Genomic Insights into Seed Germination Differences in Buffalobur (Solanum rostratum Dunal) under Contrasting GA and ABA Availability
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Seed Germination Assays
2.3. RNA Extraction and Quality Evaluation
2.4. Library Generation and RNA-Seq
2.5. Differentially Expressed Gene Analysis
2.6. Quantitative Real-Time PCR (qPCR)
2.7. EBM Extraction and Determination
2.8. Puncture Force Measurement
2.9. Statistical Analyses
3. Results
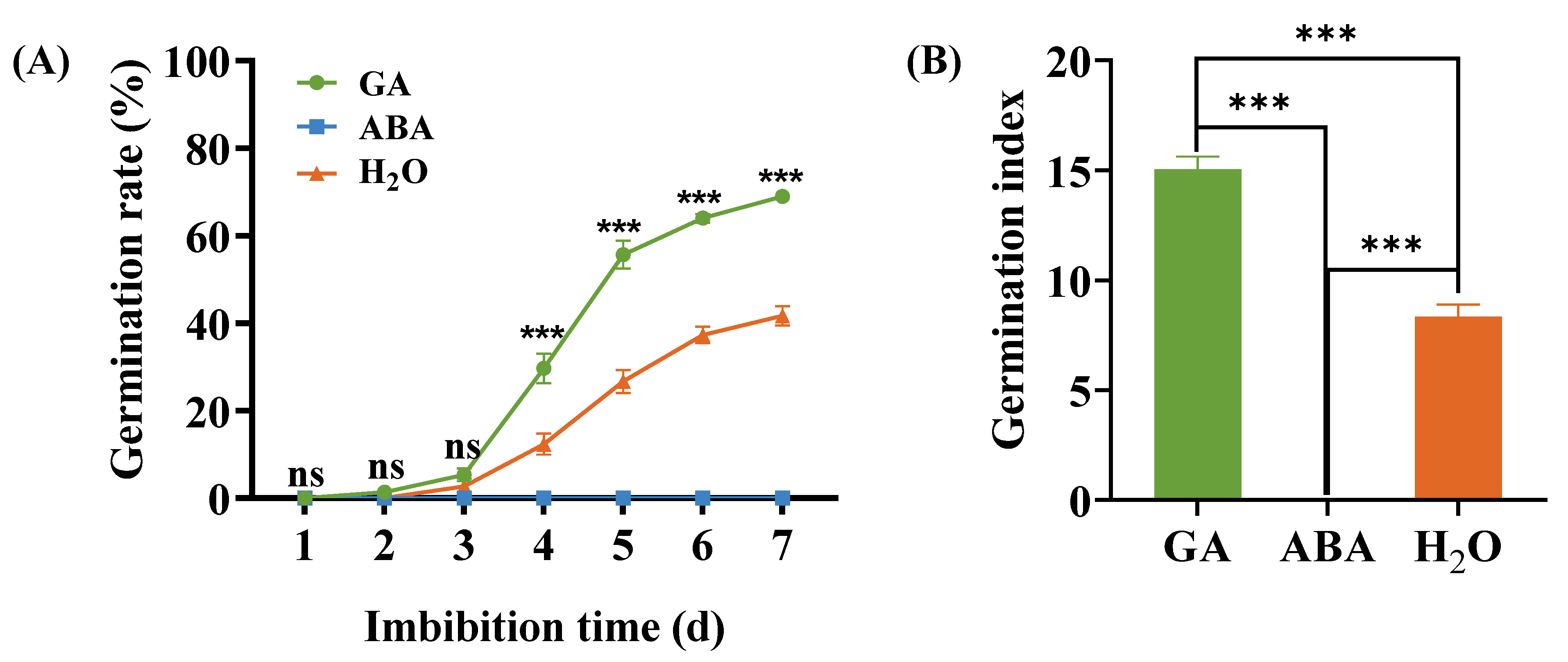
3.1. Buffalobur Seed Germination under GA and ABA Imbibition
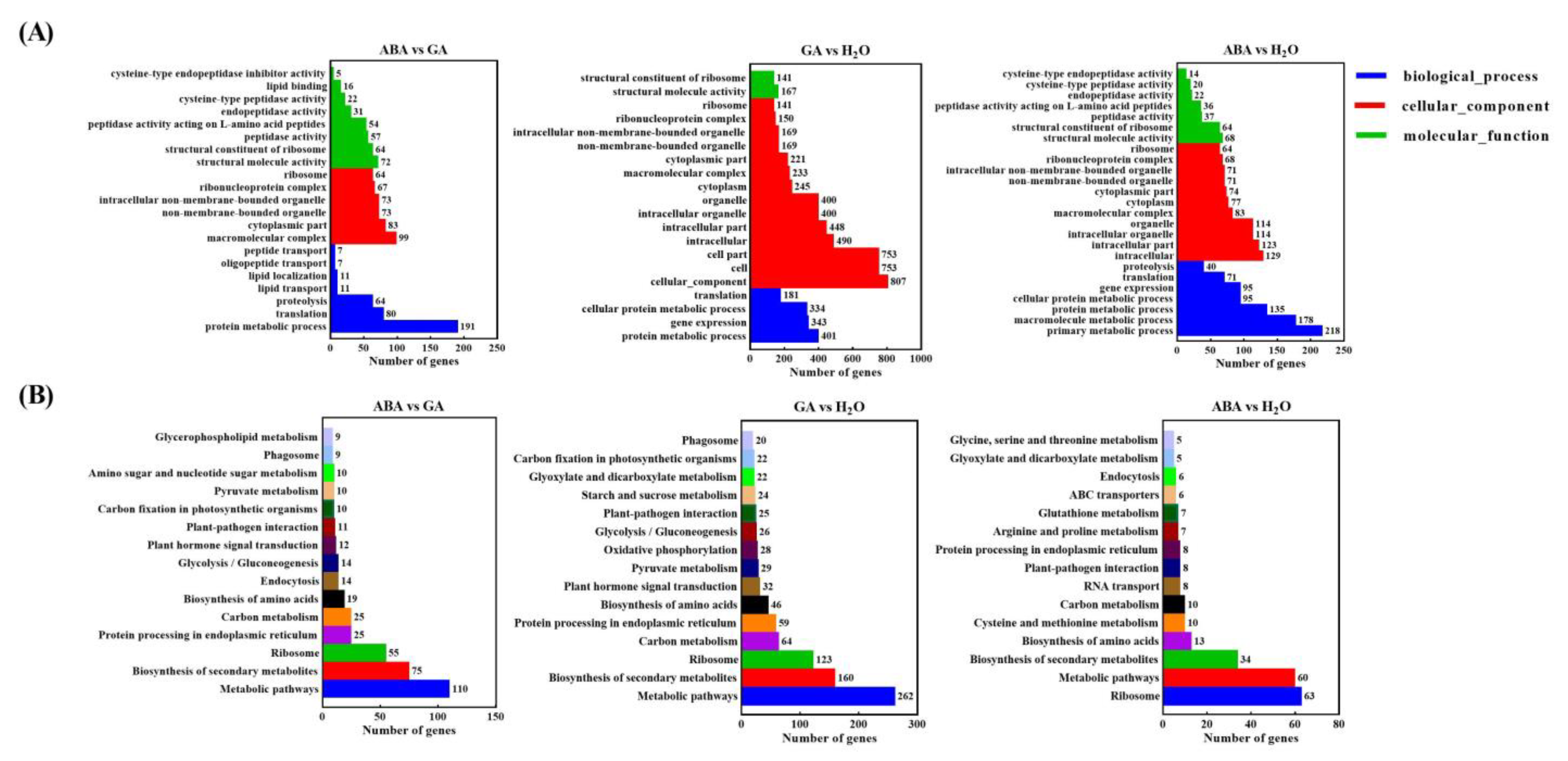
3.2. Unigenes and Functional Annotation
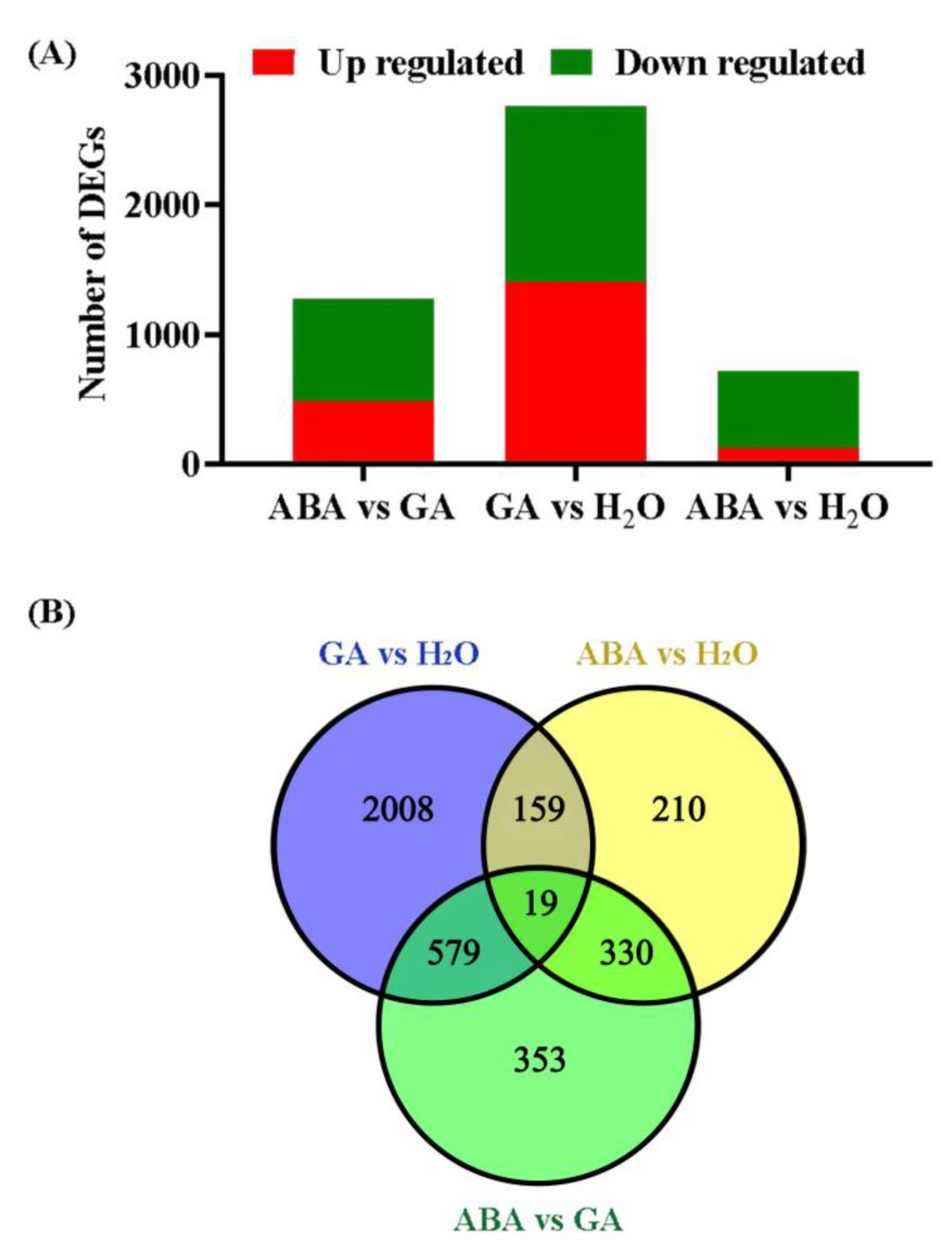
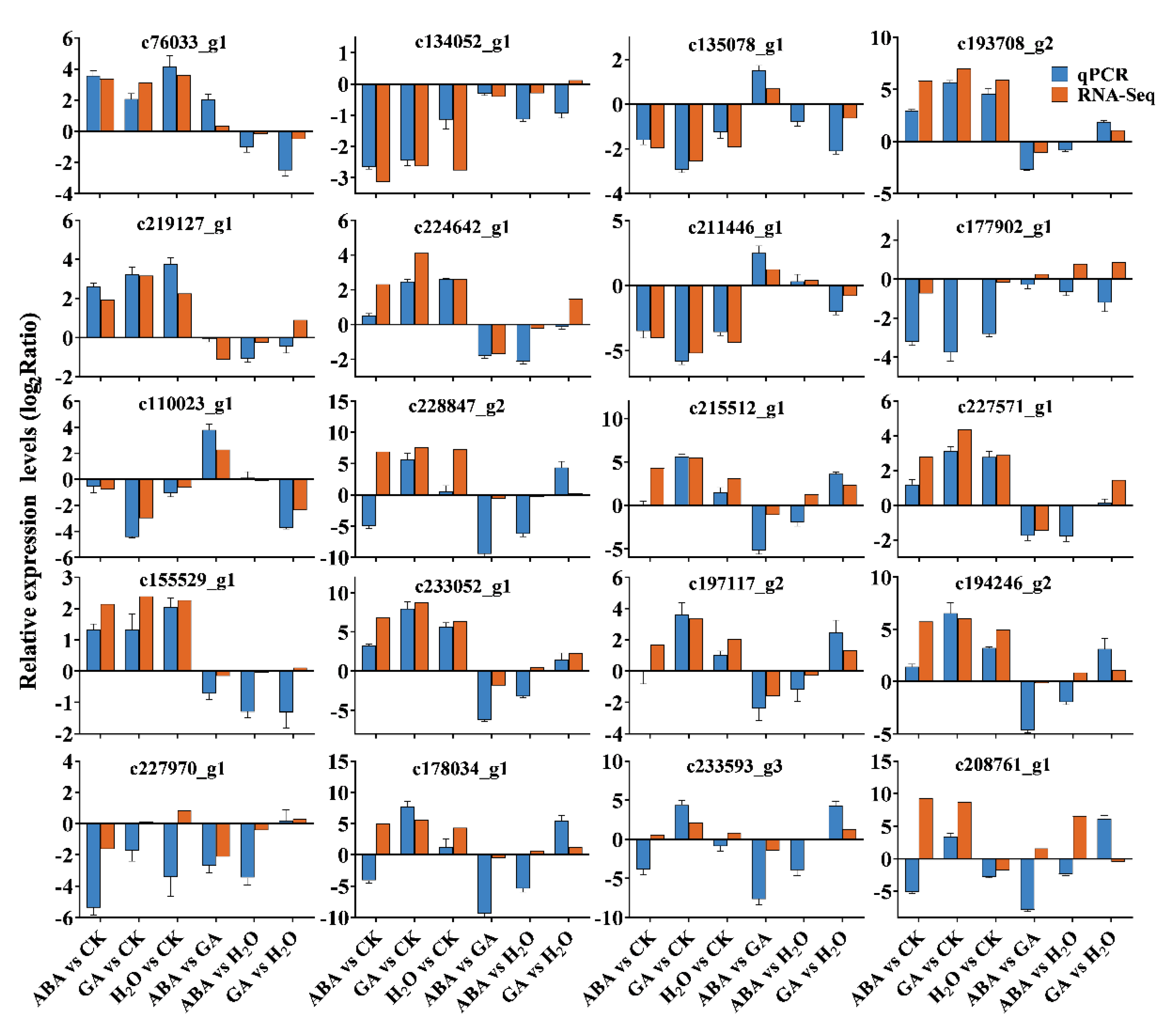
3.3. Identifying DEGs and qPCR Validations
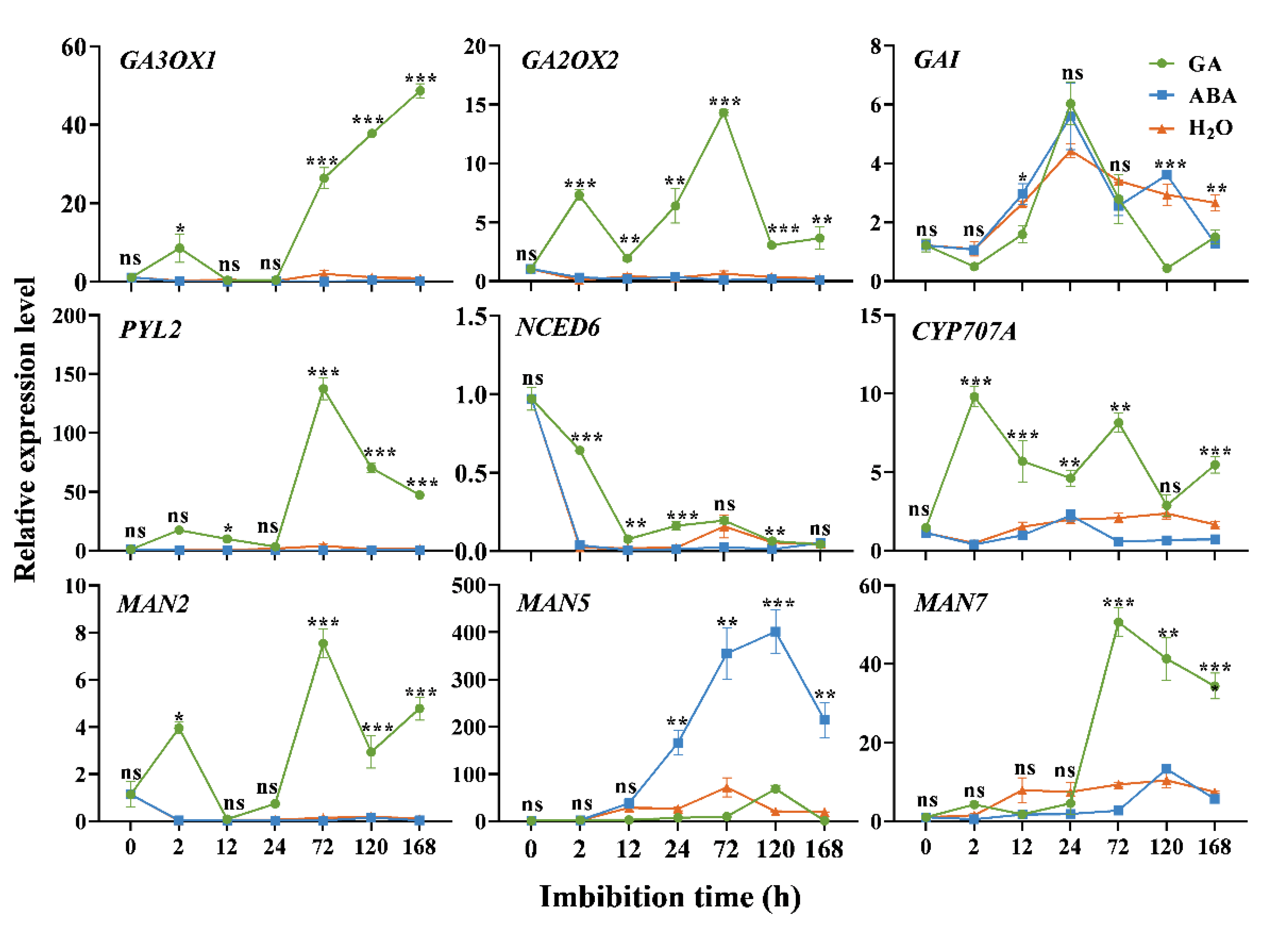
3.4. Expression Analysis of Germination-Related DEGs
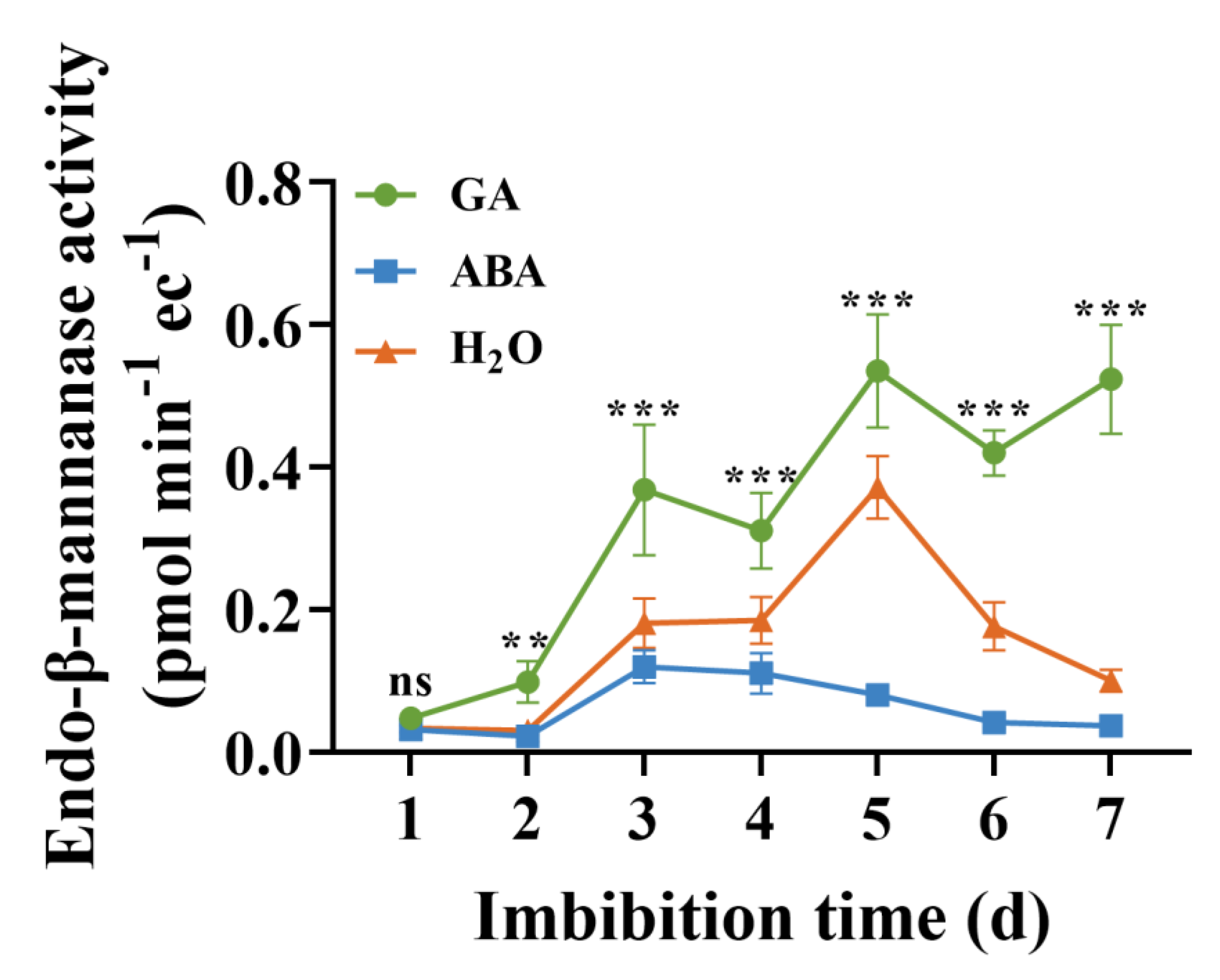
3.5. Activities of EBM
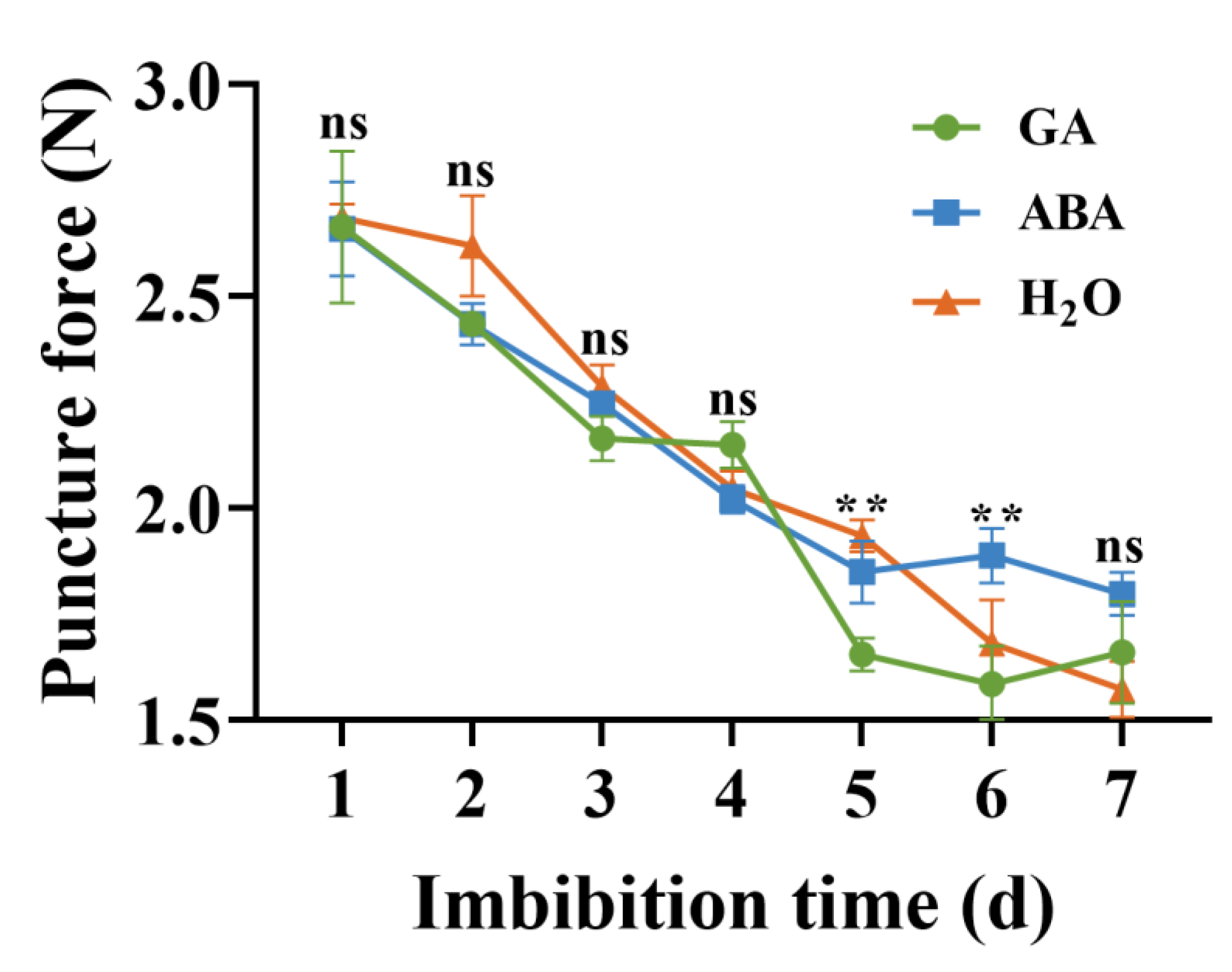
3.6. Puncture Force Measurements
4. Discussion
4.1. Response of Genes in GA Pathway during Seed Imbibition
4.2. Response of Genes in the ABA Pathway during Seed Imbibition
4.3. Response of MAN2, MAN5 and MAN7 during Seed Imbibition
4.4. Puncture Force and EBM Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, S.; Zhang, C.; Li, X.; Cui, H.; Huang, H.; Sui, B.; Meng, Q.; Zhang, H. Factors affecting buffalobur (Solanum rostratum) seed germination and seedling emergence. Weed Sci. 2009, 57, 521–525. [Google Scholar] [CrossRef]
