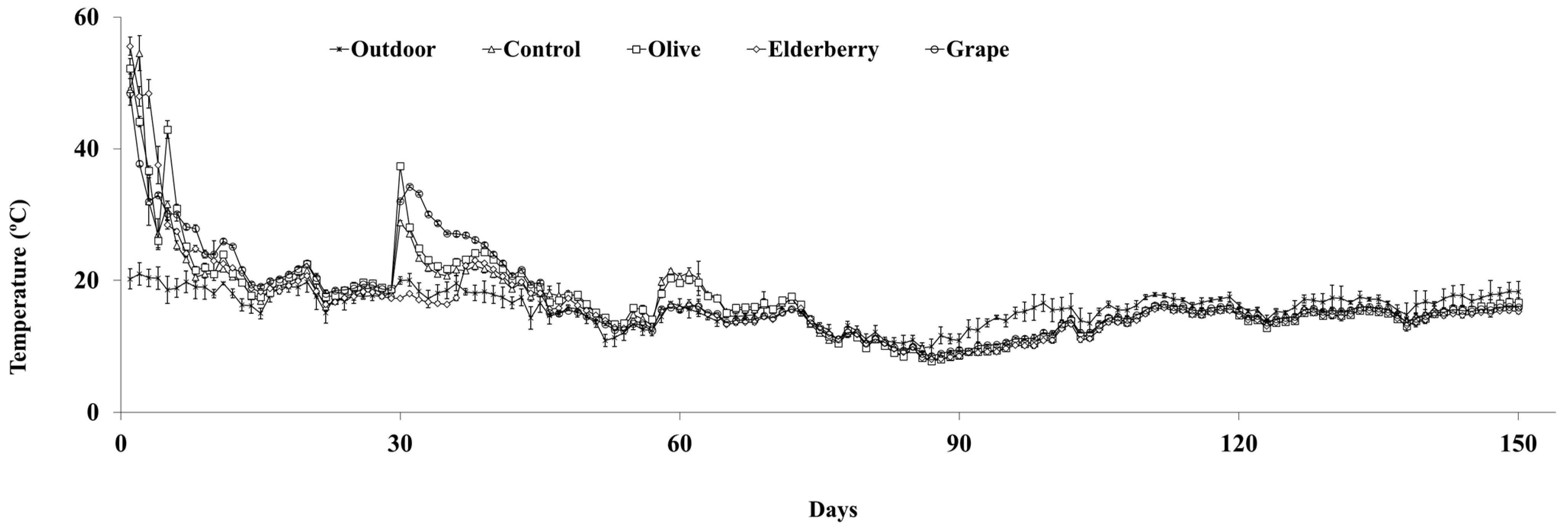
3.1. Temperature
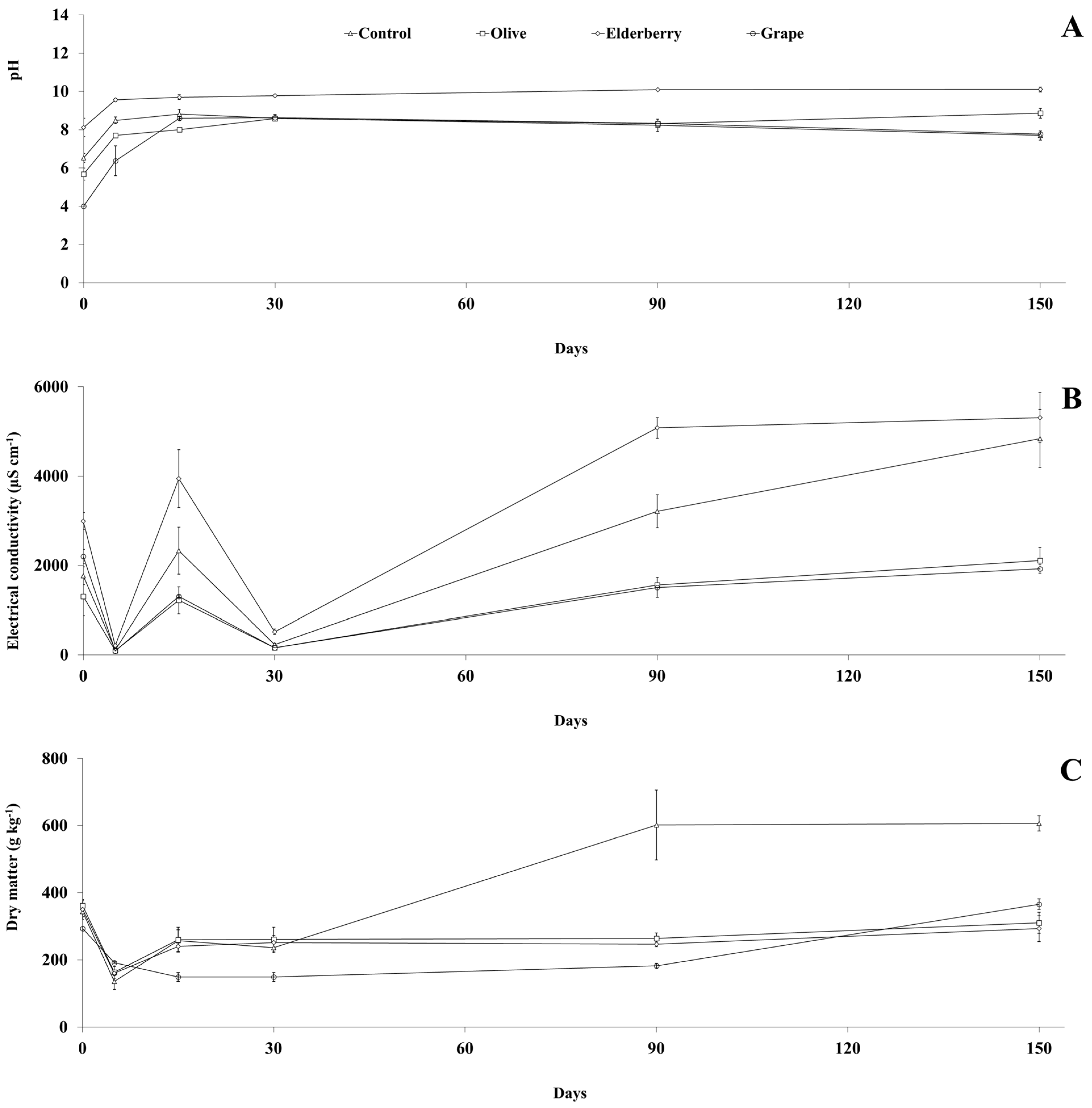
3.2. Physicochemical Parameters
3.3. Nitrogen Emissions
3.4. Carbon Emissions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czekała, W.; Janczak, D.; Pochwatka, P.; Nowak, M.; Dach, J. Gases Emissions during Composting Process of Agri-Food Industry Waste. Appl. Sci. 2022, 12, 9245. [Google Scholar] [CrossRef]
- Pinto, R.; Correia, C.; Mourão, I.; Moura, L.; Brito, L.M. Composting Waste from the White Wine Industry. Sustainability 2023, 15, 3454. [Google Scholar] [CrossRef]
- Santos, C.; Goufo, P.; Fonseca, J.; Pereira, J.L.S.; Ferreira, L.; Coutinho, J.; Trindade, H. Effect of lignocellulosic and phenolic compounds on ammonia, nitric oxide and greenhouse gas emissions during composting. J. Clean. Prod. 2018, 171, 548–556. [Google Scholar] [CrossRef]