- Mora-Carrera, E.; Castaneda-Zarate, M.; Fornoni, J.; Boege, K.; Dominguez, C.A. On the adaptive value of monomorphic versus dimorphic enantiostyly in Solanum rostratum. Ann. Bot. 2019, 123, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, M.; Tian, M.; Yuan, L.; Yu, B.; Qu, B.; An, T.; Feng, Y. Pyrrole alkaloids from Solanum rostratum and their chemical defense function against Henosepilachna vigintioctomaculata. Fitoterapia 2021, 155, 105031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, W.; Yuan, Z.; Song, Z.; Wang, Z.; Gao, J.; Fu, W.; Zhang, G. Chromosome-level genome assembly and annotation of the prickly nightshade Solanum rostratum Dunal. Sci. Data 2023, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hawthorne, D.; Qin, Y.; Pan, X.; Li, Z.; Zhu, S. Impact of climate and host availability on future distribution of Colorado potato beetle. Sci. Rep. 2017, 7, 4489. [Google Scholar] [CrossRef]
- Mayumi, V.; Lislie, S.; Mario, V.; Leonardo, D.A.; Jesús, F.G. Reproductive strategy of an invasive buzz-pollinated plant (Solanum rostratum). S. Afr. J. Bot. 2023, 162, 342–352. [Google Scholar] [CrossRef]
- Penfield, S. Seed dormancy and germination. Curr. Biol 2017, 27, R874–R878. [Google Scholar] [CrossRef] [PubMed]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef]
- Fujii, H.; Chinnusamy, V.; Rodrigues, A.; Rubio, S.; Antoni, R.; Park, S.-Y.; Cutler, S.R.; Sheen, J.; Rodriguez, P.L.; Zhu, J.-K. In vitro reconstitution of an abscisic acid signalling pathway. Nature 2009, 462, 660–664. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Wang, Y.; Liu, X.; Ma, L.; Zhang, Z.; Mu, C.; Zhang, Y.; Peng, L.; Xie, S.; et al. Initiation and amplification of SnRK2 activation in abscisic acid signaling. Nat. Commun. 2021, 12, 2456. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.; Bressan, R.A.; Song, C.; Zhu, J.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Ye, Y.; Zhou, L.; Liu, X.; Liu, H.; Li, D.; Cao, M.; Chen, H.; Xu, L.; Zhu, J.-K.; Zhao, Y. A novel chemical inhibitor of ABA signaling targets all ABA receptors. Plant Physiol. 2017, 173, 2356–2369. [Google Scholar] [CrossRef] [PubMed]
- Grappin, P.; Bouinot, D.; Sotta, B.; Miginiac, E.; Jullien, M. Control of seed dormancy in Nicotiana plumbaginifolia: Post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta 2000, 210, 279–285. [Google Scholar] [CrossRef]
- Thompson, A.J.; Jackson, A.C.; Symonds, R.C.; Mulholland, B.J.; Dadswell, A.R.; Blake, P.S.; Burbidge, A.; Taylor, I.B. Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J. 2000, 23, 363–374. [Google Scholar] [CrossRef]
- Martínez-Andújar, C.; Ordiz, M.I.; Huang, Z.; Nonogaki, M.; Beachy, R.N.; Nonogaki, H. Induction of 9-cis-epoxycarotenoid dioxygenase in Arabidopsis thaliana seeds enhances seed dormancy. Proc. Natl. Acad. Sci. USA 2011, 108, 17225–17229. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Abe, F.; Nonogaki, M.; Kanno, Y.; Seo, M.; Nonogaki, H.; Kawakami, N. Modulation of wheat grain dormancy by introducing the recombinant abscisic acid-stimulated abscisic acid biosynthesis gene. Plant Biotechnol. 2023, 40, 31–41. [Google Scholar] [CrossRef]
- Okamoto, M.; Kushiro, T.; Jikumaru, Y.; Abrams, S.R.; Kamiya, Y.; Seki, M.; Nambara, E. ABA 9’-hydroxylation is catalyzed by CYP707A in Arabidopsis. Phytochemistry 2011, 72, 717–722. [Google Scholar] [CrossRef]
- Lee, S.; Cheng, H.; King, K.E.; Wang, W.; He, Y.; Hussain, A.; Lo, J.; Harberd, N.P.; Peng, J. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 2002, 16, 646–658. [Google Scholar] [CrossRef]