- Santos, F.T.; Goufo, P.; Santos, C.; Botelho, D.; Fonseca, J.; Queirós, A.; Costa, M.S.S.M.; Trindade, H. Comparison of five agro-industrial waste-based composts as growing media for lettuce: Effect on yield, phenolic compounds and vitamin C. Food Chem. 2016, 209, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, N.; Jamuda, M.; Panda, A.K.; Samal, K.; Nayak, J.K. Emission of greenhouse gases (GHGs) during composting and vermicomposting: Measurement, mitigation, and perspectives. Energy Nexus 2022, 7, 100092. [Google Scholar] [CrossRef]
- Nordahl, S.L.; Preble, C.V.; Kirchstetter, T.W.; Scown, C.D. Greenhouse Gas and Air Pollutant Emissions from Composting. Environ. Sci. Technol. 2023, 57, 2235–2247. [Google Scholar] [CrossRef] [PubMed]
- IPCC. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Calvo Buendia, E., Tanabe, K., Kranjc, A., Baasansuren, J., Fukuda, M., Ngarize, S., Osako, A., Pyrozhenko, Y., Shermanau, P., Federici, S., Eds.; IPCC: Geneva, Switzerland, 2019; Available online: http://www.ipcc-nggip.iges.or.jp (accessed on 13 December 2023).