- Li, C.; Dong, S.; Beckles, D.M.; Miao, H.; Sun, J.; Liu, X.; Wang, W.; Zhang, S.; Gu, X. The qLTG1.1 candidate gene CsGAI regulates low temperature seed germination in cucumber. Theor. Appl. Genet. 2022, 135, 2593–2607. [Google Scholar] [CrossRef]
- Hauvermale, A.L.; Ariizumi, T.; Steber, C.M. Gibberellin signaling: A theme and variations on DELLA repression. Plant Physiol. 2012, 160, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, M.; He, D.; Yang, P. Advances on post-translational modifications involved in seed germination. Front. Plant Sci. 2021, 12, 642979. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, J.; Nakata, M.; Ito, T.; Matsushita, A.; Yamaguchi, S.; Takahashi, Y. bZIP transcription factor RSG controls the feedback regulation of NtGA20ox1 via intracellular localization and epigenetic mechanism. Plant Signal. Behav. 2011, 6, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Guo, B.; Song, F.; Peng, H.; Yao, Y.; Zhang, Y.; Sun, Q.; Ni, Z. Genome-wide identification of gibberellins metabolic enzyme genes and expression profiling analysis during seed germination in maize. Gene 2011, 482, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, S.; Rizza, A.; Martone, J.; Circelli, P.; Costantino, P.; Vittorioso, P. The Dof protein DAG1 mediates PIL5 activity on seed germination by negatively regulating GA biosynthetic gene AtGA3ox1. Plant J. 2010, 61, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef]
- Hilhorst, H.W.M.; Groot, S.P.C.; Bino, R.J. The tomato seed as a model system to study seed development and germination. Acta Bot. Neerl. 1998, 47, 169–183. [Google Scholar]