- Sayara, T.; Sánchez, A. Gaseous Emissions from the Composting Process: Controlling Parameters and Strategies of Mitigation. Processes 2021, 9, 1844. [Google Scholar] [CrossRef]
- Maeda, K.; Hanajima, D.; Morioka, R.; Toyoda, S.; Yoshida, N.; Osada, T. Mitigation of greenhouse gas emission from the cattle manure composting process by use of a bulking agent. Soil Sci. Plant Nutr. 2013, 59, 96–106. [Google Scholar] [CrossRef]
- Lim, S.L.; Lee, L.H.; Wu, T.Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: Recent overview, greenhouse gases emissions and economic analysis. J. Clean. Prod. 2016, 111, 262–278. [Google Scholar] [CrossRef]
- Santos, C.; Fonseca, J.; Aires, A.; Coutinho, J.; Trindade, H. Effect of different rates of spent coffee grounds (SCG) on composting process, gaseous emissions and quality of end-product. Waste Manag. 2017, 59, 37–47. [Google Scholar] [CrossRef]
- Pereira, J.L.S.; Perdigão, A.; Marques, F.; Wessel, D.F.; Trindade, H.; Fangueiro, D. Mitigating Ammonia and Greenhouse Gas Emissions from Stored Pig Slurry Using Chemical Additives and Biochars. Agronomy 2022, 12, 2744. [Google Scholar] [CrossRef]
- EN 13037; Soil Improvers and Growing Media—Determination of pH. European Committee for Standardization: Brussels, Belgium, 2011.
- EN 13038; Soil Improvers and Growing Media—Determination of Electrical Conductivity. European Committee for Standardization: Brussels, Belgium, 1999.
- EN 13040; Soil Improvers and Growing Media—Sample Preparation for Chemical and Physical Tests, Determination of Dry Matter Content, Moisture Content and Laboratory Compacted Bulk Density. European Committee for Standardization: Brussels, Belgium, 1999.
- EN 13654-1; Soil Improvers and Growing Media—Determination of Nitrogen—Part 1: Modified Kjeldahl Method. European Committee for Standardization: Brussels, Belgium, 2002.
- EN 13652; Soil Improvers and Growing Media—Extraction of Water Soluble Nutrients and Elements. European Committee for Standardization: Brussels, Belgium, 2002.
- ISO 19822; Fertilizers and Soil Conditioners—Determination of Humic and Hydrophobic Fulvic Acids Concentrations in Fertilizer Material. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 17126; Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Screening Test for Emergence of Lettuce Seedlings (Lactuca sativa L.). International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 16649-2; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 6579; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2002.