- Pinto, L.V.; Da Silva, E.A.; Davide, A.C.; De Jesus, V.A.; Toorop, P.E.; Hilhorst, H.W. Mechanism and control of Solanum lycocarpum seed germination. Ann. Bot. 2007, 100, 1175–1187. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Chandrasekaran, U.; Zhao, X.; Luo, X.; Wei, S.; Shu, K. Endosperm weakening: The gateway to a seed’s new life. Plant Physiol. Biochem. 2022, 178, 31–39. [Google Scholar] [CrossRef]
- Toorop, P.E.; van Aelst, A.C.; Hilhorst, H.W. The second step of the biphasic endosperm cap weakening that mediates tomato (Lycopersicon esculentum) seed germination is under control of ABA. J. Exp. Bot. 2000, 51, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qu, H.; Zhang, H.; Liu, S.; Li, Y.; Zhang, C. Hormone and RNA-seq analyses reveal the mechanisms underlying differences in seed vigour at different maize ear positions. Plant Mol. Biol. 2019, 99, 461–476. [Google Scholar] [CrossRef]
- Burks, D.J.; Azad, R.K. RNA-Seq data analysis pipelinpe for plants: Transcriptome assembly, alignment, and differential expression analysis. Methods Mol. Biol. 2022, 2396, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Li, D.; Zand, M.S.; Dye, T.D.; Goniewicz, M.L.; Rahman, I.; Xie, Z. An evaluation of RNA-seq differential analysis methods. PLoS ONE 2022, 17, e264246. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Huang, H.; Wei, S.; Liu, Y.; Jiang, C.; Zhang, J.; Zhang, C. Selection of relatively exact reference genes for gene expression studies in goosegrass (Eleusine indica) under herbicide stress. Sci. Rep. 2017, 7, 46494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, X.; Chen, J.; Huang, Z.; Huo, H.; Jiang, C.; Huang, H.; Zhang, C.; Wei, S. Selection of reference genes for qPCR normalization in buffalobur (Solanum rostratum Dunal). Sci. Rep. 2019, 9, 6948. [Google Scholar] [CrossRef] [PubMed]