- Gavilanes-Terán, I.; Jara-Samaniego, J.; Idrovo-Novillo, J.; Bustamante, M.A.; Moral, R.; Paredes, C. Windrow composting as horticultural waste management strategy—A case study in Ecuador. Waste Manag. 2016, 48, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, X.; Wu, Y.; Wang, X.; Zhao, J.; Liu, Y.; Chen, Z.; Jiang, Z.; Tian, W.; Zhang, J. Effects of thermophiles inoculation on the efficiency and maturity of rice straw composting. Bioresour. Technol. 2022, 354, 127195. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, T.; Xing, Z.; Zhang, Q.; Niu, X.; Yu, Y.; Teng, Z.; Chen, J. Enhanced lignocellulose degradation and composts fertility of cattle manure and wheat straw composting by Bacillus inoculation. J. Environ. Chem. Eng. 2023, 11, 109940. [Google Scholar] [CrossRef]
- Yu, J.; Gu, J.; Wang, X.; Lei, L.; Guo, H.; Song, Z.; Sun, W. Exploring the mechanism associated with methane emissions during composting: Inoculation with lignocellulose-degrading microorganisms. J. Environ. Manag. 2023, 325, 116421. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Y.; Liu, H.; Xie, S.; Abbas, F. Impact of different nitrogen source on the compost quality and greenhouse gas emissions during composting of garden waste. Process Saf. Environ. Prot. 2019, 124, 326–335. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Kim, S.H.; Shim, J.; Park, S.J. Composting Process and Gas Emissions during Food Waste Composting under the Effect of Different Additives. Sustainability 2020, 12, 7811. [Google Scholar] [CrossRef]
- Zhu, P.; Shen, Y.; Pan, X.; Dong, B.; Zhou, J.; Zhang, W.; Li, X. Reducing odor emissions from feces aerobic composting: Additives. RSC Adv. 2021, 11, 15977–15988. [Google Scholar] [CrossRef]
- Zenjari, B.; El Hajjouji, H.; Baddi, G.A.; Bailly, J.R.; Revel, J.C.; Nejmeddine, A.; Hafidi, M. Eliminating toxic compounds by composting olive mill wastewater-straw mixtures. J. Hazard. Mater. 2006, 138, 433–437. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.; Sun, H.; Xu, Z. Analysis of humus formation and factors for driving the humification process during composting of different agricultural wastes. Front. Environ. Sci. 2022, 10, 954158. [Google Scholar] [CrossRef]
- Yuan, J.; Chadwick, D.; Zhang, D.; Li, G.; Chen, S.; Luo, W.; Du, L.; He, S.; Peng, S. Effects of aeration rate on maturity and gaseous emissions during sewage sludge composting. Waste Manag. 2016, 56, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.J.; Xue, W.T.; Guo, H.; Li, X.R.; Zuo, G.Y.; Zhao, T.K.; Dong, H.M. The Characteristics of Carbon, Nitrogen and Sulfur Transformation During Cattle Manure Composting-Based on Different Aeration Strategies. Int. J. Environ. Res. Public Health 2019, 16, 3930. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Monedero, M.A.; Serramiá, N.; Civantos, C.G.O.; Fernandez-Hernandez, A.; Roig, A. Greenhouse gas emissions during composting of two-phase olive mill wastes with different agroindustrial by-products. Chemosphere 2010, 81, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, X.; Zhang, X.; Misselbrook, T.; Bai, Z.; Ma, L. Nitrifier denitrification dominates nitrous oxide production in composting and can be inhibited by a bioelectrochemical nitrification inhibitor. Bioresour. Technol. 2021, 341, 125851. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Duan, P.; Liu, Q.; Wang, K.; Li, D. Addition of cellulose and hemicellulose degrading strains promotes nitrous oxide emission during composting. Bioresour. Technol. 2024, 393, 130100. [Google Scholar] [CrossRef]
- Wu, C.; Li, W.; Wang, K.; Li, Y. Usage of pumice as bulking agent in sewage sludge composting. Bioresour. Technol. 2015, 190, 516–521. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Lu, J.; Zhang, X.; Li, S.; Wu, Y.; Li, G. Effects of digestion time in anaerobic digestion on subsequent digestate composting. Bioresour. Technol. 2018, 267, 117–125. [Google Scholar] [CrossRef]
- Lleó, T.; Albacete, E.; Barrena, R.; Font, X.; Artola, A.; Sánchez, A. Home and vermicomposting as sustainable options for biowaste management. J. Clean. Prod. 2013, 47, 70–76. [Google Scholar] [CrossRef]
- Preble, C.V.; Chen, S.S.; Hotchi, T.; Sohn, M.D.; Maddalena, R.L.; Russell, M.L.; Brown, N.J.; Scown, C.D.; Kirchstetter, T.W. Air pollutant emission rates for dry anaerobic digestion and composting of organic municipal solid waste. Environ. Sci. Technol. 2020, 54, 16097–16107. [Google Scholar] [CrossRef]




| Parameters | pH | EC | DM | TC | TN | C/N | NH4+ | NO3− | THS | FA | GI | Coli | Salm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dS m−1 | g kg−1 | g kg−1 DM | g kg−1 DM | mg N kg−1 DM | mg N kg−1 DM | g kg−1 DM | g kg−1 DM | % | CFU mL−1 | ||||
| Raw materials | |||||||||||||
| Straw | 5.8 ± 0.1 a | 0.3 ± 0.1 b | 230.7 ± 6.4 b | 524.0 ± 3.9 b | 37.6 ± 0.9 a | 13.9 ± 0.2 a | 162 ± 17 c | 1 ± 1 c | |||||
| Olive wastes | 4.0 ± 0.1 b | 0.1 ± 0.1 c | 156.4 ± 1.2 c | 515.6 ± 0.7 b | 13.3 ± 1.0 b | 38.7 ± 3.1 a | 413 ± 59 b | 34 ± 1 a | |||||
| Elderberry wastes | 4.0 ± 0.1 b | 0.1 ± 0.1 c | 277.9 ± 22.9 b | 532.2 ± 3.7 ab | 19.9 ± 4.9 b | 26.7 ± 9.4 a | 846 ± 149 a | 24 ± 2 b | |||||
| Grape wastes | 5.8 ± 0.1 a | 1.3 ± 0.1 a | 417.2 ± 17.1 a | 549.8 ± 10.1 a | 16.1 ± 4.5 b | 34.2 ± 18.2 a | 562 ± 96 b | 1 ± 1 c | |||||
| Initial mixtures | |||||||||||||
| Control | 6.5 ± 0.2 b | 1.8 ± 0.2 bc | 343.9 ± 15.4 a | 494.9 ± 9.2 a | 22.9 ± 3.6 a | 24.1 ± 2.4 a | 2835 ± 114 a | 0.2 ± 0.1 a | |||||
| Olive | 5.7 ± 0.3 c | 1.3 ± 0.4 c | 361.4 ± 15.0 a | 496.9 ± 5.3 a | 17.5 ± 1.0 a | 28.5 ± 0.7 a | 1 ± 1 b | 0.1 ± 0.1 a | |||||