- Harshitha, R.; Arunraj, D.R. Real-time quantitative PCR: A tool for absolute and relative quantification. Biochem. Mol. Biol. Educ. 2021, 49, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.H.; Zhang, J.H.; Zhang, Y.Y.; Wang, T.T.; Chen, R.G.; Li, H.X.; Ye, Z.B. Isolation and expression of GA 2-oxidase2 in tomato. DNA Seq. 2007, 18, 474–479. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Zhao, Y.; Zhang, J.; Li, Z. Auxin and GA signaling play important roles in the maize response to phosphate deficiency. Plant Sci. 2019, 283, 177–188. [Google Scholar] [CrossRef]
- Ariizumi, T.; Hauvermale, A.L.; Nelson, S.K.; Hanada, A.; Yamaguchi, S.; Steber, C.M. Lifting della repression of Arabidopsis seed germination by nonproteolytic gibberellin signaling. Plant Physiol. 2013, 162, 2125–2139. [Google Scholar] [CrossRef]
- Wang, Y.; He, S.; Wei, Y.; Dong, C.; Liu, L.; Jue, D.; Shi, S.; Li, W. Molecular and functional characterization of two DELLA protein-coding genes in litchi. Gene 2020, 738, 144455. [Google Scholar] [CrossRef]
- Kanno, Y.; Jikumaru, Y.; Hanada, A.; Nambara, E.; Abrams, S.R.; Kamiya, Y.; Seo, M. Comprehensive hormone profiling in developing Arabidopsis seeds: Examination of the site of ABA biosynthesis, ABA transport and hormone interactions. Plant Cell Physiol. 2010, 51, 1988–2001. [Google Scholar] [CrossRef]
- Weng, J.K.; Ye, M.; Li, B.; Noel, J.P. Co-evolution of hormone metabolism and signaling networks expands plant adaptive plasticity. Cell 2016, 166, 881–893. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, F.; Zhu, D.; Chen, X.; Kong, X.; Wu, Y.; Chen, M.; Du, J.; Qu, L.J.; Wu, Z. Progressive chromatin silencing of ABA biosynthesis genes permits seed germination in Arabidopsis. Plant Cell 2022, 34, 2871–2891. [Google Scholar] [CrossRef]
- Carrillo-Barral, N.; Matilla, A.J.; Rodríguez-Gacio, M.D.C.; Iglesias-Fernández, R. Mannans and endo-β-mannanase transcripts are located in different seed compartments during Brassicaceae germination. Planta 2018, 247, 649–661. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Iglesias-Fernández, R.; Matilla, A.J. Genes involved in ethylene and gibberellins metabolism are required for endosperm-limited germination of Sisymbrium officinale L. seeds. Planta 2010, 231, 653–664. [Google Scholar] [CrossRef]
- Yu, L.L.; Xu, F. MAN5, a glycosyl hydrolase superfamily protein, is a key factor involved in cyanide-promoted seed germination in Arabidopsis thaliana. Genes 2023, 14, 1361. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Mirwais, M.Q. Environmental regulation of weed seed dormancy and germination. Seeds 2023, 2, 259–277. [Google Scholar] [CrossRef]
| Statistic Value | |
|---|---|
| Total clean reads | 23,047,819–26,014,113 |
| Unigenes | 336,057 |
| Min sequence length | 201 |
| Median sequence length | 336 |
| Max sequence length | 16,961 |
| N50 length | 818 |
| N90 length | 248 |
| Annotated in NR | 159,827 (47.56%) |
| Annotated in NT | 122,783 (36.54%) |
| Annotated in KEGG | 63,579 (18.92%) |
| Annotated in SwissProt | 97,319 (28.96%) |
| Annotated in Pfam | 126,338 (37.59%) |
| Annotated in GO | 101,187 (30.11%) |
| Annotated in COG/KOG | 75,203 (22.38%) |
| Gene ID | Gene Name | Gene Symbol | KEGG Annotation | ||
|---|---|---|---|---|---|
| Pathway ID | KO ID | KO Description | |||
| c155529_g1 | GA-insenstive | GAI | - | - | |
| c228847_g2 | gibberellin 3-beta-dioxygenase 1 | GA3OX1 | ko00904 | K04124 | gibberellin 3-beta-dioxygenase |
| c233593_g3 | gibberellin 2-oxidase 2 | GA2OX2 | ko00904 | K04125 | gibberellin 2-oxidase |