| Elderberry | 8.1 ± 0.5 a | 3.0 ± 0.2 a | 350.2 ± 29.9 a | 407.9 ± 15.6 b | 18.2 ± 3.2 a | 23.1 ± 3.0 a | 17 ± 2 b | 0.2 ± 0.1 a | |||||
| Grape | 4.0 ± 0.1 d | 2.2 ± 0.2 b | 293.1 ± 6.9 a | 486.4 ± 13.8 a | 17.8 ± 2.8 a | 26.1 ± 1.6 a | 837 ± 724 b | 0.1 ± 0.1 a | |||||
| Final composts | |||||||||||||
| Control | 7.7 ± 0.2 c | 4.9 ± 0.7 a | 606.3 ± 22.9 a | 414.8 ± 21.4 b | 38.0 ± 3.5 a | 10.9 ± 0.1 b | 453 ± 61 b | 0.1 ± 0.1 a | 186.8 ± 19.4 a | 110.7 ± 0.9 a | 136 ± 21 a | 1 ± 1 a | Absence |
| Olive | 8.9 ± 0.3 b | 2.1 ± 0.3 b | 311.0 ± 31.3 b | 502.3 ± 7.8 a | 37.9 ± 3.4 a | 13.3 ± 0.7 ab | 276 ± 47 b | 0.1 ± 0.1 a | 220.7 ± 10.4 a | 120.7 ± 3.1 a | 96 ± 13 a | 1 ± 1 a | Absence |
| Elderberry | 10.1 ± 0.1 a | 5.3 ± 0.6 a | 293.2 ± 38.7 b | 417.3 ± 7.5 b | 35.9 ± 7.8 a | 12.1 ± 1.7 b | 911 ± 157 a | 0.1 ± 0.1 a | 100.9 ± 11.2 b | 15.8 ± 2.1 b | 104 ± 3 a | 1 ± 1 a | Absence |
| Grape | 7.8 ± 0.2 c | 1.9 ± 0.1 b | 366.1 ± 15.5 b | 522.6 ± 7.8 a | 27.3 ± 8.7 a | 20.9 ± 4.0 a | 308 ± 135 b | 0.1 ± 0.1 a | 71.6 ± 6.9 b | 24.3 ± 2.7 b | 121 ± 6 a | 1 ± 1 a | Absence |
| Parameters | Control | Olive | Elderberry | Grape |
|---|---|---|---|---|
| NH3 (mg kg−1 initial DM) | 804 ± 13 a | 407 ± 9 c | 438 ± 6 c | 614 ± 11 b |
| N2O (mg kg−1 initial DM) | 122 ± 3 a | 66 ± 1 b | 141 ± 15 a | 93 ± 2 ab |
| CO2 (g kg−1 initial DM) | 186 ± 2 a | 127 ± 2 b | 169 ± 12 ab | 172 ±9 ab |
| CH4 (mg kg−1 initial DM) | 951 ± 1 a | 644 ± 6 b | 704 ± 1 b | 936 ± 22 a |
| GWP (g CO2-eq. kg−1 initial DM) | 245 ± 3 a | 162 ± 2 b | 226 ± 16 ab | 223 ± 10 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.L.S.; Costa, T.; Figueiredo, V.; Marques, F.; Perdigão, A.; Brás, I.; Silva, M.E.F.; Wessel, D.F. Influence of Different Agro-Food Waste on Ammonia and Greenhouse Gas Emissions during Composting. Agronomy 2024, 14, 220. https://doi.org/10.3390/agronomy14010220
Pereira JLS, Costa T, Figueiredo V, Marques F, Perdigão A, Brás I, Silva MEF, Wessel DF. Influence of Different Agro-Food Waste on Ammonia and Greenhouse Gas Emissions during Composting. Agronomy. 2024; 14(1):220. https://doi.org/10.3390/agronomy14010220
Chicago/Turabian StylePereira, José L. S., Telma Costa, Vitor Figueiredo, Francisco Marques, Adelaide Perdigão, Isabel Brás, Maria E. F. Silva, and Dulcineia F. Wessel. 2024. "Influence of Different Agro-Food Waste on Ammonia and Greenhouse Gas Emissions during Composting" Agronomy 14, no. 1: 220. https://doi.org/10.3390/agronomy14010220
APA StylePereira, J. L. S., Costa, T., Figueiredo, V., Marques, F., Perdigão, A., Brás, I., Silva, M. E. F., & Wessel, D. F. (2024). Influence of Different Agro-Food Waste on Ammonia and Greenhouse Gas Emissions during Composting. Agronomy, 14(1), 220. https://doi.org/10.3390/agronomy14010220