| c178034_g1 | pyrabactin resistance-like 2 | PYL2 | ko04075 | K14496 | abscisic acid receptor PYR/PYL family |
| c227970_g1 | 9-cis-epoxycarotenoid dioxygenase 6 | NCED6 | ko00906 | K09840 | 9-cis-epoxycarotenoid dioxygenase |
| c197117_g2 | abscisic acid 8′-hydroxylase | CYP707A | ko00906 | K09843 | (+)-abscisic acid 8′-hydroxylase |
| c208761_g1 | β-mannanase 2 | MAN2 | - | - | - |
| c233052_g1 | β-mannanase 5 | MAN5 | - | - | - |
| c194246_g2 | β-mannanase 7 | MAN7 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Li, L.; Wu, K.; Zhao, D.; Yang, L.; Huang, H.; Huang, Z.; Wei, S. Genomic Insights into Seed Germination Differences in Buffalobur (Solanum rostratum Dunal) under Contrasting GA and ABA Availability. Agronomy 2024, 14, 212. https://doi.org/10.3390/agronomy14010212
Chen Z, Li L, Wu K, Zhao D, Yang L, Huang H, Huang Z, Wei S. Genomic Insights into Seed Germination Differences in Buffalobur (Solanum rostratum Dunal) under Contrasting GA and ABA Availability. Agronomy. 2024; 14(1):212. https://doi.org/10.3390/agronomy14010212
Chicago/Turabian StyleChen, Zhaoxia, Longlong Li, Kaidie Wu, Dandan Zhao, Long Yang, Hongjuan Huang, Zhaofeng Huang, and Shouhui Wei. 2024. "Genomic Insights into Seed Germination Differences in Buffalobur (Solanum rostratum Dunal) under Contrasting GA and ABA Availability" Agronomy 14, no. 1: 212. https://doi.org/10.3390/agronomy14010212
APA StyleChen, Z., Li, L., Wu, K., Zhao, D., Yang, L., Huang, H., Huang, Z., & Wei, S. (2024). Genomic Insights into Seed Germination Differences in Buffalobur (Solanum rostratum Dunal) under Contrasting GA and ABA Availability. Agronomy, 14(1), 212. https://doi.org/10.3390/agronomy14010212






